ABSTRACT
Foreskin is the principal site of heterosexual HIV-1 infection in men. However, little is known about HIV-1-specific immune responses or inflammation in foreskin. To the best of our knowledge, no previous studies have assessed immune responses to candidate HIV-1 vaccines in foreskin. Using the rhesus monkey model, we show that intramuscular immunization with adenovirus serotype 26 and 35 vectors expressing SIV antigens elicited durable SIV Gag-specific CD4+ and CD8+ T cell responses in foreskin that were detectable for more than 1 year following vaccination. Gag-specific CD4+ and CD8+ T cells were also detectable in foreskin of SIV- and SHIV-infected animals and were at least comparable in magnitude to those in peripheral blood. However, unlike peripheral blood T cells, the majority of foreskin T cells exhibited transitional memory or effector memory phenotype and expressed higher levels of the activation markers CD69, HLA-DR, and CCR5, although vaccination did not further enhance foreskin CD4+ T cell activation. These findings suggest that systemic vaccination strategies can elicit potentially important SIV-specific cellular immunity in foreskin. Further characterization of vaccine-elicited immune responses and inflammation in foreskin is warranted.
IMPORTANCE We demonstrate here the induction of SIV-specific cellular immune responses in foreskin by adenovirus serotype 26 and 35 vaccine vectors. Foreskin T cells were more activated than peripheral blood T cells, but foreskin T cells were not further activated by vaccination. These findings suggest that alternative serotype adenovirus vectors induce potentially important immune responses in foreskin.
INTRODUCTION
Foreskin is the principal site of heterosexual HIV-1 infection in men (1–7), and medical male circumcision has been shown to reduce the risk of HIV-1 acquisition (8–10). However, little is known about HIV-1-specific immune responses in foreskin. To date, only one study has assessed simian immunodeficiency virus (SIV)-specific immune responses in foreskin of SIV-infected rhesus monkeys (11), and the potential induction and phenotype of SIV- or HIV-1-specific immune responses in foreskin following vaccination has not previously been explored. In the present study, we explore the hypothesis that vaccination may elicit potentially important cellular immune responses in foreskin.
It has been shown that CD4+ T cells in foreskin in humans are highly activated, potentially contributing to the risk of HIV-1 infection by increased HIV-1 target cells (12). It is therefore important to assess both antigen-specific immune responses and inflammation in foreskin following vaccination. Previous studies have shown that vaccination of rhesus monkeys with adenovirus serotype 26 (Ad26)-based SIV vaccines elicited robust and durable SIV-specific CD4+ and CD8+ T cell responses at gastrointestinal and cervicovaginal mucosal sites (13). Unlike peripheral blood, these mucosal T cell responses were primarily transitional memory (TM) and effector memory (EM) phenotype (13). Moreover, it is possible that vaccine-elicited mucosal immune responses may have contributed to protective efficacy afforded by these vaccines against infection following mucosal SIV challenges (14). Whether similarly protective immune responses can be elicited by vaccines in foreskin is currently unknown.
Using the rhesus monkey model, we investigated whether intramuscular (i.m.) vaccination with Ad26 and Ad35 vectors expressing SIV Gag, Pol, and Env immunogens would elicit SIV-specific T cells in foreskin, and we compared the phenotype and activation status of foreskin and peripheral blood T cells in vaccinated, infected, and naive animals. We show that immunization with Ad35/Ad26-based SIV vaccines elicited durable and multifunctional SIV-specific T cells in foreskin. Furthermore, we confirm that foreskin T cells show higher levels of immune activation compared to peripheral blood T cells, but that this cellular activation was not further increased by vaccination. This is the first demonstration of vaccine-elicited SIV-specific T cells in foreskin and suggests that systemic HIV-1 and SIV vaccination strategies can elicit the potentially important adaptive immune responses in this key tissue compartment.
MATERIALS AND METHODS
Animals, immunizations, and infection.
A total of 45 adult male Indian origin rhesus monkeys (Macaca mulatta) were housed in a Biosafety Level 2 (BSL-2) facility at the New England Primate Research Center in Southborough, MA. All studies were approved by the Harvard Medical School Institutional Animal Care and Use Committee. Priming immunizations involved i.m. injection of 3 × 1010 virus particles (vp) of replication-incompetent recombinant Ad35 vectors expressing SIVmac239 Gag, Pol, and Env immunogens (Ad35 Gag/Pol/Env), followed by boosting at week 26 by i.m. injection of 3 × 1010 vp of replication-incompetent recombinant Ad26 vectors expressing SIVmac239 Gag, Pol, and Env (Ad26 Gag/Pol/Env) (n = 16). Additional monkeys were infected intrarectally with SIVsmE660 (SIV, n = 14) or SHIV-SF162P3 (SHIV, n = 8). Animals were divided into three groups: SIV or SHIV infected (n = 22), vaccinated and not infected (n = 16), and sham immunized and not infected (n = 7). Paired peripheral blood and foreskin specimens were obtained from the animals at necropsy approximately 12 months after infection or vaccination for analysis of SIV-specific CD4+ and CD8+ T cell responses, phenotype, and activation status. Sections of foreskin specimens were also evaluated by immunohistochemistry.
To evaluate the acute effects of immunization on peripheral blood and foreskin T cell activation, another cohort of 6 additional animals were immunized i.m. with 3 × 1010 vp of Ad26 Gag/Pol/Env. Day 2 (n = 3) and day 14 (n = 3) postimmunization specimens from peripheral blood and foreskin were obtained from the animals at necropsy for analysis of SIV- and Ad26 vector-specific CD4+ and CD8+ T cell responses, CD4+ T cell phenotype and activation status via multiparameter flow cytometry and immunohistochemistry. The results were compared to those of sham-immunized and uninfected (baseline) animals.
PBMC and foreskin lymphocyte isolation.
Peripheral blood mononuclear cells (PBMC) were isolated from blood samples by Ficoll density gradient sedimentation. Matched foreskin lymphocytes were isolated from foreskin specimens essentially as previously described (13, 15). Briefly, foreskin samples were cut into ∼1-mm pieces and incubated in RPMI 1640 medium containing 10% fetal bovine serum supplemented with 300 U of type IV collagenase (Sigma-Aldrich)/ml and 30 U of DNase I (Sigma-Aldrich)/ml at 37°C with shaking for 30 min. The digested foreskin samples were then homogenized and passed through a 70-μm-pore-size cell strainer (BD Falcon). Cell suspensions were then centrifuged at 695 × g for 25 min on a 35% Percoll gradient (Sigma-Aldrich). Pellets containing the lymphocytes were then collected for use in assays. Approximately one-quarter and three-quarters of the isolated foreskin mononuclear cells (typically 106 cells) were used for immunophenotyping and intracellular cytokine staining (ICS), respectively.
