Abstract
Although microscopic examination of Giemsa-stained blood smears remains the gold standard for the diagnosis of malaria, molecular detection using PCR is becoming increasingly popular. Due to discrepant PCR and microscopy results, we aimed to optimize our detection assays for Plasmodium malariae and Plasmodium ovale by sequencing the 18S rRNA region and developing a new primer and probe set for real-time quantitative PCR (qPCR). Clinical specimens positive for P. malariae (n = 15) or P. ovale (n = 33) underwent amplification and sequencing of the 18S rRNA region. Based on sequence discrepancies between our current primer/probe and clinical isolates, degenerate P. ovale primer and probe were developed to determine if their performance characteristics improved. The reference (gold) standard was microscopy. No 18S sequence heterogeneity was observed among the P. malariae isolates, and the sensitivity and specificity of our current P. malariae qPCR assay were both 100%. Compared to microscopy, the sensitivity and specificity of our current P. ovale qPCR assay were 72.7% and 100%, respectively. Five single nucleotide polymorphisms (SNPs) were identified in P. ovale. The sensitivity of the new P. ovale assay increased to 100% with 100% specificity. We therefore improved the performance characteristics of our P. ovale molecular detection assay through the development of a degenerate primer and probe set which accommodates 18S SNPs among the 2 subspecies of P. ovale. Given the suboptimal sensitivity of rapid diagnostic tests for non-falciparum malaria and the typically low parasitemia of P. malariae and P. ovale, a well-performing confirmatory molecular assay is imperative for clinical laboratories.
INTRODUCTION
There are five Plasmodium species which are known to cause human malaria, Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi (1). Although P. malariae and P. ovale occur less frequently among human populations, they are widely distributed and their diagnosis has proven challenging due to their relatively low parasitemia and the morphology of blood stages, as well as the poor sensitivity of rapid diagnostic tests in non-falciparum species (1, 2).
Microscopic examination of Giemsa-stained thick and thin blood smears, rapid diagnostic tests (RDTs), and PCR assays are the mainstays of malaria diagnostics in resourced settings; however, microscopy remains the gold standard. Diagnoses of P. malariae and P. ovale based on microscopy present challenges due to the technical expertise required, the typically low parasitemias associated with these species, and the similar morphological features to other Plasmodium species (1). PCR assays may be either genus or species specific and do not require extensive training to perform; however, significant laboratory infrastructure is required, thus limiting their use to resourced settings (3, 4). Our current molecular approach to the diagnosis of malaria involves use of one Plasmodium genus-specific and two duplexed Plasmodium species-specific real-time quantitative PCR (qPCR) assays, which can discriminate among the 4 common human malaria species, as described by Khairnar and colleagues (3). A recent case of P. ovale in which real-time quantitative PCR was indeterminate, with sequencing required to resolve the causative malaria species (5), and our own experience with discordant results using current qPCR and microscopy, highlight the challenges of laboratory diagnostics in malaria. Genetic polymorphisms between the classic (P. ovale curtisi) and variant (P. ovale wallikeri) subspecies of P. ovale may render the molecular detection of these two species difficult (6).
In the present study, we aimed to optimize the diagnostic qPCR currently used in our clinical laboratory for Plasmodium malariae and Plasmodium ovale through sequence analysis of the 18S rRNA region and develop new primers and probes which could accommodate any sequence heterogeneity.
MATERIALS AND METHODS
Specimens.
Between January 2007 and July 2013, 15 whole-blood specimens positive for P. malariae and 33 specimens positive for P. ovale were examined in our laboratory and banked at −80°C following diagnostic testing. Microscopy was performed by examination of Giemsa-stained thick and thin blood films by certified medical lab technologists. Rapid diagnostic testing was conducted with a BinaxNow malaria kit (Alere, ME) according to the manufacturer's instructions. P. falciparum, P. vivax, P. malariae, and P. ovale small-subunit rRNA DNA clones (MRA-177, MRA-178, MRA-179, MRA-180, and MR4; ATCC, Manassas, VA) were used as positive-control materials. Randomly selected banked specimens positive for P. falciparum (n = 20) (with parasitemia ranging from <0.1 to 28.6%) or P. vivax (n = 20) (with parasitemia ranging from <0.1 to 0.9%) or negative for Plasmodium (n = 20) (confirmed by microscopy and qPCR) were used as negative-control specimens for calculation of performance characteristics.
