Abstract
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 strains are major human food-borne pathogens, responsible for bloody diarrhea and hemolytic-uremic syndrome worldwide. Thus far, there is no vaccine for humans against EHEC infections. In this study, a comparative genomics analysis was performed to identify EHEC-specific antigens useful as potential vaccines. The genes present in both EHEC EDL933 and Sakai strains but absent in nonpathogenic E. coli K-12 and HS strains were subjected to an in silico analysis to identify secreted or surface-expressed proteins. We obtained a total of 65 gene-encoding protein candidates, which were subjected to immunoinformatics analysis. Our criteria of selection aided in categorizing the candidates as high, medium, and low priority. Three members of each group were randomly selected and cloned into pVAX-1. Candidates were pooled accordingly to their priority group and tested for immunogenicity against EHEC O157:H7 using a murine model of gastrointestinal infection. The high-priority (HP) pool, containing genes encoding a Lom-like protein (pVAX-31), a putative pilin subunit (pVAX-12), and a fragment of the type III secretion structural protein EscC (pVAX-56.2), was able to induce the production of EHEC IgG and sIgA in sera and feces. HP candidate-immunized mice displayed elevated levels of Th2 cytokines and diminished cecum colonization after wild-type challenge. Individually tested HP vaccine candidates showed that pVAX-12 and pVAX-56.2 significantly induced Th2 cytokines and production of fecal EHEC sIgA, with pVAX-56.2 reducing EHEC cecum colonization. We describe here a bioinformatics approach able to identify novel vaccine candidates potentially useful for preventing EHEC O157:H7 infections.
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) strains are zoonotic extracellular pathogens, members of the Shiga-toxin producing E. coli (STEC) pathogroup. EHEC causes sporadic outbreaks of diarrhea and hemorrhagic colitis, particularly in developed countries (reviewed in references 1, 2, and 3). In the United States, EHEC causes approximately 0.9 cases per 100,000 inhabitants, with a significant number of associated hospitalizations and death, particularly among children and the elderly (reviewed in references 4 and 5). E. coli O157:H7 is the serotype most commonly associated with outbreaks (6), and the expression of Shiga toxin (Stx), in addition to being linked to hemorrhagic colitis, is associated with the progression to the hemolytic-uremic syndrome (HUS), which causes renal failure and a high fatality rate (reviewed in reference 7). In addition, EHEC O157:H7 uses a type III secretion system (T3SS) to translocate effector proteins into the eukaryotic cell, causing changes in the host cytoskeleton, ultimately leading to improved bacterial adherence and colonization and, in some cases, host cell death (8). The EHEC T3SS is comprised of a basal ATP-dependent secretion apparatus, with an EscC polymer ring spanning bacterial outer membrane and a needle-like structure formed by polymers of the EscF protein and an extension structure comprised of polymerized EspA. Finally, the EspD and EspB proteins form a translocon structure in the host membrane (9–11).
Generally asymptomatic, ruminants are the principal EHEC reservoir. Contaminated meat or fresh produce resulting from animal shedding constitute an important route for human infection (12). Current prevention efforts are centered in the elimination of animal colonization, whether by vaccination or by improving sanitary and breeding practices (12, 13). Once the human infection is acquired, supportive care is provided, since antibiotic treatment could induce Shiga toxin expression. To date, two vaccines able to reduce EHEC colonization in cattle are commercially available (13, 14). Nevertheless, development of other subunit-based vaccines has been focused in the T3SS and its associated proteins, as well as Stx (4, 12). For example, inactivated Stx derivatives are able to induce Stx-neutralizing antibodies in mice (15, 16), and hybrid A-B subunit-derived Stx toxins also induce antibody production and increase survival against toxemia and EHEC challenge in vivo (17–19). Fusion proteins comprising of Stx-derived peptides and T3SS-related proteins are promising vaccine candidates. St2B-Tir-Stx1B-Zot, Stx2B-Stx1B-Int281, EspA-Stx2A1, EspA-IntiminC300-Stx2B, and Stx2B-BLS fusions have been demonstrated to reduce EHEC colonization in animal models, such as mice and goats (20–27). Overall, cumulative information indicates that mucosal delivery routes seem to be an effective way to induce immune responses to block the adhesion of EHEC in the intestine, mainly through expression of secretory immunoglobulin A (sIgA) (4).
In addition to the worldwide outbreaks caused by STEC O157:H7, this organism has come recently under renewed scientific investigation as a result of the emergence of a subpopulation of strains that have acquired critical virulence factors that contribute to more severe and lethal disease in humans (28, 29). Further, the discovery of cattle reservoirs shedding high levels of STEC O157:H7, which has been associated with the transmission between animals and across the human-animal interface (30, 31), strongly supports the idea that adoption of vaccination for livestock and/or susceptible individuals will have significant public health benefits, preventing substantial numbers of human STEC O157 cases (32). Therefore, further discovery for EHEC-specific antigens needs to be done to improve existing or to develop novel vaccines. Because the EHEC-associated disease is complex and many molecular and cellular processes affected during infection are not fully understood, it is plausible to propose that some EHEC-encoded virulence-associated proteins could have important, yet unveiled role in the immune/protective process. Therefore, in order to bypass the bias toward assaying a limited number of known virulence factors as components of a vaccine against O157:H7, we performed a genome-wide in silico search for proteins most likely to be effective as immunogenic/protective antigens. By comparative genomics, we identified EHEC-specific antigens with high probability to be exposed to the host during infection. Using an immunoinformatics approach, we further grouped our candidates into high-, medium-, and low-priority groups based on their putative antigenicity and screened a subset of them as vaccine candidates in a murine model of gastrointestinal infection. Our approach involved randomly selecting three candidates from each group which were evaluated as DNA vaccines for their capacity to induce an EHEC immune response and to reduce bacterial colonization in the murine intestine.
MATERIALS AND METHODS
Bacterial strains and media.
The prototype enterohemorrhagic E. coli O157:H7 strains 86-24 (33) and EDL933 (34) were grown in Luria-Bertani (LB) broth at 37°C. Strains bearing pVAX1 derivatives were grown in medium supplemented with kanamycin (Sigma, 25 μg/ml) as a requirement for recombinant plasmid selection.
Comparative genomics and immunoinformatics analysis.
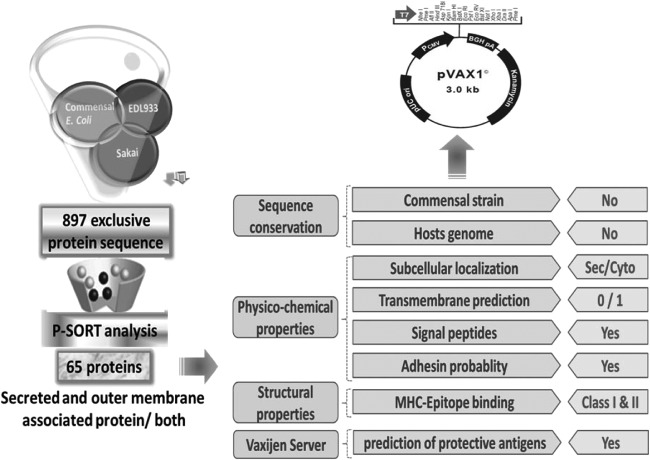
A comparative bioinformatic analysis was developed to obtain groups of orthologous proteins of E. coli O157:H7 strains that excluded orthologous proteins from other nonpathogenic (named external) strains. To achieve this, we included two E. coli O157:H7 strains (EDL933 and Sakai) and two external strains: E. coli HS and E. coli K-12 strain MG1655. The most probable set of orthologous proteins shared by the two E. coli O157:H7 strains were identified using a reciprocal best-hit criterion as follows. All of the predicted proteins of one genome were searched against the predicted proteins of the other genome and vice versa using BLAST-P with a cutoff E value of 10−12. To be included in an orthologue group, the alignment region between the subject protein and the query protein had to be at least of 80%, and there had to be at least 40% similarity for both query and target sizes. Next, the orthologs groups that contained a protein of any of the two external strains were excluded (Fig. 1). This resulted in a set of 897 groups of orthologous proteins shared only by the E. coli O157:H7 strains. The orthologue sequences corresponding to the EDL933 strain were analyzed by the P-SORT software (http://www.psort.org/psortb/) in order to predict their subcellular localization. Sixty-five proteins that were putatively secreted or outer membrane associated were selected as candidates. These proteins were extensively analyzed for critical features found in vaccine candidates, such as physicochemical properties, adhesiveness, and antigenicity and subsequently predicted for immunodominant epitopes. At least two different software programs were used for each property as described below. In most cases, the underlying principle of these programs for the same categorical property is different to ensure wide coverage and yet maintaining stringency by creating a consensus of predicted results (Table 1).
