Abstract
Protease inhibitors (PIs) targeting the hepatitis C virus (HCV) NS3 protease, such as telaprevir, have significantly improved the sustained virologic response (SVR) rates of HCV genotype 1 antiviral therapy. Given the expanding antiviral therapy regimen, fast HCV PI resistance assays are urgently needed. In this view, we have developed a novel phenotypic resistance test for HCV PIs based on in vitro synthesis of patient-derived HCV NS3 protease and subsequent enzymatic testing in a fluorescent readout. The enzymatically active HCV NS3 proteases were synthesized from PCR-derived templates by an Escherichia coli S30 extract system. Tests of the protease genes with known mutations for telaprevir resistance showed that the phenotypic resistance test was fast, with a total turnaround time of <10 h, and was fully in agreement with the previous resistance results. The initial tests with 38 treatment-naive serum samples showed that the method was significantly less laborious and faster than currently available phenotypic resistance assays of HCV NS3 PIs.
INTRODUCTION
Chronic infection with the hepatitis C virus (HCV) affects an estimated 160 million individuals worldwide (1) and leads to severe liver diseases, such as fibrosis, cirrhosis, and hepatocellular carcinoma (2). Since the early 2000s, pegylated interferon (PEG-IFN) and ribavirin (RBV) have been used in the standard of care (SOC) for treatment of chronic hepatitis C and result in a sustained virologic response (SVR) for 80% of patients infected with HCV genotype 2 or 3. However, in patients infected with genotype 1, SVR rates with the SOC reach only 42 to 46% (3, 4). In 2011, two protease inhibitors (PIs), boceprevir and telaprevir, were approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency as the first two direct-acting antivirals (DAAs) for the treatment of patients infected with chronic HCV genotype 1. Clinical studies showed that these PIs improved the SVR rates to HCV genotype 1 in treatment-naive and previously treated patients when compared to the standard dual-treatment regimen (5–8). Thus, the current SOC recommended for HCV genotype 1 infection is a triple therapy combining PEG-IFN, ribavirin, and a protease inhibitor, either boceprevir or telaprevir (9). Because of the narrow spectrums of activity and the side effects of the two approved PIs, second-generation PIs with broad genotypic coverage and a high genetic barrier for resistance are being developed actively (10). Given the expected expanding antiviral therapy regimen using the PIs, fast HCV PI resistance assays are urgently needed.
The current methods used for testing the PI resistance of HCV are normally genotypic assays based on determining the individual mutation pattern of a patient's virus population. Genotypic methods, such as population-sequencing methods (11, 12), clonal sequencing (13), the TaqMan mismatch amplification mutation assay (TaqMAMA) (14), and ultradeep pyrosequencing (15, 16), have been developed. However, due to the high replication rate of HCV and its error-prone RNA polymerase with no proofreading activity, new mutations or complex mutation patterns are commonly found in patient samples. Therefore, it is difficult to establish a well-characterized resistance mutation database and quantitatively interpret the sequencing results with the presence of new mutations or complex mutation patterns. Such shortcomings of genotyping can be complemented by phenotypic methods of testing drug susceptibility.
Several enzymatic and replicon-based phenotypic assays have been developed for assessing the PI susceptibilities of HCV through replicons with NS3 genes derived from clinical isolates (17–19) or recombinant NS3 proteases coded by the NS3 genes of HCV in patient sera (13). These assays can confirm the effects of new mutations or complex mutation patterns on HCV susceptibility to protease inhibitors, but they are laborious and time-consuming, with turnaround times ranging from a few days to weeks.
Given the long period of time for constructing the clones of NS3 protease in the current enzymatic phenotypic assays, we developed a fast phenotypic method (the total turnaround time was ca. 10 h) for assessing PI susceptibility of HCV through in vitro synthesis of NS3 proteases coded by NS3 genes derived from clinical samples using a coupled in vitro transcription/translation system and a fluorescence enzyme-kinetic protease assay. Telaprevir, boceprevir, and treatment-naive sera were used to test the performance of the method.
MATERIALS AND METHODS
Clinical samples and HCV plasmids.
A total of 38 treatment-naive serum samples with HCV RNA levels of ≥5 × 103 IU/ml were obtained from the Wuhan Medical Treatment Center (Wuhan, China). Identified samples were obtained under informed consent with approval by the Academic and Ethical Committee of the Wuhan Institute of Virology, Chinese Academy of Sciences. The subgenomic HCV replicon pFKI389neo/NS3-3′ plasmid (20) was used as the wild-type or sensitive control (Con1) and was kindly provided by Xinwen Chen from the Wuhan Institute of Virology, Chinese Academy of Sciences.
Overall procedure of the rapid phenotypic resistance testing of HCV NS3 protease inhibitors.