DNA extraction.
DNA was extracted using the DNA Minikit blood or body fluid spin protocol (Qiagen, Germantown MD). A total of 200 μl of frozen whole blood was thawed and each sample was eluted with 60 μl AE buffer and stored at −20°C prior to use.
Real-time PCR.
Our current molecular diagnostic assay includes 4 qPCRs: human beta-2-microglobulin (B2MG) extraction control, Plasmodium genus specific, P. falciparum/P. vivax species-specific duplex, and P. malariae/P. ovale species-specific duplex qPCR as previously described by Khairnar and colleagues (3, 4). The P. falciparum/P. vivax duplex qPCR was conducted with the P. malariae and P. ovale specimens to exclude mixed infections. The P. malariae/P. ovale duplex qPCR was conducted for all specimens to establish the sensitivities and specificities of the qPCR assays. All qPCR assays were run using the ABI 7900HT real-time PCR system and under the following conditions: 50°C for 2 min, 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min (45 cycles). We used 12.5 μl of TaqMan universal PCR master mix (Life Technologies) and 5 μl of DNA primers and probes with concentrations as previously reported (3) for a final volume of 25 μl per reaction. All qPCR amplification curves were analyzed using a manual threshold cycle (CT) of 0.02 and an automatic baseline. A result was called positive if the CT value was <38 in the presence of a logarithmic amplification curve.
Amplification and sequencing of the 18S rRNA region of P. malariae and P. ovale.
Endpoint PCR of the 18S rRNA region was conducted with high-fidelity polymerase AccuPrime Pfx Supermix (Life Technologies) and 200 nM (each) of the primers Plasmo 18S forward (5′-ATTCAGATGTCAGAGGTGAAATTCT-3′) and Plasmo 18S reverse (5′-TCAATCCTACTCTTGTCTTAAACTA-3′), generating a 396-bp product. The cycling conditions were 95°C for 5 min, followed by 95°C for 15 s, 58°C for 30 s, and 68°C for 30 s for 45 cycles, and then 68°C for 5 min using an ABI Veriti fast thermal cycler. Amplicons were visualized on 1% agarose gels with ethidium bromide. PCR products were purified with a QIAquick PCR purification kit (Qiagen, Germantown, MD) and eluted with 20 μl water. Purified PCR products were Sanger sequenced using the same forward and reverse primers with a BigDye Terminator v3.1 cycle sequencing kit (Life Technologies) and run according to the manufacturer's recommended conditions. Sequenced products were purified by a BigDye XTerminator (Life Technologies) and analyzed by an ABI 3130xl genetic analyzer. Forward and reverse sequences of each sample were aligned using Vector NTI software (Life Technologies). Sequences were verified using a BLAST search to further confirm the species identification and to resolve any discrepant microscopic diagnosis and species-specific qPCR results. Alignments of P. malariae and P. ovale sequences were performed using Mega5.2 software (7). Sequences were analyzed and compared with our currently used real-time qPCR P. malariae and P. ovale primers and probes (3, 4). The 18S rRNA sequences of P. falciparum, P. vivax, P. malariae, and P. ovale from GenBank were aligned using Mega5.2 software to determine if changes in primers and probes may have caused false positives (Table 1).
TABLE 1.
GenBank sequences used for sequence alignments
| Species | GenBank 18S rRNA sequence no. |
|---|---|
| P. falciparum | M19173.1, M19172.1 |
| P. vivax | X13926.1, U03080.1, U03079.1, U07368.1, U07367.1 |
| P. ovale | L48987.1 (classic), L48986.1 (classic), AB182489.1 (classic), AB182490.1 (classic), AJ001527.1 (variant), X99790.1 (variant), AB182491.1 (variant), AB182492.1 (variant), AB182493.1 (variant) |
| P. malariae | AF488000.1, AF487999.1, M54897.1 |
Limit of detection for qPCR assay.
