Abstract
Species identification of nontuberculous mycobacteria (NTM) is challenging due to the increasing number of identified NTM species and the lack of standardized testing strategies. The objectives of this study were to investigate the distribution of NTM species recovered from respiratory specimens by multigene sequence-based typing and to evaluate the clinical significance of identified species. Two hundred thirty-two consecutive clinical NTM isolates were subjected to sequencing of multiple genes, including hsp65, rpoB, and 16S-23S rRNA internal transcribed spacer (ITS) sequence. In addition, clinical data from all patients whose specimens had NTM isolates were analyzed to examine clinical virulence and treatment history. Eighteen strains from 227 isolates from 169 patients were successfully identified at the species level by multigene sequence-based typing. Mycobacterium avium complex and M. abscessus complex made up the majority of isolated NTM (88%; 199/227), followed by M. fortuitum complex (4%; 10/227). The pathogenic potential of NTM differs enormously by species, and M. avium complex and M. abscessus complex revealed especially high levels of virulence compared with findings for other NTM species. The results from our work support M. avium complex and M. abscessus complex being the most common NTM species with highly pathogenic potential isolated from clinical respiratory specimens and could be a good resource for molecular epidemiology of NTM species in South Korea.
INTRODUCTION
All members of the genus Mycobacterium, excepting M. tuberculosis and M. leprae, are considered nontuberculous mycobacteria (NTM) and are frequently isolated from environmental sources, including surface or tap water and soil (1). Currently, more than 160 mycobacterial species/subspecies are listed in the Genus Mycobacterium database (http://www.bacterio.cict.fr/m/mycobacterium.html; accessed on 22 August 2013), and the number of newly identified NTM is increasing. Recent data suggest that the incidence of lung disease caused by NTM with or without predisposing risk factors has been growing worldwide (2–6). However, epidemiologic data on the distribution and clinical significance of NTM lung disease are still scarce because NTM lung disease is not a reportable condition in most countries.
In South Korea, NTM was first described as a cause of mycobacterial lung disease in 1981 (7); since then, the frequency of isolation of NTM from clinical specimens has shown a continuous increase. Since the 2000s, the frequency of NTM isolation has been reported to be 12 to 38% of mycobacterial culture-positive specimens, and the number of patients with NTM disease has also been considerable, with a frequency of 8 to 49% of isolated NTM (8–15). The isolation of NTM species from respiratory specimens is not sufficient evidence for the diagnosis of clinically significant NTM lung disease, since there are also clinical, radiographic, and microbiological criteria (1).
Recently, various genotype-based methods for the identification of NTM have been developed to rapidly identify mycobacteria, including commercially available molecular probes, PCR-restriction fragment length polymorphism (RFLP), and sequencing (16–18). However, determining an accurate distribution of NTM can still be problematic due to the different detectable ranges and sensitivities of different methods. Moreover, given the large number of identified NTM species, the wide spectrum of NTM virulence, and the lack of standardized diagnostic strategies, it is difficult to make an accurate NTM identification. Thus, the objectives of the present study were to investigate the distribution of NTM species recovered from respiratory specimens by multigene sequence-based typing (hsp65, rpoB, and 16S-23S rRNA internal transcribed spacer [ITS] sequences) and to evaluate their clinical significance.
MATERIALS AND METHODS
Study subjects and protocols.
All clinical NTM isolates with positive mycobacterial cultures at Samsung Medical Center (a tertiary referral hospital in South Korea) during the month of July 2012 were candidates for this study. Of the NTM isolates, we included samples from newly diagnosed patients, so that those from previously diagnosed patients with NTM lung disease were excluded from this study. NTM isolates recovered from nonrespiratory specimens were also excluded. For identification of the isolated NTM, multigene sequence-based typing of hsp65, rpoB, and the ITS was performed. Based on the typing results, we analyzed the distribution of NTM species and their clinical significance. This study was approved by the Institutional Review Board of Samsung Medical Center.
Identification of NTM species.
In our laboratory, liquid and solid culture methods for mycobacterial culture were performed simultaneously with every clinical specimen, improving the sensitivity of laboratory diagnosis. Specimens from the liquid culture method were preferred for DNA extraction. If liquid culture specimens were not available, several colonies grown on Ogawa medium were selected for the procedure. Samples were heated in a boiling-water bath for 10 min and centrifuged for 3 min. The supernatant was used to perform hsp65, rpoB, and ITS sequence analysis according to the protocol in Clinical and Laboratory Standards Institute (CLSI) guideline MM18-A (19). PCR primer sets for hsp65, rpoB, and the ITS were used according to published reports (20–22). The sequences obtained were analyzed using GenBank. hsp65 gene sequences were further validated using a recently developed public database for the hsp65 gene (23).
