Abstract
Cholesterol synthesis is a highly oxygen-dependent process. Paradoxically, hypoxia is correlated with an increase in cellular and systemic cholesterol levels and risk of cardiovascular diseases. The mechanism for the increase in cholesterol during hypoxia is unclear. Hypoxia signaling is mediated through hypoxia-inducible factor 1α (HIF-1α) and HIF-2α. The present study demonstrates that activation of HIF signaling in the liver increases hepatic and systemic cholesterol levels due to a decrease in the expression of cholesterol hydroxylase CYP7A1 and other enzymes involved in bile acid synthesis. Specifically, activation of hepatic HIF-2α (but not HIF-1α) led to hypercholesterolemia. HIF-2α repressed the circadian expression of Rev-erbα, resulting in increased expression of E4BP4, a negative regulator of Cyp7a1. To understand if HIF-mediated decrease in bile acid synthesis is a physiologically relevant pathway by which hypoxia maintains or increases systemic cholesterol levels, two hypoxic mouse models were assessed, an acute lung injury model and mice exposed to 10% O2 for 3 weeks. In both models, cholesterol levels increased with a concomitant decrease in expression of genes involved in bile acid synthesis. The present study demonstrates that hypoxic activation of hepatic HIF-2α leads to an adaptive increase in cholesterol levels through inhibition of bile acid synthesis.
INTRODUCTION
Cholesterol is an essential nutrient important in many metabolic processes, and its levels are tightly regulated. Cholesterol synthesis is a highly oxygen-dependent process. The final step from lanosterol to cholesterol conversion requires nine molecules of dioxygen (1). However, systemic hypoxia is associated with an increase in cholesterol levels. Individuals living at high altitude are at high risk for coronary heart disease due to elevated low-density lipoprotein and total cholesterol levels caused by high-altitude hypoxia (2). In chronic hypoxic diseases, such as in patients with obstructive sleep apnea, no change or an increase in serum and liver cholesterol levels are observed, which can be reversed by continuous positive airway pressure (3–5). Moreover, mice exposed to chronic intermittent hypoxia (CIH) have a significant increase in total cholesterol levels (6, 7). These studies suggest that hypoxia-signaling pathways activate an adaptive response important in cholesterol homeostasis. Several mechanisms were proposed to account for hypoxia-induced dyslipidemia (8, 9). However, the mechanisms by which hypoxia regulates cholesterol homeostasis are not clear. Hypoxic signaling is mediated by an oxygen-sensitive transcription factor, hypoxia-inducible factor (HIF), consisting of two isoforms, HIF-1α and HIF-2α (10). Activation of HIF-1α induces glycolytic genes, whereas activation of HIF-2α regulates the expression of genes that are involved in fatty acid synthesis, fatty acid uptake, inflammation, fibrosis, and vascular tumors (9, 11–14).
In this study, liver hypoxia-induced dyslipidemia was shown to be associated with spontaneous hypercholesterolemia. Consistent with previous reports, the increase in hepatic cholesterol by hypoxia was not due to alterations in cholesterol synthesis, intestinal absorptive genes, or mobilization from fat stores (15, 16). Disruption of Vhl led to a HIF-2α-dependent decrease in cholesterol hydroxylases involved in the conversion of cholesterol to bile acids. To confirm that the decrease in cholesterol hydroxylase is an important mechanism to maintain cholesterol levels during hypoxia, two systemic hypoxic mouse models were used: mice exposed to normobaric 10% O2 for 3 weeks and a mouse model of lung contusion (17). Both models exhibited significantly elevated levels of serum cholesterol with a parallel decrease in expression of genes involved in bile acid synthesis. Taken together, this study demonstrates a mechanistic basis for the adaptive maintenance of cholesterol levels during hypoxia. Thus, inhibition of HIF-2α may be a relevant and novel therapeutic target to ameliorate hypercholesterolemia during hypoxia.
MATERIALS AND METHODS
Animals and diets.
VhlF/F, VhlLivKO, VhlΔInt, Vhl/ArntLivKO, Vhl/Hif1αLivKO, Vhl/Hif2αLivKO, VhlΔMϕ, and ODD-luc mice were described previously (11, 18–22). VhlΔMϕ mice were generated by crossing LysM cre mice with VhlF/F mice. All mice were fed ad libitum and kept in a 12-h dark/light cycle. Mice were fed with either a regular chow diet or an atherogenic (35 kcal% fat, 1% cholesterol, 0.5% cholic acid; Research Diets, New Brunswick, NJ), high-fat (∼45 kcal% derived from fat; Research Diets), or ethanol (6% Lieber-DeCarli diet; Bio-Serv, Frenchtown, NJ) diet. For systemic hypoxia experiments, C57BL/6 mice in standard cages were placed in a normobaric hypoxia chamber with 10% O2 for 3 weeks. Lung contusion (C57BL/6 mice) and in vivo imaging (ODD-luc mice) were performed as described previously (17, 23). All mice were sacrificed at 7:00 p.m. unless otherwise mentioned in the figure legends. All animal studies were carried out in accordance with Association for Assessment and Accreditation of Laboratory Animal Care International guidelines and approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Western blot analysis.
Microsomes and whole-cell extracts were prepared as previously described (21). Proteins were separated by SDS-PAGE. Microsomes were used for detection of CYP7A1 (Abcam, Cambridge, MA) and normalized to total protein by Coomassie blue staining.
Primary hepatocyte isolation.
For primary hepatocyte isolation, livers were perfused continuously with 10 ml of Earle's balanced salt solution (EBSS) containing 0.5 mM EGTA, followed by serum-free L-15 medium containing collagenase type II (Worthington, Invitrogen). Hepatocytes were then harvested by gentle teasing and filtered through a 100-μm filter. Viable hepatocytes were plated in 6-well plates at a cell density of 4 × 105 in Williams E medium containing 10% fetal bovine serum (FBS). Five hours after plating, dead cells were removed and media refreshed. For adenovirus treatment, the desired amounts of Ad-Cyp7a1, Ad-HNF4α, and Ad-LRH-1 virus were added after 5 h of plating and then incubated until further analysis.
Cholesterol assay.
For cholesterol extraction, 50 mg of liver or white adipose tissue was homogenized in 1 ml of chloroform-methanol (2:1). Primary hepatocytes were collected in 300 μl methanol, and then 600 μl of chloroform was added and vortexed. The homogenates were then centrifuged, and 200 μl of water was added to the supernatant and vortexed. The organic phase separated by centrifugation was dried overnight and resuspended in isopropanol. Cholesterol measurements were performed using the Cholesterol E kit (Wako Diagnostics, VA). Serum cholesterol was measured in 20 μl of serum, and lipoprotein profiles were assessed by fast performance liquid chromatography as previously described (24).
Bile acid assay.
Bile acids from liver were extracted as described by Cheng et al. (25). Specific bile salt species were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) performed on a PESCIEX API200 ESI triple-quadrupole mass spectrometer (PerkinElmer Life Sciences) as described previously (26).
RNA extraction and qPCR analysis.
RNA extraction and gene expression analysis using quantitative real-time PCR (qPCR) were performed as previously described (9). Primers are included in Table S1 in the supplemental material.
Statistical analysis.
Results are expressed as means ± standard deviations (SD). P values were calculated by independent t test, 1-way analysis of variance (ANOVA), or 2-way ANOVA.
RESULTS
Disruption of hepatic VHL results in hypercholesterolemia.