Small-subunit rRNA DNA clones MRA-179 (P. malariae) and MRA-180 (P. ovale) were 10-fold serially diluted, yielding 8 concentrations ranging from 9 to 91 million copies/reaction. Each dilution of the clones was run in triplicate. Mean CT values were plotted against the log copy number/reaction to generate an equation to calculate the limit of detection (LOD) at a CT of 38.
RESULTS
Performance of current duplex P. malariae/P. ovale qPCR assay.
Forty-eight P. ovale and P. malariae specimens were analyzed by microscopy, RDT (Binax assay), qPCR, and Sanger sequencing. Diagnosed species were identified based on the concordance of 2 out of 3 species-specific assays, microscopy, qPCR, and BLAST sequence homology. All specimens were microscopy positive; however, for a few specimens other assays were required to resolve the species. Binax had a quick turnaround time of 20 min but considerably lower sensitivity of 35.5% for P. ovale and 78.6% for P. malariae (Tables 2 and 3). DNA was extracted from P. malariae-, P. ovale-, P. falciparum-, and P. vivax-positive specimens and negative specimens, and then verified by B2MG extraction control qPCR. Twenty-four of 33 P. ovale specimens were positive for P. ovale by our current P. malariae/P. ovale species-specific duplex qPCR (Table 2) (3). All specimens except number 25 were negative for P. falciparum, P. vivax, and P. malariae. Specimen number 25 was positive for P. falciparum and P. ovale by qPCR, but microscopy, Sanger sequencing, and BLAST analysis confirmed it to be a single P. ovale infection only. All P. malariae specimens (n = 15) were positive for P. malariae by qPCR with no mixed infections observed (Table 3). Sixty P. falciparum-positive (n = 20), P. vivax-positive (n = 20) and negative specimens (n = 20) underwent P. malariae/P. ovale qPCR and all were negative. Thus, sensitivity of the current P. malariae/P. ovale duplex qPCR assay for P. ovale was 72.7% with specificity of 100%, and for P. malariae, both sensitivity and specificity were 100%. The low sensitivity and the jagged amplification curve observed in the P. ovale qPCR runs suggested some sequence heterogeneity in the regions to which the primers and probe hybridized.
TABLE 2.
Specimens positive for Plasmodium ovalea
| Specimen no. | Diagnosed species | Microscopy analysis result | Parasitemia (%) | Binaxb |
Genus qPCR, current assay (CT) | P. falciparum qPCR, current assay (CT) | P. vivax qPCR, current assay (CT) | P. malariae qPCR, current assay (CT) | P. ovale qPCR, current assay (CT) | P. malariae qPCR, new assay (CT) | P. ovale qPCR, new assay (CT) | 100% Sequence homolog in blast | P. ovale subspecies | yr of importation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | ||||||||||||||
| 1 | P. ovale | P. ovale | <0.1 | − | + | 27.6 | − | − | − | 26.4 | − | 24.5 | P. ovale | curtisi | 2007 |
| 2 | P. ovale | P. ovale | 0.3 | − | + | 23.8 | − | − | − | 28.3 | − | 23.5 | P. ovale | wallikeri | 2008 |
| 3 | P. ovale | P. ovale | 0.3 | − | + | 26.1 | − | − | − | 24.4 | − | 22.6 | P. ovale | curtisi | 2008 |
| 4 | P. ovale | P. ovale | <0.1 | NDc | ND | 26.9 | − | − | − | 33.7 | − | 26.6 | P. ovale | wallikeri | 2009 |
| 5 | P. ovale | P. ovale | <0.1 | ND | ND | − | − | − | − | 36.5 | − | 29.6 | P. ovale | wallikeri | 2009 |