FIG 1.
Schematic summary representation of the comparative genomics and immunoinformatics approaches used in the present study to identify vaccine candidates from EHEC O157:H7.
TABLE 1.
Overview of prediction programs used
| Program | Physicochemical propertiesa |
Immunological properties |
|||||
|---|---|---|---|---|---|---|---|
| Transmembrane (TM) | Signal-peptide (SP) | Subcellular localization | Adhesion probability | B-cell epitopes | T-cell epitopes | Antigenicity | |
| 1 | TMHMM | Signal-P (trained on Gram-positive bacteria) | Signal-P (trained on Gram-negative bacteria) | SPAAN | ABCPred (linear) | NetCTL (MHC-I) | VaxiJen |
| 2 | HMMTOP | PsortB | PsortB | LipoP | NetMHC-II (MHC-II) | ||
| 3 | PHOBIUS | NetChop | NetChop | Lipo | |||
The consensus of results from all programs within a given category, wherever applied, is indicated.
The transmembrane (TM) regions were predicted using TMHMM (35), HMMTOP (36), and Phobius (37). Phobius can discern TM topology and signaling peptide in a protein. It substantially reduces the errors in the predictions of these two characteristics compared to other algorithms, including TMHMM. After an initial analysis with PsortB (38), the signal or localization peptides were also predicted using SignalP (39) and NetChop (40). The presence of lipoprotein signal peptide was determined using the method described by Juncker et al. (41), which predicts both SpI and SpII signal peptidases. The Lipo program (42), which recognizes the lipo-box in protein sequences, was also used. SPAAN (43) predicts the probability of a protein being an adhesin, which often comprise important factors in bacterial virulence. VaxiJen (44), an alignment-free approach for antigen prediction, was used to score the overall antigenicity of the protein. A VaxiJen model used was “bacteria” with the threshold set to 0.5. B-cell linear epitopes were predicted using ABCPred (45), whereas NetCTL (46) and NetMHC-II (47) programs were used to predict major histocompatibility complex class I (MHC-I) and MHC-II binding peptides, respectively. The ABCPred provided an accuracy of 65.93% and equal sensitivity and specificity using a window length of 16-mer peptides with overlapping allowed. NetCTL predicts CTL epitopes restricted to 12 MHC-I supertypes, and a specificity of 97% was used. MHC-II was used with an epitope length of 15 residues against 14 HLA-DR alleles covering the nine HLA-DR, six HLA-DQ, and six HLA-DP supertypes. The overall strategy and its preferred outcome are outlined in Fig. 1.
Plasmid construction.
Plasmid pVAX1 was obtained from Invitrogen/Life Technologies (New York, NY). For our study, large candidates were subdivided in coding sequences (CDS) of a maximum 1,000 bp in length. For the high-priority pool, candidates 31, 56, and 12 were selected. Candidate 56 (EscC) was divided into two CDS. Since the amino-terminal portion of EscC is oriented toward the periplasm (10), we decided to test the fragment comprising the carboxy terminus (pVAX56.2). Candidates 43, 16, and 9 were selected for the medium-priority pool. Finally, candidate 51 and two CDS of candidate 49 were selected for the low-priority pool. The CDS for selected candidates were amplified by PCR from genomic DNA of E. coli EDL933 (O157:H7) using the corresponding forward (Fw) and reverse (Rv) primers containing HindIII and XhoI restriction sites, respectively (Table 2). The resulting fragments were digested and cloned into the HindIII and XhoI sites of pVAX1. Fw primers were designed to generate a Kozak consensus sequence (ACCATGG) at the 5′ end of each CDS. All of the clones were sequenced.
TABLE 2.
Primers used for plasmid construction
| Construct | EHEC gene inserta | Primer | Sequence (5′–3′) |
|---|---|---|---|
| pVAX-12 | Z1538 | PVAX12-Fw | ACCAAGCTTACCATGGTTTCTACTTTCAAAAAAGCAG |
| PVAX12-Rv | ACCCTCGAG TAGAGGTAGCTCAGGGTGTATTCT | ||
| pVAX-31 | Z3075 | PVAX31-Fw | ATTAAGCTTACCATGGGTAAACTTTATGCCGCCATTTTG |
| PVAX31-Rv | ATTCTCGAGTCAATGATGATGATGATGATGGAACTTATAACCGACACCCAC | ||
| pVAX-56.2 | aa 253 to 512 from escC | PVAX56.2-Fw | ACCAAGCTTACCATGGACCGCGAAATAACGATGGAT |
| PVAX56.2-Rv | ACC CTCGAGTTATTCGCTAGATGCAGATTTTATC | ||
| pVAX-43 | Z4321 | PVAX43-Fw | ATTAAGCTTACCATGGGTGGTTCAAGACTGGCTGATAATC |
| PVAX43-Rv | ATTCTCGAGTTAAAAACGATAACCAACTCCAAC | ||
| pVAX-16 | Z1908 | PVAX16-Fw | ATTAAGCTTACCATGGCTTTTTCTTTTTTTTCTACAAAACCCATACC |
| PVAX16-Rv | ATTCTCGAGTTATCCGCCCGCACCATTAACC | ||
| pVAX-9 | Z0981 | PVAX9-Fw | ACCAAGCTTACCATGGGTAAAGTTTGTGCAGCAA |
| PVAX9-Rw | ACCCTCGAGTCAAAATTTATAACCGACACCCAC | ||
| pVAX-51 | espB | PVAX51-Fw | ATTAAGCTTACCATGGATACTATTGATAATACTCAAG |
| PVAX51-Rv | ATTCTCGAGTCAATGATGATGATGATGATGCCCAGCTAAGCGACCCGATTG | ||
| pVAX-49.1 | aa 641 to 960 from ehaG | PVAX49.1-Fw | ACCAAGCTTACCATGGCCGATGCCGTTAACGGCTC |
| PVAX49.2-Rv | TTATCTAGACTCGAGTTACTCGGCGTTCGCAATGGTG | ||
| pVAX-49.2 | aa 1141 to 1380 from ehaG | PVAX49.2-Fw | ACCAAGCTTACCATGGAACTGCTCGGTGCATTGTCT |
| PVAX49.2-Rv | TTATCTAGACTCGAGTTAGCCGGAACCAATCGCGACG |
aa, amino acids.
Immunization protocol.
Six- to eight-week-old female BALB/c mice (Harlan Laboratories) were divided into six groups (n = 10). Mice were immunized intranasally with 20 μl of the DNA vaccines in Tris-EDTA (TE) buffer (10 μl on each nostril), arranged as follows: (i) TE buffer only, (i) TE plus cholera toxin (CT; adjuvant), (iii) pVAX vector plus CT, (iv) pVAX-high priority (HP), (v) pVAX-medium priority (MP), and (vi) pVAX-low priority (LP) vaccine candidates. All of the vaccine candidates were administered in pools of three targets (20 μg of each plasmid), along with the adjuvant CT (1 μg/μl). For the immunization, the animals were anesthetized with isoflurane and primed with final dose of 60 μg of DNA per mice, followed by boosts at 2 and 4 weeks using the same dose without CT. In the case of the individual candidate immunizations, the animals received a total of 60 μg of individual plasmid. In the CT control group, priming was with CT, followed by TE boosts. One week after the last boost, blood and fecal samples were collected to monitor the mucosal antibody response.
Challenging the immunized mice.
To determine the protective ability of the potential DNA vaccine candidates, all immunized mice were challenged with a dose of 5 × 109 CFU of the streptomycin resistant EHEC O157:H7 strain 86-24, via gavage, 2 weeks after the last boost. Two hours prior to the challenge, mice received an i.p. dose of cimetidine hydroxyzine (10 mg/ml) to reduce stomach acidity. Fecal samples were collected from each group at indicated days after infection. Stools were dissolved in 2 ml of phosphate-buffered saline (PBS), serially diluted, and plated. To recover bacteria from the intestine, the mice were euthanized at the indicated days, and ceca were excised and homogenized in 2 ml of PBS. Bacterial suspensions were serially diluted and plated. Both organ and fecal samples were plated on MacConkey agar plates containing streptomycin and then incubated at 37°C overnight prior to E. coli O157:H7 colony enumeration.