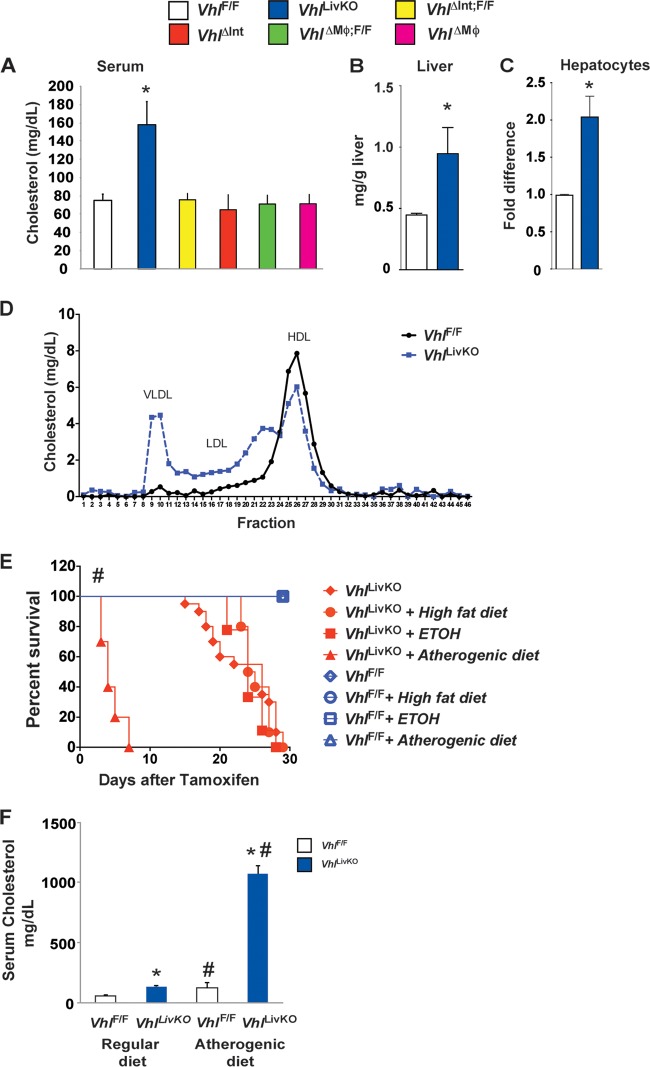
In order to elucidate the role of HIFs in cholesterol metabolism, serum cholesterol levels were assessed in mice with a conditional disruption of VHL in tissues or cells important in cholesterol homeostasis. Under normoxia, the von Hippel-Lindau tumor suppressor protein binds to hydroxylated HIFs and targets them for ubiquitination and degradation (27, 28). Genetic disruption of Vhl increases the expression of HIFs and HIF target genes (11). Disruption of VHL in the macrophage (VhlΔMϕ) or the intestine (VhlΔInt) did not affect serum cholesterol levels compared to those of wild-type littermates (VhlF/F) (Fig. 1A). However, a temporal disruption of VHL in hepatocytes (VhlLivKO) using a tamoxifen-inducible Cre recombinase (9) significantly elevated serum cholesterol (Fig. 1A), hepatic cholesterol (Fig. 1B), and cholesterol content in isolated primary hepatocytes (Fig. 1C) compared to tamoxifen-treated wild-type littermates (VhlF/F). Hypercholesterolemia is primarily associated with increases in very low-density lipoprotein (VLDL) and LDL cholesterol, while high-density lipoprotein (HDL) cholesterol was decreased in VhlLivKO mice (Fig. 1D). VhlLivKO mice on a normal chow diet die within 30 days after Vhl disruption (Fig. 1E). Feeding an atherogenic diet markedly decreased the survival of VhlLivKO mice, which was not observed in VhlLivKO mice fed a high-fat or ethanol diet (Fig. 1F). We have previously reported that disruption of Vhl in the liver results in progressive liver steatosis and fibrosis (9). Therefore, it is possible that the hypercholesterolemia augments the progressive liver disease in VhlLivKO mice. In order to investigate the effect of atherogenic diet on serum cholesterol, VhlLivKO mice were injected with tamoxifen to disrupt Vhl expression and then immediately placed on the atherogenic diet for 1 week. The atherogenic diet further augmented the serum cholesterol levels in VhlLivKO mice (Fig. 1F). Together, these data demonstrate that disruption of Vhl in the liver leads to dysregulated cholesterol homeostasis.
FIG 1.
Disruption of hepatic VHL in the liver results in hypercholesterolemia and decreased survival. (A) Serum cholesterol levels in VhlF/F, VhlLivKO, VhlΔMϕ, and VhlΔInt mice 2 weeks after tamoxifen treatment. Cholesterol content was measured in the liver (B) and primary hepatocytes (C) from VhlF/F and VhlLivKO mice. (D) Lipoprotein profile was assessed in the serum from VhlF/F and VhlLivKO mice 2 weeks after tamoxifen treatment. (E) Survival curve of VhlF/F and VhlLivKO mice fed with regular chow, high-fat, ethanol (ETOH), or atherogenic diet. (F) Serum cholesterol levels measured in VhlF/F and VhlLivKO mice on chow or atherogenic diet for 1 week. Five to seven mice were assessed per treatment group. Each bar graph represents the mean values ± SD. **, P < 0.01 versus VhlF/F; #, P < 0.01 versus regular diet.
Genes involved in de novo cholesterol synthesis in the liver and cholesterol absorption in the intestine are not altered by hepatic Vhl disruption.
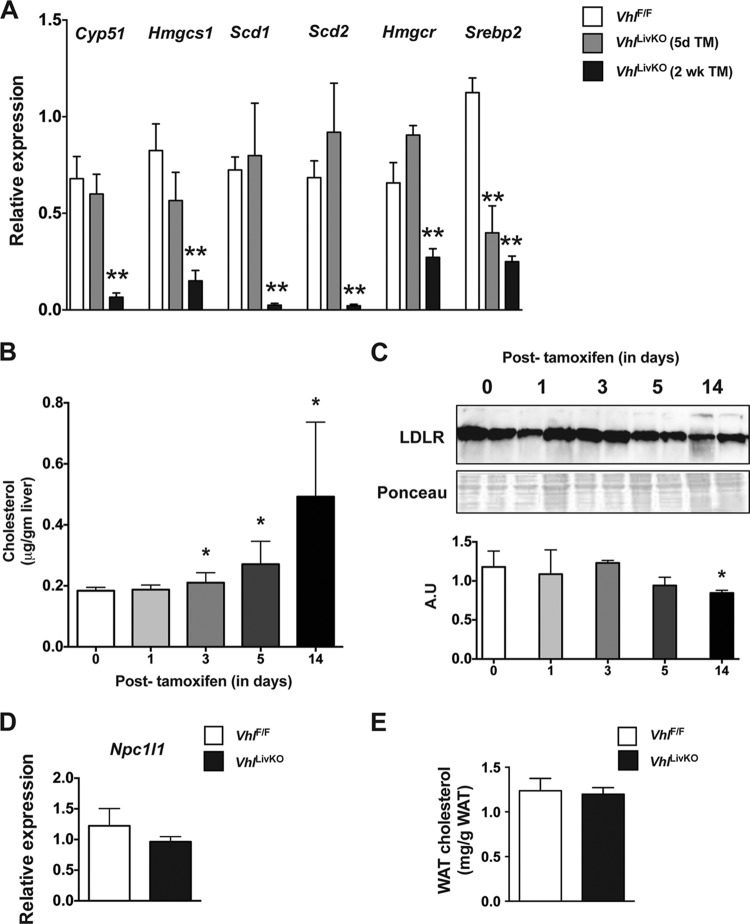
Hepatic cholesterol homeostasis is maintained by coordinated regulation of uptake, synthesis, and oxidative metabolism to bile acids (29). In order to determine the mechanism of hypercholesterolemia in VhlLivKO mice, expression of key enzymes involved in cholesterol synthesis and esterification were analyzed. Expression of Cyp51, HMG-coenzyme A (CoA) synthase (Hmgcs), HMG-CoA reductase (Hmgr), Scd1, and Scd2 were significantly repressed 2 weeks after Vhl disruption but not at 5 days (Fig. 2A). The expression of sterol regulatory element binding protein 2 (Srebp2), a transcription factor critical in cholesterol synthesis, was significantly decreased at both 5 days and 2 weeks following Vhl disruption (Fig. 2A). These data demonstrate that the cholesterol-mediated negative feedback repression on de novo synthesis is still intact (30), and cholesterol synthesis is not the major mechanism by which liver disruption of VHL induces hypercholesterolemia. Cholesterol is cleared by the liver via the LDL receptor, and loss of function or genetic ablation of the LDL receptor results in hypercholesterolemia (31). Therefore, the dynamic changes in hepatic cholesterol levels and LDL receptor expression were assessed in VhlLivKO mice. A progressive and significant increase in hepatic cholesterol levels was observed starting at 3 days following Vhl disruption (Fig. 2B); however, LDL receptor protein levels decreased significantly only 2 weeks after Vhl disruption (Fig. 2C). Loss of LDL receptor after 2 weeks could be attributed to the negative-feedback repression exerted by increased intrahepatic cholesterol levels to limit cholesterol overload (32, 33). In addition, disruption of Vhl in liver did not affect the expression of Npc1l1 (Fig. 2D), a gene involved in intestinal cholesterol absorption (34). Increasing cholesterol storage by adipose tissue improves the lipid profile in mice; on the other hand, cholesterol mobilization from adipose tissue is associated with hypercholesterolemia in humans (35, 36). The lack of a significant difference in white adipose cholesterol content between VhlLivKO and VhlF/F mice suggests that hepatic VHL disruption does not affect adipose tissue cholesterol homeostasis (Fig. 2E).
