Abstract
Limited performance data from line probe assays (LPAs), nucleic acid tests used for the rapid diagnosis of tuberculosis (TB), nontuberculosis mycobacteria (NTM), and Mycobacterium tuberculosis drug resistance are available for HIV-infected individuals, in whom paucibacillary TB is common. In this study, the strategy of testing sputum with GenoType MTBDRplus (MTBDR-Plus) and GenoType Direct LPA (Direct LPA) was compared to a gold standard of one mycobacterial growth indicator tube (MGIT) liquid culture. HIV-positive (HIV+) individuals with suspected TB from southern Africa and South America with <7 days of TB treatment had 1 sputum specimen tested with Direct LPA, MTBDR-Plus LPA, smear microscopy, MGIT, biochemical identification of mycobacterial species, and culture-based drug-susceptibility testing (DST). Of 639 participants, 59.3% were MGIT M. tuberculosis culture positive, of which 276 (72.8%) were acid-fast bacillus (AFB) smear positive. MTBDR-Plus had a sensitivity of 81.0% and a specificity of 100%, with sensitivities of 44.1% in AFB smear-negative versus 94.6% in AFB smear-positive specimens. For specimens that were positive for M. tuberculosis by MTBDR-Plus, the sensitivity and specificity for rifampin resistance were 91.7% and 96.6%, respectively, and for isoniazid (INH) they were 70.6% and 99.1%. The Direct LPA had a sensitivity of 88.4% and a specificity of 94.6% for M. tuberculosis detection, with a sensitivity of 72.5% in smear-negative specimens. Ten of 639 MGIT cultures grew Mycobacterium avium complex or Mycobacterium kansasii, half of which were detected by Direct LPA. Both LPA assays performed well in specimens from HIV-infected individuals, including in AFB smear-negative specimens, with 72.5% sensitivity for M. tuberculosis identification with the Direct LPA and 44.1% sensitivity with MTBDR-Plus. LPAs have a continued role for use in settings where rapid identification of INH resistance and clinically relevant NTM are priorities.
INTRODUCTION
Rapid laboratory identification of tuberculosis (TB) and Mycobacterium tuberculosis drug resistance are critical to ensure timely initiation of therapy, to inform appropriate TB therapy, and to facilitate infection control. Early diagnosis of TB and drug-resistant disease is of particular importance in human immunodeficiency virus (HIV)-infected individuals, as delay of therapy (1, 2) and drug-resistant TB (3) can be devastating in those with compromised immune systems. Diagnosis of TB in HIV can be a particular challenge, as 24% to 61% of HIV coinfected individuals with pulmonary TB have acid-fast bacillus (AFB)-smear-negative sputum (2). Culture-based testing for M. tuberculosis and M. tuberculosis drug resistance requires an unacceptably long turnaround for results, is limited by contamination rates, and requires considerable infrastructure and human resources.
Molecular line probe assays (LPA) permit rapid diagnosis of TB, isoniazid and rifampin resistance, and clinically relevant non-M. tuberculosis mycobacteria. In these assays, DNA or RNA is isolated from culture or direct (i.e., sputum) respiratory samples and then amplified and reverse hybridized onto a nitrocellulose strip with immobilized probes for different mycobacteria or for mutations that confer resistance. These strips can be quickly interpreted using a template, with the entire testing process taking a day or less in most cases. In 2008, the World Health Organization endorsed the use of line probe assays for detection of M. tuberculosis drug resistance; however, use was recommended on culture specimens and AFB-positive (AFB+) sputum specimens only, given the lack of data for use in AFB-negative sputum (4). Given the prevalence of paucibacillary disease in HIV-TB coinfection, it is important to understand line probe performance across the spectrum of bacillary loads in HIV-infected persons, in whom there are limited data to inform use of line probe assays.
The GenoType MTBDRplus (MTBDR-Plus) (Hain Lifesciences GmBH, Nehren, Germany) identifies rifampin (RIF) and isoniazid (INH) resistance by detecting the most common mutations of the rpoB gene and the katG and inhA genes, respectively, and can be used on both cultured and direct specimens. MTBDR-Plus LPA RIF and INH resistance results can be interpreted only if M. tuberculosis has been successfully identified by the assay probe for it. Several studies have suggested excellent performance of MTBDR-Plus in AFB-negative direct specimens with interpretable results in 80.0% to 95.8% of specimens (5, 6), but interpretable test rates have been as low as 13.7% in one series (7). Of note, these studies were conducted in populations that were either HIV uninfected or HIV status unknown. The GenoType mycobacteria direct line probe assay (Direct LPA) (also Hain Lifesciences GmbH) is another line probe assay that uses nucleic acid sequence-based amplification (NASBA) to identify the mycobacterial species-specific 23S rRNA, thus differentiating the M. tuberculosis complex from four other clinically relevant mycobacterial species, Mycobacterium avium, M. intracellulare, M. kansasii, and M. malmoense. The nontuberculosis species M. avium and M. intracellulare (collectively known as the M. avium complex [MAC]) and M. kansasii are important causes of pulmonary and disseminated disease in HIV-infected patients (8–11). Importantly, these pathogens would be missed with rapid diagnostics that identify only Mycobacterium tuberculosis. The Direct LPA is designed for use on direct sputum specimens. Data are limited for AFB-negative direct sputum samples and in HIV-infected populations, but small studies have reported sensitivities of 69.0% to 89.6% (6, 12). We sought to determine the accuracy of the MTBDR-Plus and Direct LPA in HIV-infected TB subjects, with both AFB+ and AFB-negative sputum specimens, in comparison to liquid culture. We also evaluated the incremental yield of a second Direct LPA performed on an additional sputum specimen as well as an additional MTBDR-Plus LPA performed on mycobacterial culture.
MATERIALS AND METHODS
Study population.