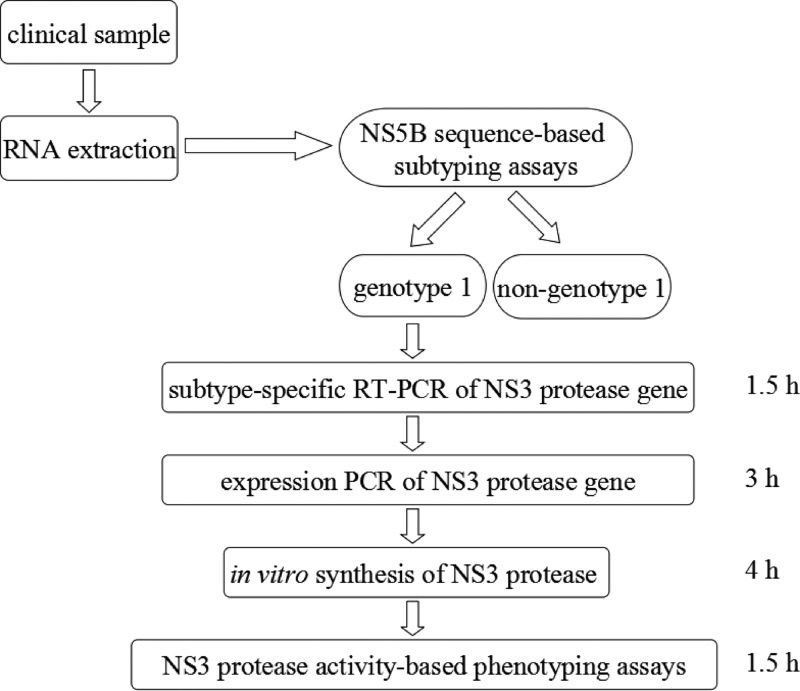
As shown in Fig. 1, the first step was RNA extraction from patient sera. Briefly, HCV RNA was extracted from 140 μl of serum using a QIAamp viral RNA kit (Qiagen, Inc., Valencia, CA). Then, the clinical samples were subtyped by an NS5b subtyping method published by the French National Research Agency on HIV/AIDS and Viral Hepatitis (ANRS) (21). The NS5b subtyping was done through a heminested PCR with the primers Pr3, Pr4, and Pr5 (Table 1). If a sample was subtyped to be HCV genotype 1, then a nested PCR was performed to prepare the DNA template for in vitro synthesis of NS3 protease. The nested PCR consisted of a one-step reverse transcription-PCR (RT-PCR) and a PCR for adding expression elements (ePCR), such as the Tac promoter, lac operator, and SD sequence required for in vitro transcription and translation. The primer pair used for the RT-PCR was F-HCV-RT (1b) and R-HCV-RT, and for the ePCR the primer pair was F-Ptac-NS3 and R-NS3 (Table 1). After the in vitro synthesis of the HCV NS3 protease by use of an Escherichia coli S30 extract system for linear templates (Promega Corporation, Madison, WI), telaprevir (or boceprevir) susceptibility of the synthesized NS3 protease in the S30 lysate was determined with a fluorescence resonance energy transfer (FRET) substrate RET S1 (Ac-DED(EDANS)EE Abuψ[COO]ASK (DABCYL)-NH2; AnaSpec, Inc., San Jose, CA) in 96-well microtiter plates (PerkinElmer, CA) as described previously (13). Before the fluorescence enzyme-kinetic protease assay, the lysate with the NS3 protease was preincubated with a cofactor peptide KK4A (KKGSVVIVGRIVLSGK) for 10 min at room temperature.
FIG 1.
Scheme of PCR-based in vitro-synthesized NS3 protease phenotyping assay.
TABLE 1.
Primer sequences used in this study
| Primer type | Primer name | Sequence (5′ to 3′) |
|---|---|---|
| Heminested PCR primers for NS5b subtyping | ||
| First step of RT-PCR | Pr3 | TATGAY ACCCGCTGY TTTGACTC |
| Pr4 | GCN GAR TAY CTVGTCATAGCCTC | |
| Second step of PCR | Pr3 | TATGAY ACCCGCTGY TTTGACTC |
| Pr5 | GCTAGTCATAGCCTCCGT | |
| Subtype-specific primers for RT-PCR | ||
| 1b | F-HCV-RT (1b) | ACCGCGGCGTGTGGGGACAT |
| R-HCV-RT | TTGCCATAGGTGGAGTACGTGATGG | |
| 2a | F-HCV-RT (2a) | ATGGAGAAGAAGGTCATCGTCTGGGG |
| R-HCV-RT | TTGCCATAGGTGGAGTACGTGATGG | |
| Primers for amplification of the NS3 protease gene | ||
| F-Ptac-NS3 | TTGACAATTAATCATCGGCTCGTATAATGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGGATCCAAATGGCGCCTATTACGGCCTACTa | |
| R-NS3 | TTAGGACCGCATAGTGGTTTCCATAGACTC | |
| Primers for site mutagenesis of different resistance variants | ||
| V36A | F-V36A | GGGAGGTCCAAGTGGCCTCCACCGCAACACAA |
| R-V36A | TTGTGTTGCGGTGGAGGCCACTTGGACCTCCC | |
| T54A | F-T54A | CAATGGCGTGTGTTGGGCTGTCTATCATGGTGC |
| R-T54A | GCACCATGATAGACAGCCCAACACACGCCATTG | |
| A156V | F-A156V | TGGGCATCTTTCGGGTTGCCGTGTGCACCC |
| R-A156V | GGGTGCACACGGCAACCCGAAAGATGCCCA | |
| A156T | F-A156T | GCTGTGGGCATCTTTCGGACTGCCGTGTGCACCCGA |
| R-A156T | TCGGGTGCACACGGCAGTCCGAAAGATGCCCACAGC |
The Ptac sequence is shown in bold type. Ptac is the abbreviation for the tac promoter, which is a strong hybrid promoter composed of the −35 region of the trp promoter and the −10 region of the lacUV5 promoter (25).