Evaluation of clinical significance of isolated NTM.
Clinical information from all patients with NTM isolates was analyzed, including age, gender, presence or absence of pulmonary symptoms and/or characteristic radiologic findings, and treatment history. Patients with NTM lung disease met the diagnostic criteria provided by the American Thoracic Society (1), including characteristic findings of NTM on high-resolution computed tomography scans, such as bilateral bronchiectasis combined with multiple small nodules and branching linear structures. Patients were further investigated for clinical virulence of isolated NTM. In the present study, the virulence, or the degree of pathogenicity as indicated by the ability of the organism to invade the tissues of the host, was defined by the proportion of patients with NTM lung disease out of all patients for whom NTM was isolated from a respiratory specimen.
RESULTS
Numbers of patients and specimens.
During the study period, a total of 2,817 clinical specimens were cultured for mycobacterium. Of these specimens, 100 samples with M. tuberculosis isolates were observed (3.5%; 100/2,817), and 367 samples of NTM isolates were observed (13.0%; 367/2,817). Of the NTM isolates, 235 (64.0%; 235/367) were newly recovered NTM samples from 175 patients and were subsequently submitted for identification. The sources of the 235 NTM isolates were 232 respiratory specimens (sputum, 95.3% [224/235]; bronchial wash, 2.1% [5/235]; or lung tissue, 1.3% [3/235]) and three nonrespiratory specimens (eye discharge, 0.4% [1/235]; and bone tissue, 0.9% [2/235]).
NTM distribution by multigene sequence-based typing.
Among the 232 NTM respiratory specimens submitted for identification, 5 specimens were excluded from analysis because of a lack of PCR product or unsatisfactory sequence quality of the target genes (n = 5; strains 14, 34, 148, 158, and 193). Thus, a total of 227 specimens from 169 patients were successfully identified at the species level by multigene sequence-based typing (97.8%; 227/232). The distribution of NTM species is summarized in Table 1. The most commonly identified organism was M. avium complex (MAC) (59.9%; 136/227), followed by M. abscessus complex (27.8%; 63/227), M. fortuitum complex (4.4%; 10/227), M. gordonae (2.2%; 5/227), M. terrae complex (1.3%; 3/227), and other NTM species. Only one M. kansasii isolate was observed (0.4%; 1/227) in the current study. Mixed isolations from two separate patients, M. massiliense (hsp65) with M. avium (rpoB) and M. abscessus (hsp65) with M. intracellulare (rpoB), were confirmed by analyzing different target gene sequences (0.8%; 2/227).
TABLE 1.
Comprehensive identification by hsp65, rpoB, and ITS sequencing
| Organism | Identificationa |
|
|---|---|---|
| No. (%) of isolatesb | No. (%) of patients | |
| M. avium complex | 136 (59.9) | 102 (60.4) |
| M. avium | 81 (35.7) | 64 (37.9) |
| M. intracellulare | 53 (23.3) | 36 (21.3) |
| M. marseillense | 1 (0.4) | 1 (0.6) |
| M. colombiense | 1 (0.4) | 1 (0.6) |
| M. abscessus complex | 63 (27.8) | 41 (24.3) |
| M. abscessus | 44 (19.4) | 25 (14.8) |
| M. massiliense | 18 (7.9) | 15 (8.9) |
| M. chelonae | 1 (0.4) | 1 (0.6) |
| M. fortuitum complex | 10 (4.4) | 9 (5.3) |
| M. fortuitum | 3 (1.3) | 3 (1.8) |
| M. peregrinum | 3 (1.3) | 2 (1.2) |
| M. conceptionense | 3 (1.3) | 3 (1.8) |
| M. porcinum | 1 (0.4) | 1 (0.6) |
| M. gordonae | 5 (2.2) | 5 (3.0) |
| M. lentiflavum | 4 (1.8) | 4 (2.4) |
| M. terrae complex | 3 (1.3) | 2 (1.2) |
| M. algericum | 2 (0.9) | 1 (0.6) |
| M. senuense | 1 (0.4) | 1 (0.6) |
| M. mucogenicum | 2 (0.9) | 2 (1.2) |
| M. kansasii | 1 (0.4) | 1 (0.6) |
| M. nebraskense | 1 (0.4) | 1 (0.6) |
| M. massiliense (hsp65) + M. avium (rpoB) | 1 (0.4) | 1 (0.6) |
| M. abscessus (hsp65) + M. intracellulare (rpoB) | 1 (0.4) | 1 (0.6) |
Total: n = 227 isolates; n = 169 patients.