This was a cross-sectional analysis of HIV-infected individuals with suspected tuberculosis enrolled from 7 sites: Rio de Janeiro, Brazil; Lima, Peru; 2 sites in Botswana (Gaborone and Molepole); and 3 sites in South Africa (1 in Johannesburg and 2 in Durban). Qualifying participants on <7 days of TB therapy were referred from clinical care to the research sites with either (i) confirmed TB (sputum AFB+ or culture positive) or (ii) probable TB (no smear- or culture-positive specimen but empirical TB treatment anticipated based on clinical symptoms). Clinical TB symptoms and signs were defined as one or more of the following: fever for >2 weeks, unintentional weight loss, night sweats, radiographic findings compatible with TB, or contact with TB-infected individuals. All participants provided one sputum specimen, with a minimum volume of 3 ml, either expectorated or induced, tested for the following: AFB smear, liquid mycobacterial culture, GenoType Direct LPA (decontaminated sediment), and GenoType MTBDR-Plus (decontaminated sediment and culture). The first 109 participants enrolled provided a second sputum specimen which was tested with AFB smear as well as Direct LPA and MTBDR-Plus LPA on decontaminated sediment. All participants with initial negative M. tuberculosis culture or with contaminated/missing results were evaluated 24 weeks after enrollment for all interim mycobacterial results and follow-up TB status. Follow-up TB+ cases were defined as those with sputum cultures positive for TB after enrollment. Lab testing occurred at one of three laboratories: (i) Contract Laboratory Services in Johannesburg, South Africa, (ii) Fundação Oswaldo Cruz Instituto de Pesquisa Clinica Evandro Chagas Laboratório de Bacteriologia, Rio de Janeiro, Brazil, and (iii) Blufstein Laboratório Clínico, Lima, Peru.
Conventional microbiology testing.
Sputum samples were digested and decontaminated with 1% N-acetyl-l-cysteine–sodium hydroxide (NALC/NaOH) as outlined by Kent and Kubica (13). After digestion and decontamination, specimens were neutralized and centrifuged at 3,000 × g for 15 min. AFB smears were prepared from decontaminated sediment with auramine and a potassium permanganate counterstain and evaluated with a Lumin portable light-emitting diode (LED) objective (40×) (LW Scientific, Lawrenceville, GA) for a minimum of 100 fields, using the WHO/International Union against Tuberculosis and Lung Disease (IUALTD) scale for fluorescence microscopy (FM) (14). Sediment was prepared for culture in mycobacterial growth indicator tube (MGIT) culture (Becton, Dickinson) according to the manufacturer's instructions (15). In cultures with mycobacterial growth, identification to the species level was performed using biochemical testing (13). Cultures in which the species was identified as M. tuberculosis underwent drug susceptibility testing using Bactec MGIT streptomycin, INH, RMP, and ethambutol (SIRE) (16), and the critical concentration was 1.0 μg/ml for RIF and 0.1 μg/ml for INH. Mycobacterial blood cultures were performed at clinician discretion using Bactec 9050 or Myco/F Lytic (both Becton, Dickinson).
Line probe assay testing.
Testing was conducted in accordance with manufacturer's recommendations and was performed by laboratory staff members trained by the manufacturer. DNA/RNA extraction, PCR, and reverse hybridization were conducted in separate rooms to minimize contamination. Tests were performed and interpreted without knowledge of culture results.
GenoType MTBDRplus (version 1.0).
Testing was performed on decontaminated sputum sediment as well as culture with confirmed TB growth. Briefly, 500 μl of decontaminated sediment was centrifuged and resuspended with 100 μl ultrapure water or 1,000 μl of bacterial suspension heat killed at 95°C for 20 min. DNA was extracted using an ultrasonic bath. Multiplex PCR was performed using HotStar Taq DNA polymerase 250 U (Qiagen GmBH, Hilden, Germany), with 40 amplification cycles for sediment and 30 cycles for cultured specimens. Hybridization was performed with a TwinCubator (Hain Lifescience GmbH). Test strips were interpreted in a two-step procedure. (i) The presence or absence of M. tuberculosis was determined and (ii) for strips with M. tuberculosis present, INH and RIF susceptibilities were interpreted. Strips that could not be definitively interpreted were repeated if possible using extracted DNA; definitive test results were reported from the second test or the final test results was reported as “indeterminate.” MTBDR-Plus drug susceptibility results were interpreted only from specimens in which M. tuberculosis was present. Strips with mixed resistance results (i.e., both wild-type and resistance mutations present) were reported as resistant for sensitivity and specificity calculations.
GenoType direct line probe (version 4.0).
RNA was extracted from a 500-μl resuspended decontaminated pellet using a magnet separator (Hain Lifescience GmbH), followed by denaturation and isothermal nucleic acid amplification performed on 10 μl purified RNA. We used 20 μl of amplified solution hybridization with a TwinCubator (Hain Lifescience GmbH). Test strips were interpreted as follows. First, the presence of the conjugate control band was confirmed, and then species identification was interpreted according to a manufacturer-provided template. Strips lacking conjugate control or lacking amplification control (in the absence of positive banding indicating species) were considered uninterpretable and the specimen was rerun if possible. As with MTBDR-Plus LPA, if repeat testing provided a definitive result, this was recorded; otherwise, final results were recorded as “indeterminate.”
Statistical methods.
Sensitivity and specificity were calculated using standard epidemiological methods. The gold standard comparator for M. tuberculosis detection was liquid culture (MGIT), for mycobacterial identification it was biochemical species determination, and for INH and RIF drug susceptibility it was MGIT SIRE testing. Because of indeterminate results, sensitivity and specificity were calculated both from an intent-to-screen approach, where indeterminate LPA test results were interpreted as false negatives, and from the subgroup for which all test results for LPA and M. tuberculosis culture were definitive. In the participants with two specimens and among those, the participants who had a negative result on the first specimen, the incremental yield was calculated as the number of second specimens that were positive divided by the number of first specimens that were negative. The 95% confidence intervals (CIs) on these measures were calculated using Wilson's score binomial method. Two-sided Fisher's exact and chi-square tests were used to compare sensitivities and specificities within subgroups, with results considered significant when alpha was <0.05; other statistical comparisons were done using two-sided Wilcoxon and chi-square tests.
The study was reviewed and approved by ethics committees at all participating sites and all participants gave written informed consent.
RESULTS
From September 2009 to October 2011, 639 eligible study participants with suspected TB provided sputum for evaluation. The median age was 36 years (interquartile range [IQR], 30, 42), 56.0% were male, and 68.5% were enrolled from Southern Africa (South Africa and Botswana) and 31.5% from South America (Brazil and Peru). The median CD4+ cell count was 151 cells/mm3 (IQR, 61, 308) with 22.4% receiving antiretroviral treatment (ART) and 23.8% having initiated TB treatment at the time of sputum collection (median 3 days [IQR, 1, 5]). Table 1 shows baseline characteristics and predictors of M. tuberculosis MGIT positivity.
TABLE 1.