Multiparameter flow cytometry.
Paired PBMC and foreskin lymphocytes were used to evaluate CD4+ and CD8+ T cell phenotype and activation status. Freshly isolated lymphocytes were stained with predetermined titers of fluorophore-tagged monoclonal antibodies (MAbs) against CD3 (SP34; Alexa 700), CD4 (L200; AmyCan), CD8 (SK1; APC-Cy7), CD28 (L293; PerCP-Cy5.5), CD95 (DX2; APC), HLA-DR (G46-6; PE-Cy7), CCR5 (3A9; PE), CCR7 (150503; FITC), and CD69 (TP1.55.3; PE-Texas Red [ECD]). The data were acquired on LSR-II analyzer (BD Biosciences). CD4+ and CD8+ T cell phenotype and activation data were analyzed by using FlowJo version 8 software (Tree Star, Inc.).
ICS assays.
ICS assays for assessment of SIV-specific CD4+ and CD8+ T cell responses in paired PBMC and foreskin specimens were performed essentially as previously described (13). Briefly, PBMC and foreskin lymphocytes were incubated for 6 h at 37°C with media, 10 pg of phorbol myristate acetate (Sigma-Aldrich)/ml, and 1 μg of ionomycin (Sigma-Aldrich)/ml or with 1 μg of pooled SIVmac239 Gag peptides/ml. Cultures contained monensin (GolgiStop; BD Biosciences), brefeldin A (GolgiPlug; BD Biosciences), and 1 μg of anti-CD49d MAb (Clone 9F10; BD Biosciences)/ml. Cells were subsequently stained for CD3, CD4, CD8, CD28, CD95, CD69, gamma interferon (IFN-γ; B27; PE-Cy7), and tumor necrosis factor alpha (TNF-α; MAb11; fluorescein isothiocyanate). All assays were performed blinded. ICS data were analyzed after subtraction of background (media) values. After background subtraction, T cells with IFN-γ or TNF-α responses of ≥0.02% were considered positive. The Boolean gating function of the FlowJo software was used to assess multifunctionality (IFN-γ and/or TNF-α secretion) of the CD4+ and CD8+ T cells.
Ad26 vector-specific CD4+ and CD8+ T cells were quantified in paired blood and foreskin specimens from the vaccinated and sham-immunized animals via ICS as previously described (16). Briefly, isolated PBMC and foreskin lymphocytes were incubated with or without stimulation using a pool of Ad26 hexon peptides (hexon; 6 h stimulation) or empty Ad26 vectors at a multiplicity of infection of 104 (Ad26; overnight stimulation). IFN-γ+ Ad26 vector-specific CD4+ and CD8+ T cells in PBMC and foreskin were subsequently determined via multiparameter flow cytometry.
IHC.
Immunohistochemistry (IHC) of foreskin tissue specimens was performed essentially as previously described (15). Briefly, immunoperoxidase staining for CD3, CD4, HLA-DR, and Langerin was performed on formalin-fixed, paraffin-embedded sections. Sections for CD3 and HLA-DR were deparaffinized and rehydrated, followed by blocking with 3% hydrogen peroxide in phosphate-buffered saline. Sections for CD4 were heated under pressure in 1 mM EDTA-0.05% Tween. Sections for Langerin were heated under pressure in Trilogy solution (Cell Marque Corp., Rocklin, CA) and then blocked using a dual endogenous enzyme block (DakoCytomation, Carpinteria, CA). A wash of Tris-buffered saline followed each step for all sections. After pretreatment, an avidin-biotin block (Invitrogen Corp., Frederick, MD) and then a Dako protein block (Dako) were conducted on all sections. Subsequently, sections were incubated with anti-human CD3 (Dako, polyclonal, 1:600, 30 min at room temperature), anti-human CD4 (Vector Laboratories [Burlingame, CA], monoclonal, 1:40, overnight in refrigerator), anti-human HLA-DR (Novocastra [Buffalo Grove, IL], monoclonal, 1:100, overnight in refrigerator), and anti-human Langerin (Abcam [Cambridge, MA], monoclonal, 1:100, 60 min at room temperature). Slides were then incubated with secondary biotinylated goat anti-rabbit antibody (Vector Laboratories,1:200, 30 min at room temperature) for CD3, biotinylated horse anti-mouse (Vector Laboratories, 1:200, 30 min at room temperature) for HLA-DR and CD4, and Dako dual link system-horseradish peroxidase (Dako) for Langerin. This was followed by a 30-min incubation at room temperature with Vectastain ABC Elite (Vector Laboratories) (CD3, CD4, and HLA-DR). All slides were developed with diaminobenzidine chromogen (Dako) and counterstained with Mayer's hematoxylin (Newcomer Supply, WI). Positive control tissues consisted of skin (Langerin) and lymph node (CD3, CD4, and HLA-DR).
Objective scoring and quantitative image analysis.
We utilized the objective histopathologic scoring criteria to evaluate stained foreskin specimens from the infected, vaccinated, and sham-vaccinated animals as previously described (15). Briefly, cells staining positive for CD3, CD4, HLA-DR, and Langerin within the epithelium and lamina propria of each foreskin specimen were quantitatively enumerated in 10 randomly selected fields at ×400 magnification using an Olympus BX41 microscope equipped with a DP25 camera (Olympus). Averaged cell count per 10 fields was obtained for each foreskin specimen and was given a score of 0 to 3, as defined in Table 1.
TABLE 1.
Objective scoring criteria for foreskina
| Score | Avg. no. of cell types at various sites |
|||||||
|---|---|---|---|---|---|---|---|---|
| CD3+ |
CD4+ |
HLA-DR |
Langerin |
|||||
| IEL | LP | IEL | LP | IEL | LP | IEL | LP | |
| 0 | <1 | <1 | 0–6 | 0–6 | <1 | <1 | ||
| 1 | 1–5 | 1–5 | <10 | <10 | 7–10 | 7–10 | 1–5 | 1–5 |
| 2 | 6–25 | 6–25 | 10–25 | 10–25 | 11–25 | 11–25 | 6–25 | 6–25 |
| 3 | >25 | >25 | >25 | >25 | >25 | >25 | >25 | >25 |
For objective scoring criteria were as follows. Numbers and distribution of cells in foreskin epithelium (intraepithelial lymphocytes [IEL]) and lamina propria (LP) were evaluated in 10 fields at ×400 magnification and assigned a score of 0 to 3 based on the indicated range.
