Abstract
A growing body of evidence has underlined the significance of endoplasmic reticulum (ER) stress in the pathogenesis of diabetes mellitus. ER oxidoreductin 1β (ERO1β) is a pancreas-specific disulfide oxidase that is known to be upregulated in response to ER stress and to promote protein folding in pancreatic β cells. It has recently been demonstrated that ERO1β promotes insulin biogenesis in β cells and thus contributes to physiological glucose homeostasis, though it is unknown if ERO1β is involved in the pathogenesis of diabetes mellitus. Here we show that in diabetic model mice, ERO1β expression is paradoxically decreased in β cells despite the indications of increased ER stress. However, overexpression of ERO1β in β cells led to the upregulation of unfolded protein response genes and markedly enlarged ER lumens, indicating that ERO1β overexpression caused ER stress in the β cells. Insulin contents were decreased in the β cells that overexpressed ERO1β, leading to impaired insulin secretion in response to glucose stimulation. These data indicate the importance of the fine-tuning of the ER redox state, the disturbance of which would compromise the function of β cells in insulin synthesis and thus contribute to the pathogenesis of diabetes mellitus.
INTRODUCTION
Diabetes mellitus has long been a worldwide threat. One of the essential aspects of diabetic pathogenesis is the progressive dysfunction of pancreatic β cells. It is widely believed that during the course of diabetes progression, insulin secretion from β cells gradually declines, eventually leading to hyperglycemia with an insufficient insulin supply to compensate for the increased insulin demand imposed by peripheral insulin resistance (1, 2). This state is called pancreatic β cell failure, the pathophysiology of which has, however, still not been fully elucidated. Endoplasmic reticulum (ER) stress is one of the strong candidates for the mechanisms underlying β cell failure (3, 4), and thus, the molecules and signaling pathways involved in the ER stress response have been intensively investigated as possible therapeutic targets for diabetes mellitus (5–7).
ER stress is known to be induced in response to multiple stimuli, all of which essentially interfere with proper protein folding in the ER. These mechanisms include impairing protein glycosylation, causing malfunctions of chaperones, or compromising oxidized protein folding, and they eventually lead to an accumulation of unfolded proteins (8, 9). Oxidized protein folding, or disulfide bond formation within a nascent polypeptide, is a facilitated process aided by protein disulfide isomerases (PDIs) (10) that is dependent on the highly oxidizing condition of the ER (11). Recently it has been reported that several ER resident proteins play essential roles in maintaining the ER oxidizing condition (12, 13), among which are a family of conserved genes termed ER oxidoreductin 1 (ERO1). ERO1p, the protein encoded by ERO1, couples the oxidizing power of molecular oxygen to generate disulfide bonds, which are eventually transferred from PDIs to client secretory proteins (11). Thus, ERO1 loss-of-function mutants of Saccharomyces cerevisiae accumulate reduced misfolded proteins in the ER (14, 15). Previous reports have shown that in S. cerevisiae, ERO transcripts are induced upon ER stress in the course of the unfolded-protein response (UPR), establishing that EROs are members of the UPR gene family (14, 15). In contrast, mammals have two isoforms of ERO, ERO1α and ERO1β, which have distinct functions with different tissue distributions (16). Importantly, only ERO1β transcripts are induced upon ER stress (16), whereas the regulation of ERO1α expression seems to be associated with hypoxia (17, 18). Furthermore, ERO1β transcripts are abundant in the pancreas (16), with preferentially higher expression in the islets than in the exocrine cells (19). Together with the facts that β cells are highly professionalized cells for insulin synthesis, with proinsulin accounting for up to 50% of the total protein (20, 21), and that the folding of proinsulin requires three intrachain disulfide bond formations (4, 22), it has been speculated that ERO1β would play significant roles in the physiological function of pancreatic β cells and not any less in the pathogenesis of diabetes mellitus.