The susceptibility results were determined based on the ratio vi/v0 of the reaction rates of the in vitro-expressed protease when telaprevir (or boceprevir) was absent (v0) and when it was present (vi). The vi/v0 ratio was calculated as vi/v0 = [vi(S) − vi(N)]/[v0(S) − v0(N)], where vi(S) is the initial rate of in vitro-synthesized protease in the presence of telaprevir (or boceprevir), vi(N) is the initial rate of negative control in the presence of telaprevir (or boceprevir), v0(S) is the initial rate of in vitro-synthesized protease in the absence of telaprevir (or boceprevir), and v0(N) is the initial rate of negative control in the absence of telaprevir (or boceprevir).
A cutoff value of vi/v0 was determined as the mean plus 3 standard deviations of the vi/v0 of a sensitive NS protease wild-type control (Con1) derived from subgenomic HCV replicon pFKI389neo/NS3-3′. The HCV virion in a serum was considered sensitive to PIs if the vi/v0 ratio of its protease was below the cutoff value (meaning that the PI can inhibit the activity of the protease as effectively as that of the Con1 protease). Otherwise, the HCV in the serum was considered resistant to PIs (meaning that the PI cannot effectively inhibit the activity of the protease). To test the reproducibility of the in vitro-synthesized protease assay, all the controls (Con1, low-resistance and high-resistance controls) were tested three times in the same batch and repeated three batches performed on different days.
In the experiments, some serum samples were subtyped to be HCV genotype 2a. The telaprevir and boceprevir susceptibilities of the NS3 proteases were also determined using the same procedure described above for HCV genotype 1, except that a subtype-specific primer pair (F-HCV-RT [2a] and R-HCV-RT) (Table 1) was used for the RT-PCR.
See Supplemental Methods in the supplemental material for the detailed conditions used for performing NS5b subtyping, RT-PCR, ePCR, in vitro synthesis of protease, and the protease activity assay.
Construction of mutated NS3 genes for coding NS3 proteases with different resistance level to PIs.
The DNA fragment encoding HCV NS3 protease was obtained by PCR from pFKI389neo/NS3-3′ and inserted into TA clone vector pGEM-T (renamed pGEM-T-Con1-NS3) for subsequent site-directed mutagenesis. Mutations in the NS3 genes which confer the NS3 proteases with low-level resistance (V36A, T54A) and high-level resistance (A156V, A156T) to telaprevir were introduced into pGEM-T-Con1-NS3 with the primers shown in Table 1 using the QuikChange site-directed mutagenesis kit (Stratagene, CA) following the manufacturer's instructions. All constructs were confirmed by sequencing.
Endogenous proteolytic activity of the lysates in different in vitro expression systems.
To test the endogenous proteolytic activity of the lysates to hydrolyze the substrate RET-S1, 10 μl of the lysates from an RTS 100 wheat germ CECF kit (5 Prime, Inc., Boulder, CO), an RTS 100 E. coli HY kit (5 Prime, Inc.), a WEPRO1240 expression kit (CellFree Sciences, Co., Ltd., Japan), or an E. coli S30 extract system for linear templates (Promega Corporation, Madison, WI) was mixed with 80 μl of a proteolytic assay buffer (50 mM HEPES with 100 mM NaCl, 20% glycerol, and 5 mM dithiothreitol [pH 7.8]). A negative control was also prepared by adding 10 μl of the proteolytic assay buffer into 80 μl of the proteolytic assay buffer. Then, the reactions were started by adding 10 μl of 2.5 μM substrate RET-S1 into each well. Finally, the fluorescence (λex [excitation], 355 nm; λem [emission], 500 nm) of each well was monitored continuously at 30°C for 2 h by a Synergy H1 hybrid reader (BioTek, USA).
RESULTS
Selection of in vitro expression system and optimization of NS3 protease synthesis.
