Abstract
PURPOSE
The purpose was to determine the interchangeability of ultrasound biomicroscopy (UBM) and anterior segment optical coherence tomography (AS-OCT) for corneal opacity depth measurement.
PATIENTS AND METHODS
Twenty-six eyes of 26 consecutive patients with corneal opacities were examined by both AS-OCT and UBM. Corneal thickness and corneal opacity depth was measured and compared. The interchangeability was determined by Bland-Altman plotting.
RESULTS
The difference in full corneal thickness and corneal opacity depth between OCT and UBM was 5±7.5 μm and −1±7.5 μm respectively. There were strong correlations and no significant differences between the paired parameters (all r>0.99, P<0.01). The limits of agreement were 5±14 μm for corneal thickness and −1±14.8 μm for corneal opacity depth.
CONCLUSION
AS-OCT and UBM maybe used interchangeably for measuring both full corneal thickness and corneal opacity depth in patients with corneal opacity.
Keywords: Ultrasound Biomicroscopy, Anterior Segment Optical Coherence Tomography, Corneal Opacity, Corneal Imaging, Cornea
INTRODUCTION
Two imaging modalities are currently available for cross-sectional corneal imaging and analysis in the presence of corneal opacity. The first introduced device, ultrasound biomicroscopy (UBM), uses ultra-high frequency acoustic waves. It has been applied in penetrating keratoplasty for patients with corneal scars.1 This is a contact modality that requires topical anesthesia and a water bath with the patient in a supine position. Another modality, anterior segment optical coherence tomography (AS-OCT), uses a near-infrared laser as its source. AS-OCT was demonstrated to be capable of clearly defining the rim of corneal opacity and thus measuring corneal opacity depth before choosing lamellar keratoplasty and phototherapeutic keratectomyin a non-contact and non-invasive way.2,3
AS-OCT and UBM have produced comparable results for measuring central corneal thickness in normal eyes.4 Based on the nature of acoustics and lasers, the unknown refractive index for pathologically altered corneal tissue (e.g., increasing corneal hydration with aqueous humor) would lead to a decrease in the refractive index and thus produce variability in measurements.5 Thus, it is unclear whether we can still use the interchangeable results as we do with normal subjects when the cornea is opaque. Therefore, the present study employed a comparative study to determine the interchangeability between AS-OCT and UBM for assessing corneal opacity depth as well as full corneal thickness for choosing the appropriate type of surgery to remove opaque corneal tissues.6
PATIENTS AND METHODS
Patients
In this study, 26 eyes of 26 consecutive patients who had corneal opacities were prospectively enrolled and examined by both AS-OCT and UBM before surgery from July 2007 to June 2008. Nine of the patients had lamellar keratoplasty (LKP) under the guidance of corneal imaging, and they were re-examined by AS-OCT two weeks after surgery. Informed consent was obtained from every participant before examination. This study was conducted at the Corneal Division of Zhongshan Ophthalmic Center at Sun Yat-sen University and approved by the Institutional Review Board.
Examinations with AS-OCT and UBM
Both examinations were performed by the same experienced operator (CXY). The OCT system (Visante AS-OCT, model 1000, Carl Zeiss Meditec International, Dublin, USA) in this study operated using an infrared light with a wavelength of 1310 nm as its light source. Both “High Resolution Quad” and “Anterior Segment Single” scan modes with resolutions of 18 μm (tissue distance equivalent) axially and 60 μm laterally were performed for each eye with fixation monitored. The high-resolution scan produceda 10 mm-long line that contained 512 A scans with a scan-acquisition time of 0.25 seconds per line, whereas the anterior segment scan produced a 16 mm-long line that contained 256 Ascans with a scan-acquisition time of 0.125 seconds per line. During examinations, patients seated with the head well positioned on the chin-and-forehead rest, looked at the center of an internal-fixation target. External fixation was used if the patient had vision impairment in the examined eye. The operator adjusted the infrared light on the center of the cornea and the pathological tissue and the noise level to obtain a clear image. A vertical flare in the central-corneal zone enabled the identification of the corneal vertex.
