Abstract
Interleukin-17A (IL-17A)-producing CD4+ T helper (Th17) cells have been shown to be essential for defense against pulmonary infection with Coccidioides species. However, we have just begun to identify the required pattern recognition receptors and understand the signal pathways that lead to Th17 cell activation after fungal infection. We previously reported that Card9−/− mice vaccinated with formalin-killed spherules failed to acquire resistance to Coccidioides infection. Here, we report that both MyD88−/− and Card9−/− mice immunized with a live, attenuated vaccine also fail to acquire protective immunity to this respiratory disease. Like Card9−/− mice, vaccinated MyD88−/− mice revealed a significant reduction in numbers of both Th17 and Th1 cells in their lungs after Coccidioides infection. Both Toll-like receptor 2 (TLR2) and IL-1 receptor type 1 (IL-1r1) upstream of MyD88 have been implicated in Th17 cell differentiation. Surprisingly, vaccinated TLR2−/− and wild-type (WT) mice showed similar outcomes after pulmonary infection with Coccidioides, while vaccinated IL-1r1−/− mice revealed a significant reduction in the number of Th17 cells in their infected lungs compared to WT mice. Thus, activation of both IL-1r1/MyD88- and Card9-mediated Th17 immunity is essential for protection against Coccidioides infection. Our data also reveal that the numbers of Th17 cells were reduced in IL-1r1−/− mice to a lesser extent than in MyD88−/− mice, raising the possibility that other TLRs are involved in MyD88-dependent Th17 immunity to coccidioidomycosis. An antimicrobial action of Th17 cells is to promote early recruitment of neutrophils to infection sites. Our data revealed that neutrophils are required for vaccine immunity to this respiratory disease.
INTRODUCTION
Coccidioides species are etiological agents of coccidioidomycosis, which is also known as San Joaquin Valley fever, a potentially life-threatening respiratory mycosis that is endemic to the southwestern United States and arid regions of Mexico and Central and South America (1). The incidence of symptomatic coccidioidomycosis in the United States increased from 2,265 reported cases in 1998 to 22,401 cases in 2011, based on data from the National Notifiable Diseases Surveillance System (NNDSS) (2). This database likely underestimates the actual burden of disease, since reporting cases of coccidioidomycosis is not mandated in every state within the known regions of endemicity. A vaccine against coccidioidomycosis would promote the well-being of at-risk populations in the United States, in addition to people who reside in arid areas of Latin America (3). Both clinical data and results of experimental animal studies have shown that T-cell immunity is essential for protection against coccidioidomycosis, and mammalian hosts with a deficiency of CD4+ T cells are at elevated risk of contracting this respiratory disease (3). Many studies of coccidioidomycosis have reported gamma interferon (IFN-γ) production as a correlate of vaccine-induced protection in mice (4, 5). Although there is controversy about the beneficial or harmful roles of interleukin-17 (IL-17) and Th17 cells in fungal infections, we have shown that murine IL-17 receptors and Th17 cells are essential for vaccine immunity to Coccidioides infection (6, 7).
Antifungal responses are initiated through host recognition of fungal pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) in innate immune cells. Host recognition of fungal invasion triggers cascades of signaling events to activate secretion of proinflammatory cytokines and induction of T-cell differentiation. Among these signaling molecules, caspase adaptor recruitment domain family member 9 (Card9) and myeloid differentiation factor 88 (MyD88) are two cytosolic adaptors that transduce signals from C-type lectin receptors (CLRs) and the Toll/IL-1 receptor (TIR) superfamily, respectively (8–10). In humans, deep dermatophytosis appears to be an important clinical manifestation of Card9 mutations (11). A mutation in Card9 results in significantly reduced numbers of Th17 cells in patients with chronic mucocutaneous candidiasis (12). In mice, Card9 has been reported to be essential for the development of Th17 cells in response to primary Candida albicans infection (13). Card9−/− mice are unable to control a subcutaneous (s.c.) injection of the live, attenuated vaccine strain of Blastomyces dermatitidis and succumb to widespread disseminated disease (14). To circumvent the potential susceptibility of Card9−/− mice to live, attenuated vaccine strains of the evaluated fungal pathogens, we have immunized them with heat-killed yeast of the species Blastomyces dermatitidis and Histoplasma capsulatum or formalin-killed spherules (FKS) of Coccidoides posadasii. Our data revealed that the Card9 axes of the signal pathway are required for the development of antigen-specific Th17 cells for these three dimorphic fungal pathogens (14).
Coccidioides spp. are diphasic fungi characterized by a parasitic cycle that is unique among the medically important fungi (15). The saprobic phase of Coccidioides grows as a filamentous (hyphal) form and produces small, dry spores (arthroconidia) that are released into the air upon disturbance of the soil. In vivo, the spores grow isotropically and develop into large, multinucleate parasitic cells (spherules; >80 μm in diameter). The latter undergo a process of segmentation of their cytoplasm, followed by differentiation of a multitude of endospores (2 to 10 μm in diameter) that are released when they enlarge and cause the spherule wall to rupture. The FKS vaccine is made of a formalin-killed mixture of spherules and endospores. Immunization of susceptible mice with the FKS vaccine has been reported to protect against a potentially lethal respiratory challenge with this pathogen (14, 16). However, we have observed an unacceptable level of inflammatory response at the injection site in mice following subcutaneous vaccination of an optimal, protective dose (3.0 mg) of the FKS vaccine (16). A genetically defined, live attenuated strain of C. posadasii (ΔT) has been generated that lost its ability to endosporulate in vivo but is able to elicit protective immunity to coccidioidomycosis in disease-susceptible mice (16). In contrast to FKS, this live vaccine shows only a minimal inflammatory response at sites of immunization in the C57BL/6 mouse model. In the present study, we investigated whether immunocompromised animals, including Card9−/−, MyD88−/−, TLR2−/−, and IL-1r1−/− mice, could control subcutaneous inoculation of the live, attenuated ΔT strain.
Dependency of MyD88 in antifungal immunity is inconsistent in murine models of fungal diseases. MyD88 knockout mice are not more susceptible to invasive aspergillosis and blood-borne disseminated paracoccidioidomycosis than are wild-type mice (17, 18). In contrast, MyD88 is essential for adaptive and innate immunity to pulmonary Blastomyces, Paracoccidioides, and Pneumocystis infections (7, 9, 19). The best-characterized PRRs upstream of MyD88 are Toll-like receptors (TLRs) that have leucine-rich repeats and share a cytoplasmic TIR domain with IL-1, IL-18, and IL-33 receptors (20). TLR2, but not TLR4, has been shown to be involved in innate recognition of Coccidioides spherules by peritoneal macrophages (21). Both TLR2 and IL-1 receptor type 1 (IL-1r1) are implicated in differentiation of Th17 cells from naive T cells (22, 23). To our knowledge, the dependency of MyD88 and its upstream TLR2 and IL-1 receptor in vaccine immunity to Coccidioides infection has not been investigated. In this study, we compared the relative contributions of MyD88 and Card9 in activation of vaccine immunity to pulmonary Coccidioides infection. We also investigated the roles of upstream TLR2 and IL-1 receptor in activation of MyD88-dependent Th responses to Coccidioides infection.
