Abstract
Osteoarticular infection is an uncommon presentation of Q fever. Positron emission tomography (PET) scanning is a valuable tool for the diagnosis of Coxiella burnetii graft prosthesis infection and endocarditis. Our objective was to test a series of culture-negative osteoarticular samples using molecular assays for Coxiella burnetii. We tested for C. burnetii by molecular assays targeting the IS1111 and the IS30A spacer regions, using culture-negative osteoarticular samples obtained in our laboratory between January 2011and December 2012. We examine a total of 1,410 osteoarticular samples, and we observed two cases of arthritis and subacromial bursitis caused by C. burnetii. The infections were localized using PET scanning, and the diagnosis was confirmed through serology. For one, a C. burnetii strain with a multispacer sequence type 8 genotype was isolated from synovial fluid culture. Q fever articular infections could be undiagnosed because of the long evolution of articular attack, and patients with high antibody titers against C. burnetii should be tested using PET scanning to localize the site of infection.
INTRODUCTION
Q fever is a worldwide zoonosis caused by Coxiella burnetii (1). Infective endocarditis is a serious complication of Q fever and can be fatal without treatment (2). The progression of infection occurs predominantly in patients with underlying cardiovascular abnormalities (2). Osteoarticular infection is an uncommon presentation of Q fever, representing approximately 2% of the reported Q fever cases in a large serologic study that extended over 14 years in France (3). Approximately 20 cases of Q fever osteoarticular infections have been reported, and chronic osteomyelitis is the most common manifestation, followed by vertebral spondylodiscitis and paravertebral abscess cases (4, 5). C. burnetii has also been identified as the infectious agent of native hip joint infections following arthroplasty resection, and recently, C. burnetii has been implicated in a prosthetic joint infection (5). To date, two cases of tenosynovitis have been reported (6).
Positron emission tomography (PET) scanning has been proposed as a promising tool for the identification of occult infectious foci, particularly in culture-negative infected cardiovascular devices, such as C. burnetii-infected vascular grafts. PET scanning has been routinely employed to identify inflammatory and infectious diseases, as inflammatory cells take up a significant amount of fluorodeoxyglucose (7). Thus, numerous case reports and a few preliminary studies have shown much promise for the use of PET scanning in the setting of vascular infections (7). PET imaging is a valuable tool for the diagnosis of C. burnetii graft prosthesis infection and has also been used for the diagnosis of C. burnetii endocarditis (8, 9). Because of its nonspecific clinical presentation, Q fever osteoarticular infection is likely underestimated, and thus the objective of the present study was to test a series of culture-negative osteoarticular samples using molecular assays for C. burnetii. We observed two C. burnetii-positive samples. For these two patients, the results of the molecular analyses were confirmed through serology, and C. burnetii active lesions were localized using PET scanning.
MATERIALS AND METHODS
Sample collections.
We retrospectively tested culture-negative osteoarticular samples obtained in our laboratory between January 2011and December 2012. All samples, cultured on Columbia 5% sheep blood agar plates (bioMérieux, Marcy l'Etoile, France), were negative after 5 days of incubation. The samples were obtained from hospitalized patients and outpatients and were sent to our laboratory under sterile conditions frozen (−20°C) or in dry ice (−80°C). All samples were handled under sterile conditions at −80°C to avoid cross-contaminations until further analysis.
Molecular assays.
DNA was extract from the samples using a QIAamp tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. The DNA extracts were used as templates in a real-time PCR (RT-PCR) assay targeting the IS1111 and the less sensitive IS30A spacer regions as previously described (10, 11). The results were considered positive when both spacer regions were confirmed through RT-PCR analysis. The quality of DNA handling and the extraction of samples were verified using RT-PCR for a housekeeping gene encoding beta-actin (12). The results were considered negative when RT-PCR analysis for C. burnetii was negative for both spacer regions and the threshold cycle (CT) values of the beta-actin control gene were ≤30.
RESULTS
We examine a total of 1,410 osteoarticular samples and observed two patients with positive RT-PCR results. The detailed histories for the patients are described below.
Case 1.
