Abstract
Aspergillus nomius and Aspergillus tamarii are Aspergillus species that phenotypically resemble Aspergillus flavus. In the last decade, a number of case reports have identified A. nomius and A. tamarii as causes of human infections. In this study, using an internal transcribed spacer, β-tubulin, and calmodulin gene sequencing, only 8 of 11 clinical isolates reported as A. flavus in our clinical microbiology laboratory by phenotypic methods were identified as A. flavus. The other three isolates were A. nomius (n = 2) or A. tamarii (n = 1). The results corresponded with those of metabolic fingerprinting, in which the A. flavus, A. nomius, and A. tamarii strains were separated into three clusters based on ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC MS) analysis. The first two patients with A. nomius infections had invasive aspergillosis and chronic cavitary and fibrosing pulmonary and pleural aspergillosis, respectively, whereas the third patient had A. tamarii colonization of the airway. Identification of the 11 clinical isolates and three reference strains by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) showed that only six of the nine strains of A. flavus were identified correctly. None of the strains of A. nomius and A. tamarii was correctly identified. β-Tubulin or the calmodulin gene should be the gene target of choice for identifying A. flavus, A. nomius, and A. tamarii. To improve the usefulness of MALDI-TOF MS, the number of strains for each species in MALDI-TOF MS databases should be expanded to cover intraspecies variability.
INTRODUCTION
In recent years, infections related to Aspergillus species have become a major focus of clinical microbiology and infectious disease studies as the number of patients infected with Aspergillus species has risen dramatically (1–5). In immunocompetent hosts, Aspergillus species rarely cause serious illnesses, except for aspergilloma in patients with preexisting chronic lung diseases and a few related chronic pulmonary diseases related to Aspergillus, including chronic cavitary pulmonary aspergillosis, chronic fibrosing aspergillosis, and chronic necrotizing aspergillosis (6). In contrast, invasive aspergillosis is one of the most important causes of morbidity and mortality in immunocompromised patients, such as those with hematological malignancies who undergo chemotherapy, marrow and solid organ transplant recipients, and HIV-positive patients (1, 7). Among the known Aspergillus species, Aspergillus fumigatus is the most common one causing infections in humans in Western countries, whereas Aspergillus flavus is as important as A. fumigatus in Hong Kong and in other Asian countries and is the second most common Aspergillus species associated with human infections in Western countries (1, 7). The other Aspergillus species commonly associated with human infections include Aspergillus niger and Aspergillus terreus (1, 7).
Traditionally, identification of Aspergillus species in clinical microbiology laboratories has relied on isolating the fungus and microscopically examining them for characteristic features. In the last decade, more widespread use of internal transcribed spacer 1 (ITS1)-5.8S-ITS2 rRNA gene cluster sequencing and sequencing of other conserved DNA regions has proved to be useful for identifying molds (8–10). In addition, it has led to the discovery of novel pathogenic fungal species (11, 12). In the last decade, a number of case reports, with the help of ITS, β-tubulin, and calmodulin gene sequencing, have identified Aspergillus nomius and Aspergillus tamarii as causes of human infections (13–18). Since A. nomius and A. tamarii are yellow and are also under the same Aspergillus section Flavi as A. flavus, we hypothesize that they can be easily misidentified as A. flavus and that a proportion of fungus isolates reported as A. flavus might be A. nomius or A. tamarii. To test this hypothesis, we performed ITS and partial β-tubulin and calmodulin gene sequencing on 11 fungal isolates reported as A. flavus in our clinical microbiology laboratory in the past 6 years. Using ITS and partial β-tubulin and calmodulin gene sequencing and metabolic fingerprinting as standards of identification, the usefulness of matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) for identifying these isolates of A. flavus, A. nomius, and A. tamarii was also examined.
MATERIALS AND METHODS
Patients and strains.
Eleven fungal isolates reported as A. flavus were retrieved from the collection in our clinical microbiology laboratory during a 6-year period (2008 to 2013). The hospital records of the three patients with A. nomius and A. tamarii infections were retrieved and analyzed. Three reference strains, including A. flavus ATCC2 04304 (Aspergillus flavus Link), A. nomius CBS 260.88T (Aspergillus nomius Kurtzman, B. W. Horn & Hesselt), and A. tamarii CBS104.13T (Aspergillus tamarii Kita, Centralbl. Bakteriol., Abt.) were purchased from the American Type Culture Collection (ATCC) and the Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre.
Phenotypic characterization.
All strains were inoculated onto Sabouraud dextrose agar (SDA) (Sigma-Aldrich, St. Louis, MO, USA) for fungal culture. Slides for microscopic examination were prepared using the agar block smear method we described recently (19).
DNA extraction and ITS, β-tubulin, and calmodulin gene sequencing.
Fungal cells on SDA were harvested with a sterilized cotton swab and suspended in 1 ml of autoclaved distilled water. DNA was then extracted from the fungal cells using the DNeasy plant mini kit according to the manufacturer's instructions (Qiagen, Hilden, Germany). The extracted DNA was eluted in 40 μl of distilled water, and 1 μl of the extracted DNA was used for PCR.