Serum and feces collection for immunoglobulin determination.
Sera were obtained by retro-orbital bleeding, clotting whole blood for 30 min at room temperature, and centrifugation at 3,000 × g for 15 min at 4°C. The resulting supernatant was collected and used for enzyme-linked immunosorbent assay (ELISA). For sIgA measurement, feces samples were weighted and diluted to 1 g/ml with PBS-phenylmethylsulfonyl fluoride. After vigorous homogenization by vortexing, feces samples were incubated for 1 h on ice and centrifuged at 4,000 rpm for 30 min at 4°C. The supernatant was collected and stored at −20°C prior to use.
Immune response. (i) ELISA.
Total serum IgG antibody responses were determined by ELISA according to the manufacturer's instructions (eBioscience). Briefly, polystyrene 96-well high-binding ELISA plates (Nunc, Denmark) were coated overnight at 4°C with capture IgG antibody. The plates were washed three times with PBS containing 0.05% (vol/vol) Tween 20 (PBS-T) and plated with blocking buffer. The diluted serum sample (1:1,000 and 1:10,000) and an IgG standard of known activity were incubated, followed by repeated washing with 0.05% PBS-T. Next, horseradish peroxidase-conjugated goat anti-mouse IgG in PBS-T (1:250) was added to the ELISA plates, followed by incubation at 37°C for 30 min, followed in turn by washing. A total of 100 μl of tetramethylbenzidine (TMB) was added to the cells, followed by incubation at room temperature for 15 min. The reaction was stopped with 100 μl of 2 M H2SO4, and the optical density at 450 nm was determined.
For the EHEC antibody response, wild-type EHEC O157:H7 was grown overnight in LB broth. Bacterial cells were pelleted (15 min at 5,000 × g) and resuspended in PBS. The bacterial suspension was pulse-sonicated on ice for 5 min. The sonicated sample was centrifuged (10,000 × g for 15 min at 4°C), and the total protein concentration in the supernatant was determined by using a bicinchoninic acid protein (BCA) assay. For ELISA, polystyrene 96-well Nunc plates were coated overnight at 4°C with 100 μl of EHEC extract (2 μg/well in coating buffer), and the assays were performed as described above.
(ii) Bio-Plex assay.
The cytokine levels in serum and feces from immunized and nonimmunized mice were measured on a Bio-Plex 200 system powered by Luminex xMAP technology (Bio-Rad, USA) using specific 23-panel group mouse assay kits (catalog no. M60009RDPD) according to the manufacturer's instructions. The cytokines, chemokines, and growth factors include interleukin-1α (IL-1α), IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12p70, IL-13, IL-17, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, KC, eotaxin, macrophage chemoattractant protein 1 (MCP-1), granulocyte colony-stimulating factor (CSF), granulocyte-CSF (GM-CSF), and RANTES. A heat map of a normalized matrix was created that correlates cytokine response pattern to stimulation by high-priority vaccine candidates. For each cytokine, means and standard deviations (SD) were calculated from their induction values (pg/ml) across the three candidates and were normalized to controls (TE and pVAX-1). The Z-score transformation was calculated for each cytokine by subtracting each induction value from the row mean and dividing by the row SD (48). Overall, the Z-score gives an estimation of the deviation of the measurement from the row mean in SD units. Each block of red or green represents a high positive or negative correlation between the cytokine production and the vaccine candidate under investigation.
Statistical analysis.
All of the statistical significance between control and vaccinated groups was assessed using SPSS software. One-way analysis of variance (ANOVA) and Student t test with threshold of P < 0.05 was used to analyze the data for colonization and antibody response. For the antibody responses the P value is indicated in the respective figures.
RESULTS
Subunit EHEC vaccine research has been mainly focused on known virulence factors. We sought to screen the genome of EHEC strains EDL933 and Sakai to identify sequences with high probability to encode protective antigens, independently of their previous assigned function. Mucosal immune responses are relatively difficult to induce by vaccine candidates due to the fact that mucosal surfaces are often in contact with nonpathogenic E. coli, which enhance the homeostatic state (reviewed in reference 49). Thus, our first criterion was to identify common EHEC antigens absent in nonpathogenic E. coli strains. For this, the set of common proteins between EDL933 and Sakai O157:H7 EHEC strains were determined by a reciprocal BLAST analysis as described in Materials and Methods. Next, DNA sequences encoding proteins present in the nonpathogenic E. coli K-12 MG1655 and commensal HS strains genomes were eliminated from the pool. This analysis rendered 897 protein sequences. It is proposed that the probabilities to provide protection increase for those antigens detected during natural infection, meaning that bacterium-exposed proteins comprise better vaccine candidates (50). In order to predict their subcellular localization, the 897 proteins selected in the previous analysis were evaluated by using the P-SORTb software. A total of 65 proteins putatively associated with the outer membrane and/or secreted were selected as vaccine candidates (Table 3). Further, we accelerated the process of identifying highly antigenic vaccine candidates by using immunoinformatics, which allowed us to assign priorities for the testing of these vaccine candidates in the mouse model of EHEC O157:H7 infection. As described in Fig. 1 and based on physicochemical properties, the proteins were prioritized on the basis of either possessing no transmembrane (TM) domains or containing one region only, having a signal peptide or whether its localization was predicted to be “secreted” and whether they display a high score for adhesiveness. Proteins that satisfy more of these characteristics were ranked higher than others. The VaxiJen server (44) predicted the protective bacterial antigens based on the overall immunogenicity score (higher was better) and also helped to rank the proteins. For B-cell epitope predictions, three parameters were calculated from the results: (i) the total number of epitopes per sequence, (ii) the total score of all epitopes combined per sequence, and (iii) the average score of an epitope. Similarly, for T-cell epitope predictions, parameters such as the total number of high-binding (HB) epitopes, the total score of HB epitopes, the percentage of HB epitopes among all epitopes predicted for a given sequence, and the number of human leukocyte antigens (HLA) alleles covered by the epitopes of a given sequence were also calculated. These parameters were also taken into account when ranking the proteins; however, a larger weight was assigned to B-cell epitopes since EHEC is an extracellular pathogen. The final ranking of all 65 proteins was conducted using a cumulative score from both physicochemical and immunological properties. Based on the combined informatics analysis, vaccine candidates were divided in three groups: high priority (HP; 17 candidates), medium priority (MP; 10 candidates), and low priority (LP; 38 candidates) (indicated in parentheses in Table 3).
TABLE 3.