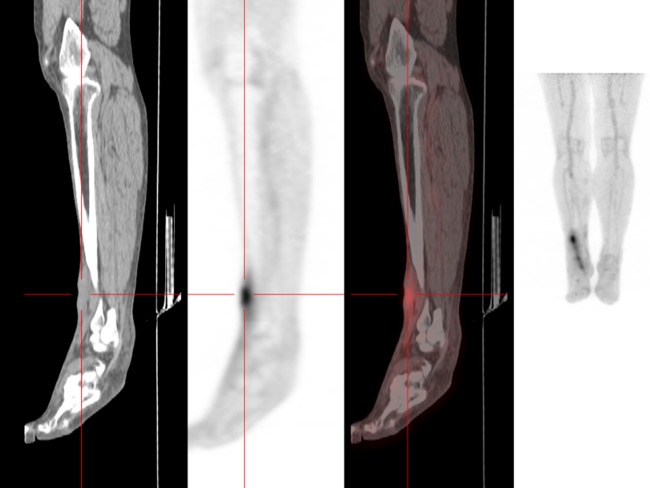
A 60-year-old man from southern France was admitted to the hospital in November 2011 for an inflammatory pain in his right ankle for 6 months. Upon examination, his right ankle was swollen and painful, with decreased joint amplitude. A tibiotarsal cyst was removed from his right ankle, and the histological analysis showed a benign cyst with hyaline necrosis of the superficial coating and fibrous remodeling of the cyst wall. Immunohistochemical staining for C. burnetii was negative, and standard culture was negative after 5 days. However, when we retrospectively examined this sample using RT-PCR, both the IS1111 and the IS30A spacer regions were positive. To confirm this result, the serum was sampled using an indirect immunofluorescence assay (IFA), which showed that the IgG, IgM, and IgA antibody titers against phase I and phase II C. burnetii antigens were positive (13) (Table 1). However, RT-PCR for C. burnetii was negative for all serum samples from this patient. Transesophageal echocardiography (TEE) did not show signs of valvulopathy. The culture of the cyst onto human embryonic lung (HEL) fibroblasts in shell vials (12) was also negative. PET scanning revealed a tibiotarsal joint fixation as the location of C. burnetii infection (Fig. 1). A course of 200 mg of oral doxycycline once per day with 200 mg of hydroxychloroquine three times per day for 18 months was introduced. Thus, the diagnosis for this patient was arthritis from C. burnetii infection.
TABLE 1.
Immunofluorescence assay, PCR, and culture results for the two patients with Q fever osteoarticular infection
| Patient and date (mo/yr) | IFA antibody titer |
RT-PCR |
Culture on articular fluid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase I |
Phase II |
Articular sample | Serum sample | ||||||
| IgG | IgM | IgA | IgG | IgM | IgA | ||||
| 1 | |||||||||
| 1/2012 | 1,600 | 0 | 400 | 3,200 | 0 | 800 | Positive | Negative | Negative |
| 4/2012 | 1,600 | 0 | 400 | 3,200 | 0 | 800 | Negative | Negative | NDa |
| 5/2012 | 1,600 | 0 | 400 | 3,200 | 0 | 800 | ND | Negative | ND |
| 2 | |||||||||
| 5/2012 | 400 | 0 | 25 | 800 | 0 | 50 | Positive | Negative | Positive |
| 6/2012 | 3,200 | 0 | 50 | 6,400 | 0 | 100 | ND | Negative | ND |
| 7/2012 | 3,200 | 0 | 50 | 6,400 | 0 | 100 | ND | Negative | ND |
ND, not done.
FIG 1.
Analysis of C. burnetii tibiotarsal fixation using PET scanning.
Case 2.
A 53-year-old woman from southern France without fever was admitted to the hospital in May 2012 for swelling of the left shoulder for 2 weeks and weight loss (approximately 15 kg) over the last 3 months (body mass index [BMI] = 17.5 kg/m2). The patient had visited an animal farm a few weeks before the swelling developed. Upon examination, her left shoulder was swollen and painful, with decreased joint amplitude and no signs of infection and lymphadenopathy. The patient had a history of psoriatic arthritis and had been treated with methotrexate (15 mg/week) for the last year. The synovial fluid analysis of the shoulder bursitis aspiration revealed nonpurulent inflammation (5 × 109/liter leukocytes with 90% polymorphonuclear cells). The blood cultures and synovial fluid were sterile after 5 days of culture. Surprisingly, the RT-PCR analysis revealed that the synovial fluid was positive for the IS1111 and the IS30A spacer regions. The IFA showed that the IgG, IgM, and IgA antibody titers against phase I and phase II C. burnetii antigens were also positive, although RT-PCR analysis for C. burnetii was negative (Table 1). C. burnetii was isolated from synovial fluid culture using the shell vial method (12). This isolate was susceptible to doxycycline (MIC = 0.25 μg/ml) (14), and multispacer sequence typing (MST) (13, 15) revealed an MST8 genotype, which is frequently detected in patients from France (13, 15). PET scanning showed high metabolic activity in the left shoulder bursitis and regional lymphadenopathy (Fig. 2). A course of 200 mg oral doxycycline once per day with 200 mg hydroxychloroquine three times per day for 18 months was introduced. The diagnosis for this patient was C. burnetii subacromial bursitis in the context of antimetabolite therapy.