FIG 2.
Cholesterol synthesis, absorption, and mobilization are not affected by the hepatic disruption of VHL. (A) qPCR analysis of cholesterol synthesis genes in VhlF/F and VhlLivKO livers 5 days or 2 weeks following tamoxifen treatment. Hepatic cholesterol (B) and LDL receptor expression (C) were analyzed and quantitated (bottom inset) in the livers of VhlLivKO mice. (D) qPCR analysis of Npc1l1 in the small intestine of VhlF/F and VhlLivKO mice 2 weeks after tamoxifen treatment. mRNA was normalized to β-actin. (E) White adipose tissue cholesterol from VhlF/F and VhlLivKO mice 2 weeks after tamoxifen treatment. Five to nine mice were assessed per treatment group. Each bar graph represents the mean values ± SD. **, P < 0.01 compared to VhlF/F mice.
CYP7A1 expression is repressed by hepatic Vhl disruption.
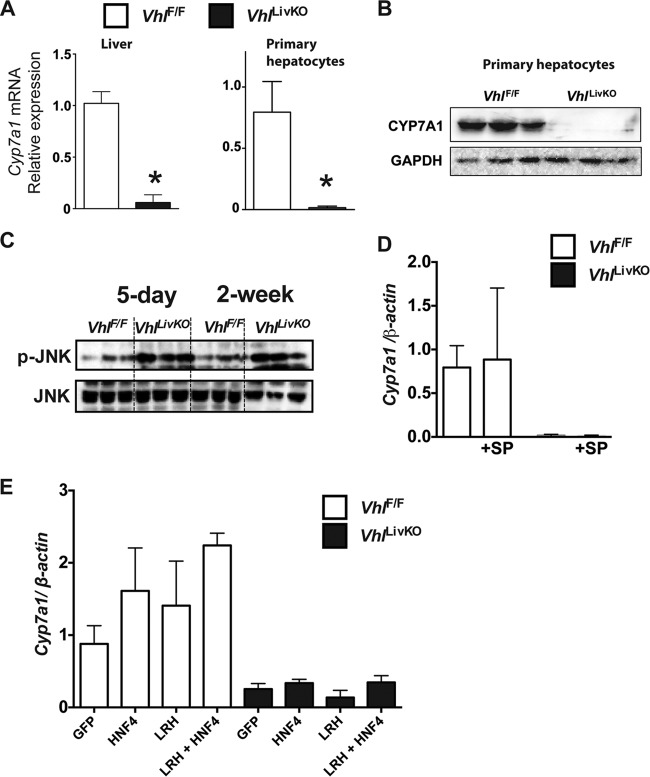
Cholesterol is cleared by the liver through conversion to bile acids (37). CYP7A1 is the major rate-limiting cholesterol hydroxylase involved in the classical pathway of bile acid synthesis. In order to assess whether hypercholesterolemia is due to altered bile acid homeostasis, CYP7A1 expression was assessed in VhlLivKO mice. Two weeks after disruption of Vhl, expression of Cyp7a1 mRNA was significantly reduced in the livers of VhlLivKO mice (Fig. 3A). Similarly, CYP7A1 mRNA and protein levels were significantly decreased in primary hepatocytes from VhlLivKO mice (Fig. 3A and B). To identify mechanisms by which disruption of Vhl repress CYP7A1 expression, several known pathways important in the regulation of CYP7A1 were assessed. Activation of JNK induces hypercholesterolemia and cholestasis (38) and is a potent inhibitor of Cyp7a1 expression (39). Consistent with inflammation in the livers of VhlLivKO mice (9), JNK phosphorylation was significantly increased at 5 days and 2 weeks after disruption of Vhl (Fig. 3C). However, JNK inhibitor (SP 600125) did not rescue Cyp7a1 mRNA levels in primary hepatocytes from VhlLivKO mice (Fig. 3D). Similarly, adenovirus-mediated overexpression of HNF-4α or LRH-1, known CYP7A1 transcriptional regulators (40), did not rescue the expression of Cyp7a1 mRNA levels in primary hepatocytes from VhlLivKO mice (Fig. 3E).
FIG 3.
Loss of VHL in liver results in repression of CYP7A1 expression. (A) qPCR analysis examining the expression of Cyp7a1 mRNA levels in the livers and primary hepatocytes of VhlLivKO mice. mRNA was normalized to β-actin. Four to six mice were assessed per treatment group. (B) Western analysis of CYP7A1 in primary hepatocytes from VhlF/F and VhlLivKO mice 2 weeks after tamoxifen treatment. (C) Western analysis examining the phosphorylation of JNK in the liver of VhlLivKO mice five days or 2 weeks after tamoxifen treatment. qPCR analysis examining the expression of Cyp7a1 mRNA levels in the primary hepatocytes of VhlLivKO mice treated with the JNK inhibitor SP 600125 (SP) (D) or with recombinant adenovirus expressing HNF-4α or LRH-1 (E). mRNA was normalized to β-actin. *, P < 0.05 versus VhlF/F mice.
Hepatic VHL disruption alters circadian oscillation of CYP7A1.
