Abstract
Mycobacterium paraffinicum has been newly recognized as a species. A case of symptomatic pulmonary infection caused by M. paraffinicum is described, and as far as we know, this is the first case of the organism as a human pathogen.
CASE REPORT
In May 2012, an 85-year-old female presented to our tertiary referral hospital with acute shortness of breath. Her medical history included hypertension, for which she was not treated. She noted progressive generalized weakness, malaise, anorexia, and a chronic cough productive of white sputum for the past 6 years. On review of systems, she reported no fevers, chills, night sweats, or hemoptysis. She denied any history of smoking (but lived with a smoker), pneumonia, or other lung infections, tuberculosis exposure, homelessness, health care work, history of imprisonment, or recent travel. On physical examination, the patient had a temperature of 37.0°C, blood pressure of 158/88 mm Hg, a heart rate of 109 beats per minute (bpm), a respiratory rate of 20, and a 93% oxygen saturation as determined by pulse oximetry on ambient air. On examination, the patient had moderate kyphosis and pectus carinatum with no cervical lymphadenopathy. There was a 3/6 holosystolic murmur present at the cardiac apex and bibasilar rales on the left lung greater than on the right with no evidence of egophony. She exhibited 5/5 strength throughout all extremities. Laboratory data were as follows: white blood cell count of 16,400/μl, with a differential of 1% band forms, 94% segmented neutrophils, 3% lymphocytes, and 2% monocytes, hemoglobin of 11.4 g/dl, platelet count of 507,000/μl, sodium at 129 meq/liter, potassium at 3.6 meq/liter, glucose at 131 mg/dl, and creatinine at 1.1 mg/dl. The patient was started on ceftriaxone and azithromycin for treatment of community-acquired pneumonia, which was consistent with her presentation. On the second day of hospitalization, a computed tomography (CT) scan of the chest, abdomen, and pelvis with contrast revealed near-complete occlusion of the left main bronchus with associated atelectasis of the lingula and cylindrical and cystic bronchiectasis within the left lower lobe and right middle lobe (Fig. 1). Also present were numerous nodules within the atelectatic lung, with central hypoattenuation, numerous additional nodules with central hypoattenuation present bilaterally, and moderate bilateral pleural effusions. A transthoracic echocardiogram was also obtained, revealing an impaired relaxation pattern of diastolic filling and elevated right ventricular systolic pressure. On the fourth day, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) of the left lower lobe, revealing severe mucous plugging of the left lung and thick mucoid secretions without evidence of endobronchial lesions, which grew Candida spp. Treatment of the BAL fluid with acid-fast (Kinyoun) stain revealed rare acid-fast bacilli. The infectious disease service was consulted, and PCR testing using a laboratory-developed test of the primary BAL sample was requested and was negative for tuberculosis (TB). Her shortness of breath improved significantly with treatment for heart failure and pneumonia, which were thought to be responsible for her acute presentation to the hospital, and she was discharged to follow up with the outpatient pulmonary service regarding culture results of the atypical mycobacterium, which was yet unidentified. The BBL MGIT (mycobacterial growth indicator tube) (Becton, Dickinson and Company, Franklin Lakes, New Jersey) was positive after 9 days, as was pinpoint growth on Middlebrook 7H11 agar, and the Accuprobe culture identification test (Gen-Probe, Inc., San Diego, CA) was negative for Mycobacterium tuberculosis and Mycobacterium avium complex (MAC). Seven days later, a yellowish tint was observed, and probes for Mycobacterium kansasii and M. gordonae were performed and were negative (Fig. 2). The isolate was sent to the National Jewish Health mycobacteriology laboratory for identification and susceptibility testing. One month after discharge, the patient was started on an airway clearance device to help mobilize secretions. Three months after discharge, the organism was identified by the National Jewish Health laboratory as Mycobacterium paraffinicum. Repeated sputum samples continued to grow M. paraffinicum, and the patient was started on an empirical regimen of azithromycin, ciprofloxacin, and linezolid. The organism demonstrated susceptibility to ciprofloxacin, clarithromycin, linezolid, and doxycycline. The mycobacterium had intermediate susceptibility to rifabutin and rifampin and was resistant to ethambutol, streptomycin, amikacin, and imipenem. It was susceptible to rifampin and ethambutol when these were tested in combination. Unfortunately, the patient was unable to tolerate the oral regimen due to nausea and vomiting and decided to discontinue treatment. A cause for her underlying bronchiectasis was never determined. She was last seen in the pulmonary clinic in September 2013, where she remained with chronic symptoms, with an occasional productive cough with green phlegm. The lowest oxygen saturation after a 6-min walk was 88%.
FIG 1.