MATERIALS AND METHODS
Fungal strains, growth conditions, and spore preparation.
The virulent fungal strain used to challenge mice in this study was a clinical isolate of C. posadasii (C735). A previously described, genetically engineered mutant (Δcts2 Δard1 Δcts3) derived from this parental strain (16) was employed as a live, attenuated vaccine and designated ΔT. Both strains were cultured on glucose-yeast extract (GYE) growth medium (1% glucose, 0.5% yeast extract, 1.5% agar) for 3 to 4 weeks at 30°C to generate a confluent layer of spores on the agar surface. Spores were harvested and suspended in phosphate-buffered saline as previously reported (6). All culturing and preparatory procedures that involved live cells of C. posadasii were conducted in a biological safety level 3 (BSL3) laboratory.
Mouse strains.
Breeding pairs of inbred stains of mice on a C57BL/6 genetic background were obtained from Jackson Laboratory unless otherwise stated. The strains included C57BL/6 (stock number 000664), B6.129P2(SJL)-Myd88tm1.1Defr/J (MyD88−/− mice; stock number 009088), and B6.129S7-IL1r1tm1Imx/J (IL-1r1−/− mice; stock number 003245). Breeding pairs of Card9−/− mice were provided by Xin Lin at the MD Anderson Cancer Center. Breeding pairs of TLR2−/− mice were a gift from Bernard Arulanandam at the University of Texas at San Antonio (UTSA). Mice were housed in a specific-pathogen-free (SPF) animal facility at UTSA and handled according to guidelines approved by the University Institutional Animal Care and Use Committee. Mice were relocated prior to vaccination and infection to an animal biosafety level 3 (ABSL3) laboratory.
Vaccination protocol and evaluation of protection.
All strains of mice were gender matched and were 8 to 12 weeks old when used in this study. Primary immunization of C57BL/6 and selected knockout mice with FKS or spores isolated from the live, attenuated strain (ΔT) was performed in the abdominal region by the s.c. route as reported previously (16). The vaccination doses were 106 FKS and 5 × 104 CFU of the ΔT vaccine in 100 μl phosphate-buffered saline (PBS). This initial immunization step was followed 14 days later with a boost of 106 FKS or 2.5 × 104 CFU of the ΔT vaccine. Control mice were immunized with PBS following the same vaccination protocol as above. Mice were challenged 4 weeks after completion of the vaccination protocol by intranasal (i.n.) instillation with approximately 80 viable spores of the virulent isolate of C. posadasii (C735) suspended in 35 μl PBS as previously reported (16). Fungal burden in the lungs and spleen was determined at 14 days postchallenge (dpc) by plating serial dilutions of separate lung and spleen homogenates on GYE agar containing 50 μg/ml chloramphenicol or 75 μg/ml hygromycin as reported elsewhere (16). The ΔT vaccine strain carrying a resistance marker to hygromycin can grow on GYE agar containing this antibiotic. The number of CFU of Coccidioides was expressed on a log scale and reported for individual mice of each group of 10 to 12 animals as previously described (16). Survival studies of vaccinated versus nonvaccinated mice were conducted over 60 days postchallenge as previously reported (16).
Intracellular cytokine staining.
Pulmonary leukocytes were isolated from lungs of both vaccinated and nonvaccinated mice at 8 and 12 days postchallenge as previously reported (6, 24). Aliquots of pulmonary leukocytes were stimulated with anti-CD3 and -CD28 in the presence of GolgiStop in 10% fetal bovine serum-complemented RPMI 1640 for 4 h at 37°C. Permeabilized cells were stained with selected fluorochrome-conjugated monoclonal antibodies (MAbs) specific for CD4, CD8, IFN-γ, or IL-17A to determine absolute numbers of the specific cytokine-producing CD4+ T cells, as previously described (6, 25). The leukocytes were gated for CD4+ CD8− T cells, and their levels of cytokine expression were determined. The absolute numbers of the specific cytokine-producing CD4+ T cells relative to the total lung-infiltrated leukocytes per lung homogenate at 8 and 12 days postchallenge were calculated by multiplying the percentage of each gated population by the total number of viable pulmonary leukocytes determined by hemocytometer counts as previously reported (6).
Depletion of neutrophils.
The monoclonal antibody against Ly6G (clone 1A8) was obtained from Bio-X-Cell (West Lebanon, NH). This rat immunoglobulin G2a (IgG2a) reacts with the Ly6G antigen expressed by murine neutrophils but not with other cell populations (26). Two groups of vaccinated and nonvaccinated C57BL/6 mice were injected intraperitoneally (i.p.) with 200 μg MAb 24 h before i.n. challenge with C. posadasii spores. This treatment was repeated every 2 days after infection until mice were sacrificed at 12 days postchallenge. Each mouse received a total of 7 injections. Control mice received equivalent amounts of normal rat IgG (Sigma Chemical Co.). The efficacy of neutrophil depletion in lungs was monitored with monoclonal antibodies against CD11b and LFA1 in a flow cytometry assay as previously described (27).
Statistical analyses.
The Student-Newman-Keuls test, a type of analysis of variance statistical test for all pairwise comparisons, was used to analyze percentages and numbers of specific cytokine-producing T cells in lungs of mice, as previously reported (25, 28). The Mann-Whitney U test was used to compare differences between the fungal burden of nonvaccinated and vaccinated mice measured as CFU, as reported previously (16). Survival data were examined by using the Kaplan-Meier test via log rank analysis to compare survival plots, as reported previously (16). A P value of <0.05 was considered statistically significant.
RESULTS
Vaccine immunity to Coccidioides infection requires both MyD88 and Card9 adaptors.
We previously reported that Th17 cells are essential for vaccine-induced protection against Coccidioides infection (6, 7). Here, we compared the relative contributions of MyD88 and Card9 in activation of vaccine immunity to pulmonary infection with Coccidioides. First, we tested whether the live, attenuated ΔT vaccine could be used to protect MyD88−/− and Card9−/− mice. Vaccination with live ΔT spores elicited a moderate inflammatory response in WT C57BL/6 and MyD88−/− mice, as revealed by slight swelling at sites of immunization and a lower concentration of neutrophils observed in paraffin sections of skin biopsies, while the ΔT-vaccinated Card9−/− mice developed severe inflammation at the injection sites, comparable to those caused by the FKS vaccine (16). We examined cultured homogenates of skin biopsies, lungs, and spleen from the mice that were immunized with a total of 7.5 × 104 spores of the ΔT strain at 4 weeks after the last vaccination. The results indicated viability of the vaccine strain at sites of immunization in the skin of mice but an absence of the hygromycin-resistant ΔT strain in the lungs and spleen. We detected a range of CFU (101.0 to 104.2) in skin biopsies of 70%, 50%, and 30% of the vaccinated Card9−/−, MyD88−/−, and wild-type mice, respectively, but none of these mice showed symptoms of coccidioidomycosis, loss of body mass, or reduced mobility. Thus, the live ΔT vaccine can be used to explore vaccine immunity to Coccidioides infection in these examined strains of knockout mice.
