Abstract
NADPH oxidase is a crucial enzyme in antimicrobial host defense and in regulating inflammation. Chronic granulomatous disease (CGD) is an inherited disorder of NADPH oxidase in which phagocytes are defective in generation of reactive oxidant intermediates. Aspergillus species are ubiquitous, filamentous fungi, which can cause invasive aspergillosis, a major cause of morbidity and mortality in CGD, reflecting the critical role for NADPH oxidase in antifungal host defense. Activation of NADPH oxidase in neutrophils can be coupled to the release of proteins and chromatin that comingle in neutrophil extracellular traps (NETs), which can augment extracellular antimicrobial host defense. NETosis can be driven by NADPH oxidase-dependent and -independent pathways. We therefore undertook an analysis of whether NADPH oxidase was required for NETosis in Aspergillus fumigatus pneumonia. Oropharyngeal instillation of live Aspergillus hyphae induced neutrophilic pneumonitis in both wild-type and NADPH oxidase-deficient (p47phox−/−) mice which had resolved in wild-type mice by day 5 but progressed in p47phox−/− mice. NETs, identified by immunostaining, were observed in lungs of wild-type mice but were absent in p47phox−/− mice. Using bona fide NETs and nuclear chromatin decondensation as an early NETosis marker, we found that NETosis required a functional NADPH oxidase in vivo and ex vivo. In addition, NADPH oxidase increased the proportion of apoptotic neutrophils. Together, our results show that NADPH oxidase is required for pulmonary clearance of Aspergillus hyphae and generation of NETs in vivo. We speculate that dual modulation of NETosis and apoptosis by NADPH oxidase enhances antifungal host defense and promotes resolution of inflammation upon infection clearance.
INTRODUCTION
NADPH oxidase is a crucial enzyme in antimicrobial host defense and in regulating inflammation. Patients with chronic granulomatous disease (CGD), an inherited disorder of NADPH oxidase in which phagocytes are defective in generation of reactive oxidant intermediates (ROIs), suffer from life-threatening bacterial and fungal infections (1). CGD is also associated with severe inflammatory complications, such as Crohn's-like inflammatory bowel disease and obstructive granulomata in the genitourinary system (2). Among CGD patients, the degree of impairment of NADPH oxidase in neutrophils correlates with mortality (3). NADPH oxidase is rapidly activated by conditions that, in nature, are associated with infectious threat, such as the ligation of specific pathogen recognition receptors by microbial products (e.g., formylated peptides and fungal cell wall beta-glucans), opsonized particles, and integrin-dependent adhesion (4–6). Activation of the phagocyte NADPH oxidase (NOX2) requires translocation of cytoplasmic subunits p47phox, p67phox, and p40phox and Rac to a membrane-bound heterodimer cytochrome comprised of gp91phox and p22phox. Molecular oxygen is converted to superoxide anion, which can be converted to downstream metabolites, including H2O2, hydroxyl anion, and hypohalous acid that damage microbes.
Invasive mold diseases, most commonly aspergillosis, are major causes of morbidity and mortality in CGD (7–11), a reflection of the critical role for NADPH oxidase in mediating antifungal host defense. Invasive aspergillosis in CGD is a disorder of host defense and of dysregulated neutrophilic inflammation. “Mulch pneumonitis” in CGD is a life-threatening hyperinflammatory response to inhalation of a large fungal inoculum and is treated with both systemic corticosteroids to limit inflammation and antifungal therapy (12). Administration of heat-killed hyphae and fungal cell wall extracts lead to robust and persistent inflammation in NADPH oxidase-deficient mice but only to mild self-limited inflammation in wild-type (WT) mice (13–15), thereby demonstrating an intrinsic role for NADPH oxidase in regulating neutrophilic inflammation in response to fungal cell wall motifs.
The kinetics and mode of neutrophil death are important for host defense, persistence versus resolution of inflammation, and the injurious phenotype of neutrophils. Mature neutrophils are likely committed to autocrine death by their constitutive coexpression of cell surface Fas and FasL via a mechanism that is mediated by the activation of caspases and suppressed by proinflammatory cytokines (16). Neutrophil extracellular traps (NETs) are defined by the extracellular release of nuclear, cytosolic, and granular proteins and chromatin that comingle in filamentous structures (17, 18). NETosis is modulated by complex intracellular signaling, which can include activation of the Raf-MEK-ERK pathway, upregulation of antiapoptotic proteins, and autophagy (19, 20). While neutrophil apoptosis represents noninflammatory physiological cell death, NETosis results in the release of antimicrobial products that likely amplify extracellular host defense but can be injurious to host tissue. The balance between NETosis versus apoptosis of neutrophils is likely to be important for defense against pathogens while averting excessive inflammatory injury (21).
There is in vitro evidence that, depending on the nature of the stimulus, neutrophil NADPH oxidase can stimulate both apoptosis and NETosis. Impaired neutrophil apoptosis and clearance likely contribute to persistent inflammation in CGD (22–24). Activation of neutrophil NADPH oxidase is also coupled to activation of neutrophil serine proteases in primary granules (25) and to the generation of NETs. NET products bind to and kill bacteria, degrade bacterial virulence factors, and target fungi (18, 26, 27). Seen in this light, NADPH oxidase in neutrophils may defend against pathogens both through the direct antimicrobial effect of ROIs and by the subsequent activation of proteases and release of NETs.
Despite strong evidence from in vitro studies that NADPH oxidase activation is required for NETosis in stimulated neutrophils (17, 18), the role for NADPH oxidase in driving NETosis in vivo is less clear. We undertook an analysis of whether NADPH oxidase was required for NETosis in Aspergillus fumigatus pneumonia. Pulmonary challenge with conidia (spores) at an inoculum sufficient to cause even mild neutrophilic inflammation in WT mice is rapidly fatal in NADPH oxidase-deficient (p47phox−/−) mice (28). In contrast, we found that oropharyngeal instillation of a live hyphal suspension resulted in hyphal deposition in alveoli and focal neutrophilic pneumonitis in both genotypes at early time points, thus permitting a comparison of the kinetics of neutrophilic accumulation and death. Both live Aspergillus hyphal challenge (29) and administration of killed hyphae (15, 30) have been used in murine models to understand immune responses to hyphae. In contrast to fungal conidia that are phagocytosed, host defense against hyphae relies on extracellular pathways. Use of hyphae as a model of fungal pneumonia enables evaluation of NADPH oxidase-dependent host defense targeted to the tissue invasive form of the pathogen, as distinguished from pathways that phagocytose and kill spores or limit their germination (15, 29, 30).
We found that NADPH oxidase was required for hyphal clearance from lungs and resolution of inflammation. Neutrophils in the lungs of WT mice showed evidence of NETosis, identified by laminar extracellular DNA stretches that colocalized with neutrophil myeloperoxidase and histone immunostaining. In contrast, NET formation was not observed in the lungs of p47phox−/− mice. Neutrophils showing nuclear decondensation, an early marker of NETosis, and no activation of caspase-3 were detected in WT mice but were virtually absent in p47phox−/− mice. In addition, the proportion of apoptotic neutrophils was similar between genotypes on day 1 after infection but significantly greater in WT mice on day 3. Consistent with these in vivo results, exposure of neutrophils ex vivo to A. fumigatus hyphae led to NADPH oxidase-dependent nuclear decondensation and NET generation. Together, our results show that NADPH oxidase is required for pulmonary clearance of Aspergillus hyphae and that NADPH oxidase modulates both NETosis and apoptosis of neutrophils in vivo. We speculate that NADPH oxidase-driven NETosis augments host defense against extracellular hyphae, while stimulation of neutrophil apoptosis promotes resolution of inflammation and averts excessive tissue injury.
MATERIALS AND METHODS
Ethical statement.
All procedures performed on animals were approved by the Institutional Animal Care and Use Committee at Roswell Park Cancer Institute and complied with the U.S. Department of Health and Human Services' Guide for the Care and Use of Laboratory Animals.
Mice.
Targeted disruption of the p47phox gene leads to a defective NADPH oxidase. Phagocytes of mice with this gene defect are not capable of producing measurable amounts of superoxide (31). p47phox−/− mice were derived from C57BL/6 and 129 intercrosses and backcrossed 14 generations (N14) in the C57BL/6 background. Age (8 to 15 weeks)- and sex-matched C57BL/6 WT mice were used as controls. Mice were bred and maintained under specific-pathogen-free conditions at the animal care facility at Roswell Park Cancer Institute, Buffalo, NY.
Administration of A. fumigatus.