The ITS region was amplified using primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (20), a segment of the β-tubulin gene was amplified using primers bT2a (5′-GGTAACCAAATCGGTGCTGCTTTC-3′) and bT2b (5′-ACCCTCAGTGTAGTGACCCTTGGC-3′) (21), and a segment of the calmodulin gene was amplified using primers cmd5 (5′-CCGAGTACAAGGAGGCCTTC-3′) and cmd6 (5′-CCGATAGAGGTCATAACGTGG-3′) (22). Each 20-μl PCR mixture contained diethyl pyrocarbonate-treated water (Invitrogen, Carlsbad, CA), fungal DNA, PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, and 3.125 mM MgCl2) (Applied Biosystems, Foster City, CA), 1 mM each primer (Invitrogen), 200 μl of each deoxyribonucleoside triphosphate, and 0.5 U of AmpliTaq Gold DNA polymerase (Applied Biosystems). The PCR mixtures were first heated at 95°C for 10 min, then heated for 40 cycles at 95°C for 30 s, at 56°C for 30 s, and at 72°C for 30 s, and finally incubated at 72°C for 10 min in an automated thermal cycler (GeneAmp PCR System 9700; Applied Biosystems).
Ten microliters of each amplified product was electrophoresed in 1.5% (wt/vol) agarose gel (SeaKem LE agarose), and the PCR products were purified using the QIAquick gel extraction kit (Qiagen, Hilden, Germany). Both strands of the PCR products were sequenced twice with an ABI Prism 3130xl DNA analyzer (Applied Biosystems) using the two PCR primers. The sequences of the PCR products were processed using BioEdit 7.1.3.0 (23) and compared with the sequences of closely related species in GenBank by multiple sequence alignment using MUSCLE 3.8 (24).
Phylogenetic characterization.
Poorly aligned or divergent regions of the aligned DNA sequences were removed using Gblocks 0.91b (25, 26) with relaxed parameters. The test for substitution model and phylogenetic tree construction, by the maximum likelihood method, were performed using MEGA 5.0.5 (27). Phylogenetic analyses included 336 nucleotide positions of the ITS, 219 nucleotide positions of the partial β-tubulin gene, and 199 nucleotide positions of the partial calmodulin gene.
Metabolic fingerprinting by UHPLC-ESI-Q-TOF MS analysis.
Metabolic fingerprinting was performed according to our previous publication, with modifications (28). Conidia of all the fungal strains at a concentration of 2 × 106 were grown in 10 ml RPMI 1640 medium (Gibco, USA) supplemented with 2% glucose at 37°C with shaking at 250 rpm for 48 h. The culture supernatant was filtered using a 0.22-μm MCE filter (Millipore, USA) and quenched immediately in liquid nitrogen for 10 min. The samples were lyophilized and stored at −80°C until use. For sample preparation for ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC) and electrospray ionization quadrupole time of flight mass spectrometry (ESI-Q-TOF MS), lyophilized samples were reconstituted in 600 μl solution containing water/acetonitrile/methanol (2:4:4 [vol/vol/vol]). For liquid chromatography, the separation was performed by an Agilent 1290 UHPLC (Agilent Technologies, USA), a Waters Acquity UPLC HSS T3 (3.0 by 100 mm, 1.8 μm) column (2.1 by 5 mm, 1.8 μm), and a VanGuard precolumn. The injection volume was 5.0 μl. The column and autosampler temperatures were maintained at 45°C and 15°C, respectively. The separation was performed at a flow rate of 0.4 ml/min under a gradient program in which mobile phase A consisted of 0.1% (vol/vol) formic acid and mobile phase B consisted of methanol. The gradient program was applied as follows: time (t) = 0 min, 1% phase B; t = 2.0 min, 1% phase B; t = 12 min, 38% phase B; t = 30 min, 99.5% phase B; t = 35 min, 99.5% phase B; t = 35.1 min, 1% phase B; and t = 38 min, 1% phase B. Detection was performed with an Agilent 6540 Q-TOF mass spectrometer (Agilent Technologies, USA) operating in the positive-ion mode using an Agilent Jet Stream electrospray ionization (ESI) source. The capillary voltage was set at 3,800 V with a nozzle voltage of 0 V. The other source conditions were kept constant in all the experiments: the gas temperature was kept at 320°C, the drying gas (nitrogen) was set at a rate of 8 liters/min, and the pressure of the nebulizer gas (nitrogen) was 40 lb/in2. The sheath gas was kept at a flow rate of 10 liters/min and was maintained at a temperature of 380°C. The voltages of the Fragmentor, Skimmer 1, and OctopoleRFPeak were 135 V, 60 V, and 500 V, respectively. The scan range was adjusted to 80 to 3,000 m/z at an acquisition rate of 2 spectra/s. The data were acquired and preprocessed using Agilent MassHunter Qualitative Analysis software version B.03.01 (Agilent Technologies, USA). Two biological replicate experiments were performed for each sample.
MALDI-TOF MS.