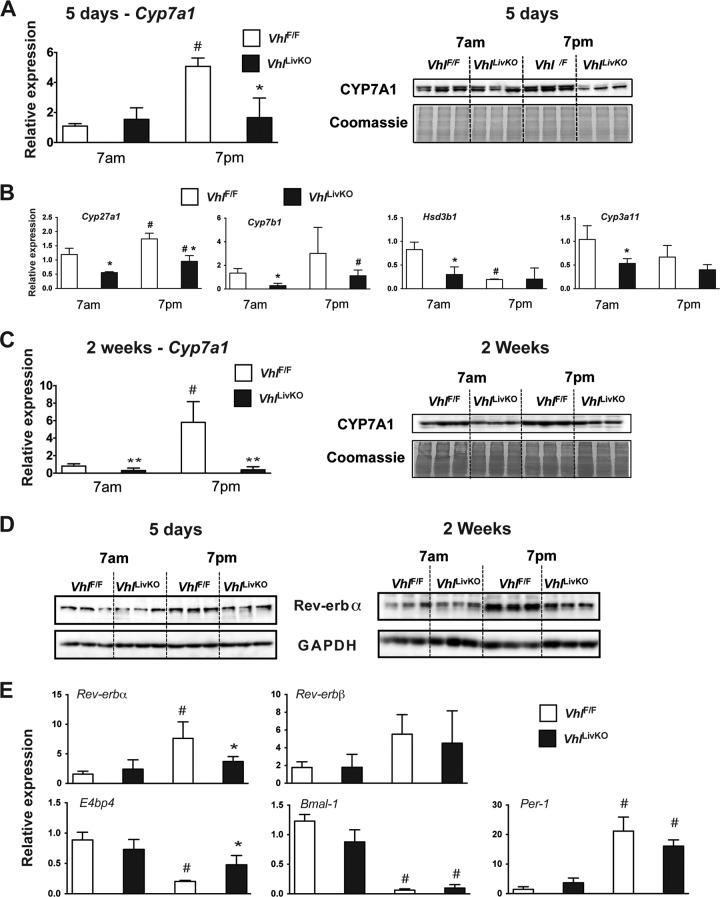
Cholesterol exhibits diurnal variation due to circadian oscillation in the expression of genes involved in cholesterol metabolism, including CYP7A1 (41). In order to investigate whether CYP7A1 expression and circadian regulation was altered by HIF activation, VhlLivKO and littermate control mice were treated with tamoxifen for 5 days and 2 weeks and killed at 7:00 a.m. and 7:00 p.m. CYP7A1 mRNA and protein levels were not changed at 7:00 a.m.; however, the circadian increase at 7:00 p.m. was significantly repressed in the VhlLivKO mice at 5 days (Fig. 4A). However, circadian expression of other cholesterol hydroxylases, such as Cyp27a1, Cyp7b1, Hsd3b1, and Cyp3a11, was not affected by disruption of Vhl (Fig. 4B). Furthermore, 2 weeks following VHL disruption, CYP7A1 mRNA and protein at 7:00 a.m. and the circadian induction at 7:00 p.m. were significantly repressed (Fig. 4C). Among the various key transcription factors that regulate circadian genes, Rev-erbα plays an essential role in the diurnal expression of Cyp7a1 (42, 43). The circadian induction of Rev-erbα is inhibited in the VhlLivKO mice at both 5 days and 2 weeks following tamoxifen treatment (Fig. 4C). Consistent with the protein expression, the circadian induction of Rev-erbα but not Rev-erbβ was significantly repressed at 5 days after disruption of Vhl (Fig. 4D). Moreover, the loss of expression of Rev-erbα in VhlLivKO mice at 7:00 p.m. resulted in a significant increase in E4bp4 mRNA levels (Fig. 4D), a negative regulator of Cyp7a1 (42). However, no difference in the expression of circadian genes such as Bmal-1 and Per-1 was observed by disruption of Vhl in liver (Fig. 4D). These data suggest that hypoxia-mediated circadian dysregulation leads to a decrease in CYP7A1 levels.
FIG 4.
Loss of VHL disrupts circadian expression of CYP7A1. qPCR and Western blot analysis of CYP7A1 in the microsome fraction (A) and qPCR analysis of cholesterol hydroxylases (B) at 7:00 a.m. and 7:00 p.m. in VhlF/F and VhlLivKO livers 5 days after Vhl disruption. (C) qPCR and Western blot analysis of CYP7A1 at 7:00 a.m. and 7:00 p.m. in VhlF/F and VhlLivKO livers 2 weeks after Vhl disruption. mRNA was normalized to β-actin, and the microsome fraction was normalized by Coomassie blue staining. Four to six mice were assessed per treatment group. (D) Western blot analysis examining the circadian expression of Rev-erbα in VhlF/F and VhlLivKO livers 5 days or 2 weeks after Vhl disruption. (E) qPCR analysis of circadian genes in VhlF/F and VhlLivKO livers 5 days after Vhl disruption. mRNA was normalized to β-actin. Each bar graph represents the mean values ± SD. *, P < 0.05 versus VhlF/F mice; **, P < 0.01 versus VhlF/F mice; #, P < 0.01 versus results at 7:00 a.m.
Vhl disruption in the liver leads to decreased expression of genes critical in bile acid synthesis.
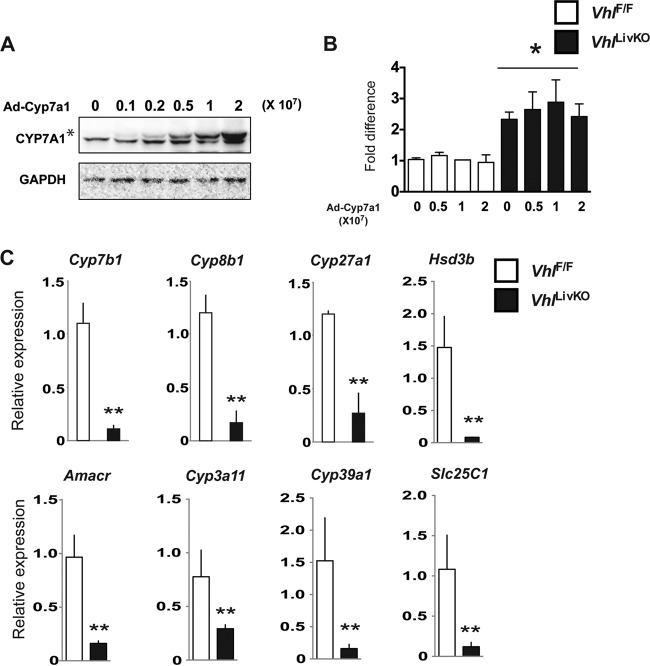
To address whether repression of CYP7A1 is the primary mechanism for the aberrant cholesterol levels in VhlLivKO mice, CYP7A1 was overexpressed in primary hepatocytes from VhlLivKO mice. Rescuing CYP7A1 did not ameliorate cholesterol levels in the primary hepatocytes from VhlLivKO mice (Fig. 5A and B). Conversion of cholesterol to bile is a multienzymatic process involving numerous cholesterol hydroxylases. Further analysis revealed that disruption of VHL in livers resulted in repression of genes encoding other key cholesterol hydroxylases, such as Cyp27a1, Cyp7b1, Cyp8b1, Hsd3b, Amacr, Cyp3a11, Cyp29a1, and Slc25c1 (Fig. 5C). Taken together, these data suggest that activation of hypoxia signaling by disruption of VHL results in a global decrease in cholesterol hydroxylases involved in bile acid synthesis.
FIG 5.
Adenoviral rescue of CYP7A1 did not ameliorate cholesterol levels in primary hepatocytes. Western blot analysis of CYP7A1 (A) and cholesterol assay in primary hepatocytes (B) treated with recombinant adenovirus expressing Cyp7a1 in primary hepatocytes from VhlF/F and VhlLivKO mice. (C) qPCR analysis of cholesterol hydroxylases in VhlF/F and VhlLivKO livers 2 weeks after Vhl disruption. mRNA was normalized to β-actin. Each bar graph represents the mean values ± SD. *, P < 0.05 versus VhlF/F mice; **, P < 0.01 versus VhlF/F mice; #, P < 0.01 versus results at 7:00 a.m.
Vhl disruption in the liver leads to decreased bile acids.