Among a total of 235 NTM isolates needing identification, 8 isolates were excluded from analysis due to either being nonrespiratory specimens (n = 3; strains 79, 80, and 81) or having no PCR product or unsatisfactory sequence quality of the target gene (n = 5; strains 14, 34, 148, 158, and 193).
Nine strains from the fifteen isolates which were relatively uncommon organisms in South Korea were categorized into species after multigene sequence-based typing. We found M. lentiflavum (1.8%; 4/227), M. marseillense (0.4%; 1/227), M. colombiense (0.4%; 1/227), M. conceptionense (1.3%; 3/227), M. peregrinum (1.3%; 3/227), M. porcinum (0.4%; 1/227), M. algericum (0.9%; 2/227), M. senuense (0.4%; 1/227), and M. negraskense (0.4%; 1/227). In the majority of cases (66.7%; 10/15), coinfection with M. tuberculosis or other NTM species, such as M. intracellulare, M. massiliense, M. avium, M. fortuitum, or M. szulgai, was found (data not shown).
Clinical significance of isolated NTM.
One hundred eleven patients met the diagnostic criteria for NTM lung disease. The average age of the patients was 61.3 ± 13.2 years (range, 24 to 92 years). Sixty-eight patients were female, and forty-three were male, yielding a female-to-male ratio of 1.6:1. The patients with NTM lung disease had infections as follows (Table 2): MAC, 65.8%, 73/111 (M. avium [36.9%; 41/111] and M. intracellulare [28.8%; 32/111]); M. abscessus complex, 28.8%, 32/111 (M. abscessus [18.9%; 21/111] and M. massiliense [9.9%; 11/111]); M. fortuitum complex, 2.7%, 3/111 (M. fortuitum [1.8%; 2/111] and M. porcinum [0.9%; 1/111]); M. kansasii, 0.9%; 1/111; and mixed infection, 1.8%; 2/111. As summarized in Table 2, virulence (B/A) is defined as the proportion of patients with NTM lung disease (B) out of all patients with isolated NTM in the present study (A). The MAC, M. abscessus complex, and M. fortuitum complex were more virulent than other species. None of the cases of NTM lung disease were caused by M. marseillense, M. colombiense, M. chelonae, M. peregrinum, M. concentionense, M. gordonae, M. lentiflavum, M. terrae complex, M. mucogenicum, or M. nebraskense. Treated individuals accounted for 91 of the patients with NTM lung disease (82.0%; 91/111), with a frequency ranging from 75.6 to 100.0% (Table 2).
TABLE 2.
Clinical significance of NTM isolated in the present study
| Organism | No. (%) of patients for whom: |
Proportion (%) with: |
|||
|---|---|---|---|---|---|
| NTM was isolated (A) | NTM lung disease was found (B) | NTM lung disease was treated (C) | Disease, B/Aa | Treatment, C/Bb | |
| M. avium complex | 102 (60.4) | 73 (65.8) | 59 (64.8) | 71.6 | 80.8 |
| M. avium | 64 (37.9) | 41 (36.9) | 31 (34.1) | 64.1 | 75.6 |
| M. intracellulare | 36 (21.3) | 32 (28.8) | 28 (30.8) | 88.9 | 87.5 |
| M. marseillense | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0.0 | NAc |
| M. colombiense | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0.0 | NA |
| M. abscessus complex | 41 (24.3) | 32 (28.8) | 27 (29.7) | 78.0 | 84.4 |
| M. abscessus | 25 (14.8) | 21 (18.9) | 17 (18.7) | 84.0 | 81.0 |
| M. massiliense | 15 (8.9) | 11 (9.9) | 10 (11.0) | 73.3 | 90.9 |
| M. chelonae | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0.0 | NA |
| M. fortuitum complex | 9 (5.3) | 3 (2.7) | 2 (2.2) | 33.3 | 66.7 |
| M. fortuitum | 3 (1.8) | 2 (1.8) | 2 (2.2) | 66.7 | 100.0 |
| M. peregrinum | 2 (1.2) | 0 (0.0) | 0 (0.0) | 0.0 | NA |
| M. conceptionense | 3 (1.8) | 0 (0.0) | 0 (0.0) | 0.0 | NA |
| M. porcinum | 1 (0.6) | 1 (0.9) | 0 (0.0) | 100.0 | NA |
| M. gordonae | 5 (3.0) | 0 (0.0) | 0 (0.0) | 0.0 | NA |
| M. lentiflavum | 4 (2.4) | 0 (0.0) | 0 (0.0) | 0.0 | NA |
| M. terrae complex | 2 (1.2) | 0 (0.0) | 0 (0.0) | 0.0 | NA |
| M. mucogenicum | 2 (1.2) | 0 (0.0) | 0 (0.0) | 0.0 | NA |
| M. kansasii | 1 (0.6) | 1 (0.9) | 1 (1.1) | 100.0 | 100.0 |
| M. nebraskense | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0.0 | NA |
| M. massiliense + M. avium | 1 (0.6) | 1 (0.9) | 1 (1.1) | 100.0 | 100.0 |
| M. abscessus + M. intracellulare | 1 (0.6) | 1 (0.9) | 1 (1.1) | 100.0 | 100.0 |
| Total | 169 (100.0) | 111 (100.0) | 91 (100.0) | 65.7 | 82.0 |
Proportion of patients with NTM lung disease among patients with isolated NTM (B/A).