To perform MALDI-TOF MS analysis, fungal cells on SDA were harvested and washed with 1 ml distilled water. The pellet was suspended in 300 μl distilled water and 700 μl absolute ethanol. The mixture was vortexed and centrifuged at 14,000 rpm for 5 min. The supernatant was removed, and the pellet was air dried for 2 h. The pellet was then resuspended in 50 μl 70% formic acid (Sigma-Aldrich, USA). An equal volume of acetonitrile (Fluka, USA) was added, followed by centrifugation at 14,000 rpm for 5 min. One microliter supernatant was transferred to an individual spot on the 48-well Vitek MS-DS disposable target slide (bioMérieux, Marcy l'Etoile, France). Each spot was covered with 1 μl ready-to-use Vitek MS-CHCA matrix (bioMérieux). The target plate was loaded into a Vitek mass spectrometer (MS) (bioMérieux) for analysis. Spectra were obtained with an accelerating voltage of 20 kV in linear mode and analyzed within the m/z range 3,000 to 15,000 Da, based on 100 measurements. All the spectra were analyzed using the Vitek MS database (Vitek MS IVD system) and SARAMIS database (Vitek MS RUO system), performed according to the manufacturer's instructions. A representative spectrum of each strain was selected for hierarchical cluster analysis with LaunchPad software version 2.8 (Shimadzu Corporation, Kyoto, Japan).
Nucleotide sequence accession numbers.
The ITS, partial β-tubulin gene, and partial calmodulin gene sequences of all clinical isolates have been deposited in GenBank under accession numbers KF562195 through KF562205, KF562206 through KF562216, and KF562217 through KF562227.
RESULTS
Patients.
Among the 11 patients, seven were male and four were female. Their median age was 69 years (range, 52 to 87 years). All 11 strains were isolated from respiratory specimens (bronchoalveolar lavage [n = 4], tracheal aspirate [n = 1], and sputum [n = 6]).
Phenotypic characteristics.
The 11 clinical isolates and the reference strains were grown on SDA for morphology-based identification. Among these 11 clinical isolates, 10 produced velvety colonies with floccose tufts and yellowish green conidia (Fig. 1A and B). The reverse of the colonies was dull yellow or orange-brown. The conidiophores were hyaline, with globose to subglobose vesicles and biseriate phialides (Fig. 1D and E). The conidia were globose to subglobose. However, one of the clinical isolates, PW2958, produced dark green-brown conidia at maturity and formed velutinous colonies with floccose tufts and showed phenotypic morphology that was the same as that of the reference strain A. tamarii CBS 104.13T (Fig. 1C). The reverse of the colony was dull yellow. The conidiophores of PW2958 were hyaline with globose vesicles and biseriate phialides producing brownish-yellow conidia (Fig. 1F).
FIG 1.
Cultures and microscopic examinations of clinical isolates on SDA after 5 days at 37°C. PW2952 (A) and PW2955 (B) produced velvety colonies with floccose tufts and yellowish green conidia. (C) PW2958 produced dark green-brown conidia at maturity and formed velutinous colonies with floccose tufts. The conidiophores of PW2952 (D) and PW2955 (E) were hyaline, with globose to subglobose vesicles and biseriate phialides. (F) The conidiophores of PW2958 were hyaline, with globose vesicles and biseriate phialides producing brownish-yellow conidia.
ITS, β-tubulin, and calmodulin gene sequencing.
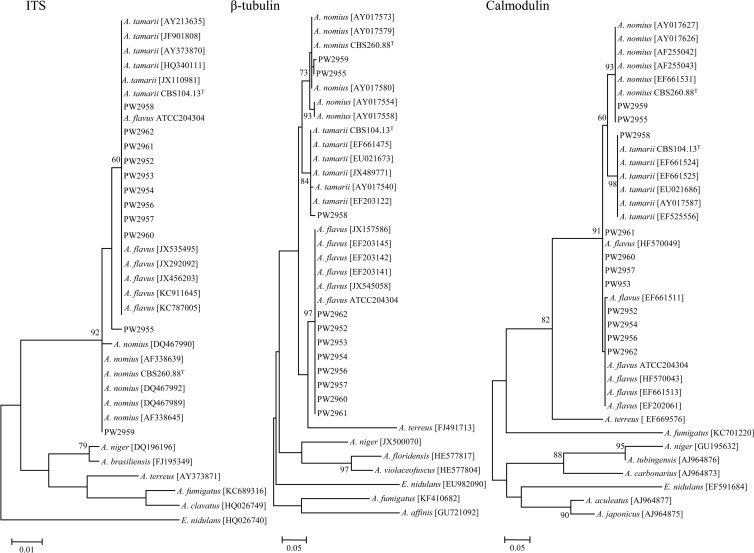
PCR of the ITS, β-tubulin, and calmodulin genes of the 11 clinical isolates and the reference strains showed bands at 500 bp to 600 bp. Sequencing and phylogenetic analysis of the ITS showed that PW2952, PW2953, PW2954, PW2956, PW2957, PW2958, PW2960, PW2961, and PW2962 were clustered with other strains of A. flavus and A. tamarii, whereas PW2959 was clustered with other strains of A. nomius (Fig. 2). Note that PW2955 did not form clear clustering with other strains of A. flavus, A. tamarii, or A. nomius (Fig. 2). Sequencing and phylogenetic analysis of the partial β-tubulin and calmodulin genes showed that PW2952, PW2953, PW2954, PW2956, PW2957, PW2960, PW2961, and PW2962 were clustered with other strains of A. flavus, PW2958 was clustered with other strains of A. tamarii, and PW2955 and PW2959 were clustered with other strains of A. nomius (Fig. 2). Using a combination of ITS, β-tubulin, and calmodulin gene sequencing, PW2952, PW2953, PW2954, PW2956, PW2957, PW2960, PW2961, and PW2962 were identified as A. flavus, PW2958 was identified as A. tamarii, and PW2955 and PW2959 were identified as A. nomius (Table 1).