A. fumigatus strain Af293 was used. Hyphae were generated in the lab of Tobias Hohl (Fred Hutchinson Cancer and Research Center, Seattle, WA) by growing the strain in Sabouraud liquid broth (5 × 105 conidia/ml) for 48 h at 37°C. The mycelia were collected on a Buchner funnel, extensively washed with phosphate-buffered saline (PBS), and sonicated to an approximate hyphal fragment size of 10 to 100 μm. Hyphal suspensions (∼750 CFU/mouse) were administered by oropharyngeal instillation. We found that this approach leads to similar degrees of fungal pneumonia in p47phox−/− mice (32) and avoids surgery. Briefly, mice were anesthetized by isoflurane inhalation using an approved chamber. After anesthesia, mice were suspended by their upper incisors from a suture thread on a 90° incline board. The tongue was gently extended, and a liquid volume (50 μl) was delivered into the distal part of the oropharynx. With the tongue extended, the animal was unable to swallow, and the liquid volume was aspirated into the lower respiratory tract. Just prior to liquid delivery, the chest was gently compressed and then released just after deposition of liquid into the oropharynx to enhance aspiration of the liquid into the lung. Mice recovered within 5 min of the procedure and were observed until they resumed normal activity.
Histopathology.
After sacrifice, mouse lungs were infused with 10% neutral buffered formalin via the trachea. Paraffin-embedded blocks were prepared and sections were stained with hematoxylin and eosin (H&E) to assess inflammation and Grocott-Gomori methenamine-silver stain (GMS) to visualize fungi. Tissues were microscopically examined for pulmonary injury, vascular invasion, and structural changes in Aspergillus hyphae. All slides were analyzed using ×40 magnification without formal morphometric analysis and blinded to genotype. The percentage of lung involved by inflammation was scored in each mouse as follows: 0, 5, and 10% and thereafter by 10% increments (e.g., 20, 30, 40, etc.). The predominant inflammatory cell type was scored.
Immunostaining of mouse tissue.
For immunostaining, specimens were processed similarly as described previously (33). Briefly, samples were deparaffinized, rehydrated in decreasing concentrations of ethanol, and subjected to antigen retrieval by cooking in 10 mM citrate buffer (pH 6.0) for 10 min. Specimens were blocked with 2% bovine serum albumin (BSA) and 36 μl of mouse immunoglobulin blocking reagent (Vector Laboratories)/ml in PBS–0.1% Triton X-100 for 1 h at room temperature. For visualization of the NETs, primary antibodies directed against myeloperoxidase (A0398; Dako) and histone H1 (clone AE-4; Acris Antibodies) diluted in blocking solution were applied overnight at 4°C. For the detection of apoptotic neutrophils, specimens were blocked with 2% BSA in PBS–0.1% Triton X-100, and primary antibodies directed against cleaved caspase-3 (catalog no. 9661; Cell Signaling Technology) and the Ly-6B.2 alloantigen (clone 7/4; AbD Serotec) were used. Primary antibodies were detected with Alexa Fluor 568- and 488-conjugated secondary antibodies (Life Technologies) diluted in 2% BSA in PBS–0.1% Triton X-100, respectively. DNA was visualized with DAPI (4′,6′-diamidino-2-phenylindole; Life Technologies), and slides were mounted with fluorescence mounting medium (Dako). An Eclipse C1 plus confocal microscope (Nikon Instruments) using a 100× oil immersion objective lens was used for image acquisition. Wavelengths of 405 nm (diode), 488 nm (Argon), and 543 nm (HeNe) were used to excite DAPI, Alexa Fluor 488 (and transmission images [trans]), and Alexa Fluor 568, respectively. Images were captured in separate passes to avoid cross talk and are presented as maximum intensity projections from Z-stacks if not otherwise stated. All images were adjusted for background fluorescence and enhanced slightly for signal intensity in NIS elements software (Nikon Instruments). Slides were analyzed for NET formation blinded to genotype.
For quantification analyses, fluorescence images were captured with an Eclipse 90i microscope (Nikon Instruments) and a Hamamatsu Orca-ER digital camera at ×60 magnification as Z-stacks covering the complete specimen depth. In overlay images of all three channels captured (DAPI, Alexa Fluor 488, and Alexa Fluor 568), the proportions of neutrophils with the following immunostaining phenotypes were quantified by manually scrolling through Z-planes: (i) decondensed nucleus, (ii) cleaved capsase-3 positive, (iii) decondensed nucleus and cleaved capsase-3 positive, and (iv) decondensed nucleus and cleaved capsase-3 negative (a marker of early stage NETosis). Neutrophils were counted manually from at least two digitized images per site (bronchioles and alveoli) and animal (n = 3) in NIS elements software (Nikon Instruments) covering between 770 and 3,600 cells per site and animal (total of between 3,500 and 6,800 cells per genotype and site). The results are expressed as number of positive cells/1,000 evaluated neutrophils.
Analysis of neutrophil NET generation and apoptosis following hyphal stimulation ex vivo.
Bone marrow neutrophils from WT and p47phox−/− mice were purified by density centrifugation as described previously (34). The purity of neutrophils is ≥85% based on cytology. Neutrophils (106 cells) were seeded onto glass coverslips (22 by 22 mm) in six-well cell culture plates in 500 μl of RPMI 1640 and incubated in a 5% CO2 incubator at 37°C for 1 h. Neutrophils were then exposed to A. fumigatus hyphae (∼750 CFU/50 μl). After either 2 or 6 h, the cells were fixed with 2% paraformaldehyde overnight at 4°C. Cells were gently washed in PBS, incubated for 1 h with anti-Ly6G (clone RB6-8C5; eBioscience) at 1 μg/ml, and then permeabilized for 5 min in permeabilization buffer (eBioscience), followed by 1 h of incubation with cleaved caspase-3 (clone D175; Cell Signaling) at 1 μg/ml. After washing, the cells were incubated with TRITC (tetramethyl rhodamine isothiocyanate)-conjugated anti-rat IgG (eBioscience) and Cy2-conjugated anti-rabbit IgG (Jackson ImmunoResearch) secondary antibodies. Coverslips were washed and mounted on slides using mounting medium with DAPI (Vectashield, 1.5 μg/ml). Fluorescence images were obtained from a TCS SP2 AOBS spectral confocal scanner mounted on a Leica DM IRE2 inverted fluorescence microscope using a ×63 oil immersion objective lens. Wavelengths of 405 nm (diode), 488 nm (argon), and 543 nm (HeNe) were used to excite DAPI (+ bright-field image [BF]), fluorescein isothiocyanate (FITC), and TRITC, respectively. Prism spectrophotometric filters ranging from 415 to 460 nm, 495 to 550 nm, and 570 to 640 nm were used to collect fluorescence emission of DAPI, FITC, and TRITC, respectively, in sequential acquisition mode. Leica confocal software was used to optimize laser settings for saturation and eliminate cross talk. Imaging fields were chosen at random and Z-sections were optimized for a number of cells. Cells were assessed by overlaying DAPI stain with BF to assess if a cell was intact or fragmented. Intact cells were quantified as neutrophils if they were both Ly6G+ and contained nuclear material by overlaying DAPI and TRITC stains. A total of 300 neutrophils per sample were counted. Nuclei of these cells were determined to be decondensed or hypersegmented compared to unstimulated neutrophils, using the same criteria that we used in in vivo studies. These cells were then assessed for caspase-3 activity by overlaying DAPI and FITC stains. The major endpoints evaluated were the presence of NETosis (yes or no) and the proportion of neutrophils with decondensed nuclei and cleaved caspase-3 negative per 300 evaluated neutrophils.
Statistical analysis.
Comparison of the proportion of neutrophils with specific immunostaining phenotypes between genotypes was assessed by using the chi-square test. The percent lung inflammation over time was compared between genotypes by two-way analysis of variance with a Bonferroni post test. Analysis was performed using Prism 5.0 software (GraphPad Software). Differences were considered significant for a two-sided P value of <0.05 and are denoted by asterisks as follows in the figures and legends: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
RESULTS
NADPH oxidase is crucial for defense against Aspergillus fumigatus hyphae.
Neutrophils from CGD patients are deficient in NET formation in vitro upon challenge with various stimuli, including pathogenic fungi (17, 18, 35). However, the role for NADPH oxidase in NET generation during fungal infection in vivo has not been investigated. Our goal was to evaluate whether NADPH oxidase was required for NET generation in pulmonary aspergillosis. To address this question, we investigated NET formation in A. fumigatus pneumonia in WT (C57BL/6) and NADPH oxidase-deficient p47phox−/− (CGD) mice. We used a hyphal challenge model instead of fungal spores because CGD mice develop severe fungal pneumonia following challenge with low spore inocula (50% lethal dose of ∼2.5 × 103 spores/mouse), while WT mice rapidly clear infection with a mild self-limited inflammatory response in lungs after challenge with >107 spores/mouse (28, 36). In contrast, we found that hyphal challenge leads to neutrophilic lung inflammation in both genotypes, enabling comparison of NETosis. In addition, the use of hyphae as a model of fungal pneumonia enables the evaluation of NADPH oxidase-dependent host defense targeted to the tissue invasive form of the pathogen.