Baseline characteristics
| Participant characteristics | Median (quartile 1, quartile 3) or n (%) for: |
Pa | |||
|---|---|---|---|---|---|
| Total (n = 639) | Data by result of MGIT culture |
||||
| Positive (n = 379) | Negative (n = 224) | Failure/missing (n = 36) | |||
| Age (yr) | 36 (30, 42) | 35 (29, 41) | 38 (33, 45) | 36 (31, 42.5) | <0.001 |
| Male | 358 (56.0) | 202 (53.3) | 129 (57.6) | 27 (75.0) | 0.306 |
| Site of enrollment | |||||
| Southern Africa | 438 (68.5) | 316 (83.4) | 101 (45.1) | 21 (58.3) | <0.001 |
| South America | 201 (31.5) | 63 (16.6) | 123 (54.9) | 15 (41.7) | |
| CD4 count at enrollmentb | |||||
| CD4 ≤ 50 cells/mm3 | 135 (21.3) | 69 (18.4) | 52 (23.5) | 14 (38.9) | 0.129 |
| CD4 ≤ 350 cells/mm3 | 503 (79.5) | 316 (84.0) | 156 (70.6) | 31 (86.1) | <0.001 |
| BMI (kg/m2)c | 20.8 (18.3, 23.4) | 20.8 (18.3, 23.1) | 21.1 (18.3, 23.7) | 20.1 (17.9, 22.2) | 0.573 |
| Abnormal CXRd | 481 (76.6) | 296 (79.6) | 157 (71.0) | 28 (80.0) | 0.018 |
| TB symptoms reportede | |||||
| Coughf | 621 (97.6) | 373 (98.9) | 215 (96.0) | 33 (94.3) | 0.016 |
| Feverg | 399 (63.0) | 222 (59.5) | 155 (69.2) | 22 (61.1) | 0.018 |
| Night sweatsh | 450 (70.8) | 274 (72.7) | 151 (67.7) | 25 (69.4) | 0.196 |
| Unintentional weight lossi | 567 (90.3) | 347 (93.8) | 189 (84.8) | 31 (88.6) | <0.001 |
| Wastingj | 350 (55.1) | 213 (56.5) | 120 (53.8) | 17 (48.6) | 0.522 |
| On ART at time of sputum collection | 143 (22.4) | 71 (18.7) | 63 (28.1) | 9 (25.0) | 0.007 |
| On TB treatment (<7 days) at time of sputum collection | 152 (23.8) | 109 (28.8) | 32 (14.3) | 11 (30.6) | <0.001 |
| AFB FM smear positivityk | 301 (47.3) | 276 (72.8) | 14 (6.3) | 11 (32.4) | <0.001 |
P values were for comparisons of MGIT culture positive versus negative. The Wilcoxon test was used for age and body mass index (BMI), and the chi-square test was used for others.
Due to missing data, sample sizes were 633, 376, 221, and 36, respectively.
Due to missing data, sample sizes were 631, 375, 221, and 35, respectively.
Due to missing data, sample sizes were 628, 372, 221, and 35, respectively.
Reported in past 30 days, any duration.
Due to missing data, sample sizes were 636, 377, 224, and 35, respectively.
Due to missing data, sample sizes were 633, 373, 224, and 36, respectively.
Due to missing data, sample sizes were 636, 377, 223, and 36, respectively.
Due to missing data, sample sizes were 628, 370, 223, and 35, respectively.
Due to missing data, sample sizes were 635, 377, 223, and 35, respectively.
Due to missing data, sample sizes were 637, 379, 224, and 34, respectively.
Figure 1 indicates the specimen flow. There were 639 specimens that yielded smear microscopy, MGIT culture, Direct LPA, and MTBDR LPA results. MGIT was positive for M. tuberculosis in 379/639 (59.3%), of which 276 (72.8%) were AFB smear positive. MGIT was positive for nontuberculosis mycobacterium (NTM) in 14/639 (2.2%) (9 MAC, 1 M. kansasii, and 4 others); no MGIT culture yielded both TB and a second mycobacterial species. Of MGIT cultures, 210/639 (32.9%) were negative for any growth, 27/639 (4.2%) were contaminated, and 9/639 (1.4%) had no results due to site/lab error. Follow-up evaluation of participants with MGIT TB-negative or contaminated/missing results identified an additional 7 (1.1%) pulmonary M. tuberculosis culture-positive cases.
FIG 1.
Specimen flow schematic.
MTBDR-Plus LPA.
MTBDR LPA was positive for M. tuberculosis in 315/639 (49.3%) specimens, negative for mycobacteria in 314 (49.1%), and had no available result in 10 (1.6%) due to test failure (7) or site/lab error (3). Table 2 provides the MTBDR LPA and MGIT results for the detection of M. tuberculosis in sputum, with MGIT as the gold standard. With the use of an intent-to-screen approach (Table 3), MTBDR correctly identified M. tuberculosis in 306 of 378 M. tuberculosis culture-positive specimens (sensitivity, 81.0% [CI, 76.7%, 84.6%]) with a specificity of 100% (224/224 [CI, 98.3%, 100%]). When evaluating the 595 specimens with interpretable results for both MGIT and MTBDR LPA, we found that sensitivity was 82.3% (306/372 [CI, 78.1%, 85.8%]) and specificity was 100% (223/223 [CI, 98.3%, 100%]). MTBDR M. tuberculosis detection varied by AFB smear status, with sensitivity of 95.6% (261/273 [CI, 92.5%, 97.5%]) in smear-positive versus 45.5% (45/99 [CI, 36.0%, 55.2%]) smear-negative specimens (Fisher's exact test, P < 0.001) (Table 3).
TABLE 2.