FIG 2.
Phylogenetic analysis of ITS (336 nucleotide positions), β-tubulin gene (219 nucleotide positions), and calmodulin gene (199 nucleotide positions) of the 11 clinical isolates, three reference strains, and other closely related species. Trees were constructed by the maximum likelihood method with 1,000 bootstrap replicates. Only bootstrap values of ≥60% are shown. Scale bars indicate the estimated number of nucleotide substitutions per 100, 20, and 20 nucleotides, respectively.
TABLE 1.
Fungal isolates reported as A. flavus and control strains used in the present study
| Strain no. | Sexa/age (yr) | Clinical specimen source | Identification by ITS, β-tubulin, and calmodulin gene sequencing | Identification by MALDI-TOF MS (% confidence level) using: |
|
|---|---|---|---|---|---|
| Vitek-MS IVD library | SARAMIS RUO library | ||||
| PW2952 | M/52 | Bronchoalveolar lavage | A. flavus | No identification | No identification |
| PW2953 | F/69 | Sputum | A. flavus | No identification | A. flavus (87.8) |
| PW2954 | M/87 | Sputum | A. flavus | No identification | A. flavus (83.1) |
| PW2955 | M/65 | Bronchoalveolar lavage | A. nomius | No identification | No identification |
| PW2956 | M/57 | Sputum | A. flavus | No identification | A. flavus (92.3) |
| PW2957 | M/85 | Sputum | A. flavus | No identification | A. flavus (99.9) |
| PW2958 | M/74 | Sputum | A. tamarii | No identification | No identification |
| PW2959 | M/64 | Bronchoalveolar lavage | A. nomius | No identification | No identification |
| PW2960 | F/70 | Bronchoalveolar lavage | A. flavus | A. flavus (97.5) | A. flavus (78.5) |
| PW2961 | F/79 | Sputum | A. flavus | No identification | No identification |
| PW2962 | F/65 | Tracheal aspirate | A. flavus | A. flavus (73.7) | A. flavus (99.1) |
| ATCC 204304 | A. flavus | A. flavus (83.3) | A. flavus (99.9) | ||
| CBS260.88T | A. nomius | No identification | No identification | ||
| CBS104.13T | A. tamarii | No identification | No identification | ||
F, female; M, male.
Metabolic fingerprinting by UHPLC-ESI-Q-TOF MS analysis.
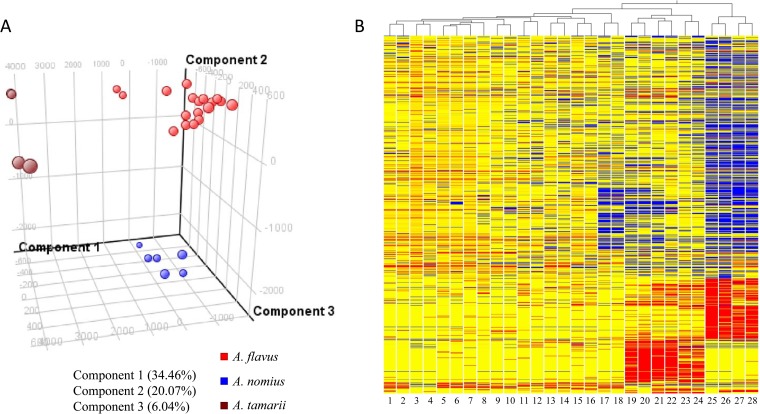
The extracellular metabolite contents of the Aspergillus isolates were examined by UHPLC-ESI-Q-TOF MS analysis. Two unsupervised multivariate clustering analyses, principal component analysis (PCA) and hierarchical clustering analysis, were performed to investigate the relationships among the isolates based on the untargeted extracellular metabolite profiles. In PCA, 60.57% of the total variance in the data was represented by the first three principal components (Fig. 3A). The three-dimensional (3D) PCA score plot revealed that the nine strains of A. flavus (PW2952, PW2953, PW2954, PW2956, PW2957, PW2960, PW2961, PW2962, and ATCC 204304), the three strains of A. nomius (PW2955, PW2959, and CBS260.88T), and the two strains of A. tamarii (PW2958 and CBS104.13T) can be distinguished from each other, but strains within the same species are closely related. The hierarchical clustering dendrogram also indicated that PW2952, PW2953, PW2954, PW2956, PW2957, PW2960, PW2961, PW2962, and ATCC 204304 possessed highly similar extracellular metabolite profiles, which are distinct from those of PW2955, PW2959, CBS260.88T, PW2958, and CBS104.13T (Fig. 3B). Moreover, PW2955, PW2959, and CBS260.88T clustered together, and PW2958 and CBS104.13T formed another cluster.