Recently, Zito et al. have reported that whole-body deletion of ERO1β specifically affects pancreatic β cells, compromising the oxidative folding of insulin and thus leading to glucose intolerance in mice (23). Another report has demonstrated that suppressed ERO1β expression in pancreatic β cells leads to an increased susceptibility to ER stress and a reduction of insulin content (24). While these data clearly indicate that ERO1β plays an important role in insulin biogenesis in β cells and contributes to physiological glucose homeostasis, it is as yet unclear how the expression or function of ERO1β is changed during pancreatic β cell failure and what its precise roles in the pathogenesis of diabetes are. Here we report that, unlike the expression of other UPR genes, that of ERO1β transcripts in the islets paradoxically declines during the course of diabetes progression despite increased ER stress. However, mice overexpressing human ERO1β specifically in pancreatic β cells showed impaired glucose tolerance due to reduced insulin secretion. In β cells overexpressing ERO1β, the expression of UPR genes was upregulated and the ER lumens were markedly enlarged, indicating that ERO1β overexpression caused ER stress in the β cells.
MATERIALS AND METHODS
Animals.
BKS.Cg-m+/+ Leprdb/J (db/db) mice and control misty/misty mice were purchased from Japan CLEA. Akita mice were purchased from Japan SLC. For the generation of hERO1βTg mice, a fusion gene was designed that comprised the rat insulin promoter and human ERO1LB cDNA coding sequences with a Flag tag at its C terminus so that its expression was targeted to β cells. The linearized construct was microinjected into the pronuclei of fertilized C57BL/6 mouse (Japan CLEA) eggs. Transgenic founder mice were identified by PCR analysis by using a primer for the Flag sequence, which was also used to determine the tissue distribution of the transgene by PCR after reverse transcription (RT). All experiments were conducted with heterozygote male mice. High-fat diet (HFD) feeding was started at 7 weeks of age where required. The Animal Care Committee of the University of Tokyo approved the animal care conditions and experimental procedures used.
Quantitative real-time PCR.
Total RNA was prepared with the RNeasy kit (Qiagen). RT reagents (Applied Biosystems) were used to prepare cDNA. Quantitative real-time PCR was performed with ABI Prism and PCR Master Mix reagent (Applied Biosystems). The sequences of the primers and probe used for the simultaneous detection of human ERO1B and mouse Ero1b were as follows: forward primer, TGGAGTTCTGGATGATTGCTT; reverse primer, TCTTCTGCCCAGAAAGGACA; probe, CGTTATTACAAGGTTAATCTGAA. All of the other primers and probes used were purchased from Applied Biosystems. The levels of mRNAs were normalized to that of cyclophilin (25).
Immunoblotting.
Immunoblotting was conducted as previously described (25). The antibodies used for immunoblotting were anti-phospho-PERK antibody (Thr980; Cell Signaling Technology); anti-phospho-eukaryotic transcription initiation factor 2 alpha subunit (anti-phospho-eIF2α) antibody (Ser51; Cell Signaling Technology); anti-4E-BP1 antibody (Cell Signaling), and anti-β-actin antibody (Sigma-Aldrich).
Metabolic assays.
A glucose tolerance test (GTT) was performed as described previously (26). The mice were fasted for 16 h, and blood samples were obtained at the indicated time points after the intraperitoneal injection of 1 g/kg body weight of d-glucose (WAKO). Blood glucose levels were checked at indicated time points (Glutest Pro; Sanwa Kagaku Kenkyusho).
Immunohistochemical and morphometric analyses of the pancreas.
Immunohistochemical and morphometric analyses of pancreas sections were performed as described earlier (27) with a slight modification. Six mice under each condition at 11, 22, and 36 weeks of age were subjected to morphometric analysis. Sections were stained with antibodies as indicated. For morphometric analysis, the images of islets were traced manually and analyzed by ImageJ software (NIH). The mean of four different sections of each pancreas was used for the analysis.
Islet isolation.
Islets were isolated by Liberase RI (Roche) with pancreatic perfusion and subsequent digestion for 24 min at 37°C (28). Islets were picked manually in Hanks' balanced salt solution (Sigma) buffer supplemented with 10% fetal calf serum and 25 mM HEPES buffer and then immediately used for further experiments, except for the pulse-chase analysis, where the islets were subjected to the experiments after overnight incubation in RPMI 1640 medium (GIBCO) supplemented with 10% (vol/vol) fetal bovine serum (GIBCO).
Glucose-stimulated insulin secretion (GSIS) assay.