Mycobacterial detection by MGIT versus line probe assays
| Sample type | MGIT results (n [%]) |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| M. tuberculosis present | NTM only | MAC or M. kansasii | Other NTM only | Contaminated | No mycobacterial growth | Not available | ||
| MTBDR-plus LPA results | ||||||||
| M. tuberculosis present | 306 (80.7) | 0 | 6 (22.2) | 0 | 3 (33.3) | 315 | ||
| No M. tuberculosis present | 66 (17.4) | 14 (100.0) | 21 (77.8) | 209 (99.5) | 4 (44.4) | 314 | ||
| Not available | 7 (1.8) | 0 | 0 | 1 (0.5) | 2 (22.2) | 10 | ||
| Total | 379 (100.0) | 14 (100.0) | 27 (100.0) | 210 (100.0) | 9 (100.0) | 639 | ||
| Direct LPA results | 0 | |||||||
| M. tuberculosis present | 334a (88.1) | 0 | 6 (22.2) | 12 (5.7) | 3 (33.3) | 355 | ||
| MAC or M. kansasii | 0 | 5 (50.0) | 1 (25.0) | 0 | 1 (0.5) | 0 | 7 | |
| Indeterminate | 3 (0.8) | 0 | 0 | 0 | 2 (1.0) | 2 (22.2) | 7 | |
| No mycobacteria present | 36 (9.5) | 5 (50.0) | 3 (75.0) | 21 (77.8) | 194 (92.4) | 2 (22.2) | 261 | |
| Not available | 6 (1.6) | 0 | 0 | 0 | 1 (0.5) | 2 (22.2) | 9 | |
| Total | 379 (100.0) | 10 (100.0) | 4 (100.0) | 27 (100.0) | 210 (100.0) | 9 (100.0) | 639 | |
M. tuberculosis and MAC were detected in 3 out of the 334 specimens by Direct LPA.
TABLE 3.
Sensitivities and specificities of line probe assays for M. tuberculosis detectiona
| Result type | Detection sensitivity (no. positive/no. detected [%]) (95% CI) |
Detection specificity (no. positive/no. detected [%]) (95% CI) |
||||
|---|---|---|---|---|---|---|
| AFB+ | AFB− | Allb | AFB+ | AFB− | All | |
| Interpretable results for both MGIT and LPA | ||||||
| Direct line probe | 260/271 (95.9) (92.9, 97.7) | 74/99 (74.7) (65.4, 82.3) | 334/370 (90.3) (86.8, 92.9) | 14/14 (100) (78.5, 100) | 195/207 (94.2) (90.1, 96.7) | 209/221 (94.6) (90.8, 96.9) |
| MTBDR-Plus (sputum) | 261/273 (95.6) (92.5, 97.5) | 45/99 (45.5) (36.0, 55.2) | 306/372 (82.3) (78.1, 85.8) | 13/13 (100) (77.2, 100) | 210/210 (100) (98.2, 100) | 223/223 (100) (98.3, 100) |
| MTBDR-Plus (culture) | 274/275 (99.6) (98.0, 99.9) | 100/103 (97.1) (91.8, 99.0) | 374/378 (98.9) (97.3, 99.6) | NAc | NAc | NAc |
| Intent-to-screen resultsb | ||||||
| Direct line probe | 260/276 (94.2) (90.8, 96.4) | 74/102 (72.5) (63.2, 80.3) | 334/378 (88.4) (84.7, 91.2) | 14/14 (100) (78.5, 100) | 198/210 (94.3) (90.3, 96.7) | 212/224 (94.6) (90.9, 96.9) |
| MTBDR-Plus (sputum) | 261/276 (94.6) (91.2, 96.7) | 45/102 (44.1) (34.9, 53.8) | 306/378 (81.0) (76.7, 84.6) | 14/14 (100) (78.5, 100) | 210/210 (100) (98.2, 100) | 224/224 (100) (98.3, 100) |
| MTBDR-Plus (culture) | 274/275 (99.6) (98.0, 99.9) | 100/103 (97.1) (91.8, 99.0) | 374/378 = 98.9) (97.3, 99.6) | NAc | NAc | NAc |
All available LPA test results were used for the calculations; indeterminate was considered false negative in the calculations.
One LPA test result was not available due to site error.
Specificity was not calculated, as LPA tests were performed on known M. tuberculosis-positive cultures only.
Of the 379 MGIT TB-positive specimens, gold standard conventional RIF and INH DST testing by MGIT was successfully performed on 374 (98.7%); an additional two specimens had only RIF DST results. Of these, 5/376 (1.3%) were RIF monoresistant, 15/374 (4.0%) were INH monoresistant, and 19/374 (5.1%) were both RIF and INH resistant (MDR) (Table 4).
TABLE 4.
Detection of rifampin and isoniazid drug resistance by culture-based drug susceptibility testing versus MTBDR line probe assay
| Drug and result | Culture-based DST results (n [%]) |
Total | |||
|---|---|---|---|---|---|
| Resistanta | Susceptible | M. tuberculosis negative | Not available | ||
| Rifampin MTBDR-Plus LPA results | |||||
| Resistant | 22 (91.7) | 12 (3.4) | 0 | 2 (5.1) | 36 |
| Susceptible | 0 | 248 (70.5) | 0 | 10 (25.6) | 258 |
| Indeterminate | 1 (4.2) | 20 (5.7) | 0 | 0 | 21 |
| M. tuberculosis negative | 1 (4.2) | 65 (18.5) | 223 (99.6) | 25 (64.1) | 314 |
| Not available | 0 | 7 (2.0) | 1 (0.4) | 2 (5.1) | 10 |
| Total | 24 (100.0) | 352 (100.0) | 224 (100.0) | 39 (100.0) | 639 |
| Isoniazid MTBDR-plus LPA results | |||||
| Resistant | 24 (70.6) | 3 (0.9) | 0 | 2 (4.8) | 29 |
| Susceptible | 4 (11.8) | 257 (75.8) | 0 | 12 (28.6) | 273 |
| Indeterminate | 1 (2.9) | 12 (3.5) | 0 | 0 | 13 |
| M. tuberculosis negative | 5 (14.7) | 60 (17.7) | 223 (99.6) | 26 (61.9) | 314 |
| Not available | 0 | 7 (2.1) | 1 (0.4) | 2 (4.8) | 10 |
| Total | 34 (100.0) | 339 (100.0) | 224 (100.0) | 42 (100.0) | 639 |
Nineteen specimens were RIF and INH resistant (MDR TB).
In an intent-to-screen analysis, MTBDR performed on sputum identified 22/24 RIF-resistant specimens (sensitivity, 91.7% [CI, 74.2%, 97.7%]) with specificity of 96.6% (339/351 [CI, 94.1%, 98.0%]). When restricted to the 282 samples with interpretable results for both MTBDR and MGIT DST, sensitivity was 100% (22/22 [CI, 85.1%, 100%]) for RIF resistance and specificity was 95.4% (248/260 [CI, 92.1%, 97.3%]). Within specimens with interpretable results, sensitivity was not affected by smear status; results were smear positive 100% (20/20 [CI, 83.9%, 100%]) versus smear negative 100% (2/2 [CI, 34.2%, 100%]) (Fig. 2). In 66 MGIT M. tuberculosis-positive specimens with interpretable DST results, MTBDR LPA did not detect M. tuberculosis and thus yielded no LPA DST test result; of these, 65 were RIF susceptible and 1 was RIF resistant. MTBDR mixed RIF resistance was present in 2 specimens, 1 RIF resistant and 1 RIF sensitive by MGIT DST.