Statistical analyses.
Immunologic parameters between PBMC and foreskin were compared using two-tailed Student t tests (GraphPad Prism 4). P values of <0.05 were considered statistically significant.
RESULTS
Foreskin is highly enriched with memory CD4+ and CD8+ T cells.
We initiated studies by evaluating expression of the costimulatory molecule CD28 and the memory marker CD95 on CD4+ and CD8+ T cells to compare distributions of naive (CD28+ CD95−), central/transitional memory (CM/TM; CD28+ CD95+), and effector memory (EM; CD28− CD95+) T cells between peripheral blood and foreskin (17) in 45 rhesus monkeys that were evaluated 1 year after infection or vaccination. We isolated ∼106 mononuclear cells per foreskin specimen, which is consistent with the relative paucity of mononuclear cells in foreskin in humans (12). We observed decreased naive CD4+ and CD8+ T cells and substantially increased CM/TM CD4+ and CD8+ T cells in foreskin compared to peripheral blood in both infected animals (Fig. 1A) and vaccinated animals (Fig. 1B; P = 0.0011 and P = 0.0038 for comparison of CM/TM CD4+ T cells and CD8+ T cells, respectively, in foreskin versus peripheral blood). This pattern was also observed in sham-immunized animals that were neither infected nor vaccinated (Fig. 1C), suggesting that the observed increase in CM/TM cells in foreskin did not result from infection or vaccination and essentially reflects the low percentage of naive T cells in this tissue compartment. The majority of CD4+ T cells in foreskin were CM/TM phenotype, whereas the CD8+ T cells in foreskin were predominantly EM phenotype (Fig. 1).
FIG 1.
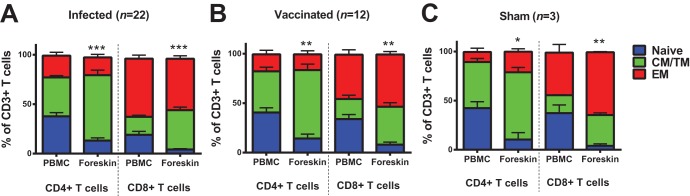
Foreskin is enriched with memory CD4+ and CD8+ T cells. CD4+ and CD8+ T cells were assessed for CD28 and CD95 expression to identify naive (CD28+ CD95−), central/transitional memory (CM/TM; CD28+ CD95+), and effector memory (EM; CD28− CD95+) T cells in paired PBMC and foreskin specimens. The proportions of naive, CM/TM, and EM CD4+ and CD8+ T cells in PBMC and foreskin are presented for infected animals (A), vaccinated animals (B), and sham-vaccinated animals (C). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (two-tailed Student t test) for comparison of the proportion of CM/TM CD4+ or CD8+ T cells between PBMC and foreskin. Error bars represent the standard errors of the mean (SEM).
We next performed more detailed phenotyping in a subset of animals. Assessing expression of the lymph node homing receptor CCR7 among the CD95+ CD4+ and CD95+ CD8+ memory T cells (18), we observed that most of the increase in CM/TM T cells in foreskin was due to an increase in the population of the transitional memory cells (TM; CD28+ CD95+ CCR7−) rather than fully differentiated central memory cells (CM; CD28+ CD95+ CCR7+) (Fig. 2). Collectively, these data suggest that foreskin is mostly populated by the TM and EM CD4+ and CD8+ T cells. These phenotypes are consistent with our previously reported T cell phenotypes at mucosal surfaces, including the gastrointestinal and cervicovaginal mucosa (13).
FIG 2.
The proportion of transitional memory T cells is higher in foreskin than peripheral blood. Memory (CD95+) T cells were assessed for CCR7 and CD28 expression in a subset of animals to identify effector memory cells (EM; CD28− CD95+ CCR7−) and differentiate transitional memory cells (TM; CD28+ CD95+ CCR7−) from fully differentiated central memory cells (CM; CD28+ CD95+ CCR7+) as previously described (18). The proportions of EM, TM, and CM CD4+ and CD8+ T cells in matched PBMC and foreskin specimens are presented for infected animals (A) and vaccinated animals (B). **, P < 0.01 (two-tailed Student t test) for comparison of the proportion of TM CD4+ or CD8+ T cells between PBMC and foreskin. Error bars represent the SEM.
CD4+ and CD8+ T cells in foreskin are activated at baseline but are not further activated by vaccination.
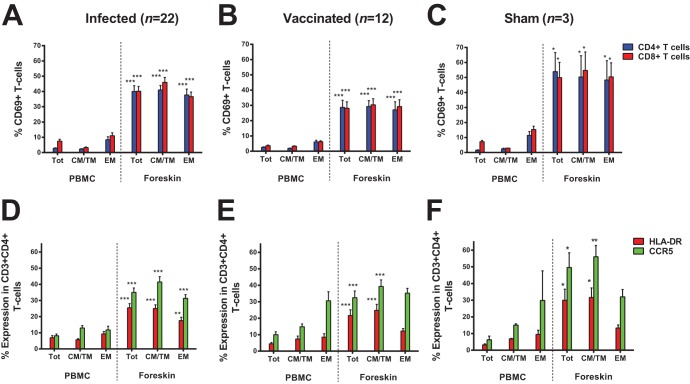
We next compared the activation status of CD4+ and CD8+ T cells between the peripheral blood and foreskin in these animals. Total (Tot), central/transitional memory (CM/TM), and effector memory (EM) T cells from foreskin showed significantly higher expression of the activation marker CD69 compared to peripheral blood T cells in infected animals (Fig. 3A; P < 0.0001 for CD69 in Tot CD4+ T cells), vaccinated animals (Fig. 3B; P < 0.0001) and sham-vaccinated animals (Fig. 3C; P = 0.0369). This increased activation status was further confirmed by elevated expression of HLA-DR and CCR5 on foreskin CD4+ T cells compared to peripheral blood CD4+ T cells from infected animals (Fig. 3D; P < 0.0001 for CCR5 in Tot CD4+ T cells), vaccinated animals (Fig. 3E; P < 0.0001), and sham-treated animals (Fig. 3F; P = 0.0452). These observations are consistent with the previously reported activated phenotype of CD4+ T cells in foreskin in human subjects (12).