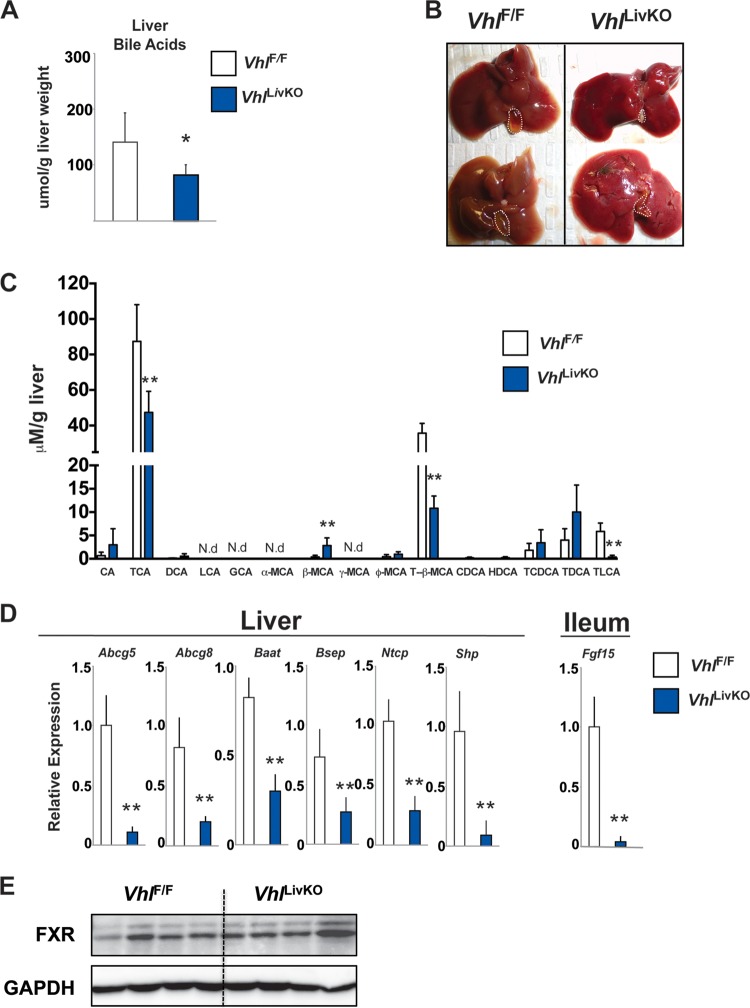
Decreases in the expression and activity of cholesterol hydroxylases decrease bile acid synthesis, leading to hypercholesterolemia. Therefore, to understand if repression of cholesterol hydroxylases by hypoxia signaling could adversely affect bile acid synthesis, the hepatic bile acid pool was measured in VhlLivKO mice. The total liver bile acid pool (Fig. 6A) and gallbladder size were significantly reduced in VhlLivKO mice (Fig. 6B). In addition, major hepatic bile acid species were significantly decreased, including the recently identified endogenous farsenoid X receptor (FXR) antagonist, tauro-β-muricholic acid (Fig. 6C) (44, 45). Bile acids inhibit their own synthesis by a negative feedback mechanism via activation of FXR (46, 47). Consistent with the decrease in its endogenous ligand, FXR target genes, such as Abcg5, Abcg8, small heterodimer partner (Shp), and bile salt export protein (Bsep) in the liver and Fgf15 in the intestines, were significantly repressed in VhlLivKO mice (Fig. 6D) without changes in FXR protein levels (Fig. 6E). In addition, the expression of bile acid-CoA:amino acid N-acyltransferase (Baat) and the bile acid transporter sodium taurocholate cotransport protein (Ntcp) are significantly reduced in the livers of VhlLivKO mice (Fig. 6D). These data demonstrate that HIF activation disrupts bile acid synthesis and signaling.
FIG 6.
Disruption of liver VHL decreases bile acid and FXR signaling. (A) Hepatic bile acids in VhlF/F and VhlLivKO mice 2 weeks after tamoxifen treatment. (B) Gross morphology of the liver and gallbladder in VhlF/F and VhlLivKO mice 2 weeks after tamoxifen treatment. (C) Bile acid species quantitation in livers of VhlF/F and VhlLivKO mice 2 weeks after tamoxifen treatment. CA, cholic acid; TCA, taurocholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; GCA, glycocholic acid; MCA, muricholic acid; T-β-MCA, tauro-β-muricholic acid; CDCA, chenodeoxycholic acid; HDCA, hyodeoxycholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid. N.D, not detected. (D) FXR target genes were assessed by qPCR in the liver and ileum of VhlF/F and VhlLivKO mice 2 weeks following tamoxifen treatment. Expression was normalized to β-actin. Five to eight mice were assessed per treatment group. Each bar graph represents the mean values ± SD. (E) Western blot analysis of FXR in livers of VhlF/F and VhlLivKO mice 2 weeks after tamoxifen treatment. *, P < 0.05 versus VhlF/F mice; **, P < 0.01 versus VhlF/F mice.
Repression of Cyp7a1 is HIF-2α dependent.
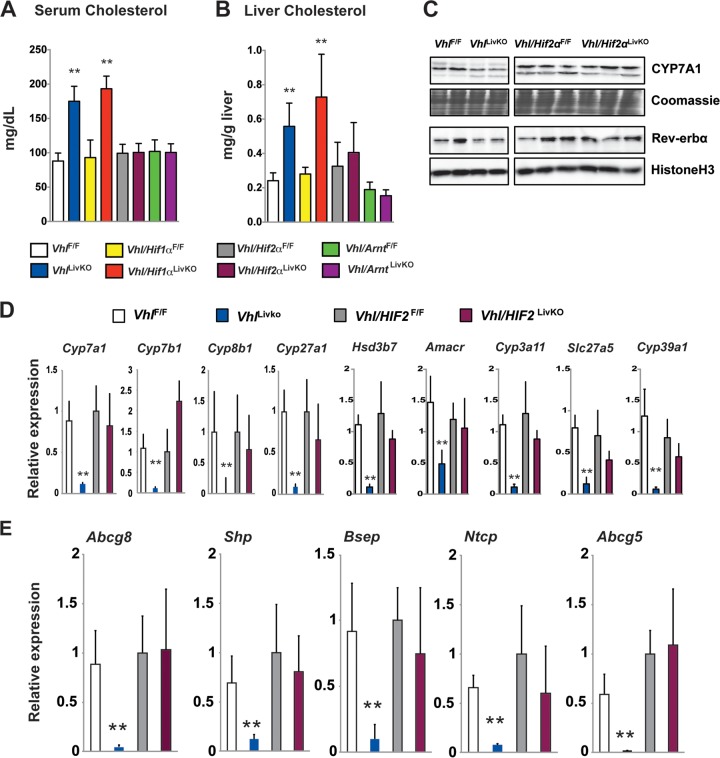
Disruption of Vhl results in the overexpression of both HIF-1α and HIF-2α (11). HIF-1α and HIF-2α exert distinct functions by regulating different subsets of genes (48). Elevation in the serum and hepatic cholesterol observed in VhlLivKO was completely abolished in mice with compound disruption of VHL and HIF-2α (Vhl/Hif-2αLivKO) and VHL and ARNT (Vhl/ArntLivKO) but not in mice with disruption of VHL and HIF-1α (Vhl/Hif-1αLivKO), demonstrating that perturbation in cholesterol levels was HIF-2α dependent (Fig. 7A and B). Concordant with the improvement in cholesterol homeostasis, CYP7A1 and Rev-erbα expression were rescued by combined disruption of VHL and HIF-2α (Fig. 7C). In addition, combined disruption of VHL and HIF-2α restored the expression of bile acid synthesis and FXR target genes (Fig. 7D and E). These data demonstrate that circadian regulation of cholesterol hydroxylases by HIF-2α plays a key role in cholesterol and bile homeostasis following hypoxia.
FIG 7.
Liver HIF-2α induces hypercholesterolemia and inhibits bile acid synthesis. Serum (A) and liver cholesterol (B) analyzed in VhlF/F, VhlLivKO, Vhl/Hif1αF/F, Vhl/Hif1αLivKO, Vhl/Hif2αF/F, and Vhl/Hif2αLivKO mice 2 weeks following tamoxifen treatment. (C) Western blot analysis examining the expression of CYP7A1 and Rev-erbα in the microsome fraction and nuclear lysates and qPCR analysis for bile acid synthesis genes (D) and FXR target genes (E) in livers of VhlF/F, VhlLivKO, Vhl/Hif2αF/F, and Vhl/Hif2αLivKO mice 2 weeks after tamoxifen treatment. Expression was normalized to β-actin. Five to nine mice were assessed per treatment group. Each bar graph represents the mean values ± SD. **, P < 0.01 versus VhlF/F mice.