EHEC O157-specific secreted and outer-membrane-associated proteins identified by P-SORT analysis
| No. (candidate type)a | GI | Annotation |
|---|---|---|
| 1 (LP)* | 12512859 | Putative fimbrial protein |
| 2 (LP)* | 12512975 | Hypothetical protein Z0266 |
| 3 (LP)* | 12513130 | Putative beta-barrel outer membrane protein |
| 4 (LP) | 12513211 | Putative structural protein (partial) |
| 5 (LP)* | 12513363 | Putative outer membrane export protein |
| 6 (LP) | 12513364 | Hypothetical protein Z0609 |
| 7 (LP) | 12513368 | Putative RTX family exoprotein |
| 8 (LP) | 12513376 | Hypothetical protein Z0639 |
| 9 (MP)* | 12513752 | Putative outer membrane protein of prophage CP-933K |
| 10 (HP) | 12514345 | Putative outer membrane protein Lom precursor of bacteriophage BP-933W |
| 11 (LP) | 12514376 | Hypothetical protein Z1516 |
| 12 (HP) | 12514403 | Putative pilin subunit |
| 13 (LP) | 12514410 | Putative member of ShlA/HecA/FhaA exoprotein family |
| 14 (HP) | 12514411 | Putative outer membrane transporter of ShlA/HecA/FhaA exoprotein family |
| 15 (HP) | 12514503 | Putative receptor |
| 16 (MP) | 12514836 | Putative tail component of prophage CP-933X |
| 17 (LP) | 12514898 | Putative outer membrane receptor, probably tonB dependent |
| 18 (LP)* | 12515102 | Putative tail component of prophage CP-933O |
| 19 (LP) | 12515159 | ORF, hypothetical protein |
| 20 (LP)* | 12515160 | Putative ATP-binding component of a transport system and adhesion protein |
| 21 (LP)* | 12515311 | Putative outer membrane protein |
| 22 (LP)* | 12515315 | Hypothetical protein Z2323 |
| 23 (MP) | 12515551 | Putative chaperone protein |
| 24 (HP)* | 13259573 | Putative Lom-like outer membrane protein of cryptic prophage CP-933P |
| 25 (LP) | 13259574 | Putative tail component of cryptic prophage CP-933P |
| 26 (LP) | 13259580 | Putative tail component of cryptic prophage CP-933P |
| 27 (LP) | 12516024 | Flagellar biosynthesis; flagellin, filament structural protein |
| 28 (LP) | 12516037 | Putative secreted protein |
| 29 (LP) | 12516039 | Putative secreted protein |
| 30 (LP) | 12516089 | Unknown protein encoded within prophage CP-933U |
| 31 (HP)a | 12516092 | Putative outer membrane protein of prophage CP-933U |
| 32 (MP) | 12516149 | Putative integrase for prophage CP-933U |
| 33 (LP) | 12516174 | Putative outer membrane receptor for iron compound or colicin |
| 34 (HP)* | 12516360 | Putative Lom-like outer membrane protein of prophage CP-933V |
| 35 (LP)* | 12516361 | Putative tail fiber protein of prophage CP-933V |
| 36 (MP) | 12516373 | Putative major tail subunit encoded within prophage CP-933V |
| 37 (LP)* | 169822942 | Putative fimbrial usher |
| 38 (LP) | 12517052 | Hypothetical protein Z3920 |
| 39 (LP)* | 12517087 | Hypothetical protein Z3954 |
| 40 (MP)* | 12517088 | Putative enzyme |
| 41 (HP) | 12517355 | Putative lipoprotein of type III secretion apparatus |
| 42 (HP) | 12517375 | Type III secretion apparatus protein |
| 43 (MP) | 12517526 | Putative PagC-like membrane protein |
| 44 (HP) | 12517607 | Putative iron compound receptor |
| 45 (HP) | 12518206 | Outer membrane heme/hemoglobin receptor |
| 46 (HP) | 12518273 | Putative fimbrial subunit |
| 47 (LP) | 12518274 | Putative fimbrial protein |
| 48 (MP) | 12518278 | Putative major fimbrial subunit |
| 49 (LP) | 12518349 | Putative adhesin |
| 50 (LP) | 12518435 | espF |
| 51 (LP) | 12518439 | Secreted protein EspB |
| 52 (LP) | 12518440 | Secreted protein ExpD |
| 53 (HP) | 12518447 | Intimin adherence protein |
| 54 (LP) | 12518449 | Putative translocated intimin receptor protein |
| 55 (LP) | 12518464 | escJ |
| 56 (HP) | 12518466 | escC |
| 57 (MP) | 12518483 | Hypothetical protein Z5142 |
| 58 (HP) | 12518576 | Putative fimbrial protein |
| 59 (HP) | 12518577 | Putative fimbrial protein |
| 60 (HP)* | 12518578 | Putative fimbrial usher |
| 61 (LP) | 12518581 | Putative major fimbrial subunit |
| 62 (MP) | 12518689 | Hypothetical protein Z5335 |
| 63 (LP) | 3822134 | Putative exoprotein precursor |
| 64 (LP) | 3822145 | Hypothetical protein |
| 65 (LP) | 3822162 | Hypothetical toxin protein |
Low-priority (LP), medium-priority (MP), and high-priority (HP) candidates are indicated. *, Final candidates analyzed further using blastp for which paralogs of some of sequences were found in recently sequenced nonpathogenic E. coli strains.
To screen the putative protective candidates as determined by their priority score, we randomly selected three candidates from each group (candidates 31, 56.2, and 12 from the HP group; candidates 43, 16, and 9 from the MP group; and candidates 51, 49.1, and 49.2 from the LP group) and cloned them into the widely used pVAX1 DNA vaccine vector (51, 52). DNA vaccine construction comprises an approach that allows the rapid testing of several vaccine candidates instead of limiting the analysis to antigens requiring optimized expression and purification. Since EHEC is an extracellular intestinal pathogen, our approach sought to induce mucosal immune responses, and DNA vaccines have been shown to induce both mucosal and systemic immune responses against pathogenic bacteria antigens when delivered by the intranasal route (53–55). During the cloning process in pVAX-1, large DNA sequences were divided in coding sequences with a maximum length of 1 kb. The constructs were pooled in their respective priority group and delivered intranasally in groups of 10 mice. A schedule of priming and two boosts was followed, with 60 μg of the pooled plasmids (20 μg of each individual construct) and cholera toxin (CT) as an adjuvant, which is further detailed in the Materials and Methods. Next, we sought to evaluate the induction of immune responses in vaccinated mice. First, we monitored the production of EHEC IgG in immunized mice by ELISA using serum samples collected 1 week after the last boost. HP and MP candidate-immunized mice presented higher levels of EHEC IgG compared to animals receiving buffer (TE), adjuvant (CT) alone, or pVAX-1 (empty vector) (Fig. 2A). Next, EHEC IgA titers in feces were determined. HP candidate-vaccinated mice elicited higher titers of EHEC sIgA compared to MP and LP candidate-immunized animals and control groups (Fig. 2B), although the titers declined with increased serum dilution. These results showed that although both HP and MP DNA vaccine pools induce EHEC antibody responses, only the HP group induced a larger amount of EHEC sIgA.
FIG 2.
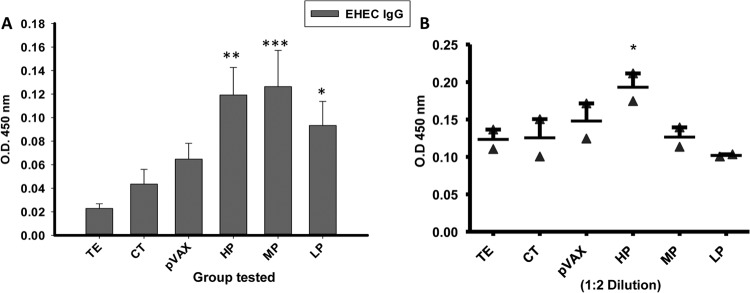
EHEC immune responses after priority groups' vaccination. (A) Anti-EHEC serum IgG following intranasal immunization. Groups of 10 mice were administered HP (pVAX-12, pVAX-31, and pVAX-56.2), MP (pVAX-43, pVAX-16, and pVAX-9), and LP (pVAX-51, pVAX49.1, and pVAX49.2) plasmids, along with CT as an adjuvant. As controls, nonimmunized mouse sera were obtained from mice receiving TE, pVAX-1, or CT. All sera were assessed using ELISA, and samples were diluted up to 1:1,000 for the assay. The results are expressed as means ± the standard deviations (SD) of duplicate values, and asterisks (*) are used to indicate statistical differences in the IgG response of HP, MP, and LP vaccine candidates compared to the TE group: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. (B) EHEC IgA measured in a fecal sample of immunized mice. Vaccinated mice were administered HP, MP, and LP vaccines, along with adjuvant (CT). Mice vaccinated with TE or pVAX-1 were used as controls. The samples were 2-fold diluted and assessed for EHEC sIgA antibody using ELISA. The results are expressed as means of duplicate values, and an asterisk (*) indicates a statistical difference (P < 0.05). HP, high priority; MP, medium priority; LP, low priority.
Two weeks after the last boost, animals were challenged with EHEC strain 86-24. The bacterial load in feces and cecum was determined from days 3 to 6 after infection. The results consistently showed a reduction pattern in the CFU recovered from feces in the HP candidate-vaccinated mice at days 4, 5, and 6 (Fig. 3A) compared to the TE buffer, CT, or pVAX-1 control groups. In addition, we observed a reduction in the LP candidate-vaccinated group at day 6, although it was less than that observed in the HP group. Cecum organ platting revealed a significant reduction of >1 log (P < 0.05) in the bacterial burden in HP candidate-vaccinated mice compared to the empty vector control group at day 3 (Fig. 3B). No reduction was observed in the ceca of MP or LP candidate-vaccinated mice. Overall, the data showed that HP plasmids were more efficient at reducing EHEC colonization in the murine model.
FIG 3.