FIG 3.
(A) Principal component analysis and (B) hierarchical clustering dendrogram of metabolic fingerprints of the 11 clinical isolates reported as A. flavus and three reference strains. Molecular features (1,016) defined by retention time and mass pair were included. The 3D PCA score plot for component PC1 versus component PC2 versus component PC3 is presented. The percentages of variance in the data set reflected by the first three PCs for each sample are shown. Hierarchical clustering of different fungal isolates is represented by a heat map and a dendrogram. Clustering was performed using Euclidean distance and average linkage. Blue and red indicate negative and positive log ratios, respectively. Two biological replicates were performed for each sample. Lanes 1 and 2, PW2952; lanes 3 and 4, PW2956; lanes 5 and 6, PW2957; lanes 7 and 8, PW2960; lanes 9 and 10, PW2962; lanes 11 and 12, ATCC 204304; lanes 13 and 14, PW2953; lanes 15 and 16, PW2954; lanes 17 and 18, PW2961; lanes 19 and 20, CBS260.88T; lanes 21 and 22, PW2955; lanes 23 and 24, PW2959; lanes 25 and 26, CBS104.13T; lanes 27 and 28, PW2958.
MALDI-TOF MS.
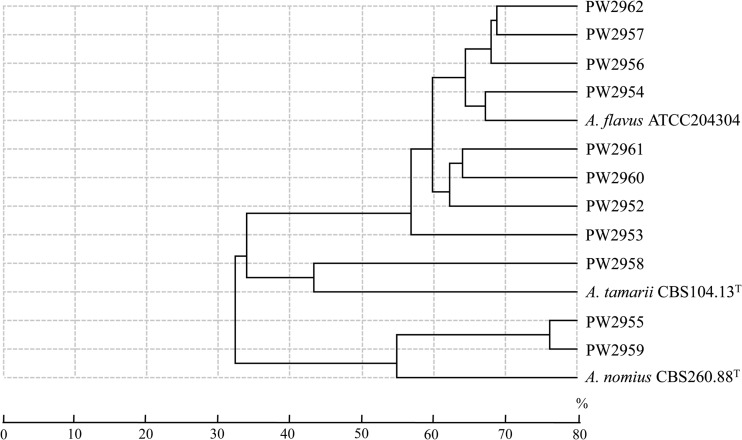
Hierarchical clustering of the MALDI-TOF MS spectra of the 11 clinical isolates reported as A. flavus and the three reference strains showed that the nine strains of A. flavus, the three strains of A. nomius, and the two strains of A. tamarii were separated into three clusters (Fig. 4). However, only six and three of the nine strains of A. flavus were identified correctly using the SARAMIS database (confidence levels, 78.5% to 99.9%) and the Vitek MS IVD database (confidence levels, 73.7% to 97.5%), respectively (Table 1). None of the strains of A. nomius and A. tamarii was correctly identified by MALDI-TOF MS.
FIG 4.
Hierarchical clustering of MALDI-TOF MS spectra of 11 clinical isolates reported as A. flavus and three reference strains. Distances are displayed in relative units.
DISCUSSION
Before the use of molecular diagnostic technologies, only A. fumigatus, A. flavus, A. niger, and A. terreus were commonly recognized as causes of aspergillosis. It was difficult for clinical microbiology laboratories to identify rarely found molds as causes of various infections. The more widespread use of ITS and other gene sequencing has enabled clinical microbiology laboratories to confidently identify molds that are rarely found in patients after preliminary morphological examination (13–18, 29). In addition, novel fungal species associated with clinical infections have been discovered (9–11). These have greatly improved our understanding in the epidemiology, laboratory diagnosis, and treatment of fungal infections. In this study, by amplifying and sequencing three independent DNA regions used commonly for fungal identification (the ITS and the β-tubulin and calmodulin genes), 3 of the 11 strains reported as A. flavus were unambiguously identified as A. nomius (n = 2) or A. tamarii (n = 1), whereas the remaining 8 strains were A. flavus. These results were also in line with those of metabolic fingerprinting, in which the A. flavus, A. nomius, and A. tamarii strains were separated into three clusters based on UHPLC MS analysis. Although A. flavus, A. nomius, and A. tamarii may show subtle differences microscopically, the recognition of such differences requires expertise, which is usually not present in most clinical microbiology laboratories. Since A. flavus, A. nomius, and A. tamarii are all yellow on SDA, A. nomius and A. tamarii are usually just reported as A. flavus, as they are rare. Since sequencing of either the β-tubulin or the calmodulin gene, but not the ITS, can identify A. flavus, A. nomius, and A. tamarii unambiguously, the β-tubulin or calmodulin gene should be the gene target of choice for differentiating these three fungal species.