Systemic hypoxia leads to hypercholesterolemia and a decrease in bile synthesis.
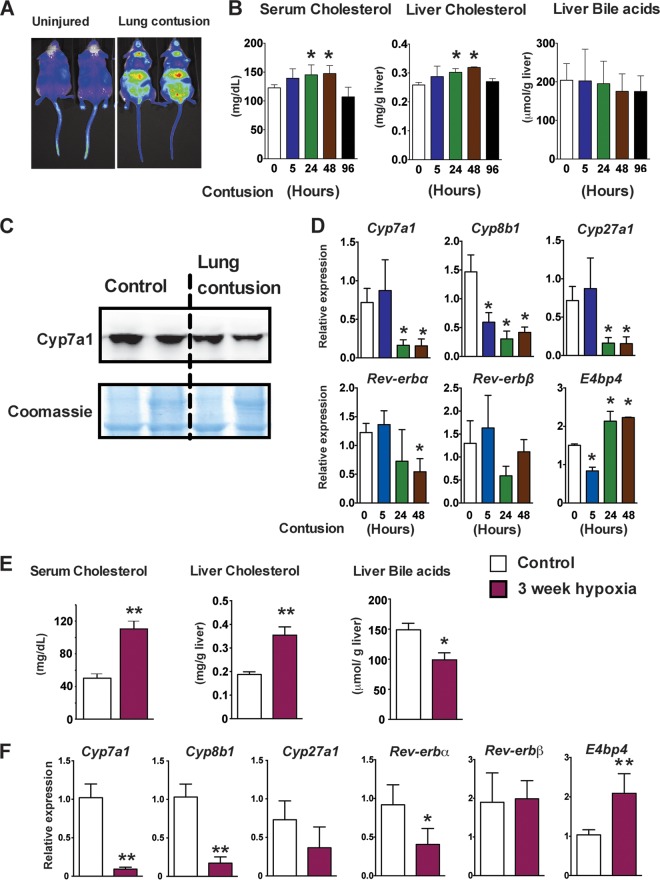
To further demonstrate that liver hypoxia signaling is a critical regulator of cholesterol homeostasis, a model of closed-chest unilateral lung contusion that rapidly induces systemic hypoxia was analyzed (49). Lung contusion induced a robust increase in systemic hypoxia 24 h following injury, as assessed in a hypoxia reporter mouse model, ODD-luc mice (Fig. 8A) (18, 23). In parallel with the systemic hypoxia, serum and liver cholesterol levels were significantly elevated at 24 and 48 h after the insult (Fig. 8B). However, the decrease in hepatic bile acid levels did not reach significance, raising the possibility that enterohepatic recycling of bile compensates for the short-term inhibition of bile acid synthesis (Fig. 8B). Systemic hypoxia decreased the levels of CYP7A1 protein in the microsomal fraction of liver 24 h after the initiation of lung contusion (Fig. 8C). In addition, mRNA levels of Cyp7a1, Cyp7b1, and Cyp27a1 were significantly decreased in livers at 24 and 48 h after lung injury (Fig. 8D). Further analysis revealed that the circadian gene Rev-erbα is significantly repressed at 48 h after lung injury with a concomitant increase in E4bp4 expression (Fig. 8D). To investigate whether chronic systemic hypoxia can affect cholesterol and bile acid homeostasis, C57BL/6 mice were exposed to 10% O2 for 3 weeks. Hypoxia significantly increased the serum and liver cholesterol levels and decreased hepatic bile acids (Fig. 8E). Moreover, the expression of cholesterol hydroxylases, such as Cyp7a1, Cyp8b1, and Cyp27a1, was significantly repressed (Fig. 8F). Similar to the lung contusion model, chronic hypoxia for 3 weeks significantly decreased Rev-erbα expression, resulting in increased expression of E4bp4 (Fig. 8F). The data demonstrate that activation of hypoxia signaling in liver increases hepatic and systemic cholesterol levels. Moreover, the data suggest that the adaptive increase in systemic cholesterol levels following hypoxia are due to a decrease in cholesterol clearance as bile acids.
FIG 8.
Chronic hypoxia and lung contusion increases cholesterol levels and decreases bile acid synthesis in mice. (A) In vivo imaging in Odd-luc mice demonstrating systemic hypoxia 24 h following lung contusion. (B) Serum cholesterol, liver cholesterol, and total liver bile acid measured at 5, 24, 48, and 96 h after induction of lung injury. (C) Western blot analysis of CYP7A1 in the microsome fractions 24 h after lung injury. Expression was normalized to Coomassie blue staining. (D) qPCR analysis in livers at 5, 24, and 48 h after lung injury. Expression was normalized to β-actin. Three to six mice were assessed per treatment group. (E) Serum cholesterol, liver cholesterol, and hepatic total bile acids measured in C57BL/6 mice exposed to 10% O2 for 3 weeks. (F) qPCR analysis of bile acid synthesis and circadian genes in mice exposed to 10% O2 for 3 weeks. Expression was normalized to β-actin. Four mice were assessed per treatment group. Each bar graph represents the mean values ± SD. *, P < 0.05 versus the control; **, P < 0.01 versus the control.
DISCUSSION
Cholesterol synthesis is a multienzymatic reaction which requires energy and oxygen. It is hypothesized that the evolution of cholesterol is directly correlated to atmospheric O2 (50). However, during hypoxia, adaptive mechanisms exist to maintain systemic cholesterol levels. Populations indigenous to high altitude, sleep apnea patients, and mouse models exposed to CIH have an increase in cholesterol levels and are at higher risk for cardiovascular diseases (2, 4, 5). It is worth noting that continuous positive airway pressure is the effective treatment for hypercholesterolemia in obstructive sleep apnea patients (3), further raising the possibility that hypoxia signaling is involved in the regulation of cholesterol homeostasis. The mechanism by which hypoxia sustains or induces systemic cholesterol levels is not well understood. Using liver-specific HIF-overexpressing (VhlLivKO) or systemic hypoxic mouse models, we demonstrate that hypoxia-mediated perturbation in cholesterol homeostasis is due to a decrease in the expression of cholesterol hydroxylases involved in bile synthesis. Moreover, the data suggest that HIF-2α plays a critical role in this mechanism by regulating circadian expression of hepatic Rev-erbα and Cyp7a1.
Hypercholesterolemia in VhlLivKO mice is not due to cholesterol biosynthesis or uptake. An increase in the intrahepatic cholesterol levels precedes the changes in LDL receptor levels, suggesting a defective cholesterol metabolism at the cellular level as the primary cause for aberrant cholesterol levels in VhlLivKO mice. However, the secondary decrease in the LDL receptor levels could further augment the increase in VLDL and LDL cholesterol, leading to hypercholesterolemia in VhlLivKO mice. CYP7A1, the key rate-limiting enzyme in bile acid synthesis, was inhibited by hypoxia and/or HIF-2α activation. Mice that overexpress CYP7A1 have increased bile acid pools and lower serum cholesterol (51, 52). On the other hand, Cyp7a1−/− mice have decreased bile acid synthesis. These studies are consistent with the data presented in the manuscript. However, Cyp7a1−/− mice do not have any overt differences in hepatic cholesterol levels compared to wild-type mice (53). Similarly, adenovirus-mediated rescue of CYP7A1 alone did not ameliorate cholesterol levels in primary hepatocytes from VhlLivKO mice. Bile acid metabolism is profoundly repressed in isolated cultured mouse primary hepatocytes compared to the liver; therefore, we cannot exclude the possibility that CYP7A1 is the primary hypoxia-repressed gene involved in hypercholesterolemia. However, a significant decrease in a battery of cholesterol hydroxylases in the liver of VhlLivKO mice suggests that global repression of other cholesterol hydroxylases, in addition to CYP7A1, is the essential mechanism that leads to dysregulated cholesterol levels following hypoxia.