FIG 3.
CD4+ and CD8+ T cells in foreskin are activated. CD4+ and CD8+ T cells from paired PBMC and foreskin specimens were assessed for expression of the activation markers CD69, HLA-DR and CCR5. The percentage expression of CD69 is presented for Total (Tot), central/transitional memory (CM/TM), and effector memory (EM) CD4+ and CD8+ T cells among infected animals (A), vaccinated animals (B), and sham-vaccinated animals (C). The percentage of expression of the activation marker HLA-DR and chemokine receptor CCR5 is presented for Total (Tot), central/transitional memory (CM/TM), and effector memory (EM) CD4+ T cells in infected animals (D), vaccinated animals (E), and sham-vaccinated animals (F). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (two-tailed Student t test) for comparison of the percentage expression of CD69, HLA-DR, or CCR5 in Tot, CM/TM, or EM T cells between PBMC and foreskin. Error bars represent the SEM.
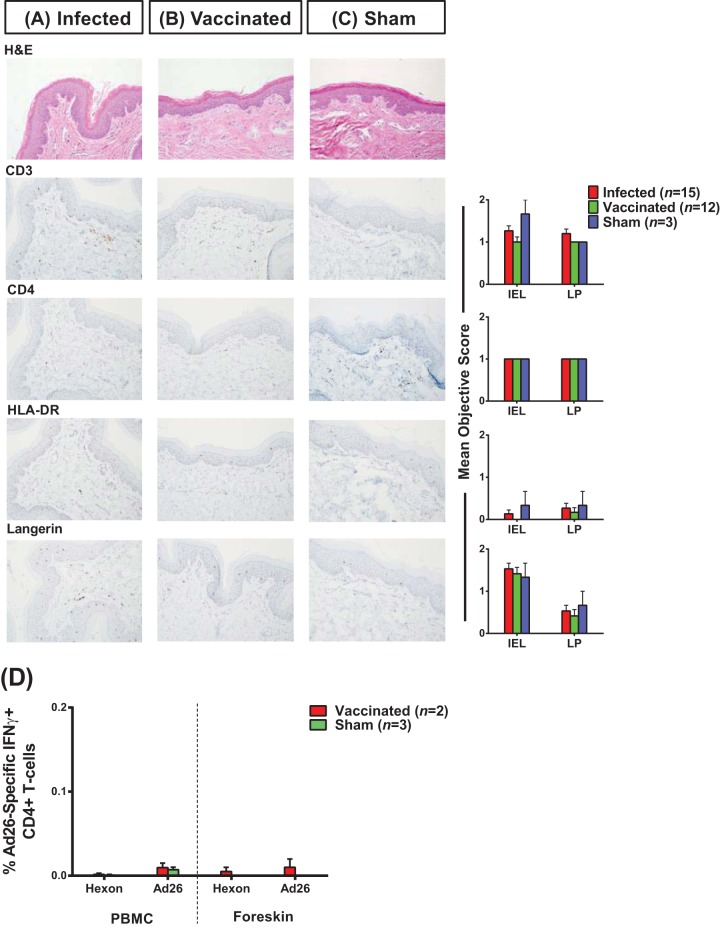
To evaluate the potential impact of immunization with Ad26 and Ad35 vectors on foreskin inflammation, we compared the degree of foreskin immune cell activation between vaccinated animals and sham-immunized controls 1 year after immunization using multiparameter flow cytometry, immunohistochemistry, and ICS. Compared to sham-treated animals, vaccinated animals did not express higher levels of CD69, HLA-DR, and CCR5 on foreskin CD4+ T cells (Fig. 3). Furthermore, we observed similar numbers and distribution of CD4+ T cells and Langerhans cells and expression of the activation marker HLA-DR in foreskin tissue sections from vaccinated and sham-treated animals via IHC (Fig. 4A to C). Moreover, using pooled Ad26 hexon peptides and whole Ad26 virus-based ICS assays, which we have shown can reliably detect Ad26 vector-specific CD4+ T cells (16), we found that Ad26 immunization of rhesus monkeys only led to marginal Ad26 vector-specific CD4+ T cells in both peripheral blood and foreskin (Fig. 4D). Collectively, these findings suggest that foreskin T cells in rhesus monkeys show high baseline activation but that vaccination did not further increase foreskin inflammation or activation 1 year following immunization.
FIG 4.
Immunization with Ad26 and Ad35 vectors did not increase foreskin inflammation 1 year after vaccination. Foreskin specimens obtained 1 year after SIV/SHIV infection or Ad35/Ad26-based SIV immunization were evaluated immunohistochemically for CD3, CD4, HLA-DR, and Langerin expression using the objective scoring criteria (Table 1) as detailed in Materials and Methods. Mean objective scores and representative immunohistochemistry slides are presented for infected animals (A), vaccinated animals (B), and sham-vaccinated animals (C). (D) The magnitudes of IFN-γ positive Ad26 vector-specific CD4+ T cells were quantified in paired peripheral blood and foreskin lymphocytes via ICS after stimulation with a pool of Ad26 hexon peptides (hexon) or empty Ad26 vectors (Ad26). IEL, intraepithelial lymphocytes; LP, lamina propria. Error bars represent the SEM.
Systemic immunization with Ad35/Ad26-based SIV vaccines and SIV infection elicit durable SIV-specific CD4+ and CD8+ T cells in foreskin.