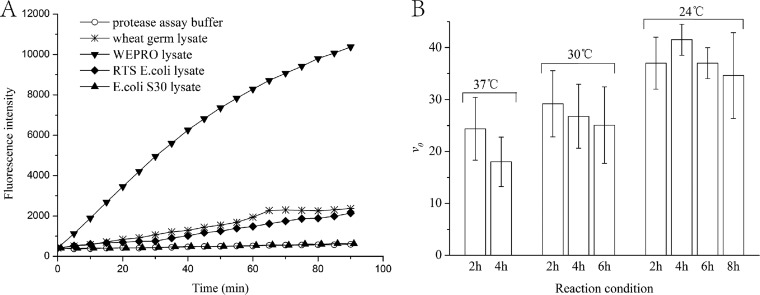
Because serine proteases are widespread in bacteria and eukaryotes, endogenous serine proteases may exist in the lysates of prokaryotic or eukaryotic cell-free expression systems, which may hydrolyze NS3/4A substrate RET-S1 and result in high fluorescent background. We tested the endogenous protease hydrolytic activity of a few commercially available in vitro expression systems. As shown in Fig. 2A, the lysates of most systems tested, such as RTS 100 wheat germ lysate and RTS 100 E. coli lysate, had significant hydrolytic activity to the NS3/4A substrate RET-S1. Only the lysate of the E. coli S30 extract system showed minimum activity to hydrolyze the substrate RET-S1, which indicated that the substrate RET-S1 could be added directly into the S30 lysate after in vitro synthesis of NS3 protease to measure the protease activity. Therefore, the E. coli S30 extract system was used throughout in the following experiments.
FIG 2.
Proteolytic activity of the lysates in commercial in vitro expression systems (A) and the initial reaction rates of the protease synthesized in vitro by E. coli S30 lysate for different times at different temperatures (B).
To achieve the highest protease expression, the protease activity was measured after incubating the E. coli S30 expression system with 8 μg of the Con1 DNA template at 37°C, 30°C, and 24°C for different times. The maximum proteolytic rate was obtained when in vitro synthesis of the NS3 protease was performed at 24°C for 4 h (Fig. 2B). Therefore, these conditions were chosen for the in vitro synthesis of NS3 protease.
Optimizing telaprevir concentration for phenotypic resistance assay.
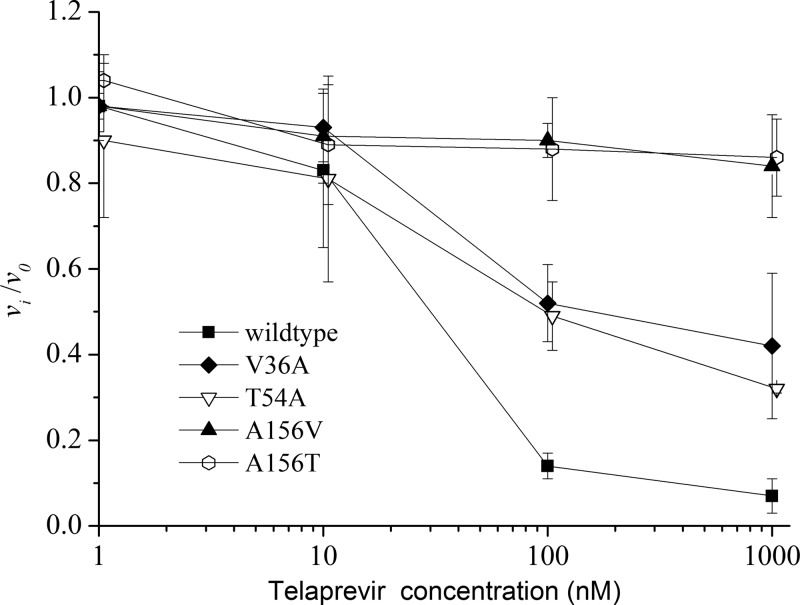
Since telaprevir is a competitive inhibitor of HCV NS3 protease, telaprevir should be used at a proper concentration in the phenotypic resistance assay. In order to select the appropriate concentration for the assay, the vi/v0 ratio of the reaction rates of the in vitro-expressed Con1 proteases, two low-level resistance variants (V36A and T54A) and two high-level resistance variants (A156V and A156T) with different concentrations of telaprevir were measured, respectively. As shown in Fig. 3, when the concentrations of telaprevir were <10 nM, the ratios did not differentiate the Con1 protease from the two low-level resistance variants and the two high-level resistance variants, which was due to the fact that low concentrations of telaprevir did not inhibit the activity of the sensitive protease or the resistance variants. When the concentrations of telaprevir were ≥100 nM, the wild-type protease and the different resistance variants were well differentiated. Because telaprevir at high concentrations might risk masking the difference between low-level and high-level resistance variants, we did not test the effects of telaprevir at concentrations >1,000 nM. Since, at a concentration of 100 nM telaprevir, wild-type protease, and low-level and high-level resistance variants were well differentiated by the vi/v0 ratio, this concentration was selected to test the susceptibility of HCV proteases derived from patient samples to telaprevir.
FIG 3.
The vi/v0 ratios of the in vitro-synthesized NS3 proteases determined under different concentrations of telaprevir.
NS5b sequence subtyping of HCV.