FIG 2.
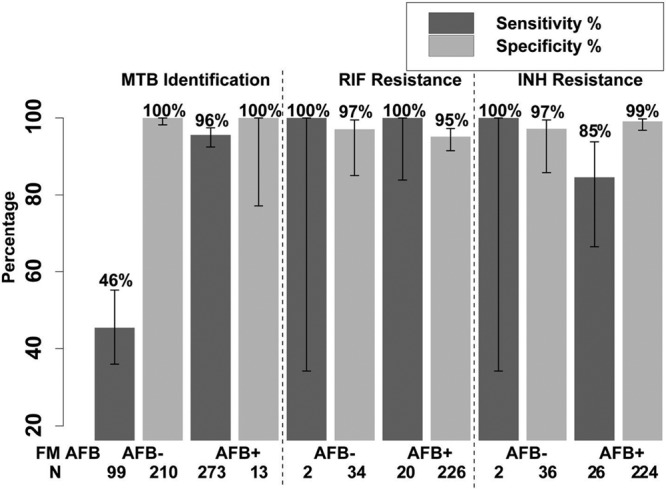
MTBDR-Plus LPA by smear status.
Overall, MTBDR detected 24/34 INH-resistant specimens (sensitivity, 70.6% [CI, 53.8%, 83.2%]) as identified by conventional DST, with specificity of 99.1% (335/338 [CI, 97.4%, 99.7%]). When restricted to specimens with both MTBDR and conventional DST results, sensitivity for INH resistance was 85.7% (24/28 [CI, 68.5%, 94.3%]) and specificity was 98.8% (257/260 [CI, 96.7%, 99.6%]). Sensitivity for INH resistance was not impacted by smear status; results were smear positive 84.6% (22/26 [CI, 66.5%, 93.8%]) versus smear negative 100% (2/2 [CI, 34.2%, 100%]); however, there were only 2 INH-resistant smear-negative specimens (Fig. 2). MTBDR LPA yielded mixed INH resistance results for 3 specimens, 2 INH resistant and 1 INH sensitive by MGIT DST.
Of the 19 specimens identified as MDR TB by MGIT DST, MDRTB LPA correctly identified 16/19 (84.2%), did not yield interpretable results in 1 (5.3%), did not detect M. tuberculosis and thus yielded no test result in 1 (5.3%), and was not MDR in 1 (5.3%; MDRTB LPA identified RIF resistance only).
Yield of second MTBDR LPA and MTBDR LPA on culture.
Performing MTBDR LPA on a second sputum sample identified 3 more MGIT M. tuberculosis-positive cases out of 109 participants with two sputa tested and 81 of these with the first tested as M. tuberculosis negative, for an incremental yield of 3.7% (CI, 1.3%, 10.3%) and identified no additional drug resistance cases. Testing MGIT M. tuberculosis-positive culture specimens with MTBDR LPA did not improve the yields of drug resistance, detecting 22/24 (91.7%) of RIF-resistant and 28/34 (82.4%) INH-resistant specimens compared to conventional DST.
Direct LPA.
The Direct LPA was positive for M. tuberculosis in 355/639 (55.6%) specimens tested, positive for M. kansasii or M. avium only in 9 (1.4%), negative for mycobacteria in 261 (40.8%), indeterminate in 7 (1.1%), and with no available result in 9 (1.4%), due to test failure (6) or site/lab error (3). Of the 7 Direct LPA indeterminate results, 3 were AFB smear positive and 4 were AFB smear negative. Direct LPA was positive for NTM in 10/639 (4 MAC, 3 M. kansasii, and 3 with mixed infections with both M. tuberculosis and MAC detected) (Table 2). Five of the 10 MAC or M. kansasii cases detected by MGIT culture were detected by Direct LPA.
Direct LPA correctly identified 334/378 (sensitivity, 88.4% [CI, 84.7%, 91.2%]) of MGIT TB positive specimens (Table 3), identifying an additional 9 specimens with M. tuberculosis in which MGIT failed, due to contamination (6) and site/lab error (3) (Table 2). Of the 591 with interpretable MGIT and Direct LPA results, the sensitivity of Direct LPA for M. tuberculosis detection by AFB smear grade was as follows (Fig. 3): smear negative 74.7% (74/99 [CI, 65.4%, 82.3%]), scanty 66.7% (10/15 [CI, 41.7%, 84.8%]), 1 + 97.1% (67/69 [CI, 90.0%, 99.2%]), 2 + 95.9% (47/49 [CI, 86.3%, 98.9%]), 3 + 98.6% (136/138 [CI, 94.9%, 99.6%]), with an overall sensitivity in smear-positive of 95.9% (260/271 [CI, 92.9%, 97.7%]) and in smear-negative specimens of 74.7% (74/99 [CI, 65.4%, 82.3%]). Of the 221 MGIT M. tuberculosis-negative specimens, Direct LPA was negative in 209 (specificity 94.6% [CI, 90.8%, 96.9%]), with slightly lower specificity in smear-negative (195/207 or 94.2% [CI, 90.1%, 96.7%]) than smear-positive specimens (14/14 or 100% [CI, 78.5%, 100%; Fisher's exact test P = 1.0 for the comparison). Direct LPA sensitivity did not differ significantly (chi-square test P = 0.46) in those with CD4+ values of ≤50 versus >50 cells/mm3 (59/67 or 88.1% versus 273/300 or 91.0%, respectively). Of the 12 MGIT TB-negative and Direct LPA M. tuberculosis-positive specimens, 9 were assessed as clinical TB (no positive culture reported but responded to empirical TB treatment) and 3 as not TB in follow-up evaluations at 24 weeks.
FIG 3.