An ultrasound biomicroscope (UBM) (Paradigm Plus Model P45, Paradigm Medical Industries, USA) with a 50-MHz transducer, providing a resolution of 50 μm and a view field of 5 mm×5 mm, was used in this study. While patients were in the supine position, each eye was anesthetized with one drop of 0.5% proparacaine hydrochloride (Alcaine eye drops, Alcon Inc., Fort Worth, Texas, USA). A suitable eyecup was inserted into the conjunctival sac to facilitate the use of a coupling solution (normal saline). The transducer was immersed in the solution and positioned properly to ensure a clear corneal image on the screen. Patients were asked to look at a fixation target on the ceiling with the other eye. Four scan directions were taken in accordance with OCT scans (the same position and direction). Each eye was examined in its axial section with the probe kept perpendicular to the corneal surface. Perpendicularity was ensured by adjusting the transducer head until the brightest reflection lines from the various corneal layers were observed in real time and with the help of an iris plane.
Measurements and Analysis of AS-OCT and UBM Images
Both AS-OCT and UBM images of horizontal and vertical directions through the corneal vertex were chosen for analysis. AS-OCT images were analyzed and the parameters were measured with built-in software. To analyze the UBM images, we developed a computer program based on Visual Basic C++ computer language to register the UBM images. The program provided tools for adjusting the signal-to-noise ratio and measuring the thickness as well as distance, and it showed a wave of signal amplitude along any vertical or horizontal line on the image (Figure 1). Each axial scan was sampled digitally at 512 points, giving an axial pixel size of 9.8 μm in air. The human operator used the computer cursor to identify the deepest rim of the corneal scar with the help of signal peaks. The computer then calculated the thickness automatically using the designed program. The thicknesses of the full corneas and corneal scars were measured perpendicular to the corneal surface by the programmer automatically. Both OCT and the UBM images were analyzed by two of the authors (ZSY and WCX) independently, and then the mean values from two analysts were used as the measurement. Depending on the location of corneal opacities, a horizontal or vertical scan line was chosen for analysis. For determining the thickness of the deepest corneal opacity in the mid-peripheral cornea, the identical of the measurement points was ensured by both the distance from visual axis to the selected point and the corneal opacity profiles. The distance from visual axis to the selected point with a clear rim of corneal opacity was determined in UBM images first and then in OCT images. For those eyes in which a clear opacity rim could not be defined in the central cornea by both devices, a para-central corneal point with a clear rim was selected for measurement comparison. For those eyes that had undergone LKP, the thickness of the (presumed healthy) deep cornea with normal light reflectivity pre-operatively and the residual native corneal stroma post-operatively were measured at the same point.
Figure 1. Illustration to define the corneal vertex and measure corneal opacity depth by the program for analyzing UBM images.
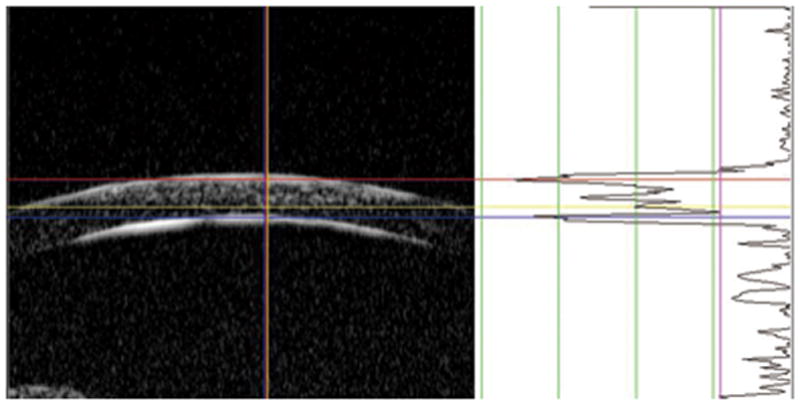
The right part of the figure presents the A-scan signal along the vertical line of the red crossing lines in the B-scan. The signal intensity was scaled to 256 steps according to its gray values. The purple line across the wave was set to judge the level of background noise signals. Based on the signal peaks of A-scan, a red line is set to measure the central anterior boundary and a blue line is set to the central posterior corneal boundary at vertex. A yellow line is measuring the central opacity depth with signal hyper-reflection. The distances between the color lines are automatically produced by the program.