In order to effectively amplify the NS3 protease gene from clinical samples, the subtype information of the HCV is needed because of the wide variety in the HCV genotypes. Since the NS5b method (21) is normally used for the subtyping of HCV, we used this method to subtype the HCV genotype in the treatment-naive sera. As shown in Fig. S1 in the supplemental material, among the 38 samples tested, 29 were found to be subtype 1b, and 9 were found to be subtype 2a.
RT-PCR and DNA sequencing results of NS3 protease genes of clinical samples.
Based on the NS5b subtype information, the NS3 protease genes in the sera were successfully amplified by the RT-PCR using the subtype-specific primers F-HCV-RT (1b) and R-HCV-RT for genotype 1 and F-HCV-RT (2a) and R-HCV-RT for genotype 2a (see Fig. S2 in the supplemental material). A comparison between the NS3 protease gene sequences of the subtype 1b sample with that of Con1 revealed significant differences in the amino acid sequences of the HCV proteases derived from different clinical samples (as shown in Table 2). There was no report on how these mutations would affect the viral resistance to telaprevir or boceprevir. Similar results were also obtained for the HCV NS3 genes derived from subtype 2a sera (as shown in Table 2) by comparing the sequences with that of strain JFH1 (GenBank accession no. AB047639).
TABLE 2.
NS5b subtyping results for clinical samples and the mutations in the corresponding NS3 protease revealed by DNA sequencing
| Sample no. | Subtype | Amino acid mutations in NS3 proteasea |
|---|---|---|
| S2546 | 1b | S7A, R26K, V48I, Q86L, S122G, V170I |
| S2925 | 1b | I18V, R26K, V48I, L94 M, V132I, S147L, A150V |
| S2964 | 1b | R26K, E32G, L94 M, D103A, S122G, V132I, S147L, A150V |
| S2979 | 1b | S7A, L14V, C16T, I18V, R26K, Q28E, V48I, C52 M, I71V, T72C, Q80K, Q86P, A87S, H110E, V116A, G124A, S125A, V132I, Y134T, L143I, L144 M, A150V, I153L, A166S, V167L, V170I, S174N |
| S2981 | 1b | S7A, L14V, C16T, I18V, R26K, Q28E, C47S, V48I, C52V, I71V, T72C, T72I, Q80K, Q86P, A87S, V107I, H110E, V116A, S122N, G124A, S125A, V132I, Y134T, L143I, L144 M, A150V, L153L, A166S, V167L, V170I, S147N |
| S3063 | 1b | R26K, V48I, S61A, L94 M, T98A, S122G, V132I, S147L, A150V, V170I |
| S3064 | 1b | I17T, R26K, L94 M, S122G, V132I, S147L, A150V |
| S11242 | 1b | V132I, S147L, A150V |
| S11329 | 1b | R26K, Q86P, T95E, S122G |
| S11367 | 1b | R26K, V48I, A59F, L64F, Q80R, L94 M, S122G, V132I, S147L, A150V |
| S11368 | 1b | S7A, R26K, Q41H, V48I, T108A |
| S11379 | 1b | R26K, S122G, V132I, S147L, A150V |
| S11451 | 1b | T22P, R26K, A59F, L64F, Q80R, L94 M, S122G, S147L, A150V |
| S11478 | 1b | R26K, V48I, I71V, Q86P, S122G |
| S11479 | 1b | R26K, V132I, S147L, A150V |
| S11542 | 1b | R26K, V48I, I71V, Q86P, T95A, S122G |
| S11543 | 1b | R26K |
| S11562 | 1b | R26K, S61A, Q80K, L94 M, L104P, D121G, S122G, V132I, S147L, A150V |
| S11564 | 1b | R26K, S122G, V132I, A150V |
| S11614 | 1b | R26K, L94 M, S122G, V132I, S147L, A150V |
| S11615 | 1b | S7A, R26K, V48I, S61P, Q86P, P89S, V132I |
| S11675 | 1b | R26K, S61T, Y105F, S122G, V132I, S147L, A150V |
| S11676 | 1b | R26K, L94 M, S122G, V132I, S147L, A150V |
| S11708 | 1b | R26K, L94 M, S122G, V132I, S147L, A150V |
| S11710 | 1b | R26K, L94 M, V132I, S147L, A150V |
| S11718 | 1b | R26K, V48I, L94 M, S122G, V132I, S147L, A150V |
| S11750 | 1b | R26K, S122G, V132I |
| S11775 | 1b | R26K, L94 M, S122G, V132I, S147 M, A150V |
| S11823 | 1b | R26K, L94 M, S122G, V132I, S147L, A150V |
| S2662 | 2a | R26K, Q29P, V33I, S40T, T47S, G66S, L67S, K98T, I132L, V150A, L153I, T174A |
| S2828 | 2a | R26K, V33I, I35V, S40T, T47S, S49A, L67S, K98T, I132L, P146S, V150A, L153I, T174A |
| S3016 | 2a | R26K, V33I, I35V, S40T, T47S, S49A, V51I, L67S, S87G, K92R, K98T, I132L, V150A, L153I, T174A |
| S11244 | 2a | A16T, R26K, V33I, I35V, S40T, T47S, Y56F, L67S, K98T, I132L, V150A, L153I |
| S11369 | 2a | R26K, V33I, I35V, S40T, T47S, S49A, Y56F, L67S, K98T, I132L, V150A, L153I, T174A |
| S11477 | 2a | R26K, V33I, I35V, S40T, T47S, L67S, S77G, K98T, V107I, N110D, A126V, I132L, P146S, V150A, L153I |
| S11480 | 2a | R26K, V33T, I35V, S40T, T47S, L67S, K98T, I132L, V150A, L153I, T174A |
| S11528 | 2a | T22S, R26K, I35V, S40T, T47S, S49A, L67S, K98T, I132L, V150A, L153I, T174A |
| S11782 | 2a | R26K, V33I, Q34R, S40T, T47S, S49A, L67S, K98T, I132L, V150A, L153I, T174A |
The sequence of Con1 NS3 protease is used as the reference sequence for comparison with the sequences derived from subtype 1b clinical samples, while the sequence of strain JFH1 NS3 protease is used as the reference sequence for comparison with the sequences derived from subtype 2a clinical samples.