Proportion of treated patients among those with NTM lung disease (C/B).
NA, not applicable.
DISCUSSION
The findings of this study support the hypothesis that the pathogenic potential of NTM differs enormously by species, and as a result, the predictive value of a positive culture for disease depends on the NTM species identified in that culture. The present study is the first to confirm the distribution and clinical significance of NTM recovered from respiratory clinical specimens by multigene sequence-based typing in South Korea.
Similar to previously published data for South Korea, MAC and M. abscessus complex make up the majority of isolated NTM (87.7%; 199/227), followed by M. fortuitum complex (4.4%; 10/227) in the current study. In Japan and many other countries, M. kansasii is endemic, but in our data, only one M. kansasii isolate was observed (0.4%; 1/227). Compared to previous studies of NTM distribution in several tertiary-care hospitals in South Korea, there were a number of differences in distribution of NTM, although the most commonly isolated NTM was generally MAC (Table 3) (8–11, 14, 15, 24, 25). In most studies using molecular methods, M. abscessus was the second-most-common species, but in a high-performance liquid chromatography (HPLC) study, where HPLC is used to identify NTM species by analyzing the mycolic acids in an organism, it ranked only 4th or 5th in prevalence among all of the isolated NTM (15, 24). Rather, M. kansasii was the 1st- or 2nd-most-common NTM species in studies using HPLC (15, 24). Considering that the studies using the HPLC method were all performed in the same region, the difference in the epidemiologic data may be representative of the significant geographic variability of NTM. Another hypothesis is that HPLC is less discriminative for the identification of closely related NTM species, although it is a highly sensitive and reliable method (1). Since the recognition of new species is difficult, HPLC analysis may be less useful in the future, since identification of NTM species will be accomplished by genotypic methods.
TABLE 3.
Current and prior studies of NTM distribution at several tertiary-care hospitals in Koreaa
| Organism | No. (%) of isolates identified for reference and method [target gene(s)] |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lee et al., 2005 (9); RFLP [rpoB] | Koh et al., 2006 (14); RFLP [rpoB] | Ryoo et al., 2008 (25); RFLP [rpoB] | Jeong et al., 2008 (15); HPLC | Yang, 2011 (8); RFLP [rpoB] | Lee et al., 2012 (11); genotyping chip [ITS] | Lee et al., 2012 (10; RFLP [rpoB] | Park et al., 2013 (24); HPLC | This study; sequencing [hsp65, rpoB, ITS] | |
| MACb | 138 (42) | 491 (32) | 6,974 (57) | 87 (23) | 74 (48) | 127 (67) | 263 (76) | 56 (42) | 134 (59) |
| M. avium | 62 (19) | 230 (15) | 1,777 (15) | 38 (10) | 67 (35) | 141 (41) | 17 (13) | 81 (36) | |
| M. intracellulare | 76 (23) | 261 (17) | 5,197 (42) | 49 (13) | 60 (32) | 122 (35) | 39 (29) | 53 (23) | |
| M. abscessus | 37 (11) | 442 (29) | 2,076 (17) | 25 (7) | 21 (14) | 31 (16) | 63 (18) | 16 (12) | 44 (19) |
| M. massiliense | 18 (8) | ||||||||
| M. chelonae | 7 (2) | 29 (1) | 123 (1) | 6 (2) | 2 (1) | 4 (2) | 1 (0.4) | ||
| M. fortuitum | 64 (15) | 268 (17) | 992 (8) | 34 (9) | 5 (3) | 10 (5) | 8 (2) | 6 (5) | 3 (1) |
| M. gordonae | 33 (10) | 188 (12) | 679 (6) | 44 (12) | 14 (9) | 1 (1) | 2 (2) | 5 (2) | |
| M. terrae | 28 (9) | 52 (3) | 8 (2) | 2 (1) | 1 (0.3) | ||||
| M. mucogenicum | 3 (1) | 14 (4) | 1 (1) | 2 (1) | |||||
| M. kansasii | 13 (4) | 27 (2) | 631 (5) | 61 (16) | 13 (8) | 9 (5) | 7 (2) | 48 (36) | 1 (0.4) |
| M. szulgai | 1 (0.4) | 36 (2) | 85 (1) | 19 (5) | 6 (4) | 1 (0.3) | 2 (2) | ||
| Othersc | 4 (1) | 15 (1) | 699 (16) | 86 (22) | 18 (12) | 5 (3) | 2 (1) | 3 (2) | 19 (7) |
| Total | 328 (96) | 1,548 (99) | 12,259 (110) | 384 (100) | 154 (100) | 189 (100) | 345 (100) | 133 (100) | 227 (100) |
Abbreviations: MAC, M. avium complex; HPLC, high-performance liquid chromatography; RFLP, restriction fragment length polymorphism.