Statistical Analysis
For data analysis, SPSS for Microsoft Windows software (version 13.0, SPSS Inc., Chicago, IL, USA) was used. The data were presented as mean ± standard deviation (SD). The mean values of full corneal thickness and corneal opacity depth measured by AS-OCT and UBM were compared using a paired two-sided t-test. In addition, the Pearson correlation coefficient (r) was also calculated to assess the correlation between the paired parameters measured with both imaging modalities. The limits of agreement (LoAs) were analyzed using a Bland-Altman plot for checking the interchangeability of these two imaging techniques.7 The LoAs were defined as mean ± 1.96 SD of the differences in measurements between the two methods. If the limits were found to be clinically relevant, the two methods could not be used interchangeably. A P value less than 0.05 was considered statistically significant.
RESULTS
The clinical features of the study population are listed in Table 1. Patient ages ranged from 14 to 70 (mean 35.2±15.3) years old. Complete cross-sectional images with clear anterior and posteriorcorneal boundaries could be obtained in all eyes by both UBM and AS-OCT, except in one eye with an intra-stromal corneal foreign body by AS-OCT (See Figure 2). The rim of corneal opacity could be clearly defined in 22 of 26 eyes at the central cornea and in all eyes at the para-central cornea by both devices, while it could not be noted in four eyes with AS-OCT and one eye with UBM. The intensity of signal reflection did not show a marked difference between corneal nebula, macula, and leucoma with either imaging technology.
Table 1.
Summary of clinical features in cases with corneal opacities (N=26).
| Characteristic | Result |
|---|---|
| Gender, (n) | |
| Male | 19 |
| Female | 7 |
| Eyes, (n) | |
| Right | 16 |
| Left | 10 |
| Cause of Corneal Opacity | |
| Corneal scar secondary to bacterial ulcer | 5 |
| Corneal scar secondary to herpes simplex keratitis | 7 |
| Corneal scar secondary to chemical burns | 8 |
| Stromal corneal dystrophy | 3 |
| Lamellar corneal graft rejection | 2 |
| Corneal foreign body | 1 |
| Intraocular pressure, (Mean±SD) (mmHg) | 14.0±3.5 |
| Degree of Corneal Opacity | |
| Nebula | 5 |
| Macula | 10 |
| Leucoma | 11 |
| With corneal neovascularization, n (%) | 10 (40%) |
Nebula means that the iris was clear seen through a corneal opacity, Macula was unclear, and Leucoma unable to be seen.
Figure 2. AS-OCT and UBM for imaging intra-stromal corneal foreign bodies (a bamboo spur).
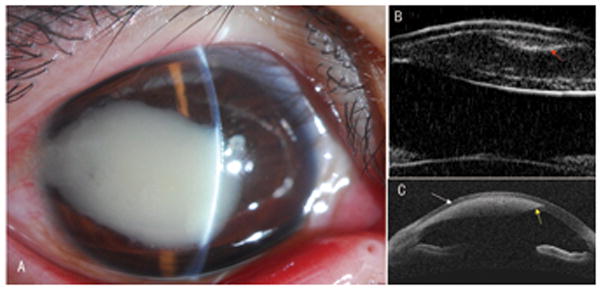
A: A 20-year-old man had a dense corneal leukoma secondary to an infiltrative reaction of an intra-stromal corneal foreign body in his right eye.
B: A UBM image showed both boundaries of the cornea and a clear curved foreign body with hyper-reflection (red arrow) beneath the anterior face of the corneal lesion. The corneal lesion presented with two surfaces in the cornea.
C: An OCT image showed the anterior face of an oval shadow with focal strong hyper-reflection inside in the corneal stroma (white arrow) and just a small part of the posterior face at the periphery (yellow arrow).
For patients with corneal opacity due to herpes simplex keratitis, chemical burns, and lamellar graft rejection, AS-OCT showed a sharper interface between the opaque corneal tissues and the adjacent normal corneal tissues than UBM in the off-center area. However, in the center, back-scattered light from deep opaque corneal tissues in the AS-OCT images usually merged together with the hyper-reflection from the normal posterior cornea when anterior corneal opacity was very close to the Descemet’s membrane. The AS-OCT images provided more details than the UBM images. For example, some granules and discrete lines with strong signal reflection identical tokeratic precipitates and corneal neovascular vessels were shown using AS-OCT but not UBM.