ePCR for adding the in vitro expression elements.
In order for the E. coli system to express protein directly from PCR products, the Tac promoter, lac operator, and SD sequence are needed to attach with the forward primers. Therefore, ePCR was performed using F-Ptac-NS3 (with a Ptac sequence) and R-NS3 as the primers using the RT-PCR amplicons of the 38 samples as the DNA templates. Figure S3 in the supplemental material shows that the ePCR effectively amplified the NS3 protease genes of all the samples.
Susceptibility testing of HCV clinical samples through in vitro-synthesized NS3 protease assay.
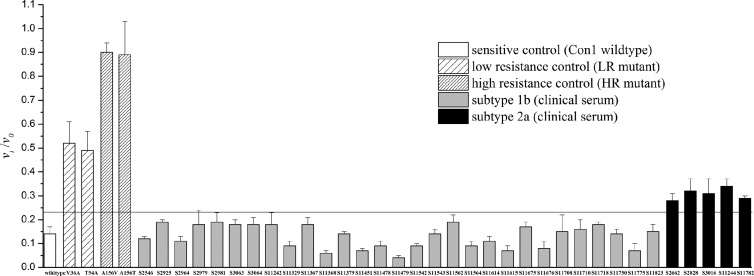
After the in vitro synthesis reactions were completed, the in vitro-synthesized protease activities in terms of the vi/v0 ratios were measured. The cutoff value used to judge telaprevir or boceprevir susceptibility was determined as the mean plus 3 standard deviations of the vi/v0 ratio of Con1 (the sensitive control). As shown in Fig. 4, among the samples, all 29 genotype 1 samples were determined as telaprevir susceptible because they exhibited vi/v0 ratios lower than the cutoff value, which means that 100 nM telaprevir inhibited the activity of these proteases as effectively as that of Con1 protease. All of the genotype 2 samples (only 5 samples are shown in Fig. 4) showed low resistance to telaprevir, since their vi/v0 ratios were slightly higher than the cutoff value (P < 0.05, Student's t test), which means that 100 nM telaprevir did not effectively inhibit the activity of these proteases as did that of the Con1 protease. Using the same procedure except replacing telaprevir with 100 nM boceprevir, all the samples tested (genotypes 1b and 2a) were determined as boceprevir susceptible because they exhibited vi/v0 ratios lower than the cutoff value (see Fig. S4 in the supplemental material), which means that 100 nM boceprevir inhibited the activity of these proteases as effectively as that of Con1 protease.
FIG 4.
The vi/v0 ratios of the in vitro-synthesized NS3 proteases determined with 100 nM telaprevir. The cutoff value is the mean plus 3 standard deviations of the vi/v0 ratio of Con1 (wild-type control). Each control and sample were tested three times.
The intra-assay and interassay reproducibilities of the in vitro-synthesized protease assay in terms of the vi/v0 ratios were found with coefficients of variation (CV) of <14% and 21%, respectively, for all the controls (Con1, two low-resistance controls, and two high-resistance controls).
DISCUSSION
HCV displays a large genetic variability due to its high replication rate and its error-prone RNA polymerase with no proofreading activity. With the wide use of protease inhibitors (PIs), it is expected that PI-resistant variants will be selected under the pressure of the antiviral agents. Rapid analysis of PI resistance of HCV in patient samples would provide valuable information for monitoring resistance development and the proper treatment of HCV infections.
The rapid phenotypic assay developed here offers a few advantages unavailable with the current methods. Compared with the current phenotypic assays based on enzymatic activity or HCV replication, the in vitro-synthesized protease assay avoids the lengthy time and steps needed in cloning the protease gene from samples and expressing the recombinant protease. The total turnaround time (from serum to susceptibility result) for the in vitro-synthesized protease assay was <10 h after finding the HCV subtype, while in the conventional enzymatic assays, at least 3 days are needed. The main obstacle for conducting the assay in a typical clinical lab is the need for molecular expertise to perform PCR. With the rapid progress in molecular diagnostics, many clinical labs are conducting molecular tests now. Therefore, the current assay has the potential to be applied in clinical labs. For those labs without molecular expertise, patient sera will need to be sent to a laboratory. An additional 1 to 2 days might be expected for sample transportation and results reporting.