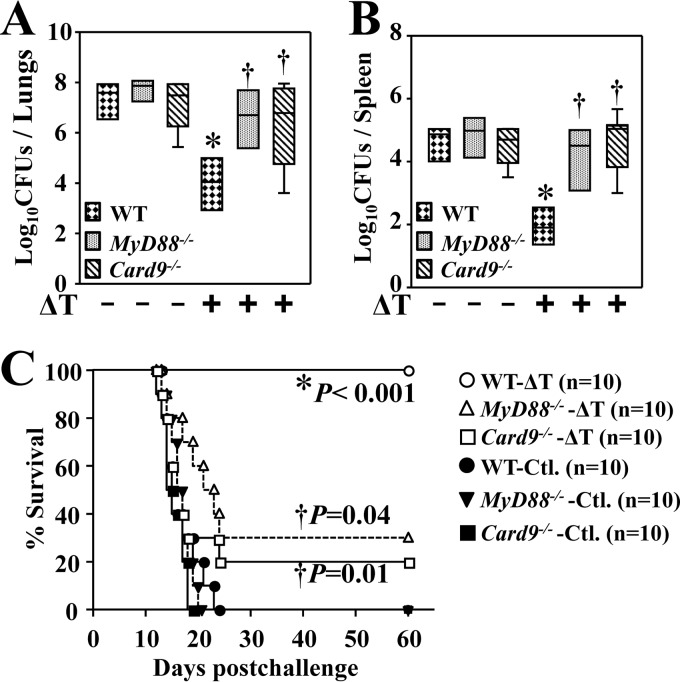
MyD88−/−, Card9−/−, and WT mice were vaccinated with the live ΔT vaccine and intranasally challenged with approximately 80 Coccidioides spores, a potentially lethal dose. The relative efficacy of protection against coccidioidomycosis was compared by measuring numbers of CFU in the lungs and spleen of these three strains of mice at 14 dpc. The mean CFU in lungs of nonvaccinated MyD88−/− and Card9−/− mice (7.7 ± 0.4 and 7.1 ± 1.1 log10, respectively [means ± standard errors of the means]) were comparable to those of wild-type mice (7.4 ± 0.8 log10) (Fig. 1A). Similarly, nonvaccinated MyD88−/− and Card9−/− mice did not show significant differences in CFU in their spleens (4.8 ± 0.6; 4.3 ± 1.2 log10) compared to reactions in nonvaccinated WT mice (4.6 ± 0.5 log10) (Fig. 1B). Both vaccinated MyD88−/− and Card9−/− mice had elevated numbers of CFU that were 2.5 logs higher than the response in vaccinated WT mice (Fig. 1A and B). Lung and spleen CFU of the ΔT-vaccinated Card9−/− mice were comparable to the results for FKS-vaccinated mice, as previously reported (14). Thus, Card9 is also required for vaccine immunity elicited by the live, attenuated vaccine. All three strains of nonvaccinated mice (controls) approached a moribund state at 12 to 24 dpc, whereas vaccinated WT mice survived for over 60 days (Fig. 1C), as previously reported (6, 16). In contrast, comparative studies revealed that the vaccinated MyD88−/− and Card9−/− mice showed 30 and 20% survival, respectively, and these rates were significantly reduced compared to those of vaccinated WT mice (P < 0.05) (Fig. 1C). These results indicated that both MyD88 and Card9 are essential for vaccine-induced resistance to Coccidioides infection.
FIG 1.
Both MyD88 and Card9 are required for vaccine-induced resistance to Coccidioides infection. The CFU of C. posadasii detected in dilution plate cultures of lung (A) and spleen (B) homogenates at 14 dpc for WT, MyD88−/−, and Card9−/− mice that were vaccinated with the ΔT vaccine (+) or injected with PBS (−) as controls are shown. All mice (n = 10 per group) were challenged by the intranasal route with 80 viable spores isolated from the virulent C735 isolate. The horizontal line within each bar indicates the mean CFU. (C) Survival plots for WT, MyD88−/−, and Card9−/− mice vaccinated with the ΔT vaccine or treated with PBS (Ctl.) as controls (n = 10). Asterisks indicate a statistically significant difference in fungal burden of the vaccinated mice compared to the nonvaccinated mice of the same strain, while the daggers indicate significantly elevated CFU in the vaccinated MyD88−/− and Card9−/− mice compared to the WT mice. The results are representative of 2 independent experiments.
MyD88 and Card9 are required for acquisition of both Th17 and Th1 cells in Coccidioides-infected lungs.
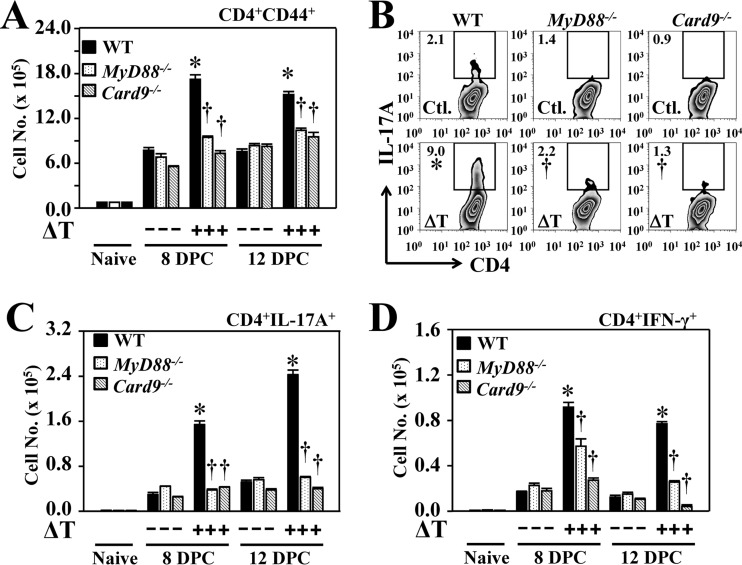
Next, we determined the numbers of activated CD4+ T cells, including Th17 and Th1 cells that had infiltrated the lungs of vaccinated and nonvaccinated MyD88−/− and Card9−/− mice at 8 and 12 dpc, and we compared the results to those of WT mice (Fig. 2A to D). Expression of CD44 on the surface of T cells is a marker of activation (6). Vaccinated MyD88−/− and Card9−/− mice had significantly reduced numbers of CD44+ CD4+ T cells in their lungs at both 8 and 12 dpc compared to vaccinated WT controls (Fig. 2A). Phenotypic analysis of activated pulmonary CD4+ T cells revealed that significantly reduced percentages and numbers of Th17 (CD4+ IL-17A+) cells were present in lungs of vaccinated MyD88−/− and Card9−/− mice than in vaccinated WT mice at both 8 and 12 dpc (Fig. 2B and C). Interestingly, marked reductions in numbers of Th1 (CD4+ IFN-γ+) cells were also observed in vaccinated MyD88−/− and Card9−/− mice compared to WT mice (Fig. 2D). Thus, both MyD88 and Card9 adaptors are required for the vaccine-induced acquisition of Th17 and Th1 cells in the lungs of Coccidioides-infected mice.