| 6 | P. ovale | P. ovale | <0.1 | − | − | 26.0 | − | − | − | 25.4 | − | 23.9 | P. ovale | curtisi | 2009 |
| 7 | P. ovale | P. ovale | <0.1 | − | − | 31.0 | − | − | − | 30.1 | − | 28.2 | P. ovale | curtisi | 2009 |
| 8 | P. ovale | P. malariae | <0.1 | − | − | 27.2 | − | − | − | − | − | 27.5 | P. ovale | wallikeri | 2009 |
| 9 | P. ovale | Psp | <0.1 | − | + | 32.3 | − | − | − | − | − | 24.1 | P. ovale | wallikeri | 2010 |
| 10 | P. ovale | P. ovale | <0.1 | − | − | 31.1 | − | − | − | 30.1 | − | 29.9 | P. ovale | curtisi | 2010 |
| 11 | P. ovale | P. ovale | <0.1 | − | − | 31.4 | − | − | − | 31.3 | − | 28.1 | P. ovale | curtisi | 2010 |
| 12 | P. ovale | P. ovale | <0.1 | − | − | 29.9 | − | − | − | 28.8 | − | 27.7 | P. ovale | curtisi | 2010 |
| 13 | P. ovale | P. ovale | <0.1 | − | − | 29.7 | − | − | − | 27.4 | − | 27.7 | P. ovale | curtisi | 2010 |
| 14 | P. ovale | P. ovale | <0.1 | − | − | 27.2 | − | − | − | 34.8 | − | 28.3 | P. ovale | wallikeri | 2011 |
| 15 | P. ovale | P. ovale | 0.1 | − | + | 26.4 | − | − | − | 25.5 | − | 24.6 | P. ovale | curtisi | 2011 |
| 16 | P. ovale | P. ovale | 0.1 | − | − | 34.7 | − | − | − | − | − | 27.5 | P. ovale | wallikeri | 2011 |
| 17 | P. ovale | P. ovale | 0.5 | − | − | 32.7 | − | − | − | 30.9 | − | 23.8 | P. ovale | wallikeri | 2011 |
| 18 | P. ovale | P. ovale | <0.1 | − | − | 33.3 | − | − | − | 32.7 | − | 31.7 | P. ovale | curtisi | 2011 |
| 19 | P. ovale | P. ovale | 0.3 | − | − | 24.4 | − | − | − | 34.5 | − | 26.8 | P. ovale | wallikeri | 2011 |
| 20 | P. ovale | Psp | 0.5 | − | + | 30.9 | − | − | − | − | − | 23.0 | P. ovale | wallikeri | 2011 |
| 21 | P. ovale | P. ovale | 0.3 | − | + | 28.4 | − | − | − | 27.0 | − | 22.4 | P. ovale | wallikeri | 2012 |
| 22 | P. ovale | P. ovale | 0.3 | − | + | 32.2 | − | − | − | 29.5 | − | 25.4 | P. ovale | wallikeri | 2012 |
| 23 | P. ovale | P. ovale | 0.2 | − | + | 26.8 | − | − | − | 26.4 | − | 24.4 | P. ovale | curtisi | 2012 |
| 24 | P. ovale | P. ovale | <0.1 | − | − | 27.7 | − | − | − | − | − | 28.2 | P. ovale | wallikeri | 2012 |
| 25 | P. ovale | P. ovale | <0.1 | − | − | 28.4 | 34.1 | − | − | 35.8 | − | 29.8 | P. ovale | wallikeri | 2012 |
| 26 | P. ovale | P. ovale | <0.1 | − | − | 33.6 | − | − | − | − | − | 31.6 | P. ovale | curtisi | 2012 |
| 27 | P. ovale | P. ovale | 0.3 | − | + | 22.9 | − | − | − | 28.5 | − | 24.0 | P. ovale | wallikeri | 2012 |
| 28 | P. ovale | Psp | <0.1 | − | − | − | − | − | − | 32.5 | − | 27.5 | P. ovale | wallikeri | 2012 |
| 29 | P. ovale | P. ovale | <0.1 | − | − | 34.7 | − | − | − | 34.1 | − | 28.1 | P. ovale | wallikeri | 2013 |
| 30 | P. ovale | P. ovale | <0.1 | − | − | 30.1 | − | − | − | 29.3 | − | 26.7 | P. ovale | curtisi | 2013 |
| 31 | P. ovale | P. ovale | <0.1 | − | − | 29.8 | − | − | − | − | − | 33.1 | P. ovale | wallikeri | 2013 |
| 32 | P. ovale | P. ovale | 0.1 | − | + | 26.2 | − | − | − | − | − | 29.7 | P. ovale | wallikeri | 2013 |
| 33 | P. ovale | P. ovale | <0.1 | − | − | 29.0 | − | − | − | − | − | 33.1 | P. ovale | wallikeri | 2013 |