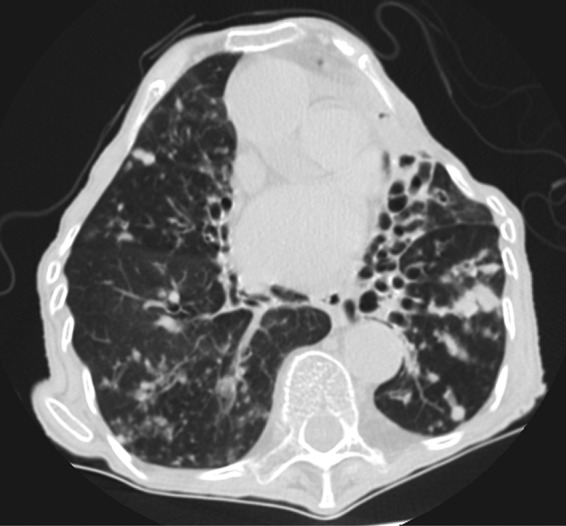
CT scan demonstrating diffuse bronchiectasis of the left lower lobe and right middle lobe.
FIG 2.
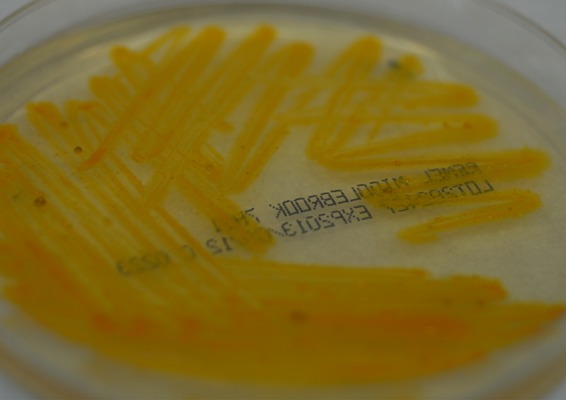
Growth of M. paraffinicum, demonstrated on Middlebrook 7H11 agar.
M. paraffinicum was initially isolated from a soil sample in 1956 (1). The organism is a long, slender, strongly acid-fast rod showing Much's granules with Ziehl-Neelsen stain, yellow, waxy, wrinkled colonies, and no growth on nutrient-agar medium, glycerol, or methane (1). Interestingly, in 1971, M. paraffinicum lost its standing as a species because it was determined that the isolate could not be reliably distinguished from Mycobacterium scrofulaceum (2). In 1991, in the fourth report of the International Working Group on Mycobacterial Taxonomy (IWGMT), it was determined that the isolate deemed M. paraffinicum had different biochemical responses from those of M. scrofulaceum and belonged to a discrete environmental species (3). In 2010, multiple molecular sequence analyses comparing M. paraffinicum to M. scrofulaceum, Mycobacterium nebraskense, and Mycobacterium seoulense via 16S rRNA gene sequences were conducted, along with hsp65 and rpoB gene sequencing, and the name M. paraffinicum was reinstated, ending what was referred to as “more than 5 decades of taxonomic confusion” (4). Along with M. paraffinicum, other species of nontuberculous mycobacteria also utilize paraffin and other complex hydrocarbons as a sole carbon source (5, 6). The reports of M. paraffinicum in the clinical setting are limited. The only study describing M. paraffinicum in a clinical setting described a pseudo-outbreak of M. paraffinicum at a university-affiliated, tertiary care facility (7, 8). M. paraffinicum was isolated from sputum and stool samples from 21 patients over 2.5 years, with identification of the hospital water system as the source of the contamination. M. paraffinicum was not implicated as a pathogen in any of the 21 patients.
Nontuberculous mycobacteria, such as M. paraffinicum, demonstrate very different susceptibility patterns that differ between species (9). In deciding antibiotic regimens for pulmonary infections caused by nontuberculous mycobacteria, it is helpful to first divide these organisms into the categories of rapidly growing mycobacteria (RGM) and slow-growing mycobacteria (SGM). Antibiotic regimens for RGM are dictated primarily by the susceptibility patterns of Mycobacterium abscessus and M. fortuitum (10, 11). Both M. abscessus and M. fortuitum exhibit inducible macrolide resistance, which is believed to work through activation of the erm gene (12). In the case of M. paraffinicum, because of its recent reclassification as a distinct species, there are no previous susceptibility data to guide therapy. Even when extrapolating from a close biological relative, M. scrofulaceum, the data remain limited. The empirical regimen for this patient, azithromycin, ciprofloxacin, and linezolid, was chosen in order to give the patient an oral regimen with activity against SGM. Many documented SGM species demonstrate susceptibility to macrolides, and these agents are now a cornerstone of most SGM regimens (9). Linezolid demonstrates high oral drug availability and good in vitro susceptibility against a broad range of SGM, but it differs depending on the strain of nontuberculous Mycobacterium that is being treated (13, 14). Finally, ciprofloxacin is relatively well tolerated with a low side effect profile and has a relatively broad coverage of the SGM group at achievable MICs (15). The choice of this regimen was corroborated when susceptibility testing returned.
Conclusion.
As far as we know, this is the first reported case of M. paraffinicum as a pathogen. Given the recent reinstatement of M. paraffinicum as a separate and distinct species, we believe that this organism represents a new addition to the list of SGM that are capable of causing human disease.