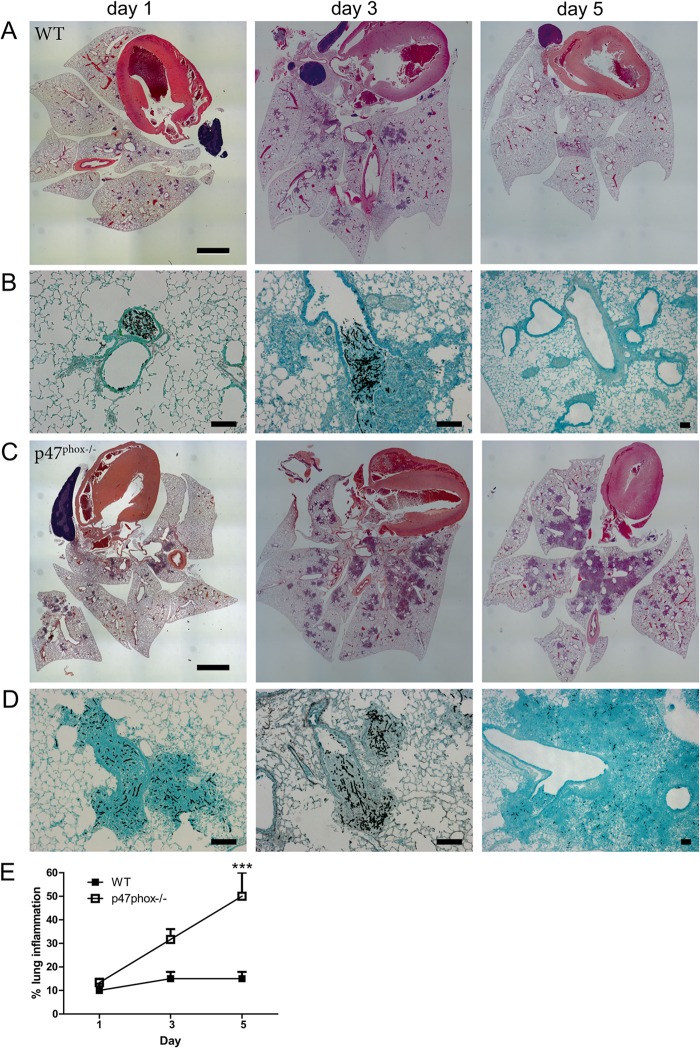
WT and p47phox−/− mice were administered a live hyphal suspension (corresponding to ∼750 CFU/mouse) by oropharyngeal instillation. Mice (n = 3 per genotype per time point) were sacrificed at 1, 3, or 5 days after infection, and lung sections were fixed at the time of harvest. The extent of inflammation and the presence of hyphae were assessed histologically by H&E and GMS staining, respectively (Fig. 1). Airway and peribronchovascular neutrophilic inflammation was similar, and hyphae were present in WT and p47phox−/− mice on day 1 after the instillation of A. fumigatus hyphae. On day 3, neutrophilic inflammation had increased in p47phox−/− mice but remained stable in WT mice. On day 5, neutrophilic inflammation had abated in WT mice with transition to a predominantly lymphohistiocytic infiltrate, in association with virtually complete clearance of hyphae. In contrast, predominantly neutrophilic lung consolidation progressed in CGD mice, in association with persistent hyphae within lung tissue (Fig. 1E). These results show that NADPH oxidase is required for clearance of Aspergillus hyphae and resolution of neutrophilic inflammation in vivo.
FIG 1.
A. fumigatus hyphae cause neutrophilic inflammation in both WT and p47phox−/− mice. WT and NADPH oxidase-deficient p47phox−/− mice were administered A. fumigatus hyphae by oropharyngeal instillation (∼750 CFU/mouse), and lungs were harvested on days 1, 3, and 5 after infection (n = 3 per genotype per time point). Representative lung histology images from WT (A and B) and p47phox−/− mice (C and D) are shown. Airway and peribronchovascular neutrophilic inflammation occurred in both genotypes on day 1 and day 3 (A and C; H&E staining). Neutrophilic inflammation had abated in WT mice by day 5 (A) but progressed in p47phox−/− mice (C). GMS staining of lung sections showed intact hyphae in WT (B) and p47phox−/− mice (D) on days 1 and 3 after instillation and almost complete clearance of hyphae on day 5 in WT mice but persistence in p47phox−/− mice. (E) Quantification of the lung inflammation in WT and p47phox−/− mice after hyphal challenge. Lung sections were analyzed using ×40 magnification without formal morphometric analysis and blinded to genotype. Scale bars, 2 mm (A and C) and 100 μm (B and D).
NADPH oxidase is required for neutrophil extracellular trap generation in pulmonary aspergillosis.
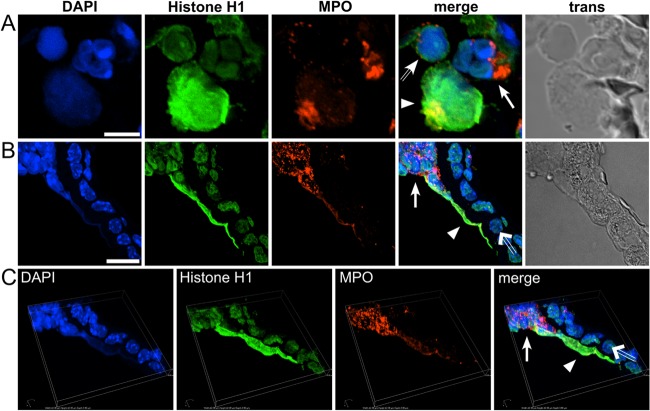
We next sought to determine whether NETs were formed by lung infiltrating neutrophils of WT and p47phox−/− mice following fungal challenge. Since neutrophilic airway inflammation was scant in WT mice at day 5, the comparison of NETs between genotypes was limited to days 1 and 3 after hyphal administration. NETs were identified using an immunofluorescence approach that showed colocalization of DNA, histones, and myeloperoxidase (MPO) in laminar extracellular stretches. NETs were observed in alveoli and within bronchi of WT mice. Figure 2A shows alveolar neutrophils at different stages of NET formation in a WT mouse at day 1 after hyphal administration. The neutrophil with diffuse DAPI staining, reflecting nuclear DNA decondensation, is an early stage of NETosis; the bona fide NET is characterized by colocalization of nuclear chromatin and MPO. Alveolar NETs occurred as solitary, rounded structures, ∼3-fold the size of an intact neutrophil. MPO colocalized partially with chromatin and was more concentrated at an edge of a NET. NETs were also visible in confocal transmission images (Fig. 2).
FIG 2.
Detection of neutrophil extracellular traps (NETs) by immunofluorescence. Neutrophils in the lungs of WT mice showed evidence of NETosis after oropharyngeal instillation of A. fumigatus hyphae (n = 3 per genotype per time point). Using representative samples, NETs were visualized by indirect immunofluorescence in sections of lung tissue using primary antibodies against histone H1 and neutrophil myeloperoxidase (MPO). Alexa Fluor 488- and 568-conjugated secondary antibodies were used for visualization of histone H1 (green channel) and MPO (red channel), respectively. (A) NETs exhibiting rounded morphology, ∼3-fold the size of an intact neutrophil, were prominent in alveoli at day 1 postinfection Three adjacent neutrophils show different stages of NET formation: (i) a non-NETotic neutrophil with intact nucleus showing typical lobulated morphology and clear separation of euchromatin and heterochromatin visualized by DAPI staining (solid arrow); (ii) a neutrophil at an early stage of NETosis characterized by loss of nuclear integrity resulting in decondensed chromatin, identified based on diffuse DAPI and anti-histone staining, but intact azurophilic granules containing MPO (hollow arrow); and (iii) a bona fide NET, identified by laminar extracellular DNA (DAPI positive) that colocalized with histone and MPO immunostaining (arrowhead). (B) NET formation by intrabronchiolar neutrophils (solid arrow), resulting in stretches of laminar DNA (arrowhead) identified by colocalization of DAPI-stained DNA, histone, and MPO. Adjacent bronchial epithelia cells (hollow arrow) are MPO negative. (C) Three-dimensional view of panel B, assembled by Z-stacking of captured images showing elongated NET structure in close proximity to the epithelial surface. The image is turned slightly counter clockwise, with the Z-axis tilted backward (bird's-eye view). Pictures were taken with a Nikon C1 confocal microscope at ×100 magnification. Scale bars, 5 μm (A) and 10 μm (B).
Alveolar NETs were prominently observed on day 1 after hyphal administration in WT mice but were less frequent on day 3. In addition to solitary alveolar NETs on day 1, NETs were found in between neutrophils at sites of neutrophil aggregation (data not shown). Neutrophil accumulation into bronchial lumens was observed on day 1 and increased on day 3 postinfection, when lumens were filled with neutrophils. Intrabronchiolar NETs were occasionally observed on day 1 (Fig. 2B and C) but were more prominent at these sites on day 3 postinfection (Fig. 3). NET formation by intrabronchiolar neutrophils was characterized by stretches of laminar DNA with colocalization of histone and MPO (Fig. 3B). No NETs or NET remnants were observed at day 5 postinfection in WT mice, when neutrophilic inflammation had abated with only few neutrophils present (not shown).
FIG 3.