P. ovale-positive specimens (n = 33) used in this study. Results from various diagnostic assays are shown. Species unidentifiable by microscopy are indicated as Psp. Species identification was based on concordance of 2 out of 3 species-specific tests, microscopy, qPCR, and BLAST sequence homology. Real-time quantitative PCR (qPCR) tests were run and compared for all 48 specimens with the protocol previously published by Khairnar et al. (current assay) (3) and a new assay with primer/probe sequences and concentrations designed in this study. Based on sequence analysis, 11 P. ovale curtisi (classic type) and 18 P. ovale wallikeri (variant type) cases were identified. Specimen numbers 5, 11, 13, and 22 were duplicate samples from the same patient above and thus were not counted as a case.
Binax T1, Plasmodium falciparum; Binax T2, Pan Plasmodium.
ND, not determined
TABLE 3.
Specimens positive for Plasmodium malariaea
| Specimen no. | Diagnosed species | Microscopy | Parasitemia (%) | Binaxb |
Genus qPCR, current assay (CT) | P. falciparum qPCR, current assay (CT) | P. vivax qPCR, current assay (CT) | P. malariae qPCR, current assay (CT) | P. ovale qPCR, current assay (CT) | P. malariae qPCR, new assay (CT) | P. ovale qPCR, new assay (CT) | 100% sequence homolog in blast | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | ||||||||||||
| 34 | P. malariae | P. malariae | <0.1 | − | + | 31.1 | − | − | 31.8 | − | 27.3 | − | P. malariae |
| 35 | P. malariae | P. malariae | 0.2 | − | + | 25.9 | − | − | 26.5 | − | 22.7 | − | P. malariae |
| 36 | P. malariae | P. malariae | <0.1 | − | + | 29.6 | − | − | 29.6 | − | 26.1 | − | P. malariae |
| 37 | P. malariae | P. malariae | <0.1 | − | + | 28.1 | − | − | 28.3 | − | 25.0 | − | P. malariae |
| 38 | P. malariae | P. malariae | <0.1 | − | + | 27.4 | − | − | 27.5 | − | 24.6 | − | P. malariae |
| 39 | P. malariae | Psp | <0.1 | − | − | 34.5 | − | − | 34.3 | − | 30.8 | − | P. malariae |
| 40 | P. malariae | Psp | <0.1 | − | − | 33.8 | − | − | 34.4 | − | 31.0 | − | P. malariae |
| 41 | P. malariae | P. malariae | <0.1 | NDc | ND | 28.8 | − | − | 29.0 | − | 25.7 | − | P. malariae |
| 42 | P. malariae | P. malariae | <0.1 | − | + | 29.3 | − | − | 29.3 | − | 26.0 | − | P. malariae |
| 43 | P. malariae | P. malariae | <0.1 | − | − | 29.6 | − | − | 30.6 | − | 26.7 | − | P. malariae |
| 44 | P. malariae | P. malariae | <0.1 | − | + | 26.5 | − | − | 26.2 | − | 22.9 | − | P. malariae |
| 45 | P. malariae | P. malariae | 0.2 | − | + | 29.8 | − | − | 30.2 | − | 25.8 | − | P. malariae |
| 46 | P. malariae | P. malariae | <0.1 | − | + | 26.3 | − | − | 26.5 | − | 24.2 | − | P. malariae |
| 47 | P. malariae | P. malariae | <0.1 | − | + | 27.9 | − | − | 28.2 | − | 24.7 | − | P. malariae |
| 48 | P. malariae | P. malariae | 0.2 | − | + | 25.4 | − | − | 24.9 | − | 23.2 | − | P. malariae |
P. malariae-positive specimens (n = 15) used in this study. Results from various diagnostic assays are shown. Species unidentifiable by microscopy are indicated as Psp. Species identification was based on concordance of 2 out of 3 species-specific tests, microscopy, qPCR, and BLAST sequence homology. Real-time quantitative PCR (qPCR) tests were run and compared for all 48 specimens with the protocol previously published by Khairnar et al. (current assay) (3) and a new assay with primer/probe sequences and concentrations designed in this study.