MAC includes 2 mycobacterial species, M. avium and M. intracellulare.
The remaining NTM species are not described in the table.
MAC includes 2 well-known NTM species, M. avium and M. intracellulare. These two species could be differentiated only by genotypic methods, not by traditional phenotypic or biochemical tests. Our data demonstrated that M. avium is more frequently isolated than M. intracellulare (35.7% [81/227] versus 23.3% [53/227]). Interestingly, the incidence of M. avium has been steadily increasing in South Korea (Table 3). It has been documented that M. intracellulare is the more virulent of the two, and infections of M. intracellulare require more intensive treatment than those of M. avium (1, 26). Although there is currently no recommendation for the routine separation of MAC isolates into M. avium and M. intracellulare, it could have important prognostic and therapeutic implications in the future.
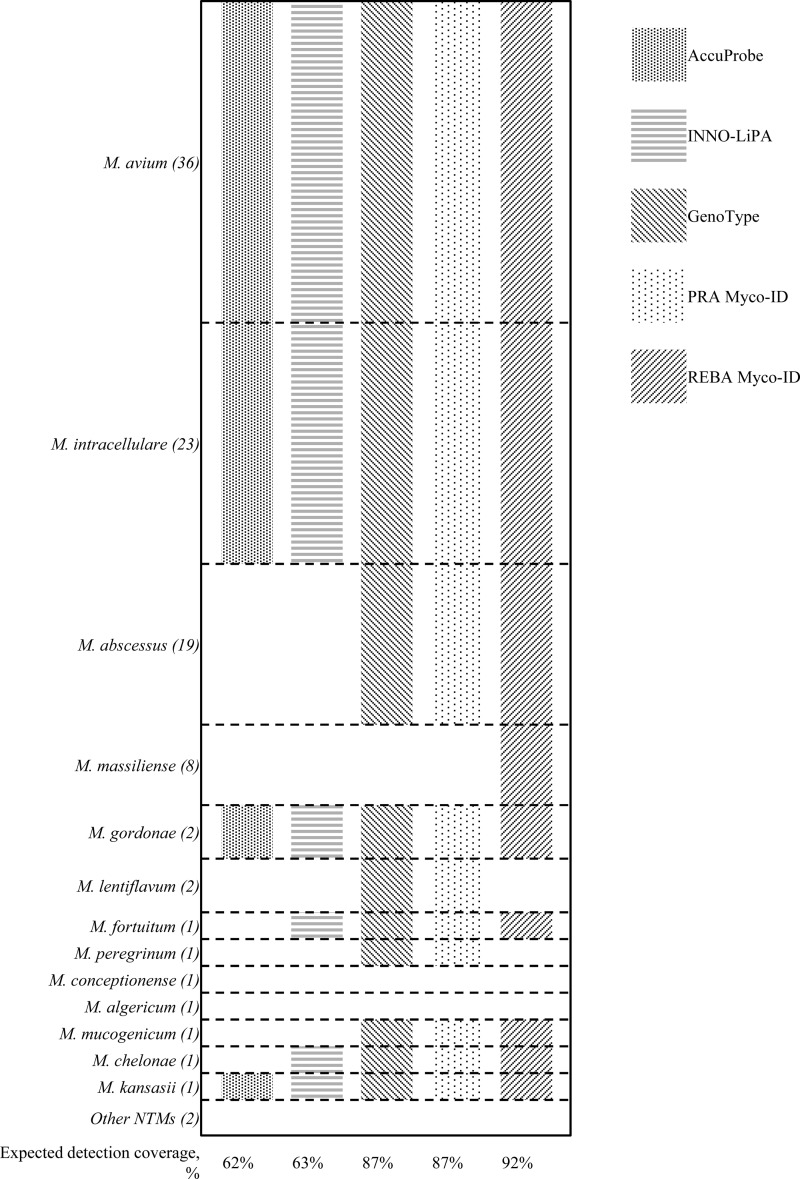
Molecular methods have replaced conventional biochemical tests for the identification of NTM. With the use of PCR-RFLP, DNA probe technology has broad commercial availability, including products from AccuProbe (Gen-Probe, San Diego, CA), INNO-LiPA (Fujirebio, Ghent, Belgium), GenoType (Hain Lifescience GmbH, Nehren, Germany), and REBA Myco-ID (YD Diagnostics, Yongin, Republic of Korea), and is one of the most widely used methods in many diagnostic laboratories (16, 27). The different detection ranges of commercial assays are illustrated in Fig. 1. Although the INNO-LiPA and GenoType assays are widely used in many laboratories worldwide, the expected coverage rate for a given specimen from this study was less than that of the REBA Myco-ID assay (INNO-LiPA, 63%, versus GenoType, 87%, versus REBA Myco-ID, 92%). In the current study, M. massiliense was relatively commonly observed (7.9%; 18/227), following M. abscessus (19.4%, 44/227). M. massiliense has been recognized as a separate species from M. abscessus, as well as having a different response to antibiotic therapy (28). REBA Myco-ID is the only commercial method with the ability to differentiate M. massiliense from M. abscessus. However, DNA probes could not identify all the NTM species recovered from clinical specimens, representing a major limitation. Furthermore, REBA Myco-ID incorrectly identified a number of NTM isolates (5.2%; 12/227) (see Tables S1 and S2 in the supplemental material). The discordant results may have originated with cross-reaction of the probe in the test, and thus the test is not able to differentiate members of closely related NTM species.
FIG 1.