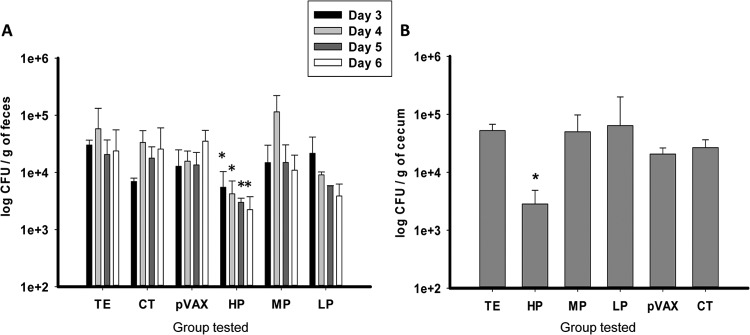
Bacterial counts in feces and cecum of EHEC-infected mice. (A) Fecal shedding of vaccinated mice with HP, MP, and LP vaccines with CT adjuvant and challenged with 109 EHEC O157:H7 for days 3 through 6 postimmunization. Feces were homogenized and plated onto MacConkey agar plates, and the numbers of CFU per gram were determined. Values are represented as means ± the SD from feces obtained from five mice, and asterisks (*) indicate statistical differences (P < 0.05). (B) Cecum segments were obtained from each mouse receiving HP, MP, and LP vaccines with CT adjuvant, infected with EHEC O157:H7. Cecum segments obtained at day 3 postinfection were homogenized and plated onto MacConkey agar plates. The ceca from mice receiving TE, pVAX-1, and CT were used as controls, and results are depicted as the log CFU/g of cecum. Means ± the SD reflect values of five mice per group, and an asterisk (*) denotes a statistically significant difference (P < 0.05).
To determine the extent of the immunogenic/protective effect of each candidate in the overall HP vaccine group, an individual plasmid vaccination experiment was performed. A total of 60 μg of pVAX-12, pVAX-31, pVAX-56.2, or TE buffer was administered to groups of mice with the same vaccination schedule as the previous experiment. To assess the immunogenicity of individual DNA vaccines, mouse sera were collected and assayed for EHEC IgG. The results showed increased EHEC IgG levels in pVAX-12. The IgG levels were also elevated with the pVAX-56.2-vaccinated group compared to the TE control group (Fig. 4A). The pVAX-31 plasmid failed to induce the production of EHEC IgG. In parallel, we measured EHEC sIgA in the feces of all vaccinated animals to evaluate mucosal immune induction. The mice immunized with pVAX-56.2 showed increases in sIgA up to the 1:8 dilution (Fig. 4B) compared to control animals. The pVAX-31 and, to a minor extent, pVAX-12 also increased the levels of sIgA in feces compared to the TE control group. Overall, pVAX-12 produced the higher induction of serum IgG, whereas pVAX-56.2 was able to stimulate high titers of both serum IgG and sIgA.
FIG 4.
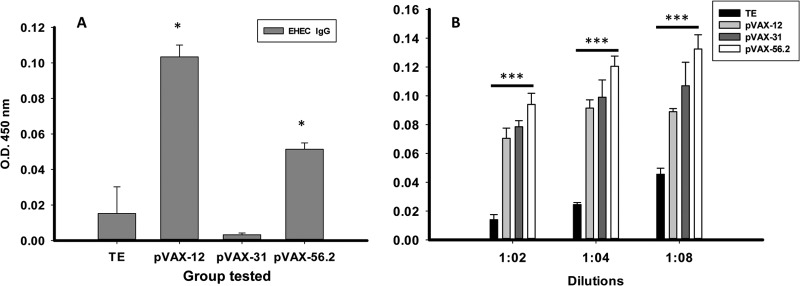
EHEC immune responses after pVAX candidate vaccination. (A) EHEC IgG antibody titers were detected in serum by ELISA. Sera were obtained from mice immunized with HP group and individual vaccine candidates: pVAX-12, pVAX-31, and pVAX-56.2 with CT adjuvant. A nonimmunized mice serum from TE group was used as control. The samples were diluted up to 1:1,000 for the assay. The results are expressed as means ± the SD of duplicate values obtained from five mice in each group, and an asterisk (*) indicates a significant difference (P < 0.05). (B) Titers of EHEC IgA measured from fecal samples of immunized mice with HP group and individual vaccine candidates: pVAX-12, pVAX-31, and pVAX-56.2 with CT adjuvant. Mice inoculated with TE were used as a control. The feces samples were serially diluted up to 1:8 for detection of sIgA antibody by ELISA. The results are expressed as means ± the SD of duplicate values from five mice in each group, and asterisks (***) indicate statistically significant differences (P < 0.05).
Next, mice vaccinated with individual candidates were challenged with EHEC 86-24 two weeks after the last boost. Daily bacterial loads in feces and cecum colonization from days 3 through 6 postinfection were assessed. The results show a clear reduction of EHEC in feces in all vaccinated groups at day 6 (Fig. 5A). No differences in cecum colonization were observed at day 3 (data not shown). However, a small reduction in EHEC CFU on the cecum from the pVAX-56.2-immunized group was detected at day 6 (Fig. 5B). Overall, pVAX-56.2 consistently induced EHEC serum IgG and fecal sIgA and reduced EHEC shedding and colonization, suggesting that candidate 56.2, which encodes the C-terminal fragment of EscC, the T3SS secretin, was the most effective candidate tested.
FIG 5.
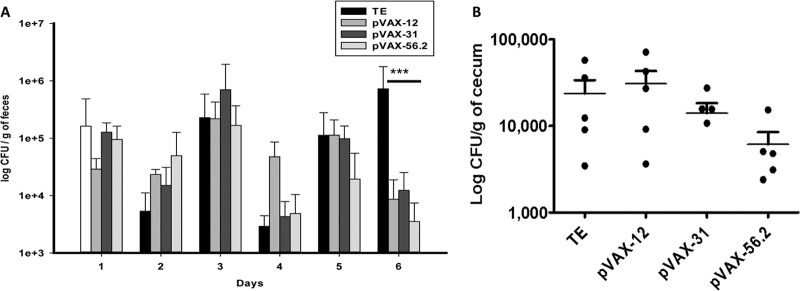
Bacterial counts in feces and cecum of EHEC-infected mice. (A) Bacterial fecal shedding was determined in mice vaccinated with pVAX-12, pVAX-31, pVAX-56.2, and HP group candidates (10 mice per group). Excretion of EHEC O157:H7 was determined as CFU/g of feces for the first 6 days postimmunization. The results are expressed as means ± the SD from five mice in each group, and asterisks (***) indicates a statistical difference (P < 0.05). (B) Cecum segments were obtained from each vaccinated mice (pVAX-12, pVAX-31, pVAX-56.2, and HP groups) challenged with 109 EHEC O157:H7. Cecum segments obtained at day 6 postinfection were homogenized and plated onto MacConkey agar. Means ± the SD of the CFU/g from 10 mice are presented.
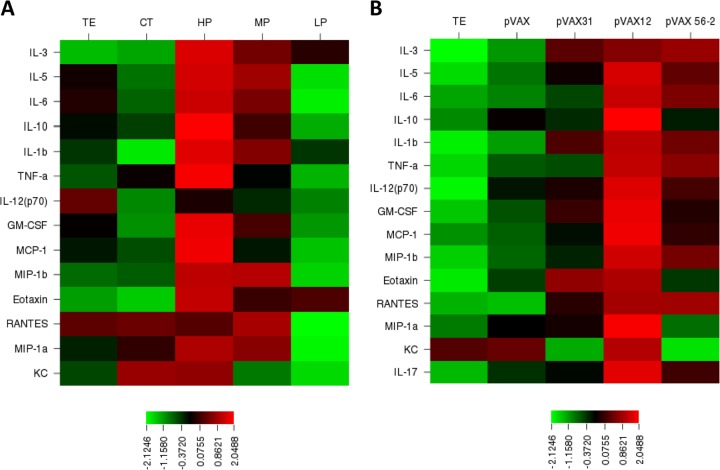
Because the cellular immune response plays a leading role in protection and long-term immune response, we also evaluated the expression of cytokines in sera of HP, MP, and LP candidate-immunized and nonimmunized mice using a Bio-Plex assay. Eleven of 23 inflammatory mediators were increased in HP candidate-immunized mice compared to nonimmunized mice, including the Th1 cytokines IL-1β and TNF-α, the Th2 cytokines IL-3, IL-5, IL-6, and IL-10, the chemokines MIP-1α, MIP-1β, eotaxin, KC, and MCP-1, and the growth factor GM-CSF (the data are summarized using heterogeneous colors between columns in the heat map in Fig. 6A). Proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 are increased at an early stage of the immune response and play a central role in the host defense mechanism. However, increased levels of Th2 cytokines, including IL-3, IL-5, IL-6, and IL-10 but not IL-4, in HP candidate-immunized mice were also detected. We propose that these Th2-type cytokine responses may account for the generation of the humoral antibody response. Sera obtained from mice vaccinated with the individual candidates pVAX-12 and pVAX-56.2 showed an increase in Th1 cytokines (IL-1β, IL-12p70, and TNF-α), Th2 cytokines (IL-3, IL-5, IL-6, and IL-10), chemokines (MIP-1β, eotaxin, MCP-1, and RANTES), and the GM-CSF growth factor compared to control mice (Fig. 6B).