Binax T1, Plasmodium falciparum; Binax T2, Pan Plasmodium.
ND, not determined.
Sequence analysis.
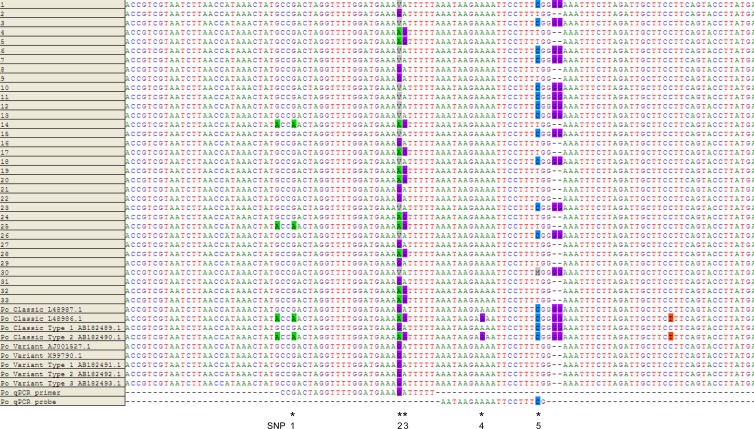
No discrepancies between the P. malariae primers and probes and P. malariae-positive specimen sequences were noted. Although an A/T mismatch to the P. malariae forward primer was found in P. malariae GenBank sequence M54897.1 (data not shown), it was not observed in any of our specimens; therefore, no modification to the primer was implemented. Sequence alignments of P. ovale specimens and GenBank sequences (Fig. 1) revealed SNPs to 3 loci to which the forward primer and 2 loci to which the probe hybridized (Fig. 1). The “V” on SNP 2 was represented by nucleotides (nt) A, C, and G. This “C” is a new nucleotide observed in this study and was included in recently submitted GenBank sequences (KC633224.1, KC633226.1, and KC866363.1). It is also observed only in P. ovale curtisi (classic) type.
FIG 1.
The 18S rRNA gene was sequenced and aligned with 4 classic and 5 variant type sequences from GenBank for 33 specimens positive for P. ovale. P. ovale forward primer and probe (nucleotide positions 1112 to 1139 and 1141 to 1159, respectively, of GenBank sequence L48987.1) sequences of the current assay were shown. The new primer and probe designed in this study with degenerate nucleotides bind to the same location. Single nucleotide polymorphisms (denoted by * and labeled as SNPs 1 to 5) were identified on 3 loci to which the primer and 2 loci to which the probe hybridized. SNP 5, along with the double-G deletions three nucleotides downstream, was used to differentiate between classic and variant types. In specimen number 30, in the SNP 5 position, although a dominant C nt was observed in the chromatogram, a minor A was also evident (denoted by an M). The dominant nt of the SNPs were indicated here; however, analysis of the peak heights of chromatograms revealed that the other polymorphic nt was also evident in SNPs 1 to 3, suggesting coinfection of more than one strain of P. ovale.