The epidemiology and clinical disease spectrum for A. nomius and A. tamarii are similar to those of A. flavus. Including the three cases in the present study, a total of 16 cases of A. nomius and A. tamarii infections have been reported in the literature (Table 2) (13–18, 29). Among the 15 strains with methods of laboratory diagnosis described in detail, all of them required molecular technologies (sequencing in 14 and high-performance liquid chromatography analysis of metabolites in 1) for identification. Among the nine patients with clinical details described, five occurred in immunocompetent patients and four in immunocompromised patients. In immunocompetent patients, A. nomius and A. tamarii were associated with keratitis, onychomycosis, and primary cutaneous aspergillosis, whereas in immunocompromised patients, including two patients in the present study (cases 4 and 5), they were associated with invasive aspergillosis or chronic cavitary and fibrosing pulmonary and pleural aspergillosis. The first patient (case 4) was a 65-year-old man with underlying diabetes mellitus and severe left lower zone bronchiectasis. He presented with community-acquired pneumonia that did not respond to intravenous meropenem and azithromycin. His condition deteriorated rapidly. A bronchoalveolar lavage specimen used for a KOH smear was positive for fungal elements and was subsequently culture positive for A. nomius. Despite intravenous liposomal amphotericin B and itraconazole treatment, the patient died of invasive aspergillosis. The second patient (case 5) was a 64-year-old man with a history of pulmonary tuberculosis (35 years ago and again 6 years ago) and bronchiectasis with left lower lobe lobectomy performed 30 years ago. He presented with blood-stained sputum. A chest radiograph revealed left upper zone infiltrate. The patient did not respond to oral amoxicillin-clavulanate. A bronchoalveolar lavage specimen was culture positive for A. nomius. Oral itraconazole was given for 6 months, and the patient's conditions improved.
TABLE 2.
Cases of A. nomius and A. tamarii infections reported in the English literature
| Infection type and case no. | Reference or source | Locality | Sexa/age (yr) | Underlying disease(s)b | Clinical specimen type | Direct microscopy result | Identification method(s) | Diagnosis | Antifungal treatment(s) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| A. nomius | ||||||||||
| 1 | Manikandan et al. (13) | India | F/64 | None | Corneal scraping | Positive | ITS, calmodulin, and β-tubulin gene sequencing | Keratitis | Topical natamycin 5%, econazole 2%, itraconazole 1%, oral ketoconazole | Corneal perforation, scleral extension |
| 2 | Zotti et al. (14) | Italy | F/53 | None | Nail | Positive | β-tubulin gene sequencing | Onychomycosis | Oral itraconazole, topical amorolfine | Cured |
| 3 | Caira et al. (15) | Italy | M/66 | AML | Sputum | Positive | ITS, calmodulin, and β-tubulin gene sequencing | Invasive aspergillosis | Voriconazole | Died |
| 4 | Present study (strain PW2955) | Hong Kong | M/65 | DM, bronchiectasis | BAL fluidc | Positive | ITS and β-tubulin gene sequencing | Invasive aspergillosis | Liposomal amphotericin B, i.v.d itraconazole | Died |
| 5 | Present study (strain PW2959) | Hong Kong | M/64 | Old TB, bronchiectasis | BAL fluid | Negative | ITS and β-tubulin gene sequencing | Chronic cavitary and fibrosing pulmonary and pleural aspergillosis | Oral itraconazole | Survived |
| A. tamarii | ||||||||||
| 6 | Kristensen et al. (16) | Denmark | M/3 | None | Nail | Positive | HPLC analysis of metabolites | Onychomycosis | Urea cream 40%, topical terbinafine | Cured |
| 7 | Kredics et al. (17) | India | F/32 | None | Corneal ulcer scraping | Positive | ITS, calmodulin and β-tubulin gene sequencing | Keratitis | Topical natamycin 5%, econazole 2%, oral ketoconazole, subconjunctival fluconazole 0.2% | Cured |
| 8–14 | Manikandan et al. 2013 (18) | India | NA | NA | Corneal ulcer scraping | NA | ITS, calmodulin, and β-tubulin gene sequencing | Keratitis | NA | NA |
| 15 | Sharma et al. (29) | India | M/65 | None | Skin nodule biopsy | Positive | Phenotypic and genotypic identifications | Primary cutaneous aspergillosis | Oral itraconazole | Cured |
| 16 | Present study (strain PW2958) | Hong Kong | M/74 | Multiple myeloma | Sputum | Negative | ITS and β-tubulin gene sequencing | Colonization | None | Survived |
F, female; M, male; NA, not applicable.
AML, acute myeloid leukemia; DM, diabetes mellitus; TB, tuberculosis.
BAL, bronchoalveolar lavage.
i.v., intravenous.