FIG 2.
MyD88 and Card9 are required for acquisition of Th17 and Th1 cells in Coccidioides-infected lungs. Fluorescence-activated cell sorting analysis of activated CD4+ T cells and IL-17A- and IFN-γ-producing Th17 and Th1 cells, respectively, were recorded and compared for lung homogenates derived from vaccinated and nonvaccinated WT, MyD88−/−, and Card9−/− mice. (A) The numbers of CD44+ CD4+ T cells were determined at 8 and 12 dpc. (B) Percentages of gated CD4+ T cells positive for IL-17A at 12 dpc. (C and D) The numbers of Th17 (C) and Th1 (D) cells were measured at 8 and 12 dpc. Data are mean values ± standard errors of the means (shown by the error bars) of 4 mice per group. The numbers of gated, specific cytokine-expressing immune cells per lung were determined by intracellular cytokine staining. Asterisks indicate significantly higher absolute numbers of the responsive T-cell phenotypes in lungs of vaccinated compared to nonvaccinated mice of the same strain, while daggers represent significant differences in the MyD88−/− and Card9−/− mice compared to WT mice. The reported results are representative of two independent experiments.
TLR2 is dispensable for vaccine immunity to Coccidioides infection.
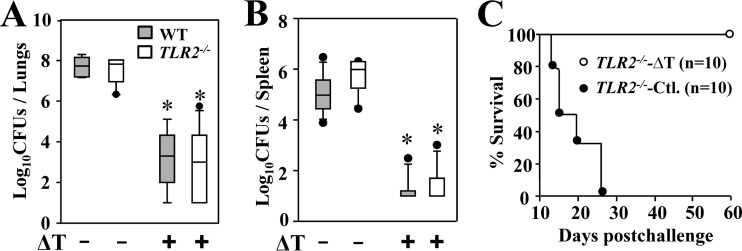
Since MyD88 and upstream TLR2 have been shown to be required for macrophages to sense Coccidioides spherules (21), we postulated that TLR2 might be essential for recognition of the live, attenuated vaccine in eliciting vaccine immunity. To test this hypothesis, we compared vaccine-induced resistance and acquisition of subtypes of CD4+ T cells in lungs and spleens of TLR2−/− and WT mice during the first 14 dpc. To our surprise, vaccinated TLR2−/− mice presented with similarly reduced levels of CFU in lungs and spleen as in vaccinated WT mice (Fig. 3A and B). In parallel, vaccinated TLR2−/− and WT mice showed 100% survival for a period of 60 days postchallenge (Fig. 3C and 1C). Phenotypic analysis of pulmonary CD4+ T cells also revealed that numbers of Th17 and Th1 cells of the vaccinated TLR2−/− mice were comparable to those of WT mice (data not shown). These results indicated that TLR2 is dispensable for pulmonary acquisition of Th17 and Th1 cells and vaccine-induced resistance to Coccidioides infection.
FIG 3.
TLR2 is dispensable for vaccine-induced resistance to Coccidioides infection. The CFU of Coccidioides detected in dilution plate cultures of lung (A) and spleen (B) homogenates at 14 dpc from WT and TLR2−/− mice that were vaccinated with the ΔT vaccine (+) or immunized with PBS (−) as controls are reported. All mice (n = 10 per group) were challenged by the intranasal route with approximately 80 viable spores isolated from the virulent C. posadasii C735 isolate. The horizontal line in each box indicates the mean CFU. (C) Survival plots for TLR2−/− mice (n = 10 per group) vaccinated with the ΔT vaccine or treated with PBS as a control (Ctl.). Asterisks indicate a statistically significant difference (P < 0.05) between CFU in the lungs of the vaccinated versus nonvaccinated mice of the same strain.
The IL-1 receptor is essential for acquisition of Th17 cells and vaccine-induced resistance to Coccidioides infection.
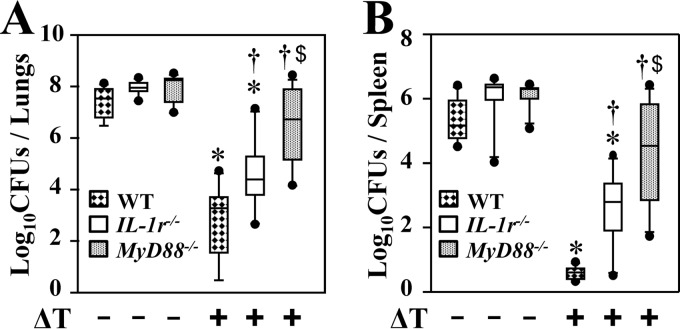
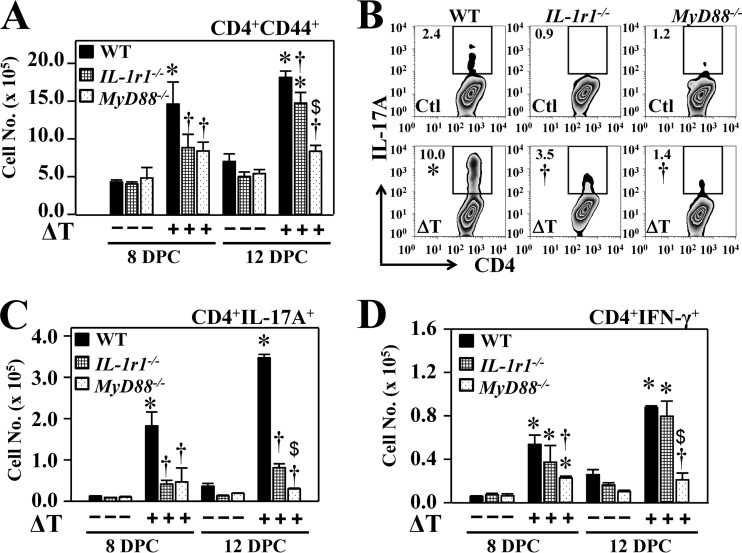
Since IL-1r1 can also transduce a signal through MyD88 to activate Th17 differentiation, we asked whether IL-1r1 contributes to vaccine-induced resistance to Coccidioides infection and acquisition of Th17 cells in the lungs. Vaccinated IL-1r1−/− mice presented with intermediate numbers of CFU in lungs and spleen compared to the numbers of CFU in vaccinated MyD88−/− and WT mice, whereas all strains of nonvaccinated mice had comparable, high numbers of CFU in lungs and spleen (Fig. 4A and B). We further measured the numbers of activated CD44+ CD4+ T cells, Th17, and Th1 cells in lungs of IL-1r1−/− mice and compared these results to those of MyD88−/− and WT mice at 8 and 12 dpc (Fig. 5A to D). Our data revealed that IL-1r1−/− mice had reduced numbers of activated CD44+ CD4+ cells at 8 and 12 dpc compared to WT mice (Fig. 5 A). Like MyD88−/− mice, vaccinated IL-1r1−/− mice revealed significantly reduced percentages and numbers of Th17 cells in their lungs at both 8 and 12 dpc compared to vaccinated WT mice (Fig. 5B and C). Interestingly, numbers of Th1 cells in lungs of vaccinated IL-1r1−/− mice were comparable to those of wild-type mice (Fig. 5D). These data suggest that IL-1r1 is essential for induction of Th17 cells and resistance to Coccidioides infection.