Schematic representation of detection ranges for NTM species by commercial assays. The expected detection coverage was calculated based on the proportions given for the identified NTM species for which data on multigene sequence-based typing were available. Other NTM species, which consisted of rarely isolated organisms (less than 2%), include M. nebraskense, M. marseillense, M. colombiense, M. porcinum, and M. senuense. The following DNA strip technology was used: AccuProbe (Gen-Probe, San Diego, CA), INNO-LiPA (Fujirebio, Ghent, Belgium), GenoType (Hain Lifescience GmbH, Nehren, Germany), and REBA Myco-ID (YD Diagnostics, Yongin, Republic of Korea). PCR-RFLP was carried out using PRA Myco-ID (YD Diagnostics, Yongin, Republic of Korea).
The pathogenic potential of NTM differs enormously by species. Notably, the more commonly recovered NTM species also show the highest virulence rates. Thus, the MAC and M. abscessus complex were more highly virulent than the other NTM species, followed by the M. fortuitum complex (72% versus 78% versus 33%). Interestingly, M. fortuitum was found to have a relatively high pathogenic potential of 66.7% in this study (Table 2). M. fortuitum is included in the rapidly growing group of mycobacteria in which visible colonies can be produced within 7 days, and many clinicians believe that M. fortuitum is not often pathogenic. However, M. fortuitum has been identified as a pathogenic NTM species in several previous studies (29–31). According to a recent study in a Japanese population, the second-most-common rapidly growing mycobacteria pathogen was M. fortuitum, following M. abscessus (31). It has been shown that lung disease caused by M. fortuitum usually occurs in patients with predisposing factors, such as malignancy, renal transplantation, chronic reflux disease, achalasia, bronchiectasis, or cystic fibrosis. In the current study, two patients with M. fortuitum lung disease showed cavitary consolidation in a chest computed tomography scan and received specific antibiotic therapy using azithromycin and moxifloxacin. In the less commonly isolated NTM species, such as M. marseillense, M. colombiense, M. algericum, M. senuense, and M. nebraskense, no patients fulfilled the diagnostic criteria for lung disease or received treatment. Several well-known environmental contaminants, M. gordonae, M. lentiflavum, M. chelonae, and M. mucogenicum, also showed no virulence in the current study. However, since our institution is a tertiary-care hospital, patients included in this study could have a higher prevalence of more advanced NTM lung disease. In addition, the results might be biased by the inclusion of a single institution. Further accumulation of data from multi-institutional studies could be helpful to overcome these limitations.