To improve the usefulness of MALDI-TOF MS, the number of strains for each species in MALDI-TOF MS databases should be expanded to cover intraspecies variability. MALDI-TOF MS has emerged as a revolutionary technique for rapid bacterial identification at a low cost and is a potentially widely used technology for bacterial identification in clinical microbiology laboratories (30, 31). In addition to bacteria, it can also be used for the identification of fungal pathogens (32, 33). In the present study, hierarchical clustering of the MALDI-TOF MS spectra showed clustering, indicating that A. flavus, A. nomius, and A. tamarii can potentially be identified to the species level. However, only six of the nine strains of A. flavus and none of the five strains of A. nomius and A. tamarii were identifiable by MALDI-TOF MS. This is in line with the results of our previous studies on bacterial identification using MALDI-TOF MS (30, 31). For example, our three Burkholderia thailandensis isolates were originally misidentified as Burkholderia pseudomallei when the database contained only one B. thailandensis spectrum, but expansion of the database with one additional B. thailandensis reference strain enabled the correct identification of two other B. thailandensis isolates (31). In order to enable MALDI-TOF MS to identify A. flavus, A. nomius, and A. tamarii confidently, adequate spectra of different strains from each species have to be included in the corresponding MALDI-TOF MS database. With the expansion of the databases, MALDI-TOF MS could be an alternative and even more user-friendly and convenient technology for the identification of molds in clinical microbiology laboratories.
ACKNOWLEDGMENTS
This work was partly supported by the Research Fund for the Control of Infectious Diseases (commissioned study) of the Health, Welfare and Food Bureau of the Hong Kong SAR government, the HKSAR Health and Medical Research Fund, a Research Grants Council grant, and the Committee for Research and Conference Grants, The University of Hong Kong.
Footnotes
Published ahead of print 22 January 2014
REFERENCES
- 1.Latge JP. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuen KY, Chan CM, Chan KM, Woo PC, Che XY, Leung AS, Cao L. 2001. Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis. J. Clin. Microbiol. 39:3830–3837. 10.1128/JCM.39.11.3830-3837.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo PC, Chan CM, Leung AS, Lau SK, Che XY, Wong SS, Cao L, Yuen KY. 2002. Detection of cell wall galactomannoprotein Afmp1p in culture supernatants of Aspergillus fumigatus and in sera of aspergillosis patients. J. Clin. Microbiol. 40:4382–4387. 10.1128/JCM.40.11.4382-4387.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo PC, Chong KT, Leung AS, Wong SS, Lau SK, Yuen KY. 2003. AFLMP1 encodes an antigenic cell wall protein in Aspergillus flavus. J. Clin. Microbiol. 41:845–850. 10.1128/JCM.41.2.845-850.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong KT, Woo PC, Lau SK, Huang Y, Yuen KY. 2004. AFMP2 encodes a novel immunogenic protein of the antigenic mannoprotein superfamily in Aspergillus fumigatus. J. Clin. Microbiol. 42:2287–2291. 10.1128/JCM.42.5.2287-2291.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denning DW, Riniotis K, Dobrashian R, Sambatakou H. 2003. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin. Infect. Dis. 37(Suppl. 3):S265–S280. 10.1086/376526 [DOI] [PubMed] [Google Scholar]
- 7.Yuen KY, Woo PC, Ip MS, Liang RH, Chiu EK, Siau H, Ho PL, Chen FF, Chan TK. 1997. Stage-specific manifestation of mold infections in bone marrow transplant recipients: risk factors and clinical significance of positive concentrated smears. Clin. Infect. Dis. 25:37–42. 10.1086/514492 [DOI] [PubMed] [Google Scholar]
- 8.Woo PC, Leung SY, To KK, Chan JF, Ngan AH, Cheng VC, Lau SK, Yuen KY. 2010. Internal transcribed spacer region sequence heterogeneity in Rhizopus microsporus: implications for molecular diagnosis in clinical microbiology laboratories. J. Clin. Microbiol. 48:208–214. 10.1128/JCM.01750-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo PC, Lau SK, Ngan AH, Tse H, Tung ET, Yuen KY. 2008. Lasiodiplodia theobromae pneumonia in a liver transplant recipient. J. Clin. Microbiol. 46:380–384. 10.1128/JCM.01137-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To KK, Lau SK, Wu AK, Lee RA, Ngan AH, Tsang CC, Ling IW, Yuen KY, Woo PC. 2012. Phaeoacremonium parasiticum invasive infections and airway colonization characterized by agar block smear and ITS and beta-tubulin gene sequencing. Diagn. Microbiol. Infect. Dis. 74:190–197. 10.1016/j.diagmicrobio.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 11.Woo PC, Lau SK, Ngan AH, Tung ET, Leung SY, To KK, Cheng VC, Yuen KY. 2010. Lichtheimia hongkongensis sp. nov., a novel Lichtheimia spp. associated with rhinocerebral, gastrointestinal, and cutaneous mucormycosis. Diagn. Microbiol. Infect. Dis. 66:274–284. 10.1016/j.diagmicrobio.