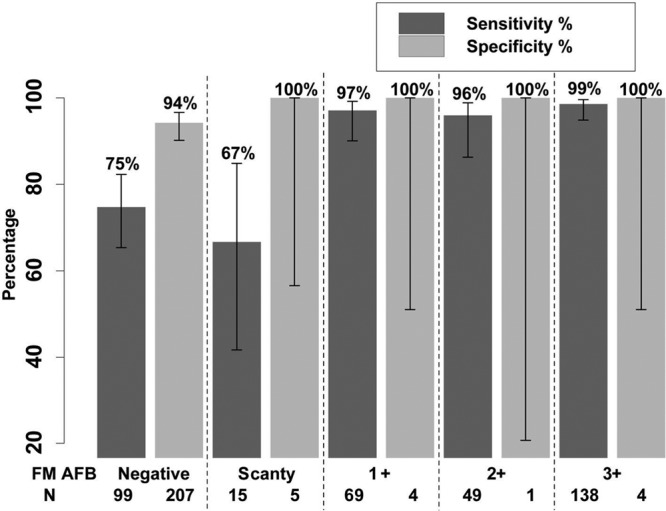
Direct LPA by smear status.
Incremental yield of second Direct LPA.
In the subset of 109 participants with two sputa tested and the first tested as M. tuberculosis-negative in 70, the second sputum with Direct LPA identified 1 additional MGIT TB+ case (incremental yield 1.4% [CI, 0.3%, 7.7%]).
DISCUSSION
This study demonstrates that MTBDR and direct line probe assays can be used effectively in HIV-infected patients undergoing TB evaluation, including those with AFB smear-negative specimens. For identification of M. tuberculosis drug resistance, MTBDR demonstrated similar performance in an HIV-infected population as has been previously shown in HIV-uninfected populations (17), detecting 91.7% of rifampin resistance and 70.6% of INH resistance. Sensitivities increased to 100% for RIF resistance and 85.7% for INH resistance when assays were restricted to samples with results interpretable by both MGIT and LPA.
When considering performance by AFB smear status, the Direct and MTBDR line probes both had excellent sensitivity of 95.6% to 95.9% on AFB+ direct sputum samples. In AFB-negative specimens, the Direct line probe identified culture-confirmed M. tuberculosis in 74.7% of AFB-negative and 66.7% of scanty smear-positive specimens, similar to the sensitivity of 68 to 75% (18) reported with Xpert MTB/RIF in AFB-negative specimens (19, 20). MTBDR was less sensitive than the Direct line probe for M. tuberculosis detection, but did identify culture-confirmed M. tuberculosis in nearly half (45.5%); these specimens were able to undergo rapid drug susceptibility testing as well. An updated version 2.0 of MTBDR-Plus was released in 2011 with reported improved sensitivity in AFB-negative specimens, ranging from 58% to 80% (21, 22), further bolstering the ability to use this assay in smear-negative specimens.
The landscape of M. tuberculosis diagnostics has undergone remarkable changes with the development and accelerated rollout of rapid, self-contained PCR platforms such as the Xpert MTB/RIF. The Xpert MTB/RIF provides an attractive alternative to line probe assays, which requires skilled laboratory staff, specialized equipment, and dedicated PCR space to reduce the risk of contamination. Given the advantages of this increasingly available technology, what is the current role of line probe assays for rapid detection of M. tuberculosis, NTM, and M. tuberculosis drug resistance? Line probe assays are still important tools for both current TB clinical care and research due to several advantageous aspects. First, the MTBDR assay is one of the few rapid diagnostics that evaluates INH susceptibility, which is not part of the current Xpert MTB/RIF test. For clinical trials of new TB treatment regimens, establishing INH susceptibility is an enrollment criterion for many drug-sensitive protocols (e.g., Evaluation of early bactericidal activity in pulmonary tuberculosis with clofazimine (C)-TMC207 (J)-PA-824 (Pa)-pyrazinamide (Z) (NC-003) [http://clinicaltrials.gov/ct2/show/NCT01691534?term=NC003&rank=1]) and MDR-TB protocols (23). Rapid identification with line probes can facilitate trial conduction and help ensure inclusion of appropriate participants. In clinical care, there has been debate about the significance of INH monoresistance and effects on TB treatment outcomes (24–27). However, a South African study (27) and a meta-analysis (26) have both demonstrated poor TB outcomes with INH monoresistance, and two studies suggest that early detection of INH resistance with modification of treatment led to better outcomes (24, 25). Further, the presence of rifampin resistance alone cannot be relied upon to predict INH resistance; INH susceptibility ranges from ≤11% to >40% of RIF-resistant isolates, depending on the setting (28, 29). Thus, there remains a need for rapid assessment of INH resistance in both research and clinical care settings. It is important to note that molecular detection methods for INH resistance detect only a portion of mutations leading to INH resistance (17), as reflected by the MTBDR sensitivity in this series of 70.6% to 85.7%. The MTBDR-Plus version 2.0 has improved INH detection, with sensitivities of 89% to 100% in recent series (21, 22).
A second advantage of line probe assays is the ability to detect nontuberculosis mycobacterium that are of particular clinical relevance to the HIV-infected population, specifically MAC and M. kansasii, and which may present similarly to or even in conjunction with TB infection. However, in this series, NTM were infrequently identified by either MGIT (2.2%) or Direct LPA (1.6%). Direct LPA identified 7 NTM (MAC or M. kansasii) as well as 3 mixed infections with MAC/M. tuberculosis. Thus, detection of NTMs may be of limited utility in a rapid M. tuberculosis diagnostic in this study's settings of particularly high TB prevalence but may have increased utility in settings with higher prevalences of MAC and/or M. kansasii (9, 30–32).
When considering line probe assay performance in comparison to culture-based methods, liquid-based mycobacterial culture is limited by contamination rates that can be as high as 14.0% to 18.6% (7, 33, 34). The line probe invalid rates were substantially lower, at 1.6% with MTBDR and 1.1% with Direct LPA, than the MGIT contamination rate in this series of 4.2%.
The study used one MGIT culture as the comparator strategy for M. tuberculosis identification, as access to culture-based M. tuberculosis evaluation is often limited by cost and access in resource-limited settings, and a single MGIT has demonstrated a higher yield for TB diagnosis than three solid-media M. tuberculosis cultures (35). Additional MGIT cultures may have identified more culture-confirmed M. tuberculosis (35); however, the specificity of the LPAs was high, suggesting that substantial TB cases were not being missed, and follow-up of available standard-of-care testing identified only 7 (1.1%) additional M. tuberculosis culture-positive cases. In addition, the study used three different labs, which has the potential to introduce bias if laboratory techniques differ across sites. To minimize this potential for bias, all sites participated in external quality assurance proficiency testing for AFB smear and mycobacterial culture, and personnel were trained by Hain technicians on correct conduct of both LPAs.