FIG 6.
Schematic heat map representation of cytokines, chemokines, and growth factors in response to vaccinated and/or infected mice. (A and B) Bio-Plex assays were performed using the sera of immunized mice administered with HP, MP, and LP candidates (A) and the individual vaccine candidates pVAX-12, pVAX-31, and pVAX-56.2 (B). Nonimmunized sera from TE and CT mice were included as controls. The data are Z-score transformed and relative to controls and are expressed as SD units from the row mean. The samples were diluted up to 1:1 for the assay. The results are expressed as means ± the SD of duplicates. Statistical significance was calculated in both the A and B groups by using one-way ANOVA. All of the cytokines presented in the heat map were significantly different (P < 0.001) compared to the TE group.
DISCUSSION
Most EHEC subunit vaccine candidates tested to date are comprised of known virulence factors, such as Stx and the T3SS-related proteins. These virulence factors are well characterized and are known to be essential for the onset of EHEC colonization and/or host damage. Further, it is well documented that the main protection mechanism for these vaccine candidates is the induction of neutralizing antibodies (4). However, the DNA sequences encoded in the genome of EHEC strains may contain unveiled gene-encoding antigenic proteins, which have not yet been investigated as vaccine candidates. Computational vaccinology tools have been proposed as a potentially powerful aid in vaccine development, particularly for new or emerging pathogens for which critical antigenic determinants and/or virulence factors knowledge is limited (56). This work combines comparative genomics and immunoinformatics analysis of available EHEC genomes in the search for vaccine candidates. This approach represent an unbiased screening method, since it examined encoded sequences irrespective of their putative or experimental function, which allowed us to discover potential candidates overlooked by other EHEC vaccine studies. Subsequently, this method led us to develop a list of 65 vaccine candidates, three of which were proven to be able to induce immune responses, and one of these also reduced EHEC colonization when delivered as a DNA vaccine. It is noteworthy that none of these three experimentally assayed candidates has been used as an EHEC vaccine before. We propose that similar comparative genomics plus immunoinformatics analysis approaches may be useful for the unbiased search of vaccine candidates in other bacterial pathogens.
Since intestinal mucosal surfaces are composed of exposed tissue in permanent contact with harmless environmental bacteria, which likely participate in the maintenance of a homeostasis state (49), immune responses are relatively difficult to induce by vaccine candidates delivered in the gastrointestinal mucosa. The HP candidates were able to induce EHEC humoral responses both in serum and in intestinal mucosa. Although some candidates assayed in mice have been shown to be protective by inducing exclusively serum responses (20, 57), most involved the production of sIgA (17, 58–61). In our case, the induction of sIgA by the HP pool correlated with a reduction in colonization, while the MP pool was also able to increase serum IgG levels but failed to reduce EHEC colonization after challenge. Assays performed with the three HP candidates individually also seem to support the role of a humoral response by the intestinal mucosa in protection, since pVAX-12 failed to protect against EHEC colonization, despite inducing a strong serum humoral response. On the other hand, pVAX-56.2, the candidate inducing the highest sIgA titers in feces, was able to cause a reduction in the bacterial load in the cecum. Nevertheless, it seems that the combined immune response induced by the three candidates is required to achieve the colonization reduction levels observed with the HP pool. Arguably, the murine model of EHEC infection possesses inherent limitations. Although Stx delivery into mice resembles human HUS damage, such as thrombocytopenia, hemolysis, and renal failure (62), EHEC lethality in mice requires large amounts of the initial inoculum and does not promote the development of attaching and effacing lesions and diarrhea, in sharp contrast to the human infection (63, 64). However, due to their affordability and ease in handling, mice have largely been used for the initial screening of vaccine candidates (4). In addition, results obtained using EHEC-vaccinated mice have resembled those obtained for more relevant animal models (57).
Although much less investigated, cellular immune responses may play a role in protection against EHEC. Our data suggest that HP candidates induced an inflammatory response, with a mixed Th1/Th2 response. As part of an effective immune response strategy, the induction of a T helper immune response is required for a robust and long-lasting humoral immune response (65). In light of this, we examined Th1 and Th2 cytokine production, which could contribute to the generation of an efficient humoral immune response. Our data suggest that HP candidates induced an inflammatory response, with mixed Th1 and Th2 cytokine production. Previously, a similar mixed Th1-Th2 response was obtained when assaying EHEC bacterial ghosts as vaccine candidates (66). However, our findings showing a high production of IL-3, IL-5, IL-16, IL-10, and TNF-α by HP candidates may indicate a Th2-dominated response for subsequent stimulation of the observed humoral immune response. A further in-depth analysis is required for the cellular mechanisms triggered by the HP pool.
Overall, our approach allowed us to identify a group of EHEC-specific vaccine candidates, some of them displaying a high potential to encode protective immunogens. This bioinformatics and initial experimental assay confirms the immunogenic and relative protective potential of a subset of the candidates and further prompts the experimental characterization of the remaining ones in order to increase the stockpile of immunogens that can become components of an efficient multisubunit vaccine for humans or a new generation of vaccines for cattle.
ACKNOWLEDGMENTS
This study and the research in the A.G.T. laboratory is supported by NIH/NIAID grant R21AI09956001.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID or NIH.
Footnotes
Published ahead of print 4 March 2014
REFERENCES
- 1.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farfan MJ, Torres AG. 2012. Molecular mechanisms that mediate colonization of Shiga toxin-producing Escherichia coli strains. Infect. Immun. 80:903–913. 10.1128/IAI.05907-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen Y, Sperandio V. 2012. Enterohemorrhagic Escherichia coli (EHEC) pathogenesis. Front. Cell Infect. Microbiol. 2:90. 10.3389/fcimb.2012.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Angulo VA, Kalita A, Torres AG. 2013. Advances in the development of enterohemorrhagic Escherichia coli vaccines using murine models of infection. Vaccine 31:3229–3235. 10.1016/j.vaccine.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks HM, Tohamy SM, Tsui F. 2013. Modeling uncertainty of estimated illnesses attributed to non-O157:H7 Shiga toxin-producing Escherichia coli and its impact on illness cost. J. Food Prot. 76:945–952. 10.4315/0362-028X.JFP-12-409 [DOI] [PubMed] [Google Scholar]
- 6.Karmali MA, Gannon V, Sargeant JM. 2010. Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol. 140:360–370. 10.1016/j.vetmic.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 7.Pacheco AR, Sperandio V. 2012. Shiga toxin in enterohemorrhagic Escherichia coli: regulation and novel anti-virulence strategies. Front. Cell Infect. Microbiol. 2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong AR, Pearson JS, Bright MD, Munera D, Robinson KS, Lee SF, Frankel G, Hartland EL. 2011. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol. Microbiol. 80:1420–1438. 10.1111/j.1365-2958.2011.07661.x [DOI] [PubMed] [Google Scholar]