Cholesterol levels exhibit circadian variation due to the diurnal expression of CYP7A1 (41). Rev-erbα is one of the key transcription factors that regulate the expression of various circadian genes. Rev-erbα knockout mice exhibit decreased bile acid levels due to loss of Cyp7a1 expression (42, 43). The current study demonstrates that activation of HIF signaling disrupts the circadian expression of Rev-erbα, resulting in increased expression of E4bp4, a negative regulator of Cyp7a1 (42). In addition, systemic hypoxia due to lung contusion or chronic hypoxia exposure of mice recapitulates the decrease in genes involved in bile acid synthesis and bile acid levels with a concomitant increase in systemic cholesterol levels. Furthermore, repression of CYP7A1 is also associated with loss of Rev-erbα and increase in E4bp4 in these hypoxic mouse models (42). The expression of other cholesterol hydroxylases was not repressed by HIF-2α through disrupting circadian regulation. This suggests that disruption of VHL inhibits bile acid synthesis, perhaps through other parallel mechanisms that need to be further investigated. Together, these results suggest that perturbation in cholesterol homeostasis following hypoxia is an adaptive mechanism to increase systemic cholesterol levels in an HIF-2α-dependent manner. The profound effect of hypoxia signaling on cholesterol metabolism is through decreasing cholesterol clearance as bile acids via disrupting the circadian expression of CYP7A1 and by repressing downstream cholesterol hydroxylases through a currently unknown mechanism.
Atherosclerosis is a well-known inflammation and lipid-associated disease, and chronic intermittent hypoxia (CIH) exacerbates disease progression (6, 7, 54). An important mechanism that contributes to atherosclerosis is recruitment of macrophages that scavenge fatty acids, resulting in the formation of foam cells. Several studies have demonstrated the importance of HIF-1α in foam cell formation and atherosclerosis (55, 56). Moreover, recently it was shown that HIF-1α induction of Angptl4 in adipose tissues contributes to the hypoxic potentiation of atherosclerosis (57). However, partial disruption of HIF-1α in adipose tissue did not improve hypercholesterolemia caused by CIH. The role of liver HIF-2α in hypoxia-mediated atherosclerosis has not yet been explored. CIH induces systemic inflammation, and recently we demonstrated that HIF-2α induces a proinflammatory response by increasing tumor necrosis factor alpha expression (58). The present work, together with our previous study (9), demonstrates that chronic hypoxic signaling through HIF-2α in the liver may initiate a proatherogenic environment by inducing inflammation and hypercholesterolemia.
The data in this study demonstrate that a decrease in cholesterol clearance as bile is the primary mechanism for the adaptive increase in liver and systemic cholesterol levels during hypoxia. Furthermore, HIF-2α regulation of the circadian gene Rev-erbα was demonstrated as a critical mechanism in cholesterol homeostasis. Since HIF-2α has an essential role in hepatic inflammation and liver cholesterol homeostasis, HIF-2α could be a therapeutic target to improve dyslipidemia and systemic inflammation in patients with increased risk of cardiovascular disease.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants (CA148828 and DK095201 to Y.M.S and HL-102013 to K.R.), the University of Michigan Gastrointestinal Peptide Center (Y.M.S.), and the National Cancer Institute Intramural Research Program (F.J.G).
We thank Phillip B. Hylemon (Virginia Commonwealth University) for the generous gift of AD-CYP7a1 and Yanqiao Zhang (Northeast Ohio Medical University) for AD-HNF4α and AD-LRH-1.
We declare no conflicts of interest.
Footnotes
Published ahead of print 13 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01441-13.
REFERENCES
- 1.Summons RE, Bradley AS, Jahnke LL, Waldbauer JR. 2006. Steroids, triterpenoids and molecular oxygen. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361:951–968. 10.1098/rstb.2006.1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherpa LY, Deji Stigum H, Chongsuvivatwong V, Luobu O, Thelle DS, Nafstad P, Bjertness E. 2011. Lipid profile and its association with risk factors for coronary heart disease in the highlanders of Lhasa, Tibet. High Alt. Med. Biol. 12:57–63. 10.1089/ham.2010.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michailidis V, Steiropoulos P, Nena E, Papanas N, Maltezos E, Bouros D. 2011. Continuous positive airway pressure treatment: effect on serum lipids in patients with obstructive sleep apnoea. Open Cardiovasc. Med. J. 5:231–238. 10.2174/1874192401105010231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. 2004. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax 59:777–782. 10.1136/thx.2003.018739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanner BM, Fluerenbrock N, Kleiber-Imbeck A, Mueller JB, Zidek W. 2001. Effect of continuous positive airway pressure therapy on infectious complications in patients with obstructive sleep apnea syndrome. Respiration 68:483–487. 10.1159/000050555 [DOI] [PubMed] [Google Scholar]
- 6.Fang G, Song D, Ye X, Mao SZ, Liu G, Liu SF. 2012. Chronic intermittent hypoxia exposure induces atherosclerosis in ApoE knockout mice: role of NF-kappaB p50. Am. J. Pathol. 181:1530–1539. 10.1016/j.ajpath.2012.07.024 [DOI] [PubMed] [Google Scholar]
- 7.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. 2010. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 209:381–386. 10.1016/j.atherosclerosis.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drager LF, Jun J, Polotsky VY. 2010. Obstructive sleep apnea and dyslipidemia: implications for atherosclerosis. Curr. Opin. Endocrinol. Diabetes Obes. 17:161–165. 10.1097/MED.0b013e3283373624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu A, Taylor M, Xue X, Matsubara T, Metzger D, Chambon P, Gonzalez FJ, Shah YM. 2011. Hypoxia-inducible transcription factor 2alpha promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology 54:472–483. 10.1002/hep.24400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang GL, Jiang BH, Rue EA, Semenza GL. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U. S. A. 92:5510–5514. 10.1073/pnas.92.12.5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haase VH, Glickman JN, Socolovsky M, Jaenisch R. 2001. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc. Natl. Acad. Sci. U. S. A. 98:1583–1588. 10.1073/pnas.98.4.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. 2006. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3:177–185. 10.1016/j.cmet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. 2009. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol. Cell. Biol. 29:4527–4538. 10.1128/MCB.00200-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B, Haase VH. 2008. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene 27:5354–5358. 10.1038/onc.2008.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albers JJ, Bierman EL. 1976. The effect of hypoxia on uptake and degradation of low density lipoproteins by cultured human arterial smooth muscle cells. Biochim. Biophys. Acta 424:422–429. 10.1016/0005-2760(76)90031-X [DOI] [PubMed] [Google Scholar]
- 16.Mukodani J, Ishikawa Y, Fukuzaki H. 1990. Effects of hypoxia on sterol synthesis, acyl-CoA:cholesterol acyltransferase activity, and efflux of cholesterol in cultured rabbit skin fibroblasts. Arteriosclerosis 10:106–110. 10.1161/01.ATV.10.1.106 [DOI] [PubMed] [Google Scholar]
- 17.Raghavendran K, Davidson BA, Helinski JD, Marschke CJ, Manderscheid P, Woytash JA, Notter RH, Knight PR. 2005. A rat model for isolated bilateral lung contusion from blunt chest trauma. Anesth. Analg. 101:1482–1489. 10.1213/01.ANE.0000180201.25746.1F [DOI] [PubMed] [Google Scholar]