The AFB+ rate in this series was high for an HIV-TB coinfected population, in which AFB− disease can be more frequent, particularly with advanced HIV. The median CD4+ cell count was 151/mm3, indicating considerable immunosuppression; however, only 18.2% of TB cases occurred in patients with CD4+ ≤50, where the highest rates of AFB smear negative disease generally occur (36). In addition, participants screened were suspected to have high-risk TB, with either demonstrated AFB+ sputum or empirical TB treatment planned, with 64.6% receiving TB treatment within 30 days of enrollment. Thus, the population may have been enriched for AFB+ specimens, given these eligibility criteria. However, nearly 100 AFB smear-negative, MGIT M. tuberculosis-positive specimens were available for analysis of LPA performance.
This study represents the largest series evaluating line probe assays in a known HIV-infected population. Our findings supports the use of the MTBDR line probe assay for rapid M. tuberculosis drug resistance identification in HIV-infected TB suspects, specifically in settings where rapid identification of INH resistance is a priority. While AFB-negative sputum decreases test sensitivity, use in this setting may still be considered, because MTBDR provided interpretable results in nearly half of smear-negative specimens. The Direct LPA has improved sensitivity over MTBDR for M. tuberculosis detection in AFB-negative specimens and performed similarly to the Xpert MTB/RIF. However, the benefit of identifying clinically relevant NTM in HIV-infected patients was not clear in this series with a low prevalence of NTM. Use of line probe assays that can detect NTM should be explored in other settings of high HIV prevalence where increased NTM prevalence may justify the additional testing.
ACKNOWLEDGMENTS
We thank the study participants, the site principal investigators and staff, and the site laboratories for their exceptional efforts to conduct the study; the data manager, Linda Wieclaw; the clinical trials specialist, Evelyn Hogg; the field representative, Holly Boyd; the study's laboratory technologist, David L. Shugarts; the laboratory data managers Courtney N. Ashton and Anthony Bloom; and the community representative, Flavia Miiro.
This work was supported by award number U01AI068636 from the National Institute of Allergy and Infectious Diseases and the Statistical and Data Management Center (SDMC) (UM1 AI068634) funded by the National Institute of Allergy and Infectious Diseases. A.F.L. reports research grant support to the University of California, San Francisco, from Cepheid.
Footnotes
Published ahead of print 15 January 2014
REFERENCES
- 1.Kang'ombe CT, Harries AD, Ito K, Clark T, Nyirenda TE, Aldis W, Nunn PP, Semba RD, Salaniponi FM. 2004. Long-term outcome in patients registered with tuberculosis in Zomba, Malawi: mortality at 7 years according to initial HIV status and type of TB. Int. J. Tuberc. Lung. Dis. 8:829–836 [PubMed] [Google Scholar]
- 2.Getahun H, Harrington M, O'Brien R, Nunn P. 2007. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 369:2042–2049. 10.1016/S0140-6736(07)60284-0 [DOI] [PubMed] [Google Scholar]
- 3.Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, Jensen P, Bayona J. 2010. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375:1830–1843. 10.1016/S0140-6736(10)60410-2 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 2008. Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR0-TB). World Health Organization, Geneva, Switzerland [Google Scholar]
- 5.Barnard M, Albert H, Coetzee G, O'Brien R, Bosman ME. 2008. Rapid Molecular screening for MDR TB in a high volume public health laboratory in South Africa. Am. J. Respir. Crit. Care Med. 177:787–792. 10.1164/rccm.200709-1436OC [DOI] [PubMed] [Google Scholar]
- 6.Neonakis IK, Gitti Z, Petinaki E, Maraki S, Spandidos DA. 2007. Evaluation of the GenoType MTBC assay for differentiating 120 clinical Mycobacterium tuberculosis complex isolates. Eur. J. Clin. Microbiol. Infect. Dis. 26:151–152. 10.1007/s10096-007-0255-y [DOI] [PubMed] [Google Scholar]
- 7.Dorman SE, Chihota VN, Lewis JJ, van der Meulen M, Mathema B, Beylis N, Fielding KL, Grant AD, Churchyard GJ. 2012. Genotype MTBDRplus for direct detection of Mycobacterium tuberculosis and drug resistance in strains from gold miners in South Africa. J. Clin. Microbiol. 50:1189–1194. 10.1128/JCM.05723-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy KD, Metchock B, Kanphukiew A, Monkongdee P, Sinthuwattanawibool C, Tasaneeyapan T, Rienthong S, Ngamlert K, Srisuwanvilai LO, Varma JK. 2008. Monitoring the performance of mycobacteriology laboratories: a proposal for standardized indicators. Int. J. Tuberc. Lung. Dis. 12:1015–1020 [PubMed] [Google Scholar]
- 9.Lan R, Yang C, Lan L, Ou J, Qiao K, Liu F, Gao Q. 2011. Mycobacterium tuberculosis and non-tuberculous mycobacteria isolates from HIV-infected patients in Guangxi, China. Int. J. Tuberc. Lung. Dis. 15:1669–1675. 10.5588/ijtld.11.0036 [DOI] [PubMed] [Google Scholar]
- 10.Marras TK, Daley CL. 2004. A systematic review of the clinical significance of pulmonary Mycobacterium kansasii isolates in HIV infection. J. Acquir. Immune. Defic. Syndr. 36:883–889. 10.1097/00126334-200408010-00001 [DOI] [PubMed] [Google Scholar]
- 11.Karakousis PC, Moore RD, Chaisson RE. 2004. Mycobacterium avium complex in patients with HIV infection in the era of highly active antiretroviral therapy. Lancet Infect. Dis. 4:557–565. 10.1016/S1473-3099(04)01130-2 [DOI] [PubMed] [Google Scholar]
- 12.Bicmen C, Gunduz AT, Coskun M, Senol G, Cirak AK, Ozsoz A. 2011. Molecular detection and identification of Mycobacterium tuberculosis complex and four clinically important nontuberculous mycobacterial species in smear-negative clinical samples by the genotype mycobacteria direct test. J. Clin. Microbiol. 49:2874–2878. 10.1128/JCM.00612-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent P, Kubica G. 1985. Public health mycobacteriology: a guide for level III laboratory. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 14.Rieder HL, Van Deun A, Kam KM, Jae Kim S, Chonde TM, Trebucq A, Urbanczik R. 2007. Priorities for tuberculosis bacteriology services in low-income countries, 2nd ed. International Union Against Tuberculosis and Lung Disease, Paris, France [Google Scholar]