- 9.Sekiya K, Ohishi M, Ogino T, Tamano K, Sasakawa C, Abe A. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. U. S. A. 98:11638–11643. 10.1073/pnas.191378598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spreter T, Yip CK, Sanowar S, André I, Kimbrough TG, Vuckovic M, Pfuetzner RA, Deng W, Yu AC, Finlay BB, Baker D, Miller SI, Strynadka NC. 2009. A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nat. Struct. Mol. Biol. 16:468–476. 10.1038/nsmb.1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tree JJ, Wolfson EB, Wang D, Roe AJ, Gally DL. 2009. Controlling injection: regulation of type III secretion in enterohaemorrhagic Escherichia coli. Trends Microbiol. 17:361–370. 10.1016/j.tim.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 12.Walle KV, Vanrompay D, Cox E. 2012. Bovine innate and adaptive immune responses against Escherichia coli O157:H7 and vaccination strategies to reduce faecal shedding in ruminants. Vet. Immunol. Immunopathol. 152:109–120. 10.1016/j.vetimm.2012.09.028 [DOI] [PubMed] [Google Scholar]
- 13.Varela NP, Dick P, Wilson J. 2013. Assessing the existing information on the efficacy of bovine vaccination against Escherichia coli O157:H7: a systematic review and meta-analysis. Zoonoses Public Health 60:253–268. 10.1111/j.1863-2378.2012.01523.x [DOI] [PubMed] [Google Scholar]
- 14.Snedeker KG, Campbell M, Sargeant JM. 2012. A systematic review of vaccinations to reduce the shedding of Escherichia coli O157 in the faeces of domestic ruminants. Zoonoses Public Health 59:126–138. 10.1111/j.1863-2378.2011.01426.x [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa S, Kawahara K, Kagami Y, Isshiki Y, Kaneko A, Matsui H, Okada N, Danbara H. 2003. Protection against Shiga toxin 1 challenge by immunization of mice with purified mutant Shiga toxin 1. Infect. Immun. 71:3235–3239. 10.1128/IAI.71.6.3235-3239.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcato P, Mulvey G, Read RJ, Vander Helm K, Nation PN, Armstrong GD. 2001. Immunoprophylactic potential of cloned Shiga toxin 2 B subunit. J. Infect. Dis. 183:435–443. 10.1086/318080 [DOI] [PubMed] [Google Scholar]
- 17.Cai K, Gao X, Li T, Wang Q, Hou X, Tu W, Xiao L, Tian M, Liu Y, Wang H. 2011. Enhanced immunogenicity of a novel Stx2Am-Stx1B fusion protein in a mice model of enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine 29:946–952. 10.1016/j.vaccine.2010.11.035 [DOI] [PubMed] [Google Scholar]
- 18.Bentancor LV, Bilen M, Brando RJ, Ramos MV, Ferreira LC, Ghiringhelli PD, Palermo MS. 2009. A DNA vaccine encoding the enterohemorrhagic Escherichia coli Shiga-like toxin 2 A2 and B subunits confers protective immunity to Shiga toxin challenge in the murine model. Clin. Vaccine Immunol. 16:712–718. 10.1128/CVI.00328-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rojas RL, Gomes PA, Bentancor LV, Sbrogio-Almeida ME, Costa SO, Massis LM, Ferreira RC, Palermo MS, Ferreira LC. 2010. Salmonella enterica serovar Typhimurium vaccine strains expressing a nontoxic Shiga-like toxin 2 derivative induce partial protective immunity to the toxin expressed by enterohemorrhagic Escherichia coli. Clin. Vaccine Immunol. 17:529–536. 10.1128/CVI.00495-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Y, Feng Y, Luo P, Gu J, Yu S, Zhang WJ, Liu YQ, Wang QX, Zou QM, Mao XH. 2009. Fusion expression and immunogenicity of EHEC EspA-Stx2Al protein: implications for the vaccine development. J. Microbiol. 47:498–505. 10.1007/s12275-009-0116-8 [DOI] [PubMed] [Google Scholar]
- 21.Gu J, Liu Y, Yu S, Wang H, Wang Q, Yi Y, Zhu F, Yu XJ, Zou Q, Mao X. 2009. Enterohemorrhagic Escherichia coli trivalent recombinant vaccine containing EspA, intimin and Stx2 induces strong humoral immune response and confers protection in mice. Microbes Infect. 11:835–841. 10.1016/j.micinf.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 22.Gu J, Ning Y, Wang H, Xiao D, Tang B, Luo P, Cheng Y, Jiang M, Li N, Zou Q, Mao X. 2011. Vaccination of attenuated EIS-producing Salmonella induces protective immunity against enterohemorrhagic Escherichia coli in mice. Vaccine 29:7395–7403. 10.1016/j.vaccine.2011.07.069 [DOI] [PubMed] [Google Scholar]
- 23.Zhang XH, He KW, Zhang SX, Lu WC, Zhao PD, Luan XT, Ye Q, Wen LB, Li B, Guo RL, Wang XM, Lv LX, Zhou JM, Yu ZY, Mao AH. 2011. Subcutaneous and intranasal immunization with Stx2B-Tir-Stx1B-Zot reduces colonization and shedding of Escherichia coli O157:H7 in mice. Vaccine 29:3923–3939. 10.1016/j.vaccine.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 24.Zhang XH, He KW, Zhao PD, Ye Q, Luan XT, Yu ZY, Wen LB, Ni YX, Li B, Wang XM, Guo RL, Zhou JM, Mao AH. 2012. Intranasal immunization with Stx2B-Tir-Stx1B-Zot protein leads to decreased shedding in goats after challenge with Escherichia coli O157:H7. Vet. Rec. 170:178. 10.1136/vr.100325 [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Cai K, Shi J, Liu H, Hou X, Tu W, Xiao L, Wang Q, Wang H. 2009. Immunogenicity of a novel Stx2B-Stx1B fusion protein in a mice model of enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine 27:2070–2076. 10.1016/j.vaccine.2009.01.115 [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Cai K, Li T, Wang Q, Hou X, Tian R, Liu H, Tu W, Xiao L, Fang L, Luo S, Liu Y, Wang H. 2011. Novel fusion protein protects against adherence and toxicity of enterohemorrhagic Escherichia coli O157:H7 in mice. Vaccine 29:6656–6663. 10.1016/j.vaccine.2011.06.106 [DOI] [PubMed] [Google Scholar]
- 27.Mejias MP, Ghersi G, Craig PO, Panek CA, Bentancor LV, Baschkier A, Goldbaum FA, Zylberman V, Palermo MS. 2013. Immunization with a chimera consisting of the B subunit of Shiga toxin type 2 and brucella lumazine synthase confers total protection against Shiga toxins in mice. J. Immunol. 191:2403–2411. 10.4049/jimmunol.1300999 [DOI] [PubMed] [Google Scholar]
- 28.Abu-Ali GS, Ouellette LM, Henderson ST, Lacher DW, Riordan JT, Whittam TS, Manning SD. 2010. Increased adherence and expression of virulence genes in a lineage of Escherichia coli O157:H7 commonly associated with human infections. PLoS One 5:e10167. 10.1371/journal.pone.0010167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neupane M, Abu-Ali GS, Mitra A, Lacher DW, Manning SD, Riordan JT. 2011. Shiga toxin 2 overexpression in Escherichia coli O157:H7 strains associated with severe human disease. Microb. Pathog. 51:466–470. 10.1016/j.micpath.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur TM, Ahmed R, Chase-Topping M, Kalchayanand N, Schmidt JW, Bono JL. 2013. Characterization of Escherichia coli O157:H7 strains isolated from supershedding cattle. Appl. Environ. Microbiol. 79:4294–4303. 10.1128/AEM.00846-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews L, Reeve R, Woolhouse ME, Chase-Topping M, Mellor DJ, Pearce MC, Allison LJ, Gunn GJ, Low JC, Reid SW. 2009. Exploiting strain diversity to expose transmission heterogeneities and predict the impact of targeting supershedding. Epidemics 1:221–229. 10.1016/j.epidem.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 32.Matthews L, Reeve R, Gally DL, Low JC, Woolhouse ME, McAteer SP, Locking ME, Chase-Topping ME, Haydon DT, Allison LJ, Hanson MF, Gunn GJ, Reid SW. 2013. Predicting the public health benefit of vaccinating cattle against Escherichia coli O157. Proc. Natl. Acad. Sci. U. S. A. 110:16265–16270. 10.1073/pnas.1304978110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarr PI, Neill MA, Clausen CR, Newland JW, Neill RJ, Moseley SL. 1989. Genotypic variation in pathogenic Escherichia coli O157:H7 isolated from patients in Washington, 1984-1987. J. Infect. Dis. 159:344–347. 10.1093/infdis/159.2.344 [DOI] [PubMed] [Google Scholar]