Advances in DNA sequencing and the increasing number of sequences available in databases have greatly improved the bacterial identification process. The use of multiple appropriate genes is gaining momentum due to the lack of genetic heterogeneity within single target genes in the genus Mycobacterium (18, 32). Many target genes, such as hsp65, rpoB, ITS, gyrB, danA, recA, and secA1, have been described (19). The gene hsp65, which is more variable than the 16S rRNA gene, is an effective target gene, especially in rapidly growing mycobacteria. A recently developed public database for hsp65 sequences makes the gene more useful for identification of NTM (23). The gene rpoB, which encodes a subunit of RNA polymerase and is widely used in NTM identification, has good power of discrimination (17). The ITS region has been suggested to represent a potential target, since it contains both conserved and highly variable signatures (33). However, the limitations of the ITS are evident because its rate of PCR amplification is rather low (91.4%; 212/232) compared to the rates for hsp65 and rpoB (98.7 [229/232] and 94% [219/232]) in the current study.
Taken together, the results from our work support that MAC and the M. abscessus complex were the most common NTM species with highly pathogenic potential isolated from clinical respiratory specimens. Molecular identification based on multigene sequence-based typing allowed for the possibility of complete coverage of isolated NTM from clinical specimens.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by a grant from the SMC-KIST Translational Research Program in 2012.
Footnotes
Published ahead of print 5 February 2014
Supplemental material in this article may be found at http://dx.doi.org/10.1128/JCM.03053-13.
REFERENCES
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 2.Marras TK, Chedore P, Ying AM, Jamieson F. 2007. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997–2003. Thorax 62:661–666. 10.1136/thx.2006.070797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Casabona N, Bahrmand AR, Bennedsen J, Thomsen VO, Curcio M, Fauville-Dufaux M, Feldman K, Havelkova M, Katila ML, Koksalan K, Pereira MF, Rodrigues F, Pfyffer GE, Portaels F, Urgell JR, Rusch-Gerdes S, Tortoli E, Vincent V, Watt B. 2004. Non-tuberculous mycobacteria: patterns of isolation. A multi-country retrospective survey. Int. J. Tuberc. Lung Dis. 8:1186–1193 [PubMed] [Google Scholar]
- 4.Billinger ME, Olivier KN, Viboud C, de Oca RM, Steiner C, Holland SM, Prevots DR. 2009. Nontuberculous mycobacteria-associated lung disease in hospitalized persons, United States, 1998–2005. Emerg. Infect. Dis. 15:1562–1569. 10.3201/eid1510.090196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan K, Wang J, Marras TK. 2007. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am. J. Respir. Crit. Care Med. 176:306–313. 10.1164/rccm.200702-201OC [DOI] [PubMed] [Google Scholar]
- 6.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K. 2010. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am. J. Respir. Crit. Care Med. 182:977–982. 10.1164/rccm.201003-0503OC [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Hong YP, Kim SC, Bai GH, Jin BW, Park CD. 1981. A case of pulmonary disease due to M. avium-intracellulare complex. Tuberc. Respir. Dis. 28:121–124 [Google Scholar]
- 8.Yang HY. 2011. Isolation trend of nontuberculosis mycobacteria at a tertiary-care hospital in 2003–2011. Kosin Med. J. 26:155–160 [Google Scholar]
- 9.Lee JY, Choi HJ, Lee H, Joung EY, Huh JW, Oh YM, Lee SD, Kim WS, Kim DS, Kim WD, Shim TS. 2005. Recovery rate and characteristics of nontuberculous mycobacterial isolates in a university hospital in Korea. Tuberc. Respir. Dis. 58:385–391 [Google Scholar]
- 10.Lee SK, Lee EJ, Kim SK, Chang J, Jeong SH, Kang YA. 2012. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand. J. Infect. Dis. 44:733–738. 10.3109/00365548.2012.681695 [DOI] [PubMed] [Google Scholar]
- 11.Lee MK, Seo YH, Jeong JH, Park PW, Kim KH, Ahn JY, Kim JY, Park JW. 2012. Nontuberculous mycobacteria isolated from respiratory specimens during recent two years: distribution and clinical significance. Korean J. Clin. Microbiol. 15:98–103. 10.5145/KJCM.2012.15.3.98 [DOI] [Google Scholar]
- 12.Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. 2010. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int. J. Tuberc. Lung Dis. 14:1069–1071 [PubMed] [Google Scholar]
- 13.Shin S, Kim EC, Yoon JH. 2006. Identification of nontuberculous mycobacteria by sequence analysis of the 16S ribosomal RNA, the heat-shock protein 65 and the RNA polymerase beta-subunit genes. Korean J. Lab. Med. 26:153–160. 10.3343/kjlm.2006.26.3.153 [DOI] [PubMed] [Google Scholar]