- 15.BD. 2002. MGIT package insert. Becton, Dickinson and Company, Sparks, MD: http://www.bd.com/ds/technicalCenter/inserts/8809501JAA(01).pdf#page=1&view=Fit [Google Scholar]
- 16.BD. 2010. MGIT SIRE package insert. Becton, Dickinson and Company, Sparks, MD: http://www.bd.com/ds/technicalCenter/inserts/8008200(201010).pdf#page=1&view=Fit [Google Scholar]
- 17.Ling DI, Zwerling AA, Pai M. 2008. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur. Respir. J. 32:1165–1174. 10.1183/09031936.00061808 [DOI] [PubMed] [Google Scholar]
- 18.Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M, Dendukuri N. 2013. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 1:CD009593 CD009593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363:1005–1015. 10.1056/NEJMoa0907847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. 2011. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 377:1495–1505. 10.1016/S0140-6736(11)60438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnard M, Gey van Pittius NC, van Helden PD, Bosman M, Coetzee G, Warren RM. 2012. The diagnostic performance of the GenoType MTBDRplus version 2 line probe assay is equivalent to that of the Xpert MTB/RIF assay. J. Clin. Microbiol. 50:3712–3716. 10.1128/JCM.01958-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crudu V, Stratan E, Romancenco E, Allerheiligen V, Hillemann A, Moraru N. 2012. First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as rifampin and isoniazid resistances. J. Clin. Microbiol. 50:1264–1269. 10.1128/JCM.05903-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, Pistorius C, Krause R, Bogoshi M, Churchyard G, Venter A, Allen J, Palomino JC, De Marez T, van Heeswijk RP, Lounis N, Meyvisch P, Verbeeck J, Parys W, de Beule K, Andries K, Mc Neeley DF. 2009. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360:2397–2405. 10.1056/NEJMoa0808427 [DOI] [PubMed] [Google Scholar]
- 24.Bang D, Andersen PH, Andersen AB, Thomsen VO. 2010. Isoniazid-resistant tuberculosis in Denmark: mutations, transmission and treatment outcome. J. Infect. 60:452–457. 10.1016/j.jinf.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 25.Cattamanchi A, Dantes RB, Metcalfe JZ, Jarlsberg LG, Grinsdale J, Kawamura LM, Osmond D, Hopewell PC, Nahid P. 2009. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin. Infect. Dis. 48:179–185. 10.1086/595689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzies D, Benedetti A, Paydar A, Royce S, Madhukar P, Burman W, Vernon A, Lienhardt C. 2009. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med. 6:e1000150. 10.1371/journal.pmed.1000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson KR, Theron D, Victor TC, Streicher EM, Warren RM, Murray MB. 2011. Treatment outcomes of isoniazid-resistant tuberculosis patients, Western Cape Province, South Africa. Clin. Infect. Dis. 53:369–372. 10.1093/cid/cir406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith SE, Kurbatova EV, Cavanaugh JS, Cegielski JP. 2012. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int. J. Tuberc. Lung. Dis. 16:203–205. 10.5588/ijtld.11.0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurbatova EV, Cavanaugh JS, Shah NS, Wright A, Kim H, Metchock B, Van Deun A, Barrera L, Boulahbal F, Richter E, Martin-Casabona N, Arias F, Zemanova I, Drobniewski F, Santos Silva A, Coulter C, Lumb R, Cegielski JP. 2012. Rifampicin-resistant Mycobacterium tuberculosis: susceptibility to isoniazid and other anti-tuberculosis drugs. Int. J. Tuberc. Lung. Dis. 16:355–357. 10.5588/ijtld.11.0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jun HJ, Jeon K, Um SW, Kwon OJ, Lee NY, Koh WJ. 2009. Nontuberculous mycobacteria isolated during the treatment of pulmonary tuberculosis. Respir. Med. 103:1936–1940. 10.1016/j.rmed.2009.05.025 [DOI] [PubMed] [Google Scholar]
- 31.Pokam BT, Asuquo AE. 2012. Acid-fast bacilli other than mycobacteria in tuberculosis patients receiving directly observed therapy short course in cross river state, Nigeria. Tuberc. Res. Treat. 2012:301056. 10.1155/2012/301056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy KD, Cain KP, Winthrop KL, Udomsantisuk N, Lan NT, Sar B, Kimerling ME, Kanara N, Lynen L, Monkongdee P, Tasaneeyapan T, Varma JK. 2012. Nontuberculous mycobacterial disease in patients with HIV in Southeast Asia. Am. J. Respir. Crit. Care Med. 185:981–988. 10.1164/rccm.201107-1327OC [DOI] [PubMed] [Google Scholar]
- 33.Chihota VN, Grant AD, Fielding K, Ndibongo B, van Zyl A, Muirhead D, Churchyard GJ. 2010. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int. J. Tuberc. Lung. Dis. 14:1024–1031 [PubMed] [Google Scholar]
- 34.Dowdy DW, Lourenco MC, Cavalcante SC, Saraceni V, King B, Golub JE, Bishai D, Durovni B, Chaisson RE, Dorman SE. 2008. Impact and cost-effectiveness of culture for diagnosis of tuberculosis in HIV-infected Brazilian adults. PLoS One 3:e4057. 10.1371/journal.pone.0004057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monkongdee P, McCarthy KD, Cain KP, Tasaneeyapan T, Nguyen HD, Nguyen TN, Nguyen TB, Teeratakulpisarn N, Udomsantisuk N, Heilig C, Varma JK. 2009. Yield of acid-fast smear and mycobacterial culture for tuberculosis diagnosis in people with human immunodeficiency virus. Am. J. Respir. Crit. Care Med. 180:903–908. 10.1164/rccm.200905-0692OC [DOI] [PubMed] [Google Scholar]
- 36.Chamie G, Luetkemeyer A, Walusimbi-Nanteza M, Okwera A, Whalen CC, Mugerwa RD, Havlir DV, Charlebois ED. 2010. Significant variation in presentation of pulmonary tuberculosis across a high resolution of CD4 strata. Int. J. Tuberc. Lung Dis. 14:1295–1302 [PMC free article] [PubMed] [Google Scholar]