For the patient with corneal opacity due to granular corneal dystrophy, clear spots or granular masses of hyper-reflection in the cornea (even on the posterior boundary) were shown using AS-OCT but not UBM. For patients with lattice corneal dystrophy, clear spots or lines with stronger hyper-reflection in the central stroma with diffuse signal hyper-reflection were noted in the AS-OCT images without a clear distance to the posterior boundary. However, a dark narrow band between the stromal area with diffuse and irregular hyper-reflection and the posterior corneal boundary was shown in the UBM images. For advanced-stagemacular corneal dystrophy, both AS-OCT and UBM showed diffuse signal hyper-reflection in the corneal stroma without a clear distance to the posterior boundary (See Figure 3).
Figure 3. AS-OCT and UBM for imaging stromal corneal dystrophy.
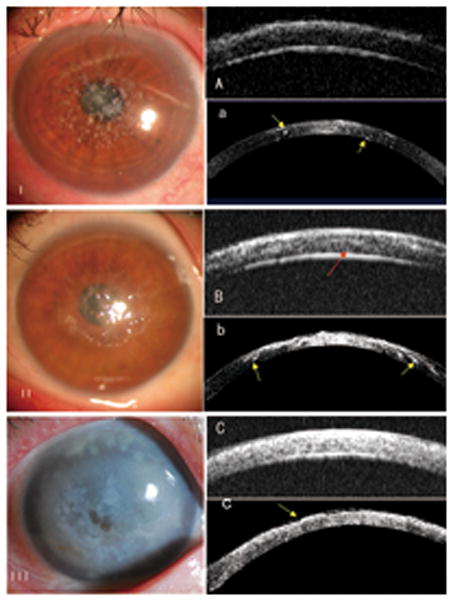
I: Many spots of hyper-reflection in the cornea were shown in the left eye of a 47-year-old woman with granular corneal dystrophy using AS-OCT (yellow arrows, a), while just diffuse hyper-reflection of the same intensity was shown using UBM (A).
II: Cloudy hyper-reflection with lines was noted in the left eye of a 47-year-old woman with lattice corneal dystrophy by AS-OCT, but no clear distance to the posterior boundary was noted (b). However, a dark narrow band between the diffuse hyper-reflection and the posterior corneal boundary was observed in the UBM images (red arrow, B).
III: For a 69-year-old woman with advanced-stage macular corneal dystrophy, both the AS-OCT image (c) and UBM image (C) showed diffuse signal hyper-reflection in the stroma without a clear distance to the posterior boundary in her right eye. However, the OCT image showed an uneven anterior surface of the cornea (yellow arrow, c).
The differences in full corneal thickness and corneal opacity depth at the deepest corneal opacity measured by AS-OCT and UBM in 25 of 26 eyes were 5±7.1 μm (95% CI: 2.3~8.2) and −1±7.5 μm (95%CI: −3.9~2.3) respectively. OCT produced greater readings for corneal thickness (t=3.665, P=0.001) while almost the same readings for corneal opacity depth measurements (t=−0.53, P=0.601). A strong correlation was noted in the paired parameter. The Pearson coefficient was higher than 0.99 for all paired parameters (P<0.001, See Table 2). In terms of the Bland-Altman analysis, the ranges of agreement, defined as 1.96 SD, were 14 μm (1.33% of the average reading) for full corneal thickness and 14.8 μm (2.07% of the average reading) for corneal opacity depth (See Figures 4 and 5). Considering the accuracy of the axial scan in the AS-OCT (±15 μm over 6 mm of tissue distance equivalent),9 the resolution of UBM of 50 μm and the properties of the magnitudes, the above LoAs were acceptable.
Table 2.
Comparison of measurement results between AS-OCT and UBM methods
| AS-OCT (μm) | UBM (μm) | Pairs (n) | Pearson Correlation | Paired t test | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| r | P | t | P | ||||
| Central Corneal Thickness | 539.6±125.4 * | 534.4±126.3 | 25 | 0.998 | 0.000 | 3.665 | 0.001 |
| Corneal Opacity Depth | 364.9±147.9 | 365.7±145.3 | 25 | 0.999 | 0.000 | −0.530 | 0.601 |
AS-OCT=anterior segment coherence tomography; UBM=ultrasound biomicroscopy;
Data were presented as Mean±standard deviation.
Figure 4.