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 12.Woo PC, Ngan AH, Tsang CC, Ling IW, Chan JF, Leung SY, Yuen KY, Lau SK. 2013. Clinical spectrum of Exophiala infections and a novel Exophiala species, Exophiala hongkongensis. J. Clin. Microbiol. 51:260–267. 10.1128/JCM.02336-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manikandan P, Varga J, Kocsube S, Samson RA, Anita R, Revathi R, Doczi I, Nemeth TM, Narendran V, Vagvolgyi C, Manoharan C, Kredics L. 2009. Mycotic keratitis due to Aspergillus nomius. J. Clin. Microbiol. 47:3382–3385. 10.1128/JCM.01051-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zotti M, Machetti M, Persi A, Barabino G, Parodi A. 2011. Onychomycosis: first case due to Aspergillus nomius. Acta Derm. Venereol. 91:591–592. 10.2340/00015555-1118 [DOI] [PubMed] [Google Scholar]
- 15.Caira M, Posteraro B, Sanguinetti M, de Carolis E, Leone G, Pagano L. 2012. First case of breakthrough pneumonia due to Aspergillus nomius in a patient with acute myeloid leukemia. Med. Mycol. 50:746–750. 10.3109/13693786.2012.660507 [DOI] [PubMed] [Google Scholar]
- 16.Kristensen L, Stenderup J, Otkjaer A. 2005. Onychomycosis due to Aspergillus tamarii in a 3-year-old boy. Acta Derm. Venereol. 85:261–262. 10.1080/00015550510025605 [DOI] [PubMed] [Google Scholar]
- 17.Kredics L, Varga J, Kocsube S, Doczi I, Samson RA, Rajaraman R, Narendran V, Bhaskar M, Vagvolgyi C, Manikandan P. 2007. Case of keratitis caused by Aspergillus tamarii. J. Clin. Microbiol. 45:3464–3467. 10.1128/JCM.00920-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manikandan P, Varga J, Kocsube S, Anita R, Revathi R, Nemeth TM, Narendran V, Vagvolgyi C, Panneer Selvam K, Shobana CS, Babu Singh YR, Kredics L. 2013. Epidemiology of Aspergillus keratitis at a tertiary care eye hospital in south India and antifungal susceptibilities of the causative agents. Mycoses 56:26–33. 10.1111/j.1439-0507.2012.02194.x [DOI] [PubMed] [Google Scholar]
- 19.Woo PC, Ngan AH, Chui HK, Lau SK, Yuen KY. 2010. Agar block smear preparation: a novel method of slide preparation for preservation of native fungal structures for microscopic examination and long-term storage. J. Clin. Microbiol. 48:3053–3061. 10.1128/JCM.00917-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics p 315–322 In Innis MA, Gelfand DH, Shinsky JJ, White TJ. (ed), PCR protocols: a guide to methods and applications. Academic Press, New York [Google Scholar]
- 21.Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong SB, Go SJ, Shin HD, Frisvad JC, Samson RA. 2005. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97:1316–1329. 10.3852/mycologia.97.6.1316 [DOI] [PubMed] [Google Scholar]
- 23.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. (Oxf.) 41:95–98 [Google Scholar]
- 24.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17:540–552. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 26.Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56:564–577. 10.1080/10635150701472164 [DOI] [PubMed] [Google Scholar]
- 27.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.To KK, Fung AM, Teng JL, Curreem SO, Lee KC, Yuen KY, Lam CW, Lau SK, Woo PC. 2013. Characterization of a Tsukamurella pseudo-outbreak by phenotypic tests, 16S rRNA sequencing, pulsed-field gel electrophoresis, and metabolic footprinting. J. Clin. Microbiol. 51:334–338. 10.1128/JCM.02845-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma S, Yenigalla BM, Naidu SK, Pidakala P. 2013. Primary cutaneous aspergillosis due to Aspergillus tamarii in an immunocompetent host. BMJ Case Rep. 2013:010128. 10.1136/bcr-2013-010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang BS, Lau SK, Teng JL, Chan TM, Chan WS, Wong TY, Tong YT, Fan RY, Yuen KY, Woo PC. 2013. Matrix-assisted laser desorption ionisation–time of flight mass spectrometry for rapid identification of Laribacter hongkongensis. J. Clin. Pathol. 66:1081–1083. 10.1136/jclinpath-2013-201651 [DOI] [PubMed] [Google Scholar]
- 31.Lau SK, Tang BS, Curreem SO, Chan TM, Martelli P, Tse CW, Wu AK, Yuen KY, Woo PC. 2012. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for rapid identification of Burkholderia pseudomallei: importance of expanding databases with pathogens endemic to different localities. J. Clin. Microbiol. 50:3142–3143. 10.1128/JCM.01349-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Carolis E, Posteraro B, Lass-Florl C, Vella A, Florio AR, Torelli R, Girmenia C, Colozza C, Tortorano AM, Sanguinetti M, Fadda G. 2012. Species identification of Aspergillus, Fusarium and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 18:475–484. 10.1111/j.1469-0691.2011.03599.x [DOI] [PubMed] [Google Scholar]
- 33.Bille E, Dauphin B, Leto J, Bougnoux ME, Beretti JL, Lotz A, Suarez S, Meyer J, Join-Lambert O, Descamps P, Grall N, Mory F, Dubreuil L, Berche P, Nassif X, Ferroni A. 2012. MALDI-TOF MS Andromas strategy for the routine identification of bacteria, mycobacteria, yeasts, Aspergillus spp. and positive blood cultures. Clin. Microbiol. Infect. 18:1117–1125. 10.1111/j.1469-0691.2011.03688.x [DOI] [PubMed] [Google Scholar]