FIG 4.
IL-1r1 is essential for vaccine-induced resistance to Coccidioides infection. The CFU of Coccidioides detected in dilution plate cultures of lungs (A) and spleen (B) homogenates at 14 dpc of WT, IL-1r1−/−, and MyD88−/− mice (n = 10 per group) vaccinated with the ΔT vaccine (+) or immunized with PBS (−) as controls are reported. Asterisks indicate statistically significant differences (P < 0.05) between vaccinated and nonvaccinated mice of the same strain, while daggers indicate significant differences between the vaccinated IL-1r1−/− and MyD88−/− mice compared to the WT mice. Dollar signs indicate significant differences between the MyD88−/− and IL-1r1−/− mice. The reported results are representative of two independent experiments.
FIG 5.
IL-1r1 is required for acquisition of Th17 cells to Coccidioides-infected lungs. Fluorescence-activated cell sorting analysis results for activated CD4+ T cells and Th17 and Th1 cells are shown for lung homogenates derived from vaccinated compared to nonvaccinated WT, IL1r1−/−, and MyD88−/− mice. (A) The numbers of CD44+ CD4+ T cells at 8 and 12 dpc; (B) percentages of gated CD4+ T cells positive for IL-17A at 12 dpc; (C and D) the numbers of IL-17A- and IFN-γ-producing Th17 and Th1 cells, respectively, are shown for the two strains of knockout mice compared to the WT mice. Asterisks indicate significantly higher absolute numbers of the responsive T-cell phenotypes in lungs of vaccinated compared to nonvaccinated mice of the same strain, while daggers represent significant differences in the IL-1r1−/− and MyD88−/− mice compared to WT mice. Dollar signs indicate significant differences between the MyD88−/− and IL-1r1−/− mice. Data are mean values ± standard errors of the means of 4 mice per group. The reported results are representative of two independent experiments.
Vaccine-induced resistance to Coccidioides infection is dependent on neutrophils.
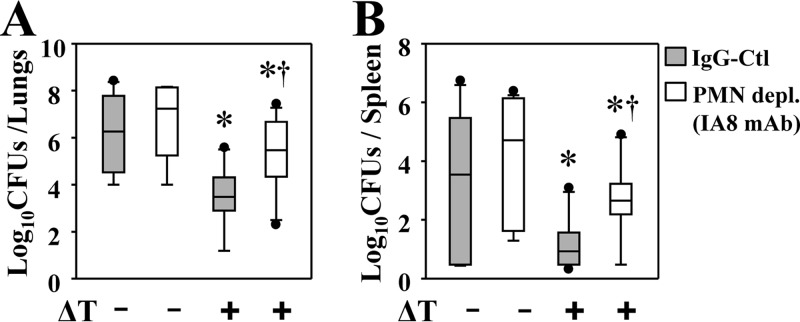
Th17 immunity can promote early infiltration and activation of phagocytic cells, especially neutrophils, at sites of infection. We previously reported that vaccinated WT mice had elevated numbers of neutrophils in lungs during the early stage (before 7 dpc) of Coccidioides infection compared to the response in nonvaccinated mice (6). We asked whether neutrophils are required for vaccine immunity to Coccidioides infection. C57BL/6 mice were treated with a monoclonal antibody (IA8 MAb) to selectively deplete Ly6G+ neutrophils during the effector phase of Coccidioides infection. Mice intraperitoneally injected with rat IgG served as controls. We confirmed an efficient depletion (95% ± 3%) of CD11b+ LFA1+ neutrophils in the lungs of both vaccinated and nonvaccinated mice at 12 dpc. Mice were subjected to fungal burden assays at this time point. Vaccinated mice that were depleted of neutrophils had 38- and 48-fold more CFU in lungs and spleen than controls given rat IgG (P < 0.05) (Fig. 6A and B), respectively. Nonvaccinated mice depleted of neutrophils showed a trend of elevated numbers of CFU in lungs and spleen compared to control littermates that received rat IgG; however, the increase was not statistically significant (Fig. 6A and B).
FIG 6.
Neutrophils are required for vaccine-induced immunity to pulmonary Coccidioides infection during the effector phase. Mice were vaccinated with the ΔT vaccine (+) or injected with PBS as a control (−). A dose of 200 μg of IA8 MAb was injected i.p. at 24 h prior to challenge and at 1, 3, 5, 7, 9, and 11 dpc to deplete neutrophils. Greater than 92% of neutrophils were depleted, as measured by flow cytometry at 12 dpc. The CFU of C. posadasii detected in dilution plate cultures of lung (A) and spleen (B) homogenates of mice at 12 dpc are shown. CFU are reported in box plots for 10 mice per group. Asterisks indicate statistically significant differences (P < 0.05) between the vaccinated and nonvaccinated mice, while the daggers indicate significant differences between neutrophil-depleted and control mice. Data are representative of two independent experiments.
DISCUSSION
The development of robust and durable vaccines requires a fundamental understanding of how protective immune responses are induced. We applied a live, attenuated vaccine (ΔT) to explore the nature of vaccine immunity in mice during the initial 2-week period after intranasal challenge with a potentially lethal dose of Coccidioides spores. The numbers of pulmonary Th1 and Th17 cells showed a progressive increase in vaccinated mice and corresponded with a reduction of fungal burden (6). Profiles of cytokines detected in lung homogenates of ΔT-vaccinated mice were indicative of a mixed Th1, Th2, and Th17 immune response. While mice lacking an IFN-γ receptor (IFN-γR) or IL-4 receptor (IL-4R) can develop comparable vaccine immunity without loss of ΔT vaccine-induced resistance, deficiency of the IL-17 receptor (IL-17RA) results in a significant increase in susceptibility to Coccidioides infection (6). Although vaccinated IL-17A−/− mice can survive for a period of 45 days postchallenge, immune-deficient mice present with significantly elevated numbers of CFU in lungs and spleen at 14 days postchallenge compared to vaccinated wild-type mice (6, 7). Furthermore, we have created a strain of TCR transgenic mice (Bd 1807) whose CD4+ T cells respond to a shared epitope among Blastomyces dermatitidis, Histoplasma capsulatum, and Coccidioides posadasii. Adoptive transfer of CD4+ T cells prepared from Bd 1807 mice confers significant reductions of fungal burden in the lungs and spleen of the recipient mice vaccinated with FKS and challenged with a potentially lethal dose of Coccidioides spores (29). On adoptive transfer into the vaccinated mice, Bd 1807 cells were activated and differentiated into Th1 cells after trafficking to the lungs upon challenge with one of these three dimorphic fungi (29). Later, we found that Bd 1807 cells could also differentiate into Th17 effector cells in lungs of the recipient mice (7). Our data suggest that vaccine-induced Th17 cells are indispensable for protective immunity to infection with Coccidioides posadasii, Blastomyces dermatitidis, and Histoplasma capsulatum (7).