- 34.Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Hebert RJ, Olcott ES, Johnson LM, Hargrett NT, Blake PA, Cohen ML. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681–685. 10.1056/NEJM198303243081203 [DOI] [PubMed] [Google Scholar]
- 35.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567–580. 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 36.Tusnady GE, Simon I. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849–850. 10.1093/bioinformatics/17.9.849 [DOI] [PubMed] [Google Scholar]
- 37.Kall L, Krogh A, Sonnhammer EL. 2007. Advantages of combined transmembrane topology and signal peptide prediction: the Phobius web server. Nucleic Acids Res. 35:W429–W432. 10.1093/nar/gkm256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, Brinkman FS. 2010. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26:1608–1615. 10.1093/bioinformatics/btq249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8:785–786. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 40.Kesmir C, Nussbaum AK, Schild H, Detours V, Brunak S. 2002. Prediction of proteasome cleavage motifs by neural networks. Protein Eng. 15:287–296. 10.1093/protein/15.4.287 [DOI] [PubMed] [Google Scholar]
- 41.Juncker AS, Willenbrock H, Von Heijne G, Brunak S, Nielsen H, Krogh A. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12:1652–1662. 10.1110/ps.0303703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berven FS, Karlsen OA, Straume AH, Flikka K, Murrell JC, Fjellbirkeland A, Lillehaug JR, Eidhammer I, Jensen HB. 2006. Analysing the outer membrane subproteome of Methylococcus capsulatus (Bath) using proteomics and novel biocomputing tools. Arch. Microbiol. 184:362–377. 10.1007/s00203-005-0055-7 [DOI] [PubMed] [Google Scholar]
- 43.Sachdeva G, Kumar K, Jain P, Ramachandran S. 2005. SPAAN: a software program for prediction of adhesins and adhesin-like proteins using neural networks. Bioinformatics 21:483–491. 10.1093/bioinformatics/bti028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doytchinova IA, Flower DR. 2007. VaxiJen: a server for prediction of protective antigens, tumour antigens, and subunit vaccines. BMC Bioinformatics 8:4. 10.1186/1471-2105-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saha S, Raghava GP. 2006. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 65:40–48. 10.1002/prot.21078 [DOI] [PubMed] [Google Scholar]
- 46.Larsen MV, Lundegaard C, Lamberth K, Buus S, Lund O, Nielsen M. 2007. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinformatics 8:424. 10.1186/1471-2105-8-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen M, Lund O. 2009. NN-align: an artificial neural network-based alignment algorithm for MHC class II peptide binding prediction. BMC Bioinformatics 10:296. 10.1186/1471-2105-10-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalita M, Tian B, Gao B, Choudhary S, Wood TG, Carmical JR, Boldogh I, Mitra S, Minna JD, Brasier AR. 2013. Systems approaches to modeling chronic mucosal inflammation. Biomed. Res. Int. 2013:1–17. 10.1155/2013/505864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cieza RJ, Cao A, Cong Y, Torres AG. 2012. Immunomodulation for GI infections. Expert Rev. Anti Infect. Ther. 10:391–400. 10.1586/eri.11.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barat S, Willer Y, Rizos K, Claudi B, Mazé A, Schemmer AK, Kirchhoff D, Schmidt A, Burton N, Bumann D. 2012. Immunity to intracellular Salmonella depends on surface-associated antigens. PLoS Pathog. 8:e1002966. 10.1371/journal.ppat.1002966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Y, Wu X, Zhang J, Xiao L, Yang Y, Bai X, Yu Q, Li Z, Bi L, Li N, Wu X. 2012. Immunogenicity and therapeutic effects of Ag85A/B chimeric DNA vaccine in mice infected with Mycobacterium tuberculosis. FEMS Immunol. Med. Microbiol. 66:419–426. 10.1111/1574-695X.12008 [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Lin L, Li N, She F. 2012. Enhancement of Helicobacter pylori outer inflammatory protein DNA vaccine efficacy by co-delivery of interleukin-2 and B subunit heat-labile toxin gene encoded plasmids. Microbiol. Immunol. 56:85–92. 10.1111/j.1348-0421.2011.00409.x [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Shi W, Yang JY, Zhou DH, Chen YQ, Zhang Y, Yang Y, He BX, Zhong MH, Li YM, Cao Y, Xiao Y, Li W, Yu J, Li YH, Fan MW, Yan HM. 2012. Flagellin-PAc fusion protein is a high-efficacy anti-caries mucosal vaccine. J. Dent. Res. 91:941–947. 10.1177/0022034512457684 [DOI] [PubMed] [Google Scholar]
- 54.Zhu C, Wang S, Hu S, Yu M, Zeng Y, You X, Xiao J, Wu Y. 2012. Protective efficacy of a Mycoplasma pneumoniae P1C DNA vaccine fused with the B subunit of Escherichia coli heat-labile enterotoxin. Can. J. Microbiol. 58:802–810. 10.1139/w2012-051 [DOI] [PubMed] [Google Scholar]
- 55.Zhu C, Wu Y, Chen S, Yu M, Zeng Y, You X, Xiao J, Wang S. 2012. Protective immune responses in mice induced by intramuscular and intranasal immunization with a Mycoplasma pneumoniae P1C DNA vaccine. Can. J. Microbiol. 58:644–652. 10.1139/w2012-041 [DOI] [PubMed] [Google Scholar]
- 56.De Groot AS, Levitz L, Ardito MT, Skowron G, Mayer KH, Buus S, Boyle CM, Martin WD. 2012. Further progress on defining highly conserved immunogenic epitopes for a global HIV vaccine: HLA-A3-restricted GAIA vaccine epitopes. Hum. Vaccine Immunother. 8:987–1000. 10.4161/hv.20528 [DOI] [PubMed] [Google Scholar]
- 57.Babiuk S, Asper DJ, Rogan D, Mutwiri GK, Potter AA. 2008. Subcutaneous and intranasal immunization with type III secreted proteins can prevent colonization and shedding of Escherichia coli O157:H7 in mice. Microb. Pathog. 45:7–11. 10.1016/j.micpath.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 58.Fan HY, Wang L, Luo J, Long BG. 2012. Protection against Escherichia coli O157:H7 challenge by immunization of mice with purified Tir proteins. Mol. Biol. Rep. 39:989–997. 10.1007/s11033-011-0824-0 [DOI] [PubMed] [Google Scholar]
- 59.Fujii J, Naito M, Yutsudo T, Matsumoto S, Heatherly DP, Yamada T, Kobayashi H, Yoshida S, Obrig T. 2012. Protection by a recombinant Mycobacterium bovis bacillus Calmette-Guerin vaccine expressing Shiga toxin 2B subunit against Shiga toxin-producing Escherichia coli in mice. Clin. Vaccine Immunol. 19:1932–1937. 10.1128/CVI.00473-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan CS, Zhou Y, Yu Y, Peng LJ, Zhao W, Zheng XL. 2011. B-cell epitope KT-12 of enterohemorrhagic Escherichia coli O157:H7: a novel peptide vaccine candidate. Microbiol. Immunol. 55:247–253. 10.1111/j.1348-0421.2011.00316.x [DOI] [PubMed] [Google Scholar]
- 61.Amani J, Mousavi SL, Rafati S, Salmanian AH. 2011. Immunogenicity of a plant-derived edible chimeric EspA, intimin, and Tir of Escherichia coli O157:H7 in mice. Plant Sci. 180:620–627. 10.1016/j.plantsci.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 62.Keepers TR, Psotka MA, Gross LK, Obrig TG. 2006. A murine model of HUS: Shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. J. Am. Soc. Nephrol. 17:3404–3414. 10.1681/ASN.2006050419 [DOI] [PubMed] [Google Scholar]
- 63.Mohawk KL, O'Brien AD. 2011. Mouse models of Escherichia coli O157:H7 infection and Shiga toxin injection. J. Biomed. Biotechnol. 2011:258185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohawk KL, Melton-Celsa AR, Zangari T, Carroll EE, O'Brien AD. 2010. Pathogenesis of Escherichia coli O157:H7 strain 86-24 following oral infection of BALB/c mice with an intact commensal flora. Microb. Pathog. 48:131–142. 10.1016/j.micpath.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srivastava IK, Liu MA. 2003. Gene vaccines. Ann. Intern. Med. 138:550–559. 10.7326/0003-4819-138-7-200304010-00011 [DOI] [PubMed] [Google Scholar]
- 66.Cai K, Gao X, Li T, Hou X, Wang Q, Liu H, Xiao L, Tu W, Liu Y, Shi J, Wang H. 2010. Intragastric immunization of mice with enterohemorrhagic Escherichia coli O157:H7 bacterial ghosts reduces mortality and shedding and induces a Th2-type dominated mixed immune response. Can. J. Microbiol. 56:389–398. 10.1139/W10-025 [DOI] [PubMed] [Google Scholar]