Compared with the genotypic assays based on identifying gene mutations, the in vitro-synthesized protease assay provides a yes/no PI resistance result by determining if the activity of the synthesized protease can be inhibited by the PI related to that of the wild-type protease regardless of mutations, which overcomes the difficult problem of interpreting resistance in genotypic assays when new mutations or complex mutation patterns exist in the clinical samples. As shown in Table 2, the mutations in the NS3 protease genes from clinical samples are quite diverse, which suggests that polymorphisms naturally exist in HCV, since all these samples were from treatment-naive sera. Therefore, the advantage of judging resistance regardless of mutations is quite important for testing drug susceptibility of HCV in real samples.
Major differences have been found between HCV genotypes in their susceptibilities and resistance development to telaprevir (22, 23). Our results here also showed that all the in vitro-synthesized proteases derived from HCV subtype 2a samples were less susceptible to telaprevir than the proteases derived from HCV subtype 1b samples, which is consistent with a previous finding (22). The power of the in vitro-synthesized protease assay to differentiate the resistance levels between HCV genotype 2 and HCV genotype 1 may indicate that the in vitro-synthesized protease assay is applicable for testing resistance variants in clinical sera.
Something else to note is that the vi/v0 ratios of proteases within the same subtype varied slightly (Fig. 4), which means different susceptibilities to telaprevir and might be due to the different mutations in the protease genes (as shown in Table 2). It may be interesting to study whether the susceptibility differences would affect the time needed to treat the HCV infections and resistance development during the therapy.
Besides telaprevir, another NS3/4A protease inhibitor, boceprevir, was approved by the FDA for the treatment of patients with chronic HCV genotype 1 infection. By using the same procedure and replacing telaprevir with boceprevir, the in vitro-synthesized protease assay can be easily adapted to test the susceptibility of HCV to boceprevir. When we compared the vi/v0 ratios of the in vitro-synthesized NS3 proteases with 100 nM boceprevir (see Fig. S4 in the supplemental material) with those with 100 nM telaprevir (Fig. 4), boceprevir showed generally higher potency than telaprevir for inhibiting the proteases of genotypes 1b and 2a, which is in agreement with previous research (24).
In conclusion, the novel in vitro-synthesized protease assay provides a fast way for phenotypic susceptibility testing of HCV to protease inhibitors. Initial studies showed that this approach has the potential to test susceptibility directly from patient sera. Because the PIs have not been used in China to treat HCV patients, we could not obtain clinical resistant HCV genotype 1 samples. Therefore, further validation of the in vitro-synthesized protease assay is needed for testing clinical resistant strains. Although more studies are needed to further validate the method, we believe the in vitro-synthesized protease assay will be quite useful for providing a guide to the treatment of HCV infections and monitoring PI resistance development during antiviral therapy.
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the financial support from the National Natural Science Foundation of China (grants 21105114 and 21075131) and the Key Laboratory on Emerging Infectious Diseases and Biosafety in Wuhan, China.
Footnotes
Published ahead of print 22 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.03257-13.
REFERENCES
- 1.Lavanchy D. 2011. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 17:107–115. 10.1111/j.1469-0691.2010.03432.x [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH. 2002. Course and outcome of hepatitis C. Hepatology 36:S21–S29. 10.1053/jhep.2002.36227, [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958–965. 10.1016/S0140-6736(01)06102-5 [DOI] [PubMed] [Google Scholar]
- 4.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975–982. 10.1056/NEJMoa020047 [DOI] [PubMed] [Google Scholar]
- 5.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R, Investigators HR. 2011. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1207–1217. 10.1056/NEJMoa1009482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP, Investigators S. 2011. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1195–1206. 10.1056/NEJMoa1010494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S, Team AS. 2011. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364:2405–2416. 10.1056/NEJMoa1012912 [DOI] [PubMed] [Google Scholar]
- 8.McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ, Team PS. 2009. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N. Engl. J. Med. 360:1827–1838. 10.1056/NEJMoa0806104 [DOI] [PubMed] [Google Scholar]