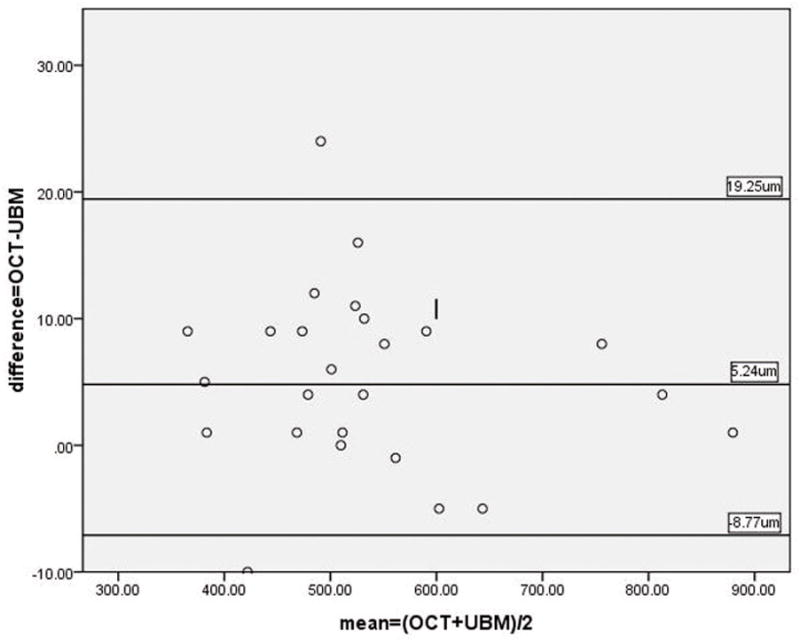
Bland-Altman plotting for the measurement of central corneal thickness between AS-OCT and UBM.
Figure 5.
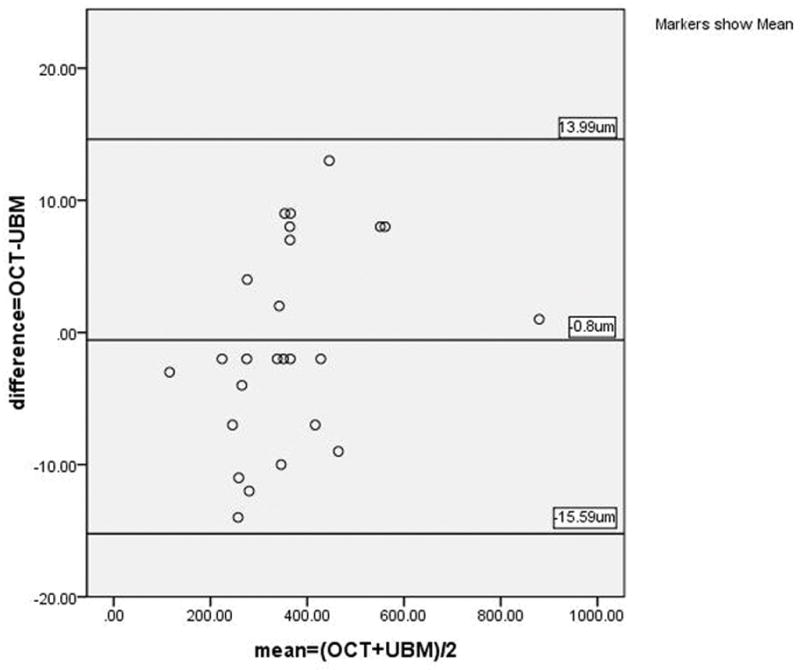
Bland-Altman plotting for the measurement of corneal opacity depth between AS-OCT and UBM.
For the eyes that under went LKP, the thickness of the residual recipient cornea post-operatively was 287±199 μm, which was 18±25 μm thinner than the thickness of the deep corneal tissues (305±192 μm) with normal signal reflection pre-operatively (n=9, t=3.128, P=0.006). This therefore indicated that the presumed overlying corneal opacities were completely removed during surgery, and this was further proved by slit lamp biomicroscopy postoperatively.
DISCUSSION
Both UBM and OCT utilize a similar imaging principle that employs back-scattered signals reflected from different layers of tissues and then reconstructs structural images. Corneal opacity exhibits signal hyper-reflectivity in both OCT and UBM imaging. Theoretically, UBM imaging reaches an axial and transverse resolution of 50 μm, while AS-OCT provides an axial resolution of 18 μm and a transverse resolution of 60 μm. In fact, they did not show sufficient resolution to visualize Bowman’s or Descemet’s membranes, both of which are approximately 10~15 μm thick. The AS-OCT images showed more detailed information and sharper rims of corneal opacity than the UBM images in this study, especially in para-central cornea.