We therefore modified the currently used P. ovale forward primer and probe sequences based on these polymorphisms and designed the new degenerate P. ovale forward primer D2 and P. ovale probe D.
Performance of new duplex P. malariae/P. ovale qPCR assay.
Modified P. ovale forward primer and probe sequences (Table 4) were tested by performing qPCR on P. falciparum, P. vivax, P. malariae, and P. ovale 18S rRNA clones. The optimized primer/probe mix in the new assay had the following concentrations: 200 nM (P. malariae forward primer), 200 nM (P. ovale forward primer D2), 200 nM (Plasmodium reverse primer), 160 nM (P. ovale probe D), and 80 nM (P. malariae probe) (Table 4). The P. malariae forward primer was increased in concentration from our current assay with the aim to maintain or improve sensitivity. These concentrations were found to have the highest sensitivity without cross-reactivity. The new assay was further validated against the same set of P. malariae-positive, P. ovale-positive, P. falciparum-positive, P. vivax-positive, and negative specimens. All P. ovale-positive specimens (n = 33) were positive with the new assay, improving sensitivity to 100% from 72.7% while maintaining 100% specificity. Using the current assay, the LOD for P. malariae was 115 copies/reaction, while the LOD for P. ovale was 452 copies/reaction. With the new assay, the LOD for P. malariae was 84 copies/reaction and the LOD for P. ovale was 477 copies/reaction. Therefore, the LOD for P. malariae improved with the new assay, while the LOD for the current and new assays were comparable for P. ovale.
TABLE 4.
P. malariae and P. ovale duplex qPCR primer/probe sequences and final concentrationsa
| Primer/probe | Sequenceb | Final concn (nM) |
|---|---|---|
| P. malariae forward primerc | CCGACTAGGTGTTGGATGATAGAGTAAA | 200 |
| P. malariae probec | 6FAM-CTATCTAAAAGAAACACTCAT-MGBNFQ | 80 |
| P. ovale forward primer D2 | CCRACTAGGTTTTGGATGAAAVRTTTTT | 200 |
| P. ovale probe D | VIC-CRAAAGGAATTYTCTTATT-MGBNFQ | 160 |
| Plasmodium reverse primerc | AACCCAAAGACTTTGATTTCTCATAA | 200 |
P. ovale degenerate (D) primer and probe were designed based on sequence heterogeneity found in this study.
R = A or G; Y = C or T; V = A or C or G. Underlining indicates degenerate nucleotides.
Sequences as previously published (4).
Classification of P. ovale as P. ovale curtisi or P. ovale wallikeri.
Based on GenBank sequences, we identified 3 loci that can distinguish P. ovale curtisi from P. ovale wallikeri denoted by SNP 5 and the double-G deletions three nucleotides downstream (Fig. 1). Using these sequence differences, we classified 13 specimens (11 cases) as P. ovale curtisi and 20 specimens (18 cases) as P. ovale wallikeri (Table 2).
DISCUSSION
The diagnosis of malaria remains challenging in settings of nonendemicity, yet, rapid and accurate diagnosis with species identification are required to inform treatment and are critical to positive patient outcomes (8, 9). Although microscopic examination of thick and thin blood smears remains the diagnostic gold standard, this technique requires considerable technologist training and ongoing internal and external proficiency assessments, and it is insensitive at very low parasitemia. RDTs have the advantage of rapid turnaround, but their excellent performance characteristics are limited to P. falciparum, with suboptimal diagnostic sensitivity for non-falciparum species, especially P. ovale and P. malariae, as reported in this study and other studies (10, 11). Molecular methods do not require highly specialized technologists and have the advantage of sensitivity and rapidity; however, their practicality is limited to well-resourced reference centers, typically in settings of nonendemicity.