FIG 2.
Analysis of C. burnetii subacromial bursitis using PET scanning.
DISCUSSION
We report 2 cases of Q fever articular infections identified through molecular analysis. The infections were localized using PET scanning, and the diagnosis was confirmed through serology and culture for patient 2. Molecular methods are critical for the detection of infectious bacteria in osteoarticular specimens. However, these bacteria are difficult to detect using a single molecular approach, and clinical microbiology laboratories commonly use universal 16S rRNA gene amplification and sequencing for the identification of the causative agents (16). Nevertheless, this method has been reported to lack sensitivity, particularly for fastidious bacteria, such as C. burnetii (16). Thus, given the low sensitivity of eubacterial PCR, infections resulting from fastidious organisms are not excluded in cases of negative 16S rRNA gene PCR results. Our specific RT-PCR assay for the detection of C. burnetii was sensitive and capable of detecting 102 bacteria/ml (10). Moreover, each positive result was confirmed through the amplification of a second specific gene of C. burnetii to avoid false-positive results, and the quality of DNA extraction was verified for all samples (12).
PET scanning has been used for the identification of infectious foci in C. burnetii vascular infections (8, 9, 17) and in a case of Q fever in the bone marrow (18). PET scanning has higher sensitivity than computed tomography (CT) scanning for the evaluation of the extent and localization of infections (8, 9, 17). In the present study, PET scanning was demonstrated as a valuable tool for the diagnosis of C. burnetii articular lesions. Indeed, C. burnetii lesions were localized on the tibiotarsal joint for patient one and on left shoulder bursitis for patient two. Thus, we suggest that PET scanning should be used as a complementary exam for patients with high C. burnetii antibody titers to localize the site of C. burnetii infection.
Articular infection is an uncommon clinical entity of Q fever, and 4 cases (including the 2 cases presented here) of C. burnetii articular infections have been reported. Landais et al. reported two cases of tenosynovitis in one male and one female patient (6). Both patients presented carpal tunnel syndrome and complained of wrist swelling and arthralgia in the hands and shoulders, without inflammatory signs. Similar to our patients, both patients had increased IgG and IgA antibodies and absent IgM antibodies (6). Persistent Q fever infections have been associated with increased levels of IgG and IgA antibodies (19, 20). Moreover, the PCR analysis of the synovial fluid collected from one patient and the peroperative samples obtained from the other patient showed positive results for C. burnetii. Despite limited data concerning therapeutic strategies for Q fever osteoarticular infections, a regimen of doxycycline and hydroxychloroquine for 18 months has been recently suggested (6, 21). Our patients have been treated with a course of doxycycline and hydroxychloroquine for 18 months.
In conclusion, Q fever articular infections may be undiagnosed because of the long evolution of articular attack, which is accompanied by a low level of biological and inflammatory signs, as well as the negative results of conventional cultures and 16S rRNA gene PCR analyses. Based on the results of the present study, we routinely employ the amplification and sequencing of the 16S rRNA gene and the fungus-specific universal internal transcribed spacer (ITS) gene as well as RT-PCR analysis of Staphylococcus aureus, C. burnetii, and Tropheryma whipplei for all culture-negative osteoarticular samples. Patients with high antibody titers against C. burnetii should be tested using PET scanning to localize the site of infection. Thus, clinicians should consider C. burnetii in the diagnosis of undetermined culture-negative articular infections with a long evolution.