We found that Card9 is an essential signal adaptor governing the activation of protective immunity elicited by both the live, attenuated vaccine (ΔT) and FKS against pulmonary Coccidioides infection (14). Dectin-1, Dectin-2, and Mincle are the best-characterized CLRs upstream of the spleen tyrosine kinase (Syk)-coupled Card9 axis of the signal cascade (30). Dectin-1 contains an immunoreceptor tyrosine-based activation motif (ITAM), whereas Dectin-2 and Mincle transduce signals through association with the ITAM-containing Fc receptor γ chain (FcRγ). Dectin-1 has been shown to be involved in innate recognition of FKS by peritoneal macrophages to produce inflammatory cytokines (21). Dectin-1 is also required for resistance to primary Coccidioides infection (31, 32). Recently, we showed that the development of both Th17 and Th1 cells was impaired in FKS-vaccinated Card9−/−, Dectin-2−/−, and FcRγ−/− mice compared to WT and Mincle−/− mice (14). In this study, we found that the live ΔT vaccine, like FKS, also activates the Card9-mediated signal pathway to elicit protective immunity to Coccidioides infection.
Our data revealed that MyD88, like Card9, is also required for vaccine-induced antifungal Th17 and Th1 responses and resistance to Coccidioides infection. Both Card9−/− and MyD88−/− knockout mice were impacted by the acquisition of Th17 cells, which are essential for vaccine immunity to Coccidioides infection (6). Similarly, antifungal Th17 and Th1 responses to pulmonary infections with Blastomyces dermatitidis and Paracoccidioides brasiliensis are also dependent on MyD88 (7, 9). Unexpectedly, our results revealed that TLR2 is not essential for vaccine immunity to Coccidioides infection, although it is required for peritoneal macrophages to secrete proinflammatory cytokines upon incubation with Coccidioides spherules in vitro (21). Comparable results were also reported for systemic Aspergillus infection and pulmonary Blastomyces dermatitidis infection, suggesting that TLR2 is dispensable during early differentiation and recruitment of CD4+ T cells in the airway (7, 33, 34). In addition to TLR2, other TLRs, including TLR4, TLR1, TLR6, and TLR9, have been implicated in innate recognition of fungal infections (35) and may compensate for the loss of TLR2 upon Coccidioides infection.
Besides serving as an immune adaptor for TLRs, MyD88 also mediates signal transduction via IL-1, IL-18, and IL-33 receptors (20, 36, 37). Both IL-1α and IL-1β interact with IL-1r1, while IL-18 and IL-33 bind to IL-18r and IL-33r, respectively. IL-1r1 is expressed on both antigen-presenting cells and T cells. Activation of IL-1r1/MyD88 can provide both extrinsic and intrinsic signals for CD4+ T cell development (37–39). IL-1α and IL-1β induce mRNA expression of hundreds of genes in multiple cell types, such as monocytes and macrophages (40). IL-1α and IL-1β induce expression of their own genes, which serves as a positive feedback loop that amplifies the IL-1 response in an autocrine or paracrine manner (38). Selective expression of receptors for an IL-1 family member has been shown in CD4+ T cells primed in the presence of Th17, Th1, or Th2 cells. IL-1r1 is required for the upregulation of IRF4 and RORC (two fundamental Th17 transcription factors) during early development and differentiation of Th17 cells (39). Activation of IL-1r1/MyD88 enhances antigen-driven expansion of Th17 cells both in vitro and in vivo (37, 41). IL-18r shows heightened expression on Th1 cells and IL-33r on Th2 cells (37). Our current model of coccidioidomycosis could not distinguish the relative contribution of the extrinsic and intrinsic roles of IL-1r1 in the development of a CD4+ T-cell response to Coccidioides infection. However, the ΔT-vaccinated IL-1r1−/− mice specifically reduced acquisition of Th17 cells, suggesting that the IL-1r/MyD88 axis of immunity is required for vaccine-induced Th17 immunity and resistance to pulmonary Coccidioides infection. Of note, our data revealed that the Th17 response was reduced in IL-1r1−/− mice to a lesser extent than in MyD88−/− mice, raising the possibility that members of the TLR family, other than TLR2, may also be involved in induction of Th17 immunity to Coccidioides infection.
Interestingly, vaccinated IL-1r1−/− mice, but not MyD88−/− mice, showed sustained acquisition of Th1 cells during the first 12 days post-Coccidioides infection. Presumably, in the absence of IL-1r1, TLRs, IL-18r, and IL-33r contribute to these MyD88-dependent CD4+ T-cell responses to Coccidioides infection. IL-18 is known to play an important role in Th1 polarization, but it also promotes production of Th2-type cytokines (e.g., IL-4, IL-5, IL-9, and IL-13) from T cells, NK cells, basophils, and mast cells (39, 42). IL-33 and IL-33r drive polarized Th2 cells to produce IL-4, IL-5, and IL-13 in mice. Future investigations of IL-18r- and IL-33-mediated signals will elucidate their roles in vaccine immunity to Coccidioides infection.
Th17 immunity promotes infiltration and activation of neutrophils as a mode of action in mediating vaccine immunity against fungal infection and inflammatory diseases (7, 43). Although the functional mechanisms of neutrophils have not yet been studied, our data suggest that neutrophils are essential for vaccine-induced resistance to Coccidioides infection. Human neutrophils have been shown to kill arthroconidia (spores) of Coccidioides (44). As the spores germinate and transform into spherules, the fungal cells progressively become larger and more resistant to the inhibitory effects of human neutrophils (45). Rupture of mature spherules and release of endospores trigger an influx of neutrophils (46). Ingestion of young spherules (spherule initials) and endospores elicits an oxidative burst, albeit to a lesser extent than that induced by spores (47). Several studies have shown that neutrophils can kill spherule initials, while mature spherules are resistant to phagocytic killing (45, 46). Neutrophils can be polarized toward proinflammatory or anti-inflammatory cells (N1 versus N2) in response to environmental signals (48). Neutrophils can also produce IL-10, a global anti-inflammatory cytokine in lung homogenates of mice during the first 14 days after Coccidioides infection (49). Thus, neutrophils have emerged as components of the effector and regulatory circuits of the innate and adaptive immune systems (50). Vaccinated and challenged C57BL/6 mice reveal early infiltration of neutrophils into lungs at 5 to 7 dpc that is sustained in moderate numbers during the first 14 dpc of Coccidioides infection (6). No severe tissue damage due to uncontrolled recruitment of neutrophils has been noted based on histopathological examination of lung tissue of such vaccinated mice. In contrast, nonvaccinated mice do not recruit polymorphonuclear lymphocytes into the lungs until 8 to 9 dpc, when Coccidioides spherules rapidly propagate and start to disseminate to extrapulmonary tissue and organs (6, 25, 28). The sharp influx of neutrophils into lungs of nonvaccinated mice that fail to develop Th17 cells after 8 days postchallenge is probably due to activation of the complement systems. Although nonvaccinated mice accumulate large numbers of neutrophils in their lungs before reaching a moribund state, partly because of their delayed recruitment, the mice are unable to inhibit reproduction of Coccidioides spherules.