Neutrophil NETosis occurs in WT mice following A. fumigatus infection. Neutrophil extracellular traps were detected in lungs of WT mice at days 1 and 3 after oropharyngeal administration of A. fumigatus hyphae (n = 3 per time point). Representative indirect immunofluorescence from a section of lung tissue at day 3 postinfection is shown. (A) NETs were most prominent at the bronchial epithelium-lumen interfaces but were also detected in between polymorphonuclear neutrophils at sites of high neutrophil density (arrowheads). NETs were identified by colocalization of extracellular laminar DNA (DAPI positive) with histone and MPO immunostaining. Borders of intraluminal neutrophils and bronchial epithelium cells (hollow arrows) were marked by a dashed line in the DAPI image. Intact intrabronchiolar neutrophils were identified based on myeloperoxidase immunostaining (solid arrow), and surrounding bronchial epithelia cells are MPO negative. (B) Magnified section of panel A, highlighting a NET (arrowhead) at the bronchial epithelium-lumen interface (the epithelium is marked by hollow arrows). Pictures were taken with a Nikon C1 confocal microscope at ×100 magnification. Scale bars, 10 μm (A) and 5 μm (B).
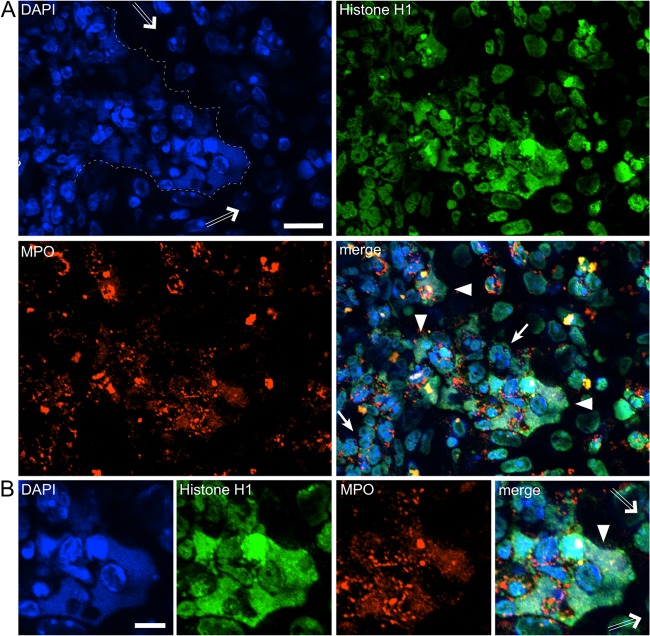
Neutrophilic lung inflammation in p47phox−/− mice significantly differed from that observed in WT mice. First, alveolar and bronchial inflammation was far more robust in p47phox−/− mice and was characterized by dense clusters of neutrophils. Neutrophilic inflammation had abated in WT mice by day 5 after Aspergillus administration but progressed in p47phox−/− mice. Second, neutrophil nuclei had the typical lobulated shape in p47phox−/− mice and, notably, we did not observe NETosis in the lungs of p47phox−/− mice at any time point examined (Fig. 4). These results show that NADPH oxidase is required for NETosis during pulmonary aspergillosis.
FIG 4.
Neutrophil NETosis is undetectable in NADPH oxidase-deficient p47phox−/− mice after A. fumigatus infection. NADPH oxidase-deficient p47phox−/− mice were administered A. fumigatus hyphae by oropharyngeal instillation (n = 3 per time point), and lungs were harvested on days 1, 3, and 5. Representative indirect immunofluorescence from sections of lung tissue stained as described in Fig. 2 was assessed. We did not observe NETosis in the lungs of p47phox−/− mice at any time point examined. Neutrophil nuclei appeared in their typical lobulated shape (solid arrows in panels A [day 1], B, [day 3], and C [day 5]) and were devoid of NETs. The bronchial epithelium is indicated by hollow arrows. Scale bars, 5 μm.
Neutrophil nuclear decondensation is increased in WT mice compared to p47phox−/− mice during pulmonary aspergillosis.
Since a NET can be a vestige of more than one neutrophil, the number of NETotic neutrophils at the terminal stages of NET release cannot be reliably quantitated with respect to cell number. Studies in vitro have shown that the early stage of NETosis involves intracellular decondensation of nuclear chromatin, followed by nuclear expansion (17, 19, 20, 37, 38). We therefore sought to determine whether NADPH oxidase would influence these attributes in vivo.
We first evaluated the average number of neutrophil nuclei containing decondensed chromatin at sites of inflammatory lesion by immunostaining. Consecutive cuts from the same specimens that were analyzed for NET formation were stained with antibodies directed against Ly-6B.2, a surface marker for neutrophils, and DNA was stained with DAPI. In addition, activation of caspase-3, an effector caspase executing programmed cell death (39) was assessed based on cleaved capase-3 immunostaining on the same sections (Fig. 5A).
FIG 5.
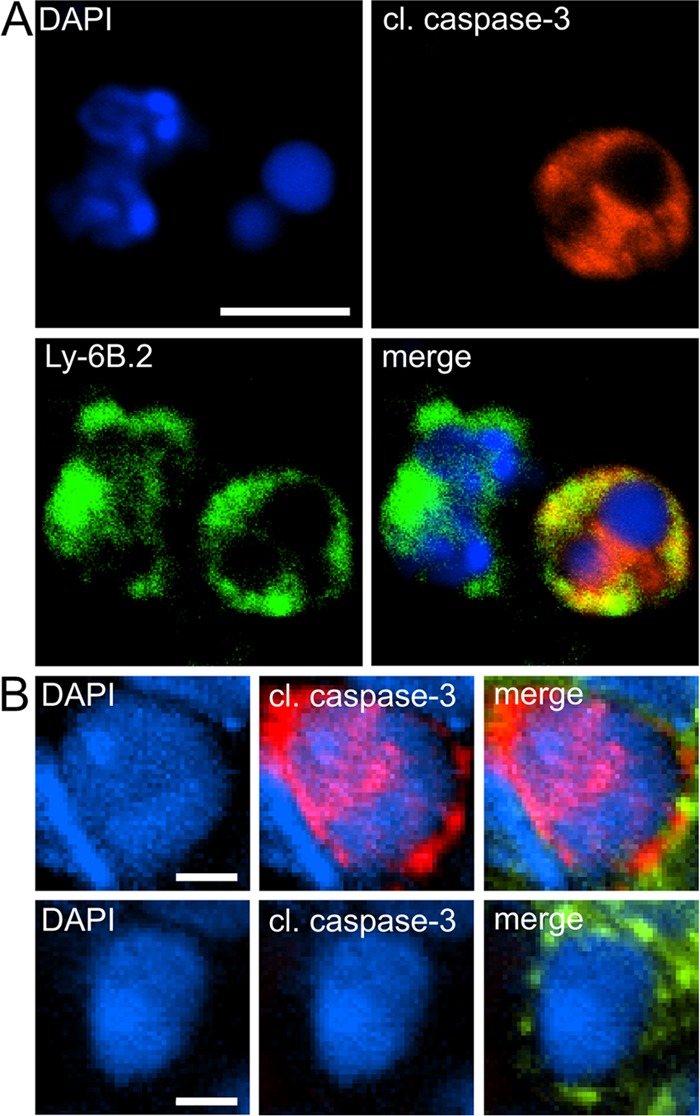
Detection of apoptotic neutrophils by immunofluorescence. Apoptotic neutrophils in lungs of WT and NADPH oxidase-deficient p47phox−/− mice at days 1 and 3 after oropharyngeal instillation of A. fumigatus hyphae (n = 3 per genotype per time point) were visualized by indirect immunofluorescence using primary antibodies against the neutrophil surface marker Ly-6B.2 and cleaved caspase-3. Alexa Fluor 488- and 568-conjugated secondary antibodies were used for visualization of Ly-6B.2 (green channel) and cleaved caspase-3 (red channel), respectively. (A) Apoptotic neutrophils were identified based on immunostaining of activated caspase-3 (red channel) and partially exhibit a pyknotic nucleus with loss of nuclear lobulation and homogeneous chromatin distribution. An adjacent intact neutrophil (left) is negative for activated caspase-3, and nuclear material is separated into euchromatin and heterochromatin. (B) Representative images of neutrophils with decondensed nuclei evaluated as apoptotic (positive for cleaved caspase-3, upper panel) or potentially NETotic (negative for cleaved caspase-3, lower panel) in quantification analyses. Pictures were taken with a Nikon C1 confocal microscope at ×100 magnification (A) and a Nikon Eclipse 90i microscope at ×60 magnification (B), respectively. Scale bars, 5 μm (A) and 2 μm (B).