Footnotes
Published ahead of print 15 January 2014
REFERENCES
- 1.Angelakis E, Raoult D. 2010. Q Fever. Vet. Microbiol. 140:297–309. 10.1016/j.vetmic.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 2.Million M, Walter G, Thuny F, Habib G, Raoult D. 2013. Evolution from acute Q fever to endocarditis is associated with underlying valvulopathy and age and can be prevented by prolonged antibiotic treatment. Clin. Infect. Dis. 57:836–844. 10.1093/cid/cit419 [DOI] [PubMed] [Google Scholar]
- 3.Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier PE, Bernit E, Stein A, Nesri M, Harle JR, Weiller PJ. 2000. Q fever 1985-1998—clinical and epidemiologic features of 1,383 infections. Medicine 79:109–123. 10.1097/00005792-200003000-00005 [DOI] [PubMed] [Google Scholar]
- 4.Merhej V, Tattevin P, Revest M, Le TB, Raoult D. 2012. Q fever osteomyelitis: a case report and literature review. Comp. Immunol. Microbiol. Infect. Dis. 35:169–172. 10.1016/j.cimid.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Tande AJ, Cunningham SA, Raoult D, Sim FH, Berbari EF, Patel R. 2013. A case of Q fever prosthetic joint infection and description of an assay for detection of Coxiella burnetii. J. Clin. Microbiol. 51:66–69. 10.1128/JCM.02352-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landais C, Fenollar F, Constantin A, Cazorla C, Guilyardi C, Lepidi H, Stein A, Rolain JM, Raoult D. 2007. Q fever osteoarticular infection: four new cases and a review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 26:341–347. 10.1007/s10096-007-0285-5 [DOI] [PubMed] [Google Scholar]
- 7.Thuny F, Gaubert JY, Jacquier A, Tessonnier L, Cammilleri S, Raoult D, Habib G. 2013. Imaging investigations in infective endocarditis: current approach and perspectives. Arch. Cardiovasc. Dis. 106:52–62. 10.1016/j.acvd.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 8.Merhej V, Cammilleri S, Piquet P, Casalta JP, Raoult D. 2012. Relevance of the positron emission tomography in the diagnosis of vascular graft infection with Coxiella burnetii. Comp. Immunol. Microbiol. Infect. Dis. 35:45–49. 10.1016/j.cimid.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 9.Wegdam-Blans MC, Stokmans RA, Tjhie JH, Korbeeck JM, Koopmans MP, Evers SM, van der Voort PH, Teijink JA. 2013. Targeted screening as a tool for the early detection of chronic Q fever patients after a large outbreak. Eur. J. Clin. Microbiol. Infect. Dis. 32:353–359. 10.1007/s10096-012-1749-9 [DOI] [PubMed] [Google Scholar]
- 10.Eldin C, Angelakis E, Renvoise A, Raoult D. 2013. Coxiella burnetii DNA, but not viable bacteria, in dairy products in France. Am. J. Trop. Med. Hyg. 88:765–769. 10.4269/ajtmh.12-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelakis E, Mediannikov O, Stein A, Bassene H, Sokhna C, Raoult D. 2014. Throat swab samples for diagnosis of Q fever. Am. J. Trop. Med. Hyg. 90:147–148. 10.4269/ajtmh.13-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angelakis E, Richet H, Rolain JM, La SB, Raoult D. 2012. Comparison of real-time quantitative PCR and culture for the diagnosis of emerging rickettsioses. PLoS Negl. Trop. Dis. 6:e1540. 10.1371/journal.pntd.0001540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelakis E, Million M, D'Amato F, Rouli L, Richet H, Stein A, Rolain JM, Raoult D. 2013. Q fever and pregnancy: disease, prevention, and strain specificity. Eur. J. Clin. Microbiol. Infect. Dis. 32:361–368. 10.1007/s10096-012-1750-3 [DOI] [PubMed] [Google Scholar]
- 14.Botelho-Nevers E, Singh S, Chiche L, Raoult D. 2013. Effect of omeprazole on vacuole size in Coxiella burnetii-infected cells. J. Infect. 66:288–289. 10.1016/j.jinf.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 15.Glazunova O, Roux V, Freylikman O, Sekeyova Z, Fournous G, Tyczka J, Tokarevich N, Kovacava E, Marrie TJ, Raoult D. 2005. Coxiella burnetii genotyping. Emerg. Infect. Dis. 11:1211–1217. 10.3201/eid1108.041354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelakis E, Roux V, Raoult D, Rolain JM. 2009. Real-time PCR strategy and detection of bacterial agents of lymphadenitis. Eur. J. Clin. Microbiol. Infect. Dis. 28:1363–1368. 10.1007/s10096-009-0793-6 [DOI] [PubMed] [Google Scholar]
- 17.Van Assen S, Houwerzijl EJ, van den Dungen JJ, Koopmans KP. 2007. Vascular graft infection due to chronic Q fever diagnosed with fusion positron emission tomography/computed tomography. J. Vasc. Surg. 46:372. 10.1016/j.jvs.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 18.Alwis L, Balan K, Wright P, Lever A, Carmichael A. 2009. Bone marrow involvement in Q fever—detection by fluorine-18-labelled fluorodeoxyglucose PET. Lancet Infect. Dis. 9:718. 10.1016/S1473-3099(09)70113-6 [DOI] [PubMed] [Google Scholar]
- 19.Raoult D. 2012. Chronic Q fever: expert opinion versus literature analysis and consensus. J. Infect. 65:102–108. 10.1016/j.jinf.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 20.Million M, Thuny F, Richet H, Raoult D. 2010. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect. Dis. 10:527–535. 10.1016/S1473-3099(10)70135-3 [DOI] [PubMed] [Google Scholar]
- 21.Angelakis E, Oddoze C, Raoult D. 2013. Vitamin D and prolonged treatment with photosensitivity-associated antibiotics. Antimicrob. Agents Chemother. 57:6409–6410. 10.1128/AAC.01969-13 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]