- 14.Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, Bai GH. 2006. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 129:341–348. 10.1378/chest.129.2.341 [DOI] [PubMed] [Google Scholar]
- 15.Jeong J, Kim SR, Chang CL, Lee SH. 2008. Identification of mycobacteria species by HPLC and species distribution during five years at Ulsan university hospital. Korean J. Lab. Med. 28:24–33. 10.3343/kjlm.2008.28.1.24 [DOI] [PubMed] [Google Scholar]
- 16.Makinen J, Sarkola A, Marjamaki M, Viljanen MK, Soini H. 2002. Evaluation of genotype and LiPA MYCOBACTERIA assays for identification of Finnish mycobacterial isolates. J. Clin. Microbiol. 40:3478–3481. 10.1128/JCM.40.9.3478-3481.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. 2000. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J. Clin. Microbiol. 38:2966–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devulder G, Perouse de Montclos M, Flandrois JP. 2005. A multigene approach to phylogenetic analysis using the genus Mycobacterium as a model. Int. J. Syst. Evol. Microbiol. 55:293–302. 10.1099/ijs.0.63222-0 [DOI] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2008. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing, Approved guideline MM18-A. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 20.Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BJ, Lee KH, Park BN, Kim SJ, Bai GH, Kim SJ, Kook YH. 2001. Differentiation of mycobacterial species by PCR-restriction analysis of DNA (342 base pairs) of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 39:2102–2109. 10.1128/JCM.39.6.2102-2109.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frothingham R, Wilson KH. 1993. Sequence-based differentiation of strains in the Mycobacterium avium complex. J. Bacteriol. 175:2818–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai J, Chen Y, Lauzardo M. 2011. Web-accessible database of hsp65 sequences from Mycobacterium reference strains. J. Clin. Microbiol. 49:2296–2303. 10.1128/JCM.02602-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JS, Choi JI, Lim JH, Ahn JJ, Jegal Y, Seo KW, Ra SW, Jeon JB, Lee SH, Kim SR, Jeong J. 2013. The combination of real-time PCR and HPLC for the identification of non-tuberculous mycobacteria. Ann. Lab. Med. 33:349–352. 10.3343/alm.2013.33.5.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryoo SW, Shin S, Shim MS, Park YS, Lew WJ, Park SN, Park YK, Kang S. 2008. Spread of nontuberculous mycobacteria from 1993 to 2006 in Koreans. J. Clin. Lab. Anal. 22:415–420. 10.1002/jcla.20278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, Shin SJ, Kwon OJ. 2012. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest 142:1482–1488. 10.1378/chest.12-0494 [DOI] [PubMed] [Google Scholar]
- 27.Padilla E, Gonzalez V, Manterola JM, Perez A, Quesada MD, Gordillo S, Vilaplana C, Pallares MA, Molinos S, Sanchez MD, Ausina V. 2004. Comparative evaluation of the new version of the INNO-LiPA Mycobacteria and GenoType Mycobacterium assays for identification of Mycobacterium species from MB/BacT liquid cultures artificially inoculated with mycobacterial strains. J. Clin. Microbiol. 42:3083–3088. 10.1128/JCM.42.7.3083-3088.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am. J. Respir. Crit. Care Med. 183:405–410. 10.1164/rccm.201003-0395OC [DOI] [PubMed] [Google Scholar]
- 29.Simons S, van Ingen J, Hsueh PR, Van Hung N, Dekhuijzen PN, Boeree MJ, van Soolingen D. 2011. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg. Infect. Dis. 17:343–349. 10.3201/eid1703100604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S, Suh GY, Chung MP, Kim H, Kwon OJ, Lee KS, Lee NY, Koh WJ. 2008. Clinical significance of Mycobacterium fortuitum isolated from respiratory specimens. Respir. Med. 102:437–442. 10.1016/j.rmed.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 31.Yano Y, Kitada S, Mori M, Kagami S, Taguri T, Uenami T, Namba Y, Yoneda T, Yokota S, Maekura R. 2013. Pulmonary disease caused by rapidly growing mycobacteria: a retrospective study of 44 cases in Japan. Respiration 85:305–311. 10.1159/000339631 [DOI] [PubMed] [Google Scholar]
- 32.Macheras E, Roux AL, Ripoll F, Sivadon-Tardy V, Gutierrez C, Gaillard JL, Heym B. 2009. Inaccuracy of single-target sequencing for discriminating species of the Mycobacterium abscessus group. J. Clin. Microbiol. 47:2596–2600. 10.1128/JCM.00037-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth A, Reischl U, Streubel A, Naumann L, Kroppenstedt RM, Habicht M, Fischer M, Mauch H. 2000. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J. Clin. Microbiol. 38:1094–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.