In our center, PCR is used to resolve cases difficult to speciate by microscopy, those with discordant or unusual RDT patterns, and those in which clinical suspicion is high, but microscopy and RDT are noncontributory. We noted several cases of microscopy and qPCR discordance and sought to optimize our molecular detection assays for P. malariae and P. ovale. Our new assay demonstrated superior sensitivity for P. ovale and improved LOD for P. malariae, without compromising specificity. With the recognition of 2 subspecies of P. ovale, both of which were imported to Ontario over our enrollment period, using a diagnostic assay that can reliably detect both strains is imperative to appropriate clinical management (6). In addition, using an assay that can distinguish the 2 subspecies may become epidemiologically important as well, given new published information regarding their differing latencies (12).
In summary, we improved our molecular detection algorithm for P. malariae and P. ovale by optimizing primers and probe concentrations and developing a new degenerate primer and probe set for P. ovale based on sequence analysis of the 18S region and accommodation of known and newly identified SNPs.
ACKNOWLEDGMENTS
We thank MR4 for providing us with Plasmodium small-subunit rRNA clones contributed by Peter A. Zimmerman.
This work was funded by Public Health Ontario.
Footnotes
Published ahead of print 15 January 2014
REFERENCES
- 1.Obare P, Ogutu B, Adams M, Odera JS, Lilley K, Dosoo D, Adhiambo C, Owusu-Agyei S, Binka F, Wanja E, Johnson J. 2013. Misclassification of Plasmodium infections by conventional microscopy and the impact of remedial training on the proficiency of laboratory technicians in species identification. Malar. J. 12:113. 10.1186/1475-2875-12-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins WE, Jeffery GM. 2007. Plasmodium malariae: parasite and disease. Clin. Microbiol. Rev. 20:579–592. 10.1128/CMR.00027-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khairnar K, Martin D, Lau R, Ralevski F, Pillai DR. 2009. Multiplex real-time quantitative PCR, microscopy and rapid diagnostic immuno-chromatographic tests for the detection of Plasmodium spp: performance, limit of detection analysis and quality assurance. Malar. J. 8:284. 10.1186/1475-2875-8-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. 2009. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J. Clin. Microbiol. 47:975–980. 10.1128/JCM.01858-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen R, Feghali K, Alemayehu S, Komisar J, Hang J, Weina PJ, Coggeshall P, Kamau E, Zapor M. 2013. Use of qPCR and genomic sequencing to diagnose Plasmodium ovale wallikeri malaria in a returned soldier in the setting of a negative rapid diagnostic assay. Am. J. Trop. Med. Hyg. 89:501–506. 10.4269/ajtmh.12-0724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauffe F, Desplans J, Fraisier C, Parzy D. 2012. Real-time PCR assay for discrimination of Plasmodium ovale curtisi and Plasmodium ovale wallikeri in the Ivory Coast and in the Comoros Islands. Malar. J. 11:307. 10.1186/1475-2875-11-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kain KC, MacPherson DW, Kelton T, Keystone JS, Mendelson J, MacLean JD. 2001. Malaria deaths in visitors to Canada and in Canadian travellers: a case series. CMAJ 164:654–659 [PMC free article] [PubMed] [Google Scholar]
- 9.Suh KN, Kain KC, Keystone JS. 2004. Malaria. CMAJ. 170:1693–1702. 10.1503/cmaj.1030418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maltha J, Gillet P, Jacobs J. 2013. Malaria rapid diagnostic tests in endemic settings. Clin. Micro. Infect. 19:399–407. 10.1111/1469-0691.12151 [DOI] [PubMed] [Google Scholar]
- 11.Maltha J, Gillet P, Jacobs J. 2013. Malaria rapid diagnostic tests in travel medicine. Clin. Micro. Infect. 19:408–415. 10.1111/1469-0691.12152 [DOI] [PubMed] [Google Scholar]
- 12.Nolder D, Oguike MC, Maxwell-Scott H, Niyazi HA, Smith V, Chiodini PL, Sutherland CJ. 2013. An observational study of malaria in British travellers: Plasmodium ovale wallikeri and Plasmodium ovale curtisi differ significantly in the duration of latency. BMJ Open 3:e002711. 10.1136/bmjopen-2013-002711 [DOI] [PMC free article] [PubMed] [Google Scholar]