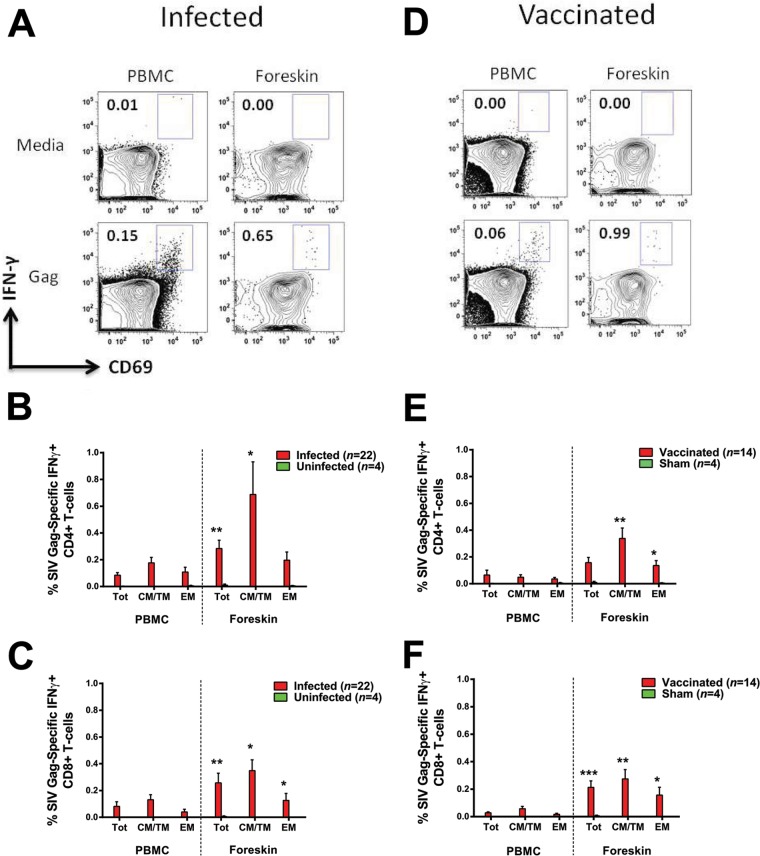
SIV-specific CD8+ T cells in foreskin have only been described in one prior study in SIV-infected rhesus monkeys infected by the penile route (11). To determine whether systemic SIV infection would similarly result in SIV-specific CD4+ and CD8+ T cells in foreskin, we assessed SIV Gag-specific CD4+ and CD8+ T cell responses in foreskin and peripheral blood of SIV- and SHIV-infected animals infected by the intrarectal route. We detected SIV Gag-specific CD4+ and CD8+ T cells in the foreskin of infected animals (Fig. 5A) at magnitudes that were comparable or higher than those observed in peripheral blood (Fig. 5B and C).
FIG 5.
Infection and vaccination elicit SIV Gag-specific CD4+ and CD8+ T cells in foreskin. Paired PBMC and foreskin lymphocytes obtained 1 year after SIV/SHIV infection or Ad35/Ad26-based SIV immunization were assessed for SIV-specific CD4+ and CD8+ T cell responses via ICS using pooled SIVmac239 Gag peptides. Flow panels in panel A depict presence of SIV Gag-specific CD8+ T cells in PBMC and foreskin of one representative SIV-infected animal. The magnitude of SIV Gag-specific CD4+ and CD8+ T cell responses in all SIV/SHIV-infected animals is quantified for both CD4+ T cells and CD8+ T cells in panels B and C, respectively. Similarly, flow panels in panel D depict presence of SIV Gag-specific CD8+ T cells in the PBMC and foreskin of a representative vaccinated animal. The magnitude of SIV Gag-specific CD4+ and CD8+ T cell responses in all vaccinated animals is quantified for both CD4+ T cells and CD8+ T cells in panels E and F, respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (two-tailed Student t test) for comparison of the magnitude of SIV Gag-specific responses between PBMC and foreskin in Total (Tot), central/transitional memory (CM/TM), or effector memory (EM) CD4+ or CD8+ T cells. Error bars represent the SEM.
It is currently unknown whether vaccination elicits SIV/HIV-1-specific T cells in foreskin. To investigate whether i.m. immunization of rhesus monkeys would induce SIV-specific immune responses in foreskin, we evaluated SIV Gag-specific CD4+ and CD8+ T cells in foreskin and peripheral blood of 14 rhesus monkeys 1 year following i.m. immunization with Ad35 and Ad26 vectors expressing SIV Gag, Pol, and Env antigens. SIV Gag-specific T cells were observed in peripheral blood in 10 of 14 immunized animals. Robust SIV Gag-specific CD4+ and CD8+ T cells were observed in foreskin in 9 of 14 animals at this late time point after Ad35/Ad26-based SIV immunization (Fig. 5D). Moreover, vaccine-elicited SIV-specific CD4+ and CD8+ T cells in foreskin were comparable or higher in magnitude than those in peripheral blood (Fig. 5E and F). These responses were durable since they were detected ∼1 year after vaccination.
Memory phenotype and functionality of SIV Gag-specific CD4+ and CD8+ T cells in foreskin.
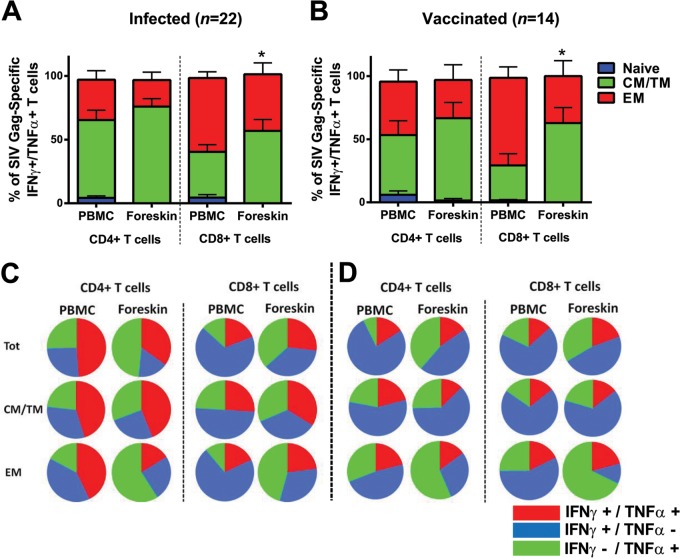
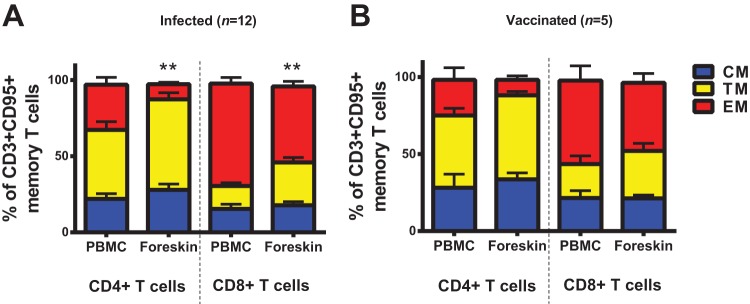
We sought to characterize the phenotype and functionality of the SIV Gag-specific CD4+ and CD8+ T cells in foreskin elicited 1 year after infection or vaccination. Compared to peripheral blood, the Gag-specific CD4+ and CD8+ T cells in foreskin were primarily CM/TM but also EM phenotype in both infected (Fig. 6A) and vaccinated rhesus monkeys (Fig. 6B). Gag-specific CD4+ and CD8+ T cells in foreskin demonstrated levels of both IFN-γ and TNF-α secretion largely similar to those observed in peripheral blood after infection (Fig. 6C) and vaccination (Fig. 6D). In contrast, the proportion of the monofunctional TNF-α-secreting Gag-specific T cells in foreskin were higher than those in peripheral blood, which were primarily monofunctional IFN-γ-secreting cells, specifically in the EM compartments of both CD4+ and CD8+ T cells (Fig. 6C and D; P = 0.0068 and P = 0.0018 for comparison of Gag-specific TNF-α+ EM CD8+ T cells in infected and vaccinated animals, respectively, in foreskin versus peripheral blood).