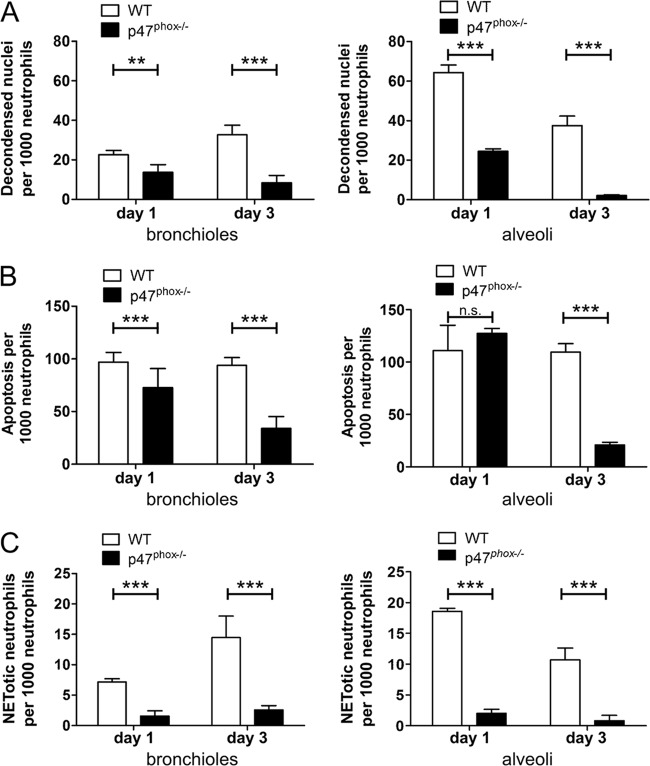
Decondensed neutrophil nuclei—characterized by the loss of a clear separation of euchromatin and heterochromatin—were identified based on diffuse DAPI staining in Ly-6B.2-positive cells. Representative images used for evaluation are presented in Fig. 5B. Cells with decondensed nuclei were counted from at least two different images from similar inflammatory lesions in bronchioles and alveoli that were analyzed for NET formation on day 1 and day 3 after hyphal administration (n = 3 mice per genotype per time point) and are presented as number per 1,000 neutrophils. Whereas nuclear decondensation was observed in neutrophils in the airways of WT mice at both time points (day 1, bronchioles [2.26% ± 0.37%] and alveoli [6.44% ± 0.67%]; day 3, bronchioles [3.26% ± 0.85%] and alveoli [3.75% ± 0.82%]), the proportion of neutrophils with nuclear decondensation was significantly lower at day 1 postinfection and hardly detectable at day 3 postinfection in p47phox−/− mice (day 1, bronchioles [1.36% ± 0.69%] and alveoli [2.46% ± 0.21%]; day 3, bronchioles [0.84% ± 0.66%] and alveoli [0.22% ± 0.06%]) (Fig. 6A). These results demonstrate that nuclear decondensation can be quantitated at a single-cell level in inflamed tissue and provide further support for the requirement of NADPH oxidase to NET generation during fungal pneumonia.
FIG 6.
Quantitative approach to evaluate NET formation. We visually counted different populations of neutrophils from immunofluorescence images of infected lung tissue at day 1 and day 3 (n = 3 per genotype per time point). Both bronchial and alveolar sites of neutrophilic inflammation were evaluated blinded for genotype. Nuclei were counted from sections of at least two fluorescence images for each animal (n = ∼4,000 cells per strain and site), and mean number per 1,000 neutrophils is indicated. (A) Total numbers of decondensed neutrophil nuclei identified based on diffuse DAPI staining (see Fig. 5B). (B) Numbers of apoptotic cells based on cleaved caspase-3 staining regardless of nuclear morphology. (C) Numbers of decondensed neutrophil nuclei identified based on diffuse DAPI staining that did not stain for cleaved caspase-3 and are most likely committed to undergo NETosis. The data are means ± the standard deviations of three biological replicates. The proportions between grouped data from each genotype were compared using a chi-square test.
NADPH oxidase promotes apoptosis of neutrophils in lungs following Aspergillus challenge.
In addition to NET formation, neutrophils with an aberrant nuclear morphology might also be committed to undergo apoptosis, which is likewise characterized by the loss of nuclear lobulation. In contrast to NETosis, apoptosis is characterized by chromatin condensation rather than decondensation. However, these nuclear morphologies may not be easily distinguished at early stages of neutrophil death in fixed tissue samples. Therefore, we evaluated apoptotic cells at the sites evaluated for decondensed neutrophil nuclei by immunofluorescence detection of active caspase-3 (Fig. 5). Activation of caspases was not observed during NET formation by our group and others (17, 20, 38, 40). Thus, we reasoned that nuclear decondensation in the absence of caspase activation might be an attribute of neutrophils that are prone to undergo NETosis. To test this hypothesis, we determined the number of apoptotic neutrophils in relation to the total number of decondensed nuclei and the number of decondensed nuclei that were devoid of an apoptotic staining (Fig. 6B and C).
A similar proportion of apoptotic cells was observed at day 1 postinfection in both genotypes (WT, bronchioles [9.7% ± 1.6%] and alveoli [11.09% ± 4.22%]; p47phox−/−, bronchioles [7.27% ± 3.12%] and alveoli [12.74% ± 0.81%]). However, the proportion of neutrophils undergoing apoptosis at day 3 remained constant in WT mice but was significantly reduced in p47phox−/− mice (WT, bronchioles [9.38% ± 1.31%] and alveoli [10.69% ± 1.38%]; p47phox−/−, bronchioles [3.4% ± 1.97%] and alveoli [2.1% ± 0.41%]) (Fig. 6B). We also observed neutrophils that exhibited decondensed nuclear chromatin but were not positive for activated caspase-3 in WT animals (day 1, bronchioles [0.72% ± 0.09%] and alveoli [1.86% ± 0.09%]; day 3, bronchioles [1.45% ± 0.61%] and alveoli [1.07% ± 0.34%]). This cell type was virtually absent in p47phox−/− mice at any time point analyzed (day 1, bronchioles [0.16% ± 0.15%] and alveoli [0.2% ± 0.12%]; day 3, bronchioles [0.26% ± 0.12%] and alveoli [0.08% ± 0.14%]) (Fig. 6C).
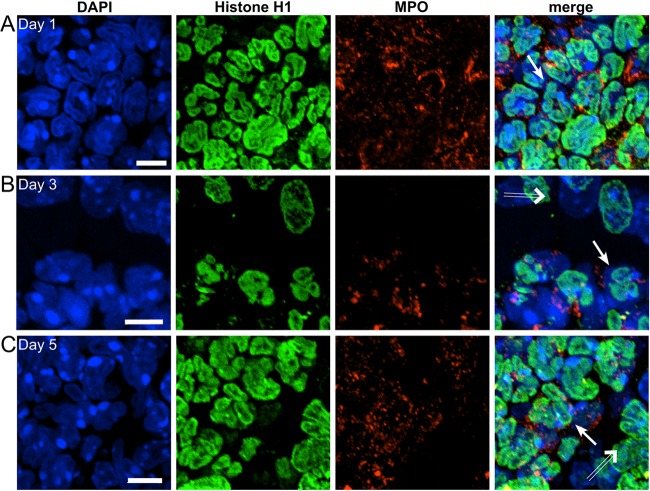
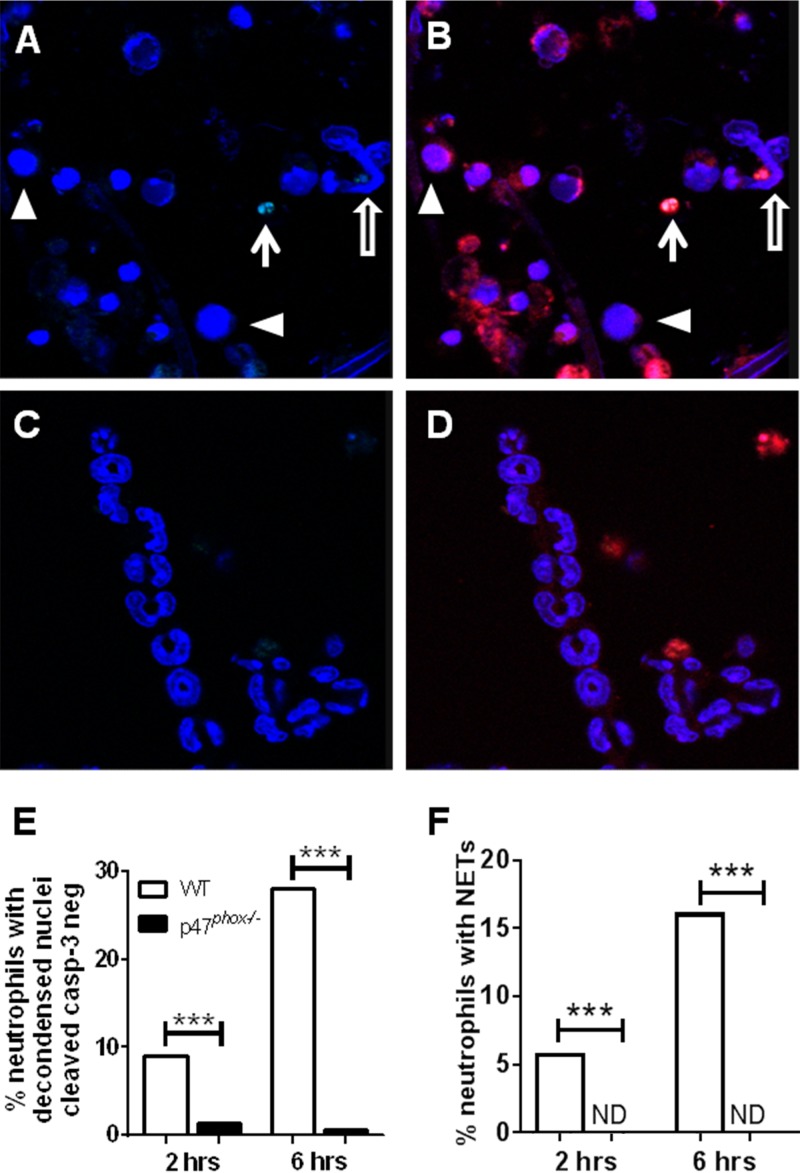
These findings suggest that decondensed neutrophil nuclei that do not exhibit caspase activation can serve as an indirect measure to quantify neutrophils that are committed to undergo NET formation. The presence of neutrophils that exhibit decondensed nuclear chromatin but are negative for an apoptotic marker in WT mice and the considerably lower frequency of these cells in p47phox−/− mice support our qualitative observation that NADPH oxidase is required for NET formation during pulmonary fungal infection. Realizing that neutrophilic responses in vivo could be modulated by several factors (e.g., the age of the neutrophils, the time of the recruitment of neutrophils to the lung in relation to lung harvest, and the cytokine milieu), we conducted ex vivo studies to evaluate the role of NADPH oxidase in neutrophil nuclear decondensation and NET generation following hyphal stimulation. WT and p47phox−/− bone marrow-purified neutrophils were stimulated with A. fumigatus hyphae or vehicle and fixed at 2 or 6 h after stimulation. The proportions of WT neutrophils with decondensed nuclei and cleaved capase-3 negative were 27/300 (9%) at 2 h and 84/300 (28%) at 6 h after hyphal stimulation. In contrast, virtually all p47phox−/− neutrophils had a hypersegmented appearance, with only ∼1% showing nuclear decondensation and cleaved caspase-3 negative (Fig. 7). NETs were visualized in WT neutrophils at 2 h (17/300; 5.7%) and at 6 h (48/300; 16%) but not in p47phox−/− neutrophils (Fig. 7). Cleaved caspase-3 was not detected in NETs under these conditions. By assuming that a NET is a vestige of a single neutrophil, this scoring approach may underestimate the proportion of NETotic neutrophils. Although this experiment does not enable assessment of the fate of individual neutrophils over time, our in vivo and ex vivo results show the requirement for NADPH oxidase for A. fumigatus hypha-induced NET generation and support cleaved caspase-3-negative neutrophils with decondensed nuclei as quantitative markers for NET generation.