Both ligands of Toll/TIRs and CLRs are of growing interest as adjuvants to enhance vaccine efficacy (51). Wüthrich and colleagues have also shown that addition of IL-1 to weak antigens can enhance vaccine capacity to induce protection against lethal Blastomyces dermatitidis infection in mice and is far more effective than lipopolysaccharide (52). Our data suggest that an adjuvant system composed of ligands to stimulate both Card9- and MyD88-mediated signals is worthy of investigation. For example, porous glucan particles that consist primarily of β-1,3-d-glucans and allow for carrying pathogen-specific antigens and Toll/TIR ligands, such as CpG oligonucleotides and/or IL-1, have been exploited as receptor-targeted vaccine delivery systems (53). We postulate that this glucan particle-based vaccine platform, which delivers antigens and provides adjuvanticity, can be used to induce robust Th17 and Th1 immunity to combat Coccidioides infection.
ACKNOWLEDGMENTS
This work was supported by research grants from the National Institutes of Health, NIAID (R01 AI-071118 and RO1 AI-093553 to G.T.C. and M.W., respectively). Additional support was provided by the Margaret Batts Tobin Foundation, San Antonio, TX.
We are thankful to Xin Lin at the MD Anderson Cancer Center, Houston, TX, for approving the use of breeder pairs of Card9−/− mice. We also thank Natalia Castro-Lopez for her technical assistance in determinations of fungal burden in Coccidioides-infected mice.
Footnotes
Published ahead of print 10 March 2014
REFERENCES
- 1.Fisher MC, Koenig GL, White TJ, Taylor JW. 2002. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 94:73–84. 10.2307/3761847 [DOI] [PubMed] [Google Scholar]
- 2.Frieden TR, Jaffe HW, Stephens JW, Cardo JM, Zaza S. 2013. Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb. Mortal. Wkly. Rep. 62:217–221 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6212a1.htm [PMC free article] [PubMed] [Google Scholar]
- 3.Cole GT, Hurtgen BJ, Hung CY. 2012. Progress toward a human vaccine against coccidioidomycosis. Curr. Fungal Infect. Rep. 6:235–244. 10.1007/s12281-012-0105-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole GT, Xue JM, Okeke CN, Tarcha EJ, Basrur V, Schaller RA, Herr RA, Yu JJ, Hung CY. 2004. A vaccine against coccidioidomycosis is justified and attainable. Med. Mycol. 42:189–216. 10.1080/13693780410001687349 [DOI] [PubMed] [Google Scholar]
- 5.Cox RA, Magee DM. 2004. Coccidioidomycosis: host response and vaccine development. Clin. Microbiol. Rev. 17:804–839. 10.1128/CMR.17.4.804-839.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung CY, Gonzalez A, Wuthrich M, Klein BS, Cole GT. 2011. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect. Immun. 79:4511–4522. 10.1128/IAI.05726-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wüthrich M, Gern B, Hung C-Y, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole GT, Klein B. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest. 121:554–568. 10.1172/JCI43984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond RA, Saijo S, Iwakura Y, Brown GD. 2011. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur. J. Immunol. 41:276–281. 10.1002/eji.201041252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loures FV, Pina A, Felonato M, Feriotti C, de Araujo EF, Calich VL. 2011. MyD88 signaling is required for efficient innate and adaptive immune responses to Paracoccidioides brasiliensis infection. Infect. Immun. 79:2470–2480. 10.1128/IAI.00375-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wuthrich M, Deepe GS, Jr, Klein B. 2012. Adaptive immunity to fungi. Annu. Rev. Immunol. 30:115–148. 10.1146/annurev-immunol-020711-074958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, Migaud M, Taibi L, Ammar-Khodja A, Stambouli OB, Guellil B, Jacobs F, Goffard J-C, Schepers K, del Marmol V, Boussofara L, Denguezli M, Larif M, Bachelez H, Michel L, Lefranc G, Hay R, Jouvion G, Chretien F, Fraitag S, Bougnoux M-E, Boudia M, Abel L, Lortholary O, Casanova J-L, Picard C, Grimbacher B, Puel A. 2013. Deep dermatophytosis and inherited Card9 deficiency. N. Engl. J. Med. 369:1704–1714. 10.1056/NEJMoa1208487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glocker E-O, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschläger N, Gross O, Ruland J, Grimbacher B. 2009. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361:1727–1735. 10.1056/NEJMoa0810719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeibundGut-Landmann S, Grosz O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638. 10.1038/ni1460 [DOI] [PubMed] [Google Scholar]
- 14.Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, Cole GT, Klein BS, Wüthrich M. 2014. C-type lectin receptors differentially induce Th17 cells and vaccine immunity to the endemic mycosis of North America. J. Immunol. 192:1107–1119. 10.4049/jimmunol.1302314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole G, Xue J, K S, Borra P, Borra R, Tarcha E, Schaller R, Yu J-J, Hung C-Y. 2006. Virulence mechanisms of Coccidioides, p 363–391 In Heitman J, Filler S, Edwards J, Mitchell A. (ed), Molecular principles of fungal pathogenesis. ASM Press, Washington, DC [Google Scholar]
- 16.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. 2009. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect. Immun. 77:3196–3208. 10.1128/IAI.00459-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez A, Yanez A, Gozalbo D, Gil ML. 2008. MyD88 is dispensable for resistance to Paracoccidioides brasiliensis in a murine model of blood-borne disseminated infection. FEMS Immunol. Med. Microbiol. 54:365–374. 10.1111/j.1574-695X.2008.00487.x [DOI] [PubMed] [Google Scholar]
- 18.Dubourdeau M, Athman R, Balloy V, Huerre M, Chignard M, Philpott DJ, Latgé J-P, Ibrahim-Granet O. 2006. Aspergillus fumigatus induces innate immune responses in aveolar macrophages through the MAPK pathway independently of TLR2 and TLR4. J. Immunol. 177:3994–4001 [DOI] [PubMed] [Google Scholar]
- 19.Bello-Irizarry SN, Wang J, Olsen K, Gigliotti F, Wright TW. 2012. The alveolar epithelial cell chemokine response to Pneumocystis requires adaptor molecule MyD88 and interleukin-1 receptor but not Toll-Like receptor 2 or 4. Infect. Immun. 80:3912–3920. 10.1128/IAI.00708-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 [DOI] [PubMed] [Google Scholar]
- 21.Viriyakosol S, Fierer J, Brown GD, Kirkland TN. 2005. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect. Immun. 73:1553–1560. 10.1128/IAI.73.3.1553-1560.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30:576–587. 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyirenda MH, Sanvito L, Darlington PJ, O'Brien K, Zhang G-X, Constantinescu CS, Bar-Or A, Gran B. 2011. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J. Immunol. 187:2278–2290. 10.4049/jimmunol.1003715 [DOI] [PubMed] [Google Scholar]