In the current and reported studies, the normal central corneal stroma was observed as a reverse wedge-shaped pattern in OCT with a posterior increase of central reflectivity.2,8 This pattern of signal reflection renders OCT systems unable to differentiate the posterior rim of corneal opacity from the normal posterior corneal stromain the central corneal when the opacity is very close to Descemet’s membrane. Our results showed that UBM could not always detect this small difference either in this condition. Maybe a newly developed full range spectral domain OCT system will show the Descemet’s membrane and the small difference in contour for imaging eyes with corneal opacity.9 However, due to its acoustic wave nature, UBM was able to bypass a dense opacity and then image all of the cornea layers, including a foreign body in the corneal stroma.
According to the user’s manual from manufactory, OCT measures thinner than ultrasound pachymetry for corneal thickness in 63 normal eyes (−15.0±13 μm).10 OCT measurement was also well correlated with UBM measurements for central corneal thickness in normal eyes.4 However, the authors did not supply the difference and employ Bland-Altman analysis to investigate the interchangeability between these two devices. Meanwhile, a difference of 16.11 μm was thought to be an acceptable LoA between the Visante OCT system and the Artemis 2 ultrasound scanning system (50 MHz) for central corneal thickness measurements in 20 normal eyes.11 According to our limited knowledge, there is no reported study in PubMed for comparison in corneal thickness especially corneal opacity depth between OCT and UBM in eyes with corneal opacity. When comparing Visante OCT with ultrasound pachymetry (50 MHZ probe) for corneal thickness in eyes with corneal opacities, a difference of 13.6±38 μm was reported.2 According to the calculation, the LoAs (1.96 SD) should be 74 μm. This difference was clinically relevant and unacceptable for determining interchangeability of AS-OCT and ultrasound pachymetry, though the authors believed the results were highly correlated. In this study, we analyzed the interchangeability of Visante OCT and Paradigm UBM for measuring corneal thickness as well as corneal opacity depth in eyes with corneal opacities. Our results indicated that the interchangeability of these two systems is clinically acceptable for both parameters. OCT gave a slightly greater reading than UBM for corneal thickness measurement in eyes with corneal opacity as in normal eyes of the above studies, and the same reading for corneal opacity depth.
The clear recipient corneal beds post-operatively were 18±25 μm thinner than those of (presumed healthy) deep corneas pre-operatively, we could roughly judge the accuracy of AS-OCT measurements for corneal opacity depth. However, we could not determine whether OCT or UBM was more precise for measuring corneal opacity depth due to the lack of a gold-standard method for comparison and a lack of any topography-guided ablation with an investigational laser system for the precise removal of corneal opacity in this study. Light micrographs of fixed tissue might be considered a standard reference for comparison. However, this method also has possible limitations due to high individual variability and possible artificial changes in tissue morphology.12 Although overall changes in corneal thickness and the marked pathological changes were easily determined by OCT or UBM, the precise location of intra-corneal tissue boundaries and fine adjacent substructures was difficult to define, especially in the UBM images. The interpretation of imaging features is based on presumptive correlations with known clinical features. Moreover, the assessment of optical density and reflectivity is qualitative and insufficient to distinguish tissue types. Although the reproducibility of both instruments for measuring central corneal thickness was reported excellent in normal eyes,13,14 we did not investigate the reproducibility of both devices for imaging opaque corneas in this study.
In conclusion, both AS-OCT and UBM could offer an objective way of recording and measuring the depth of corneal opacities. AS-OCT and UBM’s corneal structure imaging abilities have significant applications for anterior lamellar keratoplasty, although they are unable to provide cellular-level resolution or to define the rim of deep corneal opacity satisfactorily. The Visante AS-OCT system and Paradigm ultrasound system may be used interchangeably for measuring both corneal thickness and corneal opacity depth in eyes with corneal opacity. As a non-contact imaging device, AS-OCT allows for safe and comfortable in vitro imaging in the early post-operative period.
Acknowledgments
This study was partly supported by the “Fundamental Research Funds for the Central Universities” in China.