FIG 7.
Aspergillus hypha-induced neutrophil nuclear decondensation and NETosis are NADPH oxidase dependent. Bone marrow-purified WT and p47phox−/− neutrophils were stimulated with A. fumigatus hyphae or vehicle, followed by fixation at 2 or 6 h. Cells were stained with DAPI (blue channel), Ly6G (red channel), and cleaved caspase-3 (green channel). Representative images from 2 h are shown. A total of 300 neutrophils (Ly6G+) per genotype per stimulation condition were analyzed. (A and B) WT neutrophils with DAPI staining (A) and an overlay of all three stains (B) showing populations with nuclear decondensation (arrowheads), bona fide NETs (hollow arrows), and rare apoptotic neutrophils (arrow). (C and D) In contrast, p47phox−/− neutrophils had almost uniformly hypersegmented nuclei. Vehicle-treated neutrophils from both genotypes had the expected hypersegmented appearance (data not shown). (E) The percentage of total neutrophils with decondensed nuclei and that are cleaved capasase-3 negative was significantly greater in hypha-stimulated WT versus p47phox−/− neutrophils at both time points. (F) Bona fide NETs were observed in hypha-stimulated WT neutrophils but not detected (ND) in p47phox−/− neutrophils. NETs did not exhibit detectable cleaved caspase-3 staining. Comparison between genotypes was performed by using a chi-square test.
DISCUSSION
Using an NADPH oxidase-deficient mouse model that mimics CGD, we used different approaches to qualitatively and quantitatively assess NET formation in Aspergillus fumigatus pneumonia, a frequent cause of death in patients suffering from this disease. Oropharyngeal instillation of a live hyphal suspension resulted in hyphal deposition in alveoli and focal neutrophilic pneumonitis in both WT and CGD genotypes at early time points, thus permitting a comparison of the kinetics of neutrophilic accumulation and death, as well as hyphal clearance. Use of hyphae as a model of fungal pneumonia enables evaluation of NADPH oxidase-dependent host defense targeted to the tissue invasive form of the pathogen, as distinguished from pathways that kill spores or limit transition from spores to the hyphal stage. We found that NADPH oxidase was required for hyphal clearance. Fungal disease had resolved in WT mice by day 5 after infection but progressed in p47phox−/− mice. In addition, NADPH oxidase was required for NET generation. In WT mice, neutrophils in the lungs showed evidence of NETosis, identified by laminar extracellular DNA stretches that colocalized with neutrophil myeloperoxidase and histone, whereas NETotic neutrophils were not identified in the lungs of p47phox−/− mice. Since the detection of NETs relies on visual identification, we acknowledge the possibility of rare NETotic events that were not observed. In addition to being required for NETosis, NADPH oxidase also increased the proportion of apoptotic neutrophils at day 3 after hyphal administration. A limitation of this experiment is that confounding variables such as differences in neutrophil age and density can potentially affect the proportion of apoptotic neutrophils (41). Together, these results show that NADPH oxidase is required for pulmonary clearance of Aspergillus hyphae and generation of NETs and likely also limits neutrophilic inflammation by accelerating apoptosis.
Consistent with prior in vitro studies showing that neutrophil-mediated damage of Aspergillus hyphae is augmented by NADPH oxidase (42–44), our results show that NADPH oxidase targets A. fumigatus hyphae during pulmonary infection. However, the requirement for NADPH oxidase for neutrophil-mediated damage varies among different Aspergillus strains (45), reflecting the importance of both NADPH oxidase-dependent and -independent pathways. Conceivably, Aspergillus strains may differ in their ability to induce NET generation in neutrophils, and the virulence of Aspergillus strains might be influenced by their ability to induce NETs in vivo. In contrast to fungal conidia that are phagocytosed, host defense against hyphae relies on extracellular pathways. A. fumigatus hyphae have been shown to be the most potent inducers of NET formation in human neutrophils in vitro compared to resting and swollen conidia (46). Calprotectin is a NET constituent that mediates nutritional immunity by sequestering divalent metal ions and targets Candida and Aspergillus species (33, 47). Based on these results, we speculate that NADPH oxidase may damage A. fumigatus hyphae through a direct injurious effect of ROIs and indirectly by promoting NET generation. It is appealing to assume that NETs diminish dissemination of Aspergillus hyphae by entrapment and growth inhibition. Although the proportion of NETotic neutrophils in WT mice at fixed time points following fungal challenge is low, the considerably large areas of NETs (Fig. 2) may serve an important function in limiting hyphal tissue invasion. Studies of isolated neutrophils and experimental pulmonary aspergillosis point to NETs restricting hyphal growth rather than having a major role in fungal killing (18, 46, 47). Further studies are required to elucidate whether the major role of NETs is to trap versus directly kill pathogens in vivo.
While neutrophil apoptosis leads to noninflammatory physiological cell death, NETosis results in the extracellular release of proteases and other injurious neutrophil constituents that can exacerbate inflammatory injury (48–50). Apoptosis in neutrophils is the default mode of death and is modulated via NADPH oxidase-dependent and -independent pathways, depending on the nature of the stimulus (22, 51). Accelerating neutrophil death and clearance are likely to be important modes by which NADPH oxidase limits acute inflammation. In contrast, NETosis can be triggered by exposure of neutrophils to numerous stimuli, including bacteria and fungi and their products, proinflammatory cytokines, and complement-mediated opsonization (17, 27, 52–55). We speculate that NADPH oxidase may augment extracellular antifungal host defense through NETosis and promote resolution of inflammation after fungal infection through stimulation of apoptosis.
Quantification of NET release bears some inherent challenges due to the fragility of these structures. Nuclear expansion has been used earlier as a marker and quantitative measure for NET formation in vitro (19, 37, 38). We adapted this approach for in vivo analysis of nuclear expansion together with assessment of apoptosis. Quantitative evaluation of nuclear decondensation at similar sites that were analyzed for NET formation revealed a significantly reduced number of decondensed nuclei in CGD compared to WT mice. Apoptosis is also characterized by loss of nuclear lobulation. Indeed, evaluation of apoptotic neutrophils in the same lesions that were analyzed for chromatin decondensation revealed that a large proportion of neutrophil nuclei with homogenous chromatin distribution was apoptotic (based on cleaved caspase-3 immunostaining). Observations from our group and others support the notion that caspases are not activated during NETosis (17, 20, 38). Whereas neutrophils with decondensed nuclei and negative for cleaved caspase-3 were found in WT mice, they were virtually absent in CGD mice. Consistent with these in vivo findings, WT neutrophils exposed to A. fumigatus hyphae ex vivo had nuclear decondensation and formed NETs, while p47phox−/− neutrophils had a virtually uniform hypersegmented nuclear morphology. The percentage of decondensed, cleaved-caspase-3-negative nuclei of WT neutrophils was ex vivo larger than in vivo, which might stem from different surrounding milieus, nevertheless emphasizing the necessity for in vivo quantification. A limitation of this approach is that we cannot track the fate of individual neutrophils regarding the development of bona fide NETs. Together, direct visualization of NETs shows a requirement for NADPH oxidase in NETosis during pulmonary aspergillosis, and quantification of early NETotic markers (decondensed nuclei and negative for cleaved caspase-3 immunostaining) in pulmonary aspergillosis and in ex vivo studies provides additional confirmatory results.