- 9.European Association for the study of the Liver. 2012. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J. Hepatol. 57:167–185. 10.1016/j.jhep.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 10.Clark VC, Peter JA, Nelson DR. 2013. New therapeutic strategies in HCV: second-generation protease inhibitors. Liver Int. 33(Suppl. 1):80–84. 10.1111/liv.12061 [DOI] [PubMed] [Google Scholar]
- 11.Bartels DJ, Zhou Y, Zhang EZ, Marcial M, Byrn RA, Pfeiffer T, Tigges AM, Adiwijaya BS, Lin C, Kwong AD, Kieffer TL. 2008. Natural prevalence of hepatitis C virus variants with decreased sensitivity to NS3.4A protease inhibitors in treatment-naive subjects. J. Infect. Dis. 198:800–807. 10.1086/591141 [DOI] [PubMed] [Google Scholar]
- 12.Kieffer TL, Kwong AD, Picchio GR. 2010. Viral resistance to specifically targeted antiviral therapies for hepatitis C (STAT-Cs). J. Antimicrob. Chemother. 65:202–212. 10.1093/jac/dkp388 [DOI] [PubMed] [Google Scholar]
- 13.Sarrazin C, Kieffer TL, Bartels D, Hanzelka B, Muh U, Welker M, Wincheringer D, Zhou Y, Chu HM, Lin C, Weegink C, Reesink H, Zeuzem S, Kwong AD. 2007. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 132:1767–1777. 10.1053/j.gastro.2007.02.037 [DOI] [PubMed] [Google Scholar]
- 14.Curry S, Qiu P, Tong X. 2008. Analysis of HCV resistance mutations during combination therapy with protease inhibitor boceprevir and PEG-IFN alpha-2b using TaqMan mismatch amplification mutation assay. J. Virol. Methods 153:156–162. 10.1016/j.jviromet.2008.07.020 [DOI] [PubMed] [Google Scholar]
- 15.Thomas XV, de Bruijne J, Sullivan JC, Kieffer TL, Ho CK, Rebers SP, de Vries M, Reesink HW, Weegink CJ, Molenkamp R, Schinkel J. 2012. Evaluation of persistence of resistant variants with ultra-deep pyrosequencing in chronic hepatitis C patients treated with telaprevir. PLoS One 7:e41191. 10.1371/journal.pone.0041191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca-Coronado S, Escobar-Gutierrez A, Ruiz-Tovar K, Cruz-Rivera MY, Rivera-Osorio P, Vazquez-Pichardo M, Carpio-Pedroza JC, Ruiz-Pacheco JA, Cazares F, Vaughan G. 2012. Specific detection of naturally occurring hepatitis C virus mutants with resistance to telaprevir and boceprevir (protease inhibitors) among treatment-naive infected individuals. J. Clin. Microbiol. 50:281–287. 10.1128/JCM.05842-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X, Bae A, Liu S, Yang H, Sun SC, Harris J, Delaney W, Miller M, Mo H. 2009. Development of a replicon-based phenotypic assay for assessing the drug susceptibilities of HCV NS3 protease genes from clinical isolates. Antiviral Res. 81:166–173. 10.1016/j.antiviral.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 18.Sheaffer AK, Lee MS, Hernandez D, Chaniewski S, Yu F, Falk P, Friborg J, Zhai G, McPhee F. 2011. Development of a chimeric replicon system for phenotypic analysis of NS3 protease sequences from HCV clinical isolates. Antivir. Ther. 16:705–718. 10.3851/IMP1825 [DOI] [PubMed] [Google Scholar]
- 19.Imhof I, Simmonds P. 2010. Development of an intergenotypic hepatitis C virus (HCV) cell culture method to assess antiviral susceptibilities and resistance development of HCV NS3 protease genes from HCV genotypes 1 to 6. J. Virol. 84:4597–4610. 10.1128/JVI.02698-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pei R, Zhang X, Xu S, Meng Z, Roggendorf M, Lu M, Chen X. 2012. Regulation of hepatitis C virus replication and gene expression by the MAPK-ERK pathway. Virol. Sin. 27:278–285. 10.1007/s12250-012-3257-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandres-Saune K, Deny P, Pasquier C, Thibaut V, Duverlie G, Izopet J. 2003. Determining hepatitis C genotype by analyzing the sequence of the NS5b region. J. Virol. Methods 109:187–193. 10.1016/S0166-0934(03)00070-3 [DOI] [PubMed] [Google Scholar]
- 22.Imhof I, Simmonds P. 2011. Genotype differences in susceptibility and resistance development of hepatitis C virus to protease inhibitors telaprevir (VX-950) and danoprevir (ITMN-191). Hepatology 53:1090–1099. 10.1002/hep.24172 [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Labrador FX, Moya A, Gonzalez-Candelas F. 2008. Mapping natural polymorphisms of hepatitis C virus NS3/4A protease and antiviral resistance to inhibitors in worldwide isolates. Antivir. Ther. 13:481–494 [PubMed] [Google Scholar]
- 24.Silva MO, Treitel M, Graham DJ, Curry S, Frontera MJ, McMonagle P, Gupta S, Hughes E, Chase R, Lahser F, Barnard RJ, Howe AY, Howe JA. 2013. Antiviral activity of boceprevir monotherapy in treatment-naive subjects with chronic hepatitis C genotype 2/3. J. Hepatol. 59:31–37. 10.1016/j.jhep.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 25.de Boer HA, Comstock LJ, Vasser M. 1983. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc. Natl. Acad. Sci. U. S. A. 80:21–25. 10.1073/pnas.80.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.