FIG 6.
Phenotype and functionality of SIV Gag-specific CD4+ and CD8+ T cells in foreskin. The CD4+ and CD8+ T cells in PBMC and foreskin expressing both IFN-γ and TNF-α after SIVmac239 Gag stimulation were identified via ICS using the Boolean function of FlowJo software. The proportions of naive (CD28+CD95-), central/transitional memory (CM/TM; CD28+ CD95+) and effector memory (EM; CD28− CD95+) T cells among these SIV Gag-specific IFN-γ+ TNF-α+ T cells were subsequently identified in matched PBMC and foreskin of both infected animals (A) and vaccinated animals (B). Multifunctionality (IFN-γ and/or TNF-α secretion) of all SIV Gag-specific CD4+ and CD8+ T cells from PBMC and foreskin is presented in the pie charts for both infected animals (A) and vaccinated animals (B). *, P < 0.05 (two-tailed Student t test) for comparison of the proportion of CM/TM CD8+ T cells between PBMC and foreskin. Error bars represent the SEM.
Vaccination with Ad26 vectors induced only transient activation of peripheral blood but not foreskin CD4+ T cells.
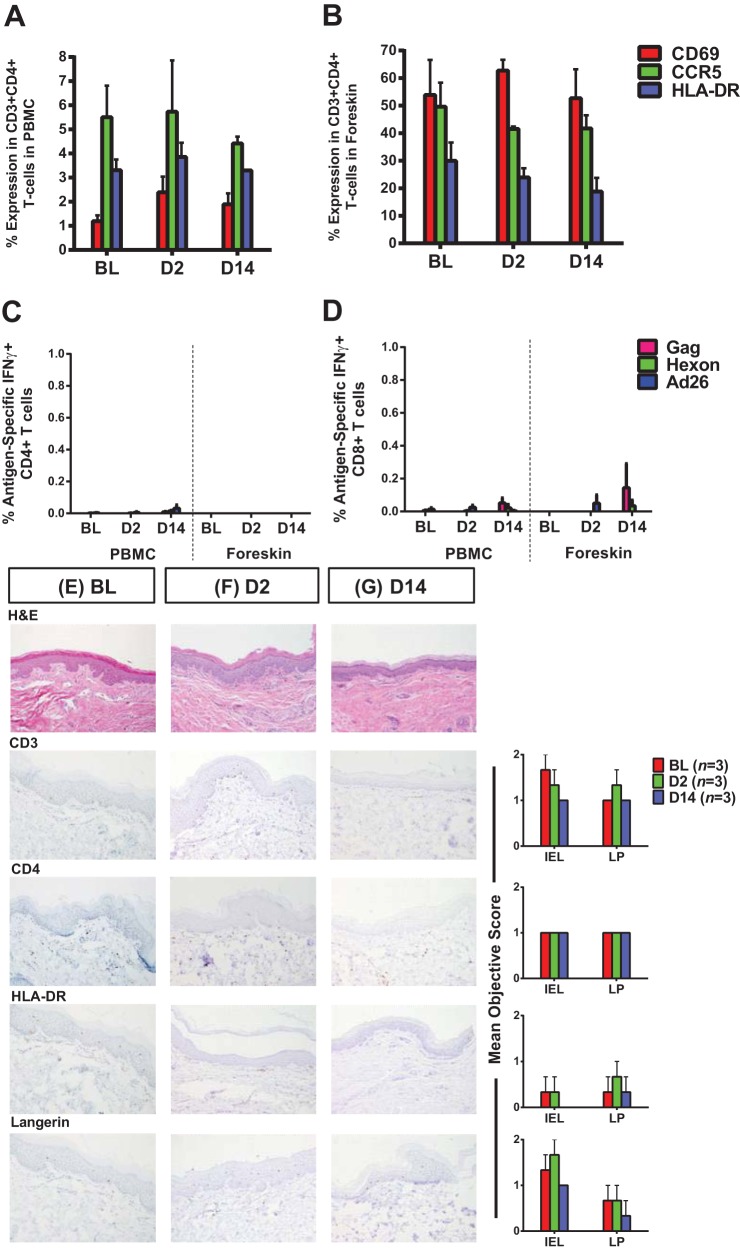
To evaluate the early effects of immunization on peripheral blood and foreskin inflammation, we immunized six rhesus monkeys with Ad26 vectors expressing SIV Gag/Pol/Env antigens and compared the degree of peripheral blood and foreskin immune cell activation between vaccinated animals and sham-vaccinated controls on days 2 and 14 after vaccination. Compared to sham-treated animals, vaccinated animals showed only minimal and transient increases in CD4+ T cell expression of CD69, HLA-DR, and CCR5 in blood on day 2 that waned by day 14 after immunization (Fig. 7A). In contrast, vaccination with Ad26 vectors did not increase CD4+ T cell activation in foreskin at any time point studied (Fig. 7B). Moreover, we observed only marginal Ad26 vector-specific CD4+ T cells in blood on day 14 after immunization (Fig. 7C). Low-level Ad26 vector-specific and SIV Gag-specific CD8+ T cells were observed in both peripheral blood and foreskin 14 days after immunization (Fig. 7D). Furthermore, we observed similar numbers and distribution of CD4+ T cells and Langerhans cells and expression of the activation marker HLA-DR in foreskin tissue sections from sham-treated and vaccinated animals 2 and 14 days after immunization via IHC (Fig. 7E, F, and G). These findings suggest that immunization with Ad26 vectors did not detectably increase foreskin inflammation or CD4+ T cell activation at these early time points following immunization.
FIG 7.