Footnotes
Published ahead of print 22 January 2014
REFERENCES
- 1.Chase HH, Davis JB, Raymond RL. 1956. Mycobacterium paraffinicum n. sp., a bacterium isolated from soil. Appl. Microbiol. 4:310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wayne LG, Dietz TM, Gernez-Rieux C, Jenkins PA, Kappler W, Kubica GP, Kwapinski JB, Meissner G, Pattyn SR, Runyon EH, Schroder KH, Silcox VA, Tacquet A, Tsukamura M, Wolinsky E. 1971. A co-operative numerical anaysis of scotochromogenic slowly growing mycobacteria. J. Gen. Microbiol. 66:255–271. 10.1099/00221287-66-3-255 [DOI] [PubMed] [Google Scholar]
- 3.Wayne LG, Good RC, Krichevsky MI, Blacklock Z, David HL, Dawson D, Gross W, Hawkins J, Levy-Frebault VV, McManus C, Portaels F, Rüsch-Gerdes S, Schrőder KH, Silcox VA, Tsukamura M, Van Den Breen L, Yakrus MA. 1991. Fourth report of the cooperative, open-ended study of slowly growing mycobacteria by the International Working Group on Mycobacterial Taxonomy. Int. J. Syst. Bacteriol. 41:463–472. 10.1099/00207713-41-4-463 [DOI] [PubMed] [Google Scholar]
- 4.Toney N, Adekambi T, Toney S, Yakrus M, Butler WR. 2010. Revival and amended description of ‘Mycobacterium paraffinicum' Davis, Chase and Raymond 1956 as Mycobacterium paraffinicum sp. nov., nom. rev. Int. J. Syst. Evol. Microbiol. 60:2307–2313. 10.1099/ijs.0.016972-0 [DOI] [PubMed] [Google Scholar]
- 5.Ollar RA, Brown S, Dale JW, Felder MS, Brown IN, Edwards FF, Armstrong D. 1991. A modified broth dilution assay for antibiotic sensitivity testing of Mycobacterium avium-intracellulare using paraffin slide cultures. Tubercle 72:198–205. 10.1016/0041-3879(91)90008-G [DOI] [PubMed] [Google Scholar]
- 6.Ollar RA, Dale JW, Felder MS, Favate A. 1990. The use of paraffin wax metabolism in the speciation of Mycobacterium avium-intracellulare. Tubercle 71:23–28. 10.1016/0041-3879(90)90056-E [DOI] [PubMed] [Google Scholar]
- 7.Wang SH, Mangino JE, Stevenson K, Yakrus MA, Cooksey R, Butler WR, Healy M, Wise MG, Schlesinger LS, Pancholi P. 2008. Characterization of “Mycobacterium paraffinicum” associated with a pseudo-outbreak. J. Clin. Microbiol. 46:1850–1853. 10.1128/JCM.02079-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SH, Pancholi P, Stevenson K, Yakrus MA, Butler WR, Schlesinger LS, Mangino JE. 2009. Pseudo-outbreak of “Mycobacterium paraffinicum” infection and/or colonization in a tertiary care medical center. Infect. Control Hosp. Epidemiol. 30:848–853. 10.1086/599071 [DOI] [PubMed] [Google Scholar]
- 9.Brown-Elliott BA, Wallace RJ., Jr 2009. Infections due to nontuberculous mycobacteria other than Mycobacterium avium-intracellulare, p 3191–3198 In Mandell GL, Bennett JE, Dolin R. (ed), Mandell, Douglas, and Bennett's Principles and practice of infectious diseases, 7th ed. Churchill Livingstone, Philadelphia, PA [Google Scholar]
- 10.Iseman MD, Marras TK. 2008. The importance of nontuberculous mycobacterial lung disease. Am. J. Respir. Crit. Care Med. 178:999–1000. 10.1164/rccm.200808-1258ED [DOI] [PubMed] [Google Scholar]
- 11.Philley JV, Griffith DE. 2013. Management of nontuberculous mycobacterial (NTM) lung disease. Semin. Respir. Crit. Care Med. 34:135–142. 10.1055/s-0033-1333575 [DOI] [PubMed] [Google Scholar]
- 12.Griffith DE. 2010. Nontuberculous mycobacterial lung disease. Curr. Opin. Infect. Dis. 23:185–190. 10.1097/QCO.0b013e328336ead6 [DOI] [PubMed] [Google Scholar]
- 13.Brown-Elliott BA, Crist CJ, Mann LB, Wilson RW, Wallace RJ., Jr 2003. In vitro activity of linezolid against slowly growing nontuberculous mycobacteria. Antimicrob. Agents Chemother. 47:1736–1738. 10.1128/AAC.47.5.1736-1738.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavusoglu C, Soyler I, Akinci P. 2007. Activities of linezolid against nontuberculous mycobacteria. New Microbiol. 30:411–414 [PubMed] [Google Scholar]
- 15.Gay JD, DeYoung DR, Roberts GD. 1984. In vitro activities of norfloxacin and ciprofloxacin against Mycobacterium tuberculosis, M. avium complex, M. chelonei, M. fortuitum, and M. kansasii. Antimicrob. Agents Chemother. 26:94–96. 10.1128/AAC.26.1.94 [DOI] [PMC free article] [PubMed] [Google Scholar]