- 24.Hung CY, Hurtgen BJ, Bellecourt M, Sanderson SD, Morgan EL, Cole GT. 2012. An agonist of human complement fragment C5a enhances vaccine immunity against Coccidioides infection. Vaccine 30:4681–4690. 10.1016/j.vaccine.2012.04.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, Cole GT. 2012. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against coccidioidomycosis. Infect. Immun. 80:3960–3974. 10.1128/IAI.00566-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70. 10.1189/jlb.0407247 [DOI] [PubMed] [Google Scholar]
- 27.Nanjappa SG, Heninger E, Wuthrich M, Gasper DJ, Klein BS. 2012. Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS Pathog. 8(7):e1002771. 10.1371/journal.ppat.1002771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez A, Hung CY, Cole GT. 2011. Absence of phagocyte NADPH oxidase 2 leads to severe inflammatory response in lungs of mice infected with Coccidioides. Microb. Pathog. 51:432–441. 10.1016/j.micpath.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wuthrich M, Hung CY, Gern BH, Pick-Jacobs JC, Galles KJ, Filutowicz HI, Cole GT, Klein BS. 2011. A TCR transgenic mouse reactive with multiple systemic dimorphic fungi. J. Immunol. 187:1421–1431. 10.4049/jimmunol.1100921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardison SE, Brown GD. 2012. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 13:817–822. 10.1038/ni.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.del Pilar Jimenez-Alzate M, Viriyakosol S, Walls L, Datta SK, Kirkland T, Heinsbroek SEM, Brown G, Fierer J. 2008. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of Dectin-1 (Clec7a). Genes Immun. 9:338–348. 10.1038/gene.2008.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viriyakosol S, Jimenez MP, Gurney MA, Ashbaugh ME, Fierer J. 2013. Dectin-1 is required for resistance to coccidioidomycosis in mice. mBio 4(1):e00597–e00612. 10.1128/mBio.00597-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier A, Kirschning CJ, Nikolaus T, Wagner H, Heesemann J, Ebel F. 2003. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell. Microbiol. 5:561–570. 10.1046/j.1462-5822.2003.00301.x [DOI] [PubMed] [Google Scholar]
- 34.Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant'Angelo DB, Pamer EG. 2006. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity 25:665–675. 10.1016/j.immuni.2006.08.016 [DOI] [PubMed] [Google Scholar]
- 35.Brown GD. 2011. Innate antifungal immunity: the key role of phagocytes. Annu. Rev. Immunol. 29:1–21. 10.1146/annurev-immunol-030409-101229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber A, Wasiliew P, Kracht M. 2010. Interleukin-1 (IL-1) pathway. Sci. Signal. 3:cm1. 10.1126/scisignal.3105cm1 [DOI] [PubMed] [Google Scholar]
- 37.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. 2009. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. U. S. A. 106:13463–13468. 10.1073/pnas.0906988106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinarello CA. 2009. Immunological and inflammatory functions of theinterleukin-1 family. Annu. Rev. Immunol. 27:519–550. 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- 39.Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. 2013. IL-1 and T helper immune responses. Front. Immunol. 4:182. 10.3389/fimmu.2013.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allantaz F, Chaussabel D, Stichweh D, Bennett L, Allman W, Mejias A, Ardura M, Chung W, Smith E, Wise C, Palucka K, Ramilo O, Punaro M, Banchereau J, Pascual V. 2007. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. J. Exp. Med. 204:2131–2144. 10.1084/jem.20070070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee W-W, Kang SW, Choi J, Lee S-H, Shah K, Eynon EE, Flavell RA, Kang I. 2010. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood 115:530–540. 10.1182/blood-2009-08-236521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawada M, Kawayama T, Imaoka H, Sakazaki Y, Oda H, Takenaka S-i, Kaku Y, Azuma K, Tajiri M, Edakuni N, Okamoto M, Kato S, Hoshino T. 2013. IL-18 induces airway hyperresponsiveness and pulmonary inflammation via CD4+ T cell and IL-13. PLoS One 8(1):e54623. 10.1371/journal.pone.0054623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA. 2010. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115:335–343. 10.1182/blood-2009-04-216085 [DOI] [PubMed] [Google Scholar]
- 44.Galgiani JN, Payne CM, Jones JF. 1984. Human polymorphonuclear-leukocyte inhibition of incorporation of chitin precursors into mycelia of Coccidioides immitis. J. Infect. Dis. 149:404–412 [DOI] [PubMed] [Google Scholar]
- 45.Galgiani JN. 1986. Inhibition of different phases of Coccidioides immitis by human neutrophils or hydrogen peroxide. J. Infect. Dis. 153:217–222 [DOI] [PubMed] [Google Scholar]
- 46.Frey CL, Drutz DJ. 1986. Influence of fungal surface components on the interaction of Coccidioides immitis with polymorphonuclear neutrophils. J. Infect. Dis. 153:933–943 [DOI] [PubMed] [Google Scholar]
- 47.Galgiani JN. 1995. Differences in oxidant release by human polymorphonuclear leukocytes produced by stimulation with different phases of Coccidioides immitis. J. Infect. Dis. 172:199–203 [DOI] [PubMed] [Google Scholar]
- 48.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. 2009. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16:183–194. 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung C-Y, Castro-Lopez N, Cole GT. 2014. Vaccinated C57BL/6 mice develop protective and memory T cell responses to Coccidioides posadasii in the absence of IL-10. Infect. Immun. 82:903–913. 10.1128/IAI.01148-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C, Mantovani A. 2013. Neutrophils in innate and adaptive immunity. Semin. Immunopathol. 35:377–394. 10.1007/s00281-013-0374-8 [DOI] [PubMed] [Google Scholar]
- 51.Coffman RL, Sher A, Seder RA. 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33:492–503. 10.1016/j.immuni.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wüthrich M, LeBert V, Galles K, Hu-Li J, Ben-Sasson SZ, Paul WE, Klein BS. 2013. Interleukin 1 enhances vaccine-induced antifungal T-helper 17 cells and resistance against Blastomyces dermatitidis infection. J. Infect. Dis. 208:1175–1182. 10.1093/infdis/jit283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cole GT, Hung C-Y, Sanderson SD, Hurtgen BJ, Wüthrich M, Klein BS, Deepe GS, Ostroff GR, Levitz SM. 2013. Novel strategies to enhance vaccine immunity against coccidioidomycosis. PLoS Pathog. 9(12):e1003768. 10.1371/journal.ppat.1003768 [DOI] [PMC free article] [PubMed] [Google Scholar]