Vaccination with Ad26 vectors induced only transient activation of peripheral blood but not foreskin CD4+ T cells. CD4+ T cell expression of CD69, CCR5, and HLA-DR in paired peripheral blood (A) and foreskin (B) specimens were evaluated at baseline (sham-immunized uninfected animals, n = 3), 2 days (n = 3), and 14 days (n = 3) after immunization with Ad26 vectors expressing SIV Gag/Pol/Env immunogens by using multiparameter flow cytometry. The magnitudes of IFN-γ-positive SIV Gag-specific and Ad26 vector-specific CD4+ (C) and CD8+ T cells (D) were quantified in peripheral blood and foreskin lymphocytes via ICS after stimulation with a pool of SIVmac239 Gag peptides (Gag), Ad26 hexon peptides (hexon), or empty Ad26 vectors (Ad26). Immunohistochemically stained foreskin tissue sections were evaluated for CD3, CD4, HLA-DR, and Langerin expression using the objective scoring criteria (Table 1) as detailed in Materials and Methods. Mean objective scores and representative IHC slides are presented for the animals at baseline (E), 2 days postvaccination (F), and 14 days postvaccination (G). BL, baseline; D2, day 2 postvaccination; D14, day 14 postvaccination; IEL, intraepithelial lymphocytes; LP, lamina propria. Error bars represent the SEM.
DISCUSSION
Elucidating the mechanisms of HIV-1 infection through foreskin will be critical for our understanding of heterosexual transmission in men. Using the rhesus monkey model, we show that systemic immunization with Ad35/Ad26-based SIV vaccines elicited durable SIV-specific CD4+ and CD8+ T cell responses in foreskin at magnitudes at least comparable with those found in peripheral blood. Moreover, although foreskin T cells showed considerable baseline immune activation, vaccination did not further enhance foreskin inflammation or T cell activation. These findings demonstrate that Ad35 and Ad26 vaccine vectors elicit durable virus-specific cellular immune responses in foreskin.
The RV144 HIV-1 vaccine trial showed a modest degree of protective efficacy against heterosexual acquisition of HIV-1 in both men and women (19). The extent to which vaccine-elicited HIV-1-specific immune responses in foreskin may have played a role, however, remains unknown. To our knowledge, our study is the first to evaluate vaccine-elicited CD4+ and CD8+ T cells in foreskin. These foreskin responses were robust in magnitude and durable for at least 1 year after immunization. Similar to our previous observations with gastrointestinal and cervicovaginal mucosa (13), the Ad35/Ad26 vaccine-elicited SIV-specific CD4+ and CD8+ T cells in foreskin were primarily TM and EM phenotype capable of secreting both IFN-γ and TNF-α, suggesting that they may be poised for rapid and potent responses against virus challenges (13, 20, 21). However, it remains unclear to what extent the observed vaccine-elicited SIV-specific immune responses protect against SIV infection through foreskin. Further research into this question is therefore warranted.
To date, there has been only one report of the presence of SIV-specific CD8+ T cells in foreskin in SIV-infected rhesus monkeys (11). Consistent with this initial report, we also observed the presence of SIV-specific CD4+ and CD8+ T cells in the foreskin of SIV- and SHIV-infected animals. Of note, SIV infection was performed via penile foreskin SIV exposure in the previous report (11), and thus our findings confirm and extend these previous data by showing SIV-specific CD4+ and CD8+ T cell responses in foreskin following intrarectal SIV and SHIV infection. Intriguingly, as observed previously (11), SIV-specific CD4+ and CD8+ T cells in foreskin appeared to secrete more TNF-α than SIV-specific T cells in peripheral blood. The reason for this observation remains unclear but may relate in part to the response of immune cells to foreskin microbiota (22–24).
Foreskin CD4+ T cells in humans are highly activated, potentially increasing the risk of HIV-1 acquisition in men (12). A trend toward increased HIV-1 acquisition among uncircumcised and Ad5 seropositive vaccine recipients in the Merck phase 2b Step HIV-1 vaccine trial was observed for at least 18 months after vaccination and raised a hypothesis that the vaccine might have increased inflammation in foreskin and/or other portals of entry (25, 26). However, Ad26 and Ad35 vectors are substantially biologically different from Ad5 vectors in terms of tropism, cellular receptor usage, innate immune profile, and adaptive immune phenotypes (16, 27–30). Moreover, we did not evaluate the effects of Ad5 vectors in the present study. Consistent with our prior observations (16), we observed only a transient increase in CD4+ T cell activation in peripheral blood that waned by day 14 after immunization with Ad26 vectors. We observed a high degree of baseline CD4+ T cell activation in the foreskin in rhesus monkeys, but immunization with Ad26 and Ad35 vectors did not further increase the number or activation status of CD4+ T cells in foreskin at any time points studied. In addition, only marginal vector-specific CD4+ T cells were detected in the peripheral blood and foreskin of the immunized animals. We evaluated the early effects of immunization on inflammation using animals that received a single immunization with Ad26 vectors and long-term effects following heterologous Ad35 prime-Ad26 boost immunization. Thus, to complement these studies, future studies should evaluate the early effects of Ad26 boost on systemic and foreskin inflammation in Ad35 prime-immunized rhesus monkeys. Furthermore, important differences exist between the rhesus monkey model and humans, and thus clinical studies are required to assess the extent of mucosal and foreskin inflammation in humans following vaccination.
In summary, we have demonstrated for the first time that i.m. vaccination induces durable SIV-specific CD4+ and CD8+ T cells in foreskin in rhesus monkeys. These SIV-specific CD4+ and CD8+ T cells were comparable or higher in magnitude with those in peripheral blood, exhibited primarily TM and EM phenotypes, and secreted more TNF-α than IFN-γ. Our data confirm prior studies by showing that foreskin is enriched with highly activated CD4+ T cells expressing the SIV coreceptor CCR5, although foreskin CD4+ T cell activation was not further increased by i.m. immunization with Ad35/Ad26 vectors. Overall, our findings suggest that systemic Ad35/Ad26-based HIV-1 vaccination strategies may elicit potentially important HIV-1-specific CD4+ and CD8+ T cells in foreskin.
ACKNOWLEDGMENTS
We thank Robyn Hamel, Faye Stephens, Kathryn Kelly, Lisa Dunne, Hui-Wen Chang, Heather Lynn Knight, and Michelle Lifton for generous advice and assistance. We thank Elizabeth Curran for assistance with tissue procurement.
We acknowledge support from the National Institutes of Health (grants AI060354, AI096040, AI095985, and AI078526), the Bill and Melinda Gates Foundation (OPP1040741 and OPP1033091), and the Ragon Institute of MGH, MIT, and Harvard.