- 18.Safran M, Kim WY, O'Connell F, Flippin L, Gunzler V, Horner JW, Depinho RA, Kaelin WG., Jr 2006. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc. Natl. Acad. Sci. U. S. A. 103:105–110. 10.1073/pnas.0509459103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah YM, Ito S, Morimura K, Chen C, Yim SH, Haase VH, Gonzalez FJ. 2008. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology 134:2036–2048. 10.1053/j.gastro.2008.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. 2009. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 9:152–164. 10.1016/j.cmet.2008.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, Shah YM. 2011. Hypoxia-inducible factor-2alpha mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 140:2044–2055. 10.1053/j.gastro.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue X, Taylor M, Anderson E, Hao C, Qu A, Greenson JK, Zimmermann EM, Gonzalez FJ, Shah YM. 2012. Hypoxia-inducible factor-2alpha activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 72:2285–2293. 10.1158/0008-5472.CAN-11-3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson ER, Taylor M, Xue X, Martin A, Moons DS, Omary MB, Shah YM. 2012. The hypoxia-inducible factor-C/EBPalpha axis controls ethanol-mediated hepcidin repression. Mol. Cell. Biol. 32:4068–4077. 10.1128/MCB.00723-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu A, Shah YM, Manna SK, Gonzalez FJ. 2012. Disruption of endothelial peroxisome proliferator-activated receptor gamma accelerates diet-induced atherogenesis in LDL receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 32:65–73. 10.1161/ATVBAHA.111.239137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng J, Krausz KW, Li F, Ma X, Gonzalez FJ. 2013. CYP2E1-dependent elevation of serum cholesterol, triglycerides, and hepatic bile acids by isoniazid. Toxicol. Appl. Pharmacol. 266:245–253. 10.1016/j.taap.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. 2007. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 48:2664–2672. 10.1194/jlr.M700330-JLR200 [DOI] [PubMed] [Google Scholar]
- 27.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr 2001. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464–468. 10.1126/science.1059817 [DOI] [PubMed] [Google Scholar]
- 28.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. 2001. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472. 10.1126/science.1059796 [DOI] [PubMed] [Google Scholar]
- 29.Brown MS, Goldstein JL. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331–340. 10.1016/S0092-8674(00)80213-5 [DOI] [PubMed] [Google Scholar]
- 30.Bricker LA, Weis HJ, Siperstein MD. 1972. In vivo demonstration of the cholesterol feedback system by means of a desmosterol suppression technique. J. Clin. Investig. 51:197–205. 10.1172/JCI106804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein JL, Brown MS. 2009. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29:431–438. 10.1161/ATVBAHA.108.179564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma PT, Gil G, Sudhof TC, Bilheimer DW, Goldstein JL, Brown MS. 1986. Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc. Natl. Acad. Sci. U. S. A. 83:8370–8374. 10.1073/pnas.83.21.8370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell DW, Yamamoto T, Schneider WJ, Slaughter CJ, Brown MS, Goldstein JL. 1983. cDNA cloning of the bovine low density lipoprotein receptor: feedback regulation of a receptor mRNA. Proc. Natl. Acad. Sci. U. S. A. 80:7501–7505. 10.1073/pnas.80.24.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy E, Spahis S, Sinnett D, Peretti N, Maupas-Schwalm F, Delvin E, Lambert M, Lavoie MA. 2007. Intestinal cholesterol transport proteins: an update and beyond. Curr. Opin. Lipidol. 18:310–318. 10.1097/MOL.0b013e32813fa2e2 [DOI] [PubMed] [Google Scholar]
- 35.Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. 2007. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J. Nutr. 137:1776–1782 [DOI] [PubMed] [Google Scholar]
- 36.Swaner JC, Connor WE. 1975. Hypercholesterolemia of total starvation: its mechanism via tissue mobilization of cholesterol. Am. J. Physiol. 229:365–369 [DOI] [PubMed] [Google Scholar]
- 37.Duane WC. 2009. Bile acids: developments new and very old. J. Lipid Res. 50:1507–1508. 10.1194/jlr.E900004-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chai J, He Y, Cai SY, Jiang Z, Wang H, Li Q, Chen L, Peng Z, He X, Wu X, Xiao T, Wang R, Boyer JL, Chen W. 2012. Elevated hepatic multidrug resistance-associated protein 3/ATP-binding cassette subfamily C 3 expression in human obstructive cholestasis is mediated through tumor necrosis factor alpha and c-Jun NH2-terminal kinase/stress-activated protein kinase-signaling pathway. Hepatology 55:1485–1494. 10.1002/hep.24801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T, Jahan A, Chiang JY. 2006. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology 43:1202–1210. 10.1002/hep.21183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kir S, Zhang Y, Gerard RD, Kliewer SA, Mangelsdorf DJ. 2012. Nuclear receptors HNF4alpha and LRH-1 cooperate in regulating Cyp7a1 in vivo. J. Biol. Chem. 287:41334–41341. 10.1074/jbc.M112.421834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noshiro M, Usui E, Kawamoto T, Kubo H, Fujimoto K, Furukawa M, Honma S, Makishima M, Honma K, Kato Y. 2007. Multiple mechanisms regulate circadian expression of the gene for cholesterol 7alpha-hydroxylase (Cyp7a), a key enzyme in hepatic bile acid biosynthesis. J. Biol. Rhythms 22:299–311. 10.1177/0748730407302461 [DOI] [PubMed] [Google Scholar]
- 42.Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, Fontaine C, Derudas B, Bauge E, Havinga R, Bloks VW, Wolters H, van der Sluijs FH, Vennstrom B, Kuipers F, Staels B. 2008. Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology 135:689–698. 10.1053/j.gastro.2008.05.035 [DOI] [PubMed] [Google Scholar]
- 43.Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Lo Sasso G, Moschetta A, Schibler U. 2009. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 7:e1000181. 10.1371/journal.pbio.1000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. 2013. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 4:2384. 10.1038/ncomms3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. 2013. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17:225–235. 10.1016/j.cmet.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 46.Chiang JY, Kimmel R, Weinberger C, Stroup D. 2000. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J. Biol. Chem. 275:10918–10924. 10.1074/jbc.275.15.10918 [DOI] [PubMed] [Google Scholar]
- 47.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744. 10.1016/S0092-8674(00)00062-3 [DOI] [PubMed] [Google Scholar]
- 48.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. 2003. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell. Biol. 23:9361–9374. 10.1128/MCB.23.24.9361-9374.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, Knight PR. 2009. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock 32:122–130. 10.1097/SHK.0b013e31819c385c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galea AM, Brown AJ. 2009. Special relationship between sterols and oxygen: were sterols an adaptation to aerobic life? Free Radic. Biol. Med. 47:880–889. 10.1016/j.freeradbiomed.2009.06.027 [DOI] [PubMed] [Google Scholar]
- 51.Li T, Francl JM, Boehme S, Chiang JY. 2013. Regulation of cholesterol and bile acid homeostasis by the CYP7A1/SREBP2/miR-33a axis. Hepatology 58:1111–1121. 10.1002/hep.26427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li T, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G, Ellis E, Chiang JY. 2011. Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 53:996–1006. 10.1002/hep.24107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwarz M, Russell DW, Dietschy JM, Turley SD. 1998. Marked reduction in bile acid synthesis in cholesterol 7alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 39:1833–1843 [PubMed] [Google Scholar]
- 54.Drager LF, Polotsky VY, Lorenzi-Filho G. 2011. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest 140:534–542. 10.1378/chest.10-2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bostrom P, Magnusson B, Svensson PA, Wiklund O, Boren J, Carlsson LM, Stahlman M, Olofsson SO, Hulten LM. 2006. Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler. Thromb. Vasc. Biol. 26:1871–1876. 10.1161/01.ATV.0000229665.78997.0b [DOI] [PubMed] [Google Scholar]
- 56.Jiang G, Li T, Qiu Y, Rui Y, Chen W, Lou Y. 2007. RNA interference for HIF-1alpha inhibits foam cells formation in vitro. Eur. J. Pharmacol. 562:183–190. 10.1016/j.ejphar.2007.01.066 [DOI] [PubMed] [Google Scholar]
- 57.Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, Schwartz AR, Halberg N, Scherer PE, Semenza GL, Powell DR, Polotsky VY. 2013. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am. J. Respir. Crit. Care Med. 188:240–248. 10.1164/rccm.201209-1688OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM. 2013. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145:831–841. 10.1053/j.gastro.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.