The requirement for NADPH oxidase in NETosis is dependent on the stimulus (38, 52, 56–59). H2O2 and hypochlorous acid, ROI metabolites generated by NADPH oxidase activation in neutrophils, trigger NET release (17, 60–62). Neutrophils from NADPH oxidase-deficient mice were unable to form NETs in response to Candida albicans (35). Neutrophils from CGD patients are defective in NETosis, and gene therapy results in restored NETosis in NADPH oxidase-competent neutrophils in vitro (18). There are very limited data that address the role for NADPH oxidase in NET generation in vivo. Soluble immune complexes induce NETosis independently of NADPH oxidase (63). In pneumococcal lung infection, NETosis of BALF-recovered neutrophils was reduced, but not eliminated, in NADPH oxidase-deficient mice (55). In this model, NADPH oxidase may trigger NETosis directly or through stimulation of IFN-γ, which was shown to augment NETosis (55). Extracellular matrix components have been shown to be necessary for NET formation in response to fungal pathogens in a ROI-independent manner in vitro (64). However, our studies in vivo did not show NET formation by extravasated neutrophils in lung extracellular matrix in the absence of a functional NADPH oxidase.
Our results demonstrate a crucial contribution of NADPH oxidase in both the clearance of Aspergillus hyphae and for NETosis during pulmonary infection. Further studies are required to delineate the relative importance of ROI-mediated fungal damage versus NETosis in promoting Aspergillus clearance. In addition to its host defense function, we and others have found that NADPH oxidase can modulate oxidant-sensitive pathways that limit lung inflammation and injury following challenge with both microbial products and direct caustic insult (14, 15, 30, 65–68). Delineating mechanisms by which NADPH oxidase modulates the extent of neutrophilic inflammation and the mode of neutrophil death may identify new therapeutic approaches to limit inflammatory injury.
ACKNOWLEDGMENTS
We acknowledge Åsa and Jonathan Gilthorpe for reagents and assistance in immunostaining and Patrik Rydén for statistical advice.
M.R., M.J.G., N.G.A., B.H.S., and C.F.U. conceived and designed the experiments. M.R. and M.J.G. performed the experiments. M.R., M.J.G., A.C.D., B.H.S., and C.F.U. analyzed the data. M.R., B.H.S., and C.F.U. wrote the paper.
This study was supported by National Institute of Allergy and Infectious Disease grant R01AI079253 to B.H.S. and the Swedish Research Council VR-M (K2012-99X-21961-01-3), the Laboratory for Molecular Medicine Sweden (MIMS), and the Medial Faculty Umeå (316-886-10) to C.F.U. M.R. was funded by a postdoctoral fellowship of the Kempe Foundation. This research was supported, in part, by an NCI Cancer Center support grant to Roswell Park Cancer Institute (CA016056).
Footnotes
Published ahead of print 18 February 2014
REFERENCES
- 1.Segal BH, Veys P, Malech H, Cowan MJ. 2011. Chronic granulomatous disease: lessons from a rare disorder. Biol. Blood Marrow Transplant. 17:S123–S131. 10.1016/j.bbmt.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marciano BE, Rosenzweig SD, Kleiner DE, Anderson VL, Darnell DN, Anaya-O'Brien S, Hilligoss DM, Malech HL, Gallin JI, Holland SM. 2004. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics 114:462–468. 10.1542/peds.114.2.462 [DOI] [PubMed] [Google Scholar]
- 3.Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DA, Soule BP, Zarember KA, Malech HL, Holland SM, Gallin JI. 2010. Residual NADPH oxidase and survival in chronic granulomatous disease. N. Engl. J. Med. 363:2600–2610. 10.1056/NEJMoa1007097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham DB, Stephenson LM, Lam SK, Brim K, Lee HM, Bautista J, Gilfillan S, Akilesh S, Fujikawa K, Swat W. 2007. An ITAM-signaling pathway controls cross-presentation of particulate but not soluble antigens in dendritic cells. J. Exp. Med. 204:2889–2897. 10.1084/jem.20071283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. 2002. Syk is required for integrin signaling in neutrophils. Immunity 16:547–558. 10.1016/S1074-7613(02)00303-5 [DOI] [PubMed] [Google Scholar]
- 6.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107–1117. 10.1084/jem.20021787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segal BH, DeCarlo ES, Kwon-Chung KJ, Malech HL, Gallin JI, Holland SM. 1998. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore, MD) 77:345–354. 10.1097/00005792-199809000-00004 [DOI] [PubMed] [Google Scholar]
- 8.Grimm MJ, Vethanayagam RR, Almyroudis NG, Lewandowski D, Rall N, Blackwell TS, Segal BH. 2011. Role of NADPH oxidase in host defense against aspergillosis. Med. Mycol. 49(Suppl 1):S144–S149. 10.3109/13693786.2010.487077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallin JI, Alling DW, Malech HL, Wesley R, Koziol D, Marciano B, Eisenstein EM, Turner ML, DeCarlo ES, Starling JM, Holland SM. 2003. Itraconazole to prevent fungal infections in chronic granulomatous disease. N. Engl. J. Med. 348:2416–2422. 10.1056/NEJMoa021931 [DOI] [PubMed] [Google Scholar]
- 10.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. 2000. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine (Baltimore, MD) 79:155–169. 10.1097/00005792-200005000-00003 [DOI] [PubMed] [Google Scholar]
- 11.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Espanol T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia MJ, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. 2009. Chronic granulomatous disease: the European experience. PLoS One 4:e5234. 10.1371/journal.pone.0005234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui S, Anderson VL, Hilligoss DM, Abinun M, Kuijpers TW, Masur H, Witebsky FG, Shea YR, Gallin JI, Malech HL, Holland SM. 2007. Fulminant mulch pneumonitis: an emergency presentation of chronic granulomatous disease. Clin. Infect. Dis. 45:673–681. 10.1086/520985 [DOI] [PubMed] [Google Scholar]
- 13.Schappi M, Deffert C, Fiette L, Gavazzi G, Herrmann F, Belli D, Krause KH. 2008. Branched fungal beta-glucan causes hyperinflammation and necrosis in phagocyte NADPH oxidase-deficient mice. J. Pathol. 214:434–444. 10.1002/path.2298 [DOI] [PubMed] [Google Scholar]
- 14.Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, Feminella J, Dennis CG, Vethanayagam RR, Yull FE, Capitano M, Wallace PK, Minderman H, Christman JW, Sporn MB, Chan J, Vinh DC, Holland SM, Romani LR, Gaffen SL, Freeman ML, Blackwell TS. 2010. NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One 5:e9631. 10.1371/journal.pone.0009631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC. 1997. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 185:207–218. 10.1084/jem.185.2.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. 1996. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J. Exp. Med. 184:429–440. 10.1084/jem.184.2.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231–241. 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J. 2009. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114:2619–2622. 10.1182/blood-2009-05-221606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. 2011. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 7:75–77. 10.1038/nchembio.496 [DOI] [PubMed] [Google Scholar]
- 20.Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, Noppen S, Delforge M, Willems J, Vandenabeele P. 2011. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 21:290–304. 10.1038/cr.2010.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almyroudis NG, Grimm MJ, Davidson BA, Rohm M, Urban CF, Segal BH. 2013. NETosis and NADPH oxidase: at the intersection of host defense, inflammation, and injury. Front. Immunol. 4:45. 10.3389/fimmu.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA, Mayadas TN. 1996. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 5:653–666. 10.1016/S1074-7613(00)80278-2 [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, Henson PM, Bratton DL. 2009. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood 113:2047–2055. 10.1182/blood-2008-05-160564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanmun D, Witasp E, Jitkaew S, Tyurina YY, Kagan VE, Ahlin A, Palmblad J, Fadeel B. 2009. Involvement of a functional NADPH oxidase in neutrophils and macrophages during programmed cell clearance: implications for chronic granulomatous disease. Am. J. Physiol. Cell Physiol. 297:C621–C631. 10.1152/ajpcell.00651.2008 [DOI] [PubMed] [Google Scholar]
- 25.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291–297. 10.1038/416291a [DOI] [PubMed] [Google Scholar]
- 26.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. 2006. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 8:668–676. 10.1111/j.1462-5822.2005.00659.x [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 28.Vethanayagam RR, Almyroudis NG, Grimm MJ, Lewandowski DC, Pham CT, Blackwell TS, Petraitiene R, Petraitis V, Walsh TJ, Urban CF, Segal BH. 2011. Role of NADPH oxidase versus neutrophil proteases in antimicrobial host defense. PLoS One 6:e28149. 10.1371/journal.pone.0028149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SJ, Burdick MD, Brix WK, Stoler MH, Askew DS, Strieter RM, Mehrad B. 2010. Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J. Immunol. 185:6190–6197. 10.4049/jimmunol.1002064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. 2008. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451:211–215. 10.1038/nature06471 [DOI] [PubMed] [Google Scholar]