The authors declare no financial conflict of interest.
Footnotes
Published ahead of print 15 January 2014
REFERENCES
- 1.Dinh MH, Fahrbach KM, Hope TJ. 2011. The role of the foreskin in male circumcision: an evidence-based review. Am. J. Reprod. Immunol. 65:279–283. 10.1111/j.1600-0897.2010.00934.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahrbach KM, Barry SM, Anderson MR, Hope TJ. 2010. Enhanced cellular responses and environmental sampling within inner foreskin explants: implications for the foreskin's role in HIV transmission. Mucosal Immunol. 3:410–418. 10.1038/mi.2010.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischetti L, Barry SM, Hope TJ, Shattock RJ. 2009. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS 23:319–328. 10.1097/QAD.0b013e328321b778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganor Y, Zhou Z, Tudor D, Schmitt A, Vacher-Lavenu MC, Gibault L, Thiounn N, Tomasini J, Wolf JP, Bomsel M. 2010. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol. 3:506–522. 10.1038/mi.2010.32 [DOI] [PubMed] [Google Scholar]
- 5.McCoombe SG, Short RV. 2006. Potential HIV-1 target cells in the human penis. AIDS 20:1491–1495. 10.1097/01.aids.0000237364.11123.98 [DOI] [PubMed] [Google Scholar]
- 6.Morris BJ, Wamai RG. 2012. Biological basis for the protective effect conferred by male circumcision against HIV infection. Int. J. STD AIDS 23:153–159. 10.1258/ijsa.2011.011228 [DOI] [PubMed] [Google Scholar]
- 7.Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, Shiver JW, Robert-Guroff M, Robertson MN, McChesney MB, Gilbert PB, Miller CJ. 2012. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J. Virol. 86:2239–2250. 10.1128/JVI.06175-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. 2005. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2:e298. 10.1371/journal.pmed.0020298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, Williams CF, Campbell RT, Ndinya-Achola JO. 2007. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 369:643–656. 10.1016/S0140-6736(07)60312-2 [DOI] [PubMed] [Google Scholar]
- 10.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, Kiwanuka N, Moulton LH, Chaudhary MA, Chen MZ, Sewankambo NK, Wabwire-Mangen F, Bacon MC, Williams CF, Opendi P, Reynolds SJ, Laeyendecker O, Quinn TC, Wawer MJ. 2007. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369:657–666. 10.1016/S0140-6736(07)60313-4 [DOI] [PubMed] [Google Scholar]
- 11.Rothaeusler K, Ma ZM, Qureshi H, Carroll TD, Rourke T, McChesney MB, Miller CJ. 2012. Antiviral antibodies and T cells are present in the foreskin of simian immunodeficiency virus-infected rhesus macaques. J. Virol. 86:7098–7106. 10.1128/JVI.00410-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prodger JL, Gray R, Kigozi G, Nalugoda F, Galiwango R, Hirbod T, Wawer M, Hofer SO, Sewankambo N, Serwadda D, Kaul R. 2012. Foreskin T-cell subsets differ substantially from blood with respect to HIV coreceptor expression, inflammatory profile, and memory status. Mucosal Immunol. 5:121–128. 10.1038/mi.2011.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. 2011. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J. Virol. 85:11007–11015. 10.1128/JVI.05346-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93. 10.1038/nature10766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masek-Hammerman K, Li H, Liu J, Abbink P, La Porte A, O'Brien KL, Whitney JB, Carville A, Mansfield KG, Barouch DH. 2010. Mucosal trafficking of vector-specific CD4+ T lymphocytes following vaccination of rhesus monkeys with adenovirus serotype 5. J. Virol. 84:9810–9816. 10.1128/JVI.01157-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Rhee EG, Masek-Hammerman K, Teigler JE, Abbink P, Barouch DH. 2012. Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J. Virol. 86:10862–10865. 10.1128/JVI.00928-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168:29–43 [DOI] [PubMed] [Google Scholar]
- 18.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M, Jr, Lifson JD, Maino VC, Axthelm MK, Villinger F. 2006. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J. Clin. Invest. 116:1514–1524. 10.1172/JCI27564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220. 10.1056/NEJMoa0908492 [DOI] [PubMed] [Google Scholar]
- 20.Derrick SC, Yabe IM, Yang A, Morris SL. 2011. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine 29:2902–2909. 10.1016/j.vaccine.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 22.Liu CM, Hungate BA, Tobian AA, Serwadda D, Ravel J, Lester R, Kigozi G, Aziz M, Galiwango RM, Nalugoda F, Contente-Cuomo TL, Wawer MJ, Keim P, Gray RH, Price LB. 2013. Male circumcision significantly reduces prevalence and load of genital anaerobic bacteria. mBio 4:e00076-13. 10.1128/mBio.00076-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peiser M, Koeck J, Kirschning CJ, Wittig B, Wanner R. 2008. Human Langerhans cells selectively activated via Toll-like receptor 2 agonists acquire migratory and CD4+ T cell stimulatory capacity. J. Leukoc. Biol. 83:1118–1127. 10.1189/jlb.0807567 [DOI] [PubMed] [Google Scholar]
- 24.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. 1991. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc. Natl. Acad. Sci. U. S. A. 88:4220–4224. 10.1073/pnas.88.10.4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894–1905. 10.1016/S0140-6736(08)61592-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams WC, Gujer C, McInerney G, Gall JG, Petrovas C, Karlsson Hedestam GB, Koup RA, Lore K. 2011. Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation. Proc. Natl. Acad. Sci. U. S. A. 108:7499–7504. 10.1073/pnas.1017146108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havenga MJ, Lemckert AA, Ophorst OJ, van Meijer M, Germeraad WT, Grimbergen J, van Den Doel MA, Vogels R, van Deutekom J, Janson AA, de Bruijn JD, Uytdehaag F, Quax PH, Logtenberg T, Mehtali M, Bout A. 2002. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J. Virol. 76:4612–4620. 10.1128/JVI.76.9.4612-4620.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Ewald BA, Lynch DM, Denholtz M, Abbink P, Lemckert AA, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, Barouch DH. 2008. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82:4844–4852. 10.1128/JVI.02616-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teigler JE, Iampietro MJ, Barouch DH. 2012. Vaccination with adenovirus serotypes 35, 26, and 48 elicits greater innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J. Virol. 86:9590–9598. 10.1128/JVI.00740-12 [DOI] [PMC free article] [PubMed] [Google Scholar]