Footnotes
Proprietary Interests: Dr. D. Huang receives a royalty from an optical coherence tomography-related patent licensed to Carl Zeiss Meditec by the Massachusetts Institute of Technology. Dr. D. Huang has a significant financial interest in Optovue, a company that may have a commercial interest in the results of this research and technology. This potential individual conflict of interest has been reviewed and managed by the Oregon Health & Science University. Other authors have no proprietary interest in the topic of this manuscript.
Prior Presentations: The manuscript was not presented at any meeting.
Contributor Information
Shi-you Zhou, Zhongshan Ophthalmic Center at Sun Yat-sen University, The State Key Laboratory of Ophthalmology, Guangzhou, China 510060.
Chun-xiao Wang, Zhongshan Ophthalmic Center at Sun Yat-sen University, The State Key Laboratory of Ophthalmology, Guangzhou, China 510060.
Xiao-yu Cai, Zhongshan Ophthalmic Center at Sun Yat-sen University, The State Key Laboratory of Ophthalmology, Guangzhou, China 510060.
David Huang, Casey Eye Institute, Oregon Health & Science University, Portland, Oregon, USA.
Yi-zhi Liu, Email: gdeyeb@yahoo.com.cn, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China 510060, Tel: 8620-87330294, Fax: 8620-87333271.
References
- 1.Milner MS, Liebmann JM, Tello C, Speaker MG, Ritch R. High-resolution ultrasound biomicroscopy of the anterior segment in patients with dense corneal scars. Ophthalmic Surg. 1994;25:284–287. [PubMed] [Google Scholar]
- 2.Khurana RN, Li Y, Tang M, Lai MM, Huang D. High-speed optical coherence tomography of corneal opacities. Ophthalmology. 2007;114:1278–1285. doi: 10.1016/j.ophtha.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Lim LS, Aung HT, Aung T, Tan DT. Corneal imaging with anterior segment optical coherence tomography for lamellar keratoplasty procedures. Am J Ophthalmol. 200;145:81–90. doi: 10.1016/j.ajo.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Dada T, Sihota R, Gadia R, Aggarwal A, Mandal S, Gupta V. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for assessment of the anterior segment. J Cataract Refract Surg. 2007;33:837–840. doi: 10.1016/j.jcrs.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Maurice DM. The structure and transparency of the cornea. J Physiol. 1957;136:263–268. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81:184–188. doi: 10.1136/bjo.81.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 8.Wirbelauer C, Scholz C, Hoerauf H, et al. Untersuchungen der Hornhaut mittels optischer Kohärenztomographie. Ophthalmologe. 2001;98:151–156. doi: 10.1007/s003470170176. [DOI] [PubMed] [Google Scholar]
- 9.Hall RC, Mohamed FK, Htoon HM, Tan DT, Mehta JS. Laser in situ keratomileusis flap measurements: Comparison between observers and between spectral-domain and time-domain anterior segment optical coherence tomography. Cataract Refract Surg. 2011;37:544–551. doi: 10.1016/j.jcrs.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 10.Carl Zeiss Meditec Inc. Visante OCT user manual English. 2006. 01, Part number: 60899–1, Rev. A. Specifications 9-1, Appendix D-2. [Google Scholar]
- 11.Piñero DP, Plaza AB, Alió JL. Anterior segment biometry with 2 imaging technologies: very-high-frequency ultrasound scanning versus optical coherence tomography. J Cataract Refract Surg. 2008;34:95–102. doi: 10.1016/j.jcrs.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Wirbelauer C, Winkler J, Bastian GO, Häberle H, Pham DT. Histopathological correlation of corneal diseases with optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2002;240:727–734. doi: 10.1007/s00417-002-0518-3. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed S, Lee GK, Rao SK, et al. Repeatability and reproducibility of pachymetric mapping with Visante anterior segment-optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:5499–5504. doi: 10.1167/iovs.07-0591. [DOI] [PubMed] [Google Scholar]
- 14.Urbak SF, Pedersen JK, Thorsen TT. Ultrasound biomicroscopy. II. Intraobserver and interobserver reproducibility of measurements. Acta Ophthalmol Scand. 1998;76:546–549. doi: 10.1034/j.1600-0420.1998.760507.x. [DOI] [PubMed] [Google Scholar]