- 31.Jackson SH, Gallin JI, Holland SM. 1995. The p47phox mouse knockout model of chronic granulomatous disease. J. Exp. Med. 182:751–758. 10.1084/jem.182.3.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimm MJ, Vethanayagam RR, Almyroudis NG, Dennis CG, Khan AN, D'Auria AC, Singel KL, Davidson BA, Knight PR, Blackwell TS, Hohl TM, Mansour MK, Vyas JM, Rohm M, Urban CF, Kelkka T, Holmdahl R, Segal BH. 2013. Monocyte- and macrophage-targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice. J. Immunol. 190:4175–4184. 10.4049/jimmunol.1202800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5:e1000639. 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizzolla A, Hultqvist M, Nilson B, Grimm MJ, Eneljung T, Jonsson IM, Verdrengh M, Kelkka T, Gjertsson I, Segal BH, Holmdahl R. 2012. Reactive oxygen species produced by the NADPH oxidase 2 complex in monocytes protect mice from bacterial infections. J. Immunol. 188:5003–5011. 10.4049/jimmunol.1103430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. 2009. Mouse neutrophil extracellular traps in microbial infections. J. Innate Immun. 1:181–193. 10.1159/000205281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimm MJ, Vethanayagam RR, Almyroudis NG, Dennis CG, Khan AN, D'Auria AC, Singel KL, Davidson BA, Knight PR, Blackwell TS, Hohl TM, Mansour MK, Vyas JM, Rohm M, Urban CF, Kelkka T, Holmdahl R, Segal BH. 2013. Monocyte- and macrophage-targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice. J. Immunol. 190:4175–4184. 10.4049/jimmunol.1202800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. 2010. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191:677–691. 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farley K, Stolley JM, Zhao P, Cooley J, Remold-O'Donnell E. 2012. A serpinB1 regulatory mechanism is essential for restricting neutrophil extracellular trap generation. J. Immunol. 189:4574–4581. 10.4049/jimmunol.1201167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanghavi DM, Thelen M, Thornberry NA, Casciola-Rosen L, Rosen A. 1998. Caspase-mediated proteolysis during apoptosis: insights from apoptotic neutrophils. FEBS Lett. 422:179–184. 10.1016/S0014-5793(98)00004-0 [DOI] [PubMed] [Google Scholar]
- 40.Fadeel B, Ahlin A, Henter JI, Orrenius S, Hampton MB. 1998. Involvement of caspases in neutrophil apoptosis: regulation by reactive oxygen species. Blood 92:4808–4818 [PubMed] [Google Scholar]
- 41.Hannah S, Nadra I, Dransfield I, Pryde JG, Rossi AG, Haslett C. 1998. Constitutive neutrophil apoptosis in culture is modulated by cell density independently of β2 integrin-mediated adhesion. FEBS Lett. 421:141–146. 10.1016/S0014-5793(97)01551-2 [DOI] [PubMed] [Google Scholar]
- 42.Rex JH, Bennett JE, Gallin JI, Malech HL, Melnick DA. 1990. Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J. Infect. Dis. 162:523–528. 10.1093/infdis/162.2.523 [DOI] [PubMed] [Google Scholar]
- 43.Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI. 2007. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J. Immunol. 178:6367–6373 [DOI] [PubMed] [Google Scholar]
- 44.Chang YC, Segal BH, Holland SM, Miller GF, Kwon-Chung KJ. 1998. Virulence of catalase-deficient Aspergillus nidulans in p47phox−/− mice: implications for fungal pathogenicity and host defense in chronic granulomatous disease. J. Clin. Invest. 101:1843–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henriet SS, Hermans PW, Verweij PE, Simonetti E, Holland SM, Sugui JA, Kwon-Chung KJ, Warris A. 2011. Human leukocytes kill Aspergillus nidulans by reactive oxygen species-independent mechanisms. Infect. Immun. 79:767–773. 10.1128/IAI.00921-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, Jeron A, Latge JP, Brakhage AA, Gunzer M. 2010. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 6:e1000873. 10.1371/journal.ppat.1000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. 2011. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin dependent. J. Allergy Clin. Immunol. 127:1243–1252. 10.1016/j.jaci.2011.01.021 [DOI] [PubMed] [Google Scholar]
- 48.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. 2012. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 122:2661–2671. 10.1172/JCI61303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, Cifuni SM, Fuchs TA, von Andrian UH, Hartwig JH, Aster RH, Wagner DD. 2012. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 119:6335–6343. 10.1182/blood-2012-01-405183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 179:199–210. 10.1016/j.ajpath.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geering B, Gurzeler U, Federzoni E, Kaufmann T, Simon HU. 2011. A novel TNFR1-triggered apoptosis pathway mediated by class IA PI3Ks in neutrophils. Blood 117:5953–5962. 10.1182/blood-2010-11-322206 [DOI] [PubMed] [Google Scholar]
- 52.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. 2012. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18:1386–1393. 10.1038/nm.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, Yamamoto N, Akira S. 2012. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 12:109–116. 10.1016/j.chom.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 54.Martinelli S, Urosevic M, Daryadel A, Oberholzer PA, Baumann C, Fey MF, Dummer R, Simon HU, Yousefi S. 2004. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J. Biol. Chem. 279:44123–44132. 10.1074/jbc.M405883200 [DOI] [PubMed] [Google Scholar]
- 55.Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, Dittmer DP, Doerschuk CM. 2011. Interferon-gamma production by neutrophils during bacterial pneumonia in mice. Am. J. Respir. Crit. Care Med. 183:1391–1401. 10.1164/rccm.201004-0592OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. 2012. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 92:841–849. 10.1189/jlb.1211601 [DOI] [PubMed] [Google Scholar]
- 57.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185:7413–7425. 10.4049/jimmunol.1000675 [DOI] [PubMed] [Google Scholar]
- 58.Arai Y, Nishinaka Y, Arai T, Morita M, Mizugishi K, Adachi S, Takaori-Kondo A, Watanabe T, Yamashita K. 2013. Uric acid induces NADPH oxidase-independent neutrophil extracellular trap formation. Biochem. Biophys. Res. Commun. 443:556–561. 10.1016/j.bbrc.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 59.Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo DG, Park JJ, Moskowitz SM, Malech HL, Leto TL. 2013. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PLoS One 8:e54205. 10.1371/journal.pone.0054205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. 2010. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207:1853–1862. 10.1084/jem.20100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer LJ, Cooper PR, Ling MR, Wright HJ, Huissoon A, Chapple IL. 2012. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin. Exp. Immunol. 167:261–268. 10.1111/j.1365-2249.2011.04518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akong-Moore K, Chow OA, von Kockritz-Blickwede M, Nizet V. 2012. Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS One 7:e42984. 10.1371/journal.pone.0042984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen K, Nishi H, Travers R, Tsuboi N, Martinod K, Wagner DD, Stan R, Croce K, Mayadas TN. 2012. Endocytosis of soluble immune complexes leads to their clearance by FcγRIIIB but induces neutrophil extracellular traps via FcγRIIA in vivo. Blood 120:4421–4431. 10.1182/blood-2011-12-401133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Byrd AS, O'Brien XM, Johnson CM, Lavigne LM, Reichner JS. 2013. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J. Immunol. 190:4136–4148. 10.4049/jimmunol.1202671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han W, Li H, Cai J, Gleaves LA, Polosukhin VV, Segal BH, Yull FE, Blackwell TS. 2013. NADPH oxidase limits lipopolysaccharide-induced lung inflammation and injury in mice through reduction-oxidation regulation of NF-κB activity. J. Immunol. 190:4786–4794. 10.4049/jimmunol.1201809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han W, Li H, Segal BH, Blackwell TS. 2012. Bioluminescence imaging of NADPH oxidase activity in different animal models. J. Vis. Exp. 68:e3925. 10.3791/3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davidson BA, Vethanayagam RR, Grimm MJ, Mullan BA, Raghavendran K, Blackwell TS, Freeman ML, Ayyasamy V, Singh KK, Sporn MB, Itagaki K, Hauser CJ, Knight PR, Segal BH. 2013. NADPH oxidase and Nrf2 regulate gastric aspiration-induced inflammation and acute lung injury. J. Immunol. 190:1714–1724. 10.4049/jimmunol.1202410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Segal BH, Grimm MJ, Khan AN, Han W, Blackwell TS. 2012. Regulation of innate immunity by NADPH oxidase. Free Radic. Biol. Med. 53:72–80. 10.1016/j.freeradbiomed.2012.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]