Freshly isolated islets were maintained in Krebs-Ringer bicarbonate (KRB) buffer (129 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 5 mM NaHCO3, 10 mM HEPES [adjusted to pH 7.4]) containing 0.2% bovine serum albumin supplemented with 2.8 mM glucose for 30 min at 37°C. The islets were then incubated for 30 min in the same buffer containing 22.4 mM glucose or 50 mM KCl as indicated. For the MIN6 β cell experiment, cells were cultured in Dulbecco's modified Eagle's medium (GIBCO) supplemented with 10% (vol/vol) fetal bovine serum (GIBCO). The cells were incubated with KRB buffer with 2.8 mM glucose for 60 min at 37°C, and then the medium was changed to KRB buffer with 22.4 mM glucose for further incubation for 30 min. For measurement of insulin content, insulin was extracted from islets or cultured cells by overnight incubation with acid ethanol at −20°C. Insulin concentrations were measured with an insulin radioimmunoassay kit (Institute of Isotopes) according to the manufacturer's instructions.
Electron microscopy.
Two mice of each genotype were anesthetized and subjected to cardiac perfusion with 0.1 M sodium phosphate buffer (pH 7.2) containing 2% glutaraldehyde and 2% paraformaldehyde. The pancreas was excised from each mouse, cut into small pieces, and immersed overnight in the same fixative. The tissue was then exposed to 2% osmium tetroxide, stained with 2% uranyl acetate, dehydrated with ethanol, and embedded in Epon812 (TAAB). Thin sections were stained with uranyl acetate and lead citrate before examination with a Hitachi 7100 electron microscope (Hitachi). The quantification of ER luminal areas was done by a previously described method (29) in which 22 to 34 pictures were taken per animal and then by using a double-lattice test system with a spacing of 1 cm, the points that fell on the ER lumen were counted. The ratio of the points falling on the ER lumen to the points falling in the entire 20-by-20 double lattice was recorded as the ER luminal area percentage.
Detection of superoxide.
Superoxide was detected in frozen pancreas sections with dihydroethidium (DHE; 10 μmol/liter) in phosphate-buffered saline (PBS) for 30 min at 37°C in a humidified chamber protected from light. DNA-bound ethidium bromide, which was formed from DHE on reaction with superoxide, was detected as red fluorescence (30).
Generation and infection of adenoviruses.
Adenovirus encoding human ERO1β was generated according to the manufacturer's protocol (TaKaRa Biotechnology) by using the same construct as that used to generate hERO1βTg mice. An adenovirus encoding LacZ was purchased from TaKaRa Biotechnology and used as the negative control. Prior to use, all adenoviruses were purified on a cesium chloride gradient and dialyzed into PBS plus 10% glycerol. MIN6 β cells were infected with the adenoviruses at a multiplicity of infection (MOI or number of viral particles per cell) of 3,000 PFU/cell. The cells were subjected to experiments 48 h after adenovirus infection.
Pulse-chase analysis.
A total of 65 islets were preincubated in 500 μl of methionine- and cysteine-free RPMI 1640 medium (GIBCO) for 1 h and then labeled in the same medium containing [35S]methionine-cysteine (EXPRE35S35S protein labeling mix; PerkinElmer) at a concentration of 10 μCi/ml for 30 min. When necessary, a subsequent radiolabel-free chase was performed with complete RPMI medium supplemented with 10% (vol/vol) fetal bovine serum (GIBCO) after the islets were washed twice with the same medium, and islets were frozen in liquid nitrogen at the indicated times. The lysates were immunoprecipitated with anti-insulin antibody (ab8304 insulin plus proinsulin antibody; Abcam). Immunoprecipitated proteins were resolved by Tricine-SDS-PAGE with 15% polyacrylamide gel and detected by autoradiography with a phosphorimager (Typhoon FLA 7000; GE Healthcare).
Statistical analysis.
Statistical analysis was performed by using the paired two-sample t test for means. Analysis of variance (ANOVA) and Tukey's post hoc analyses were used when more than two groups were compared. Repeated-measures ANOVA was used for analyzing the results of metabolic assays. Statistical significance was accepted at P values <0.05.
RESULTS
ERO1β expression was decreased in the islets of diabetic model mice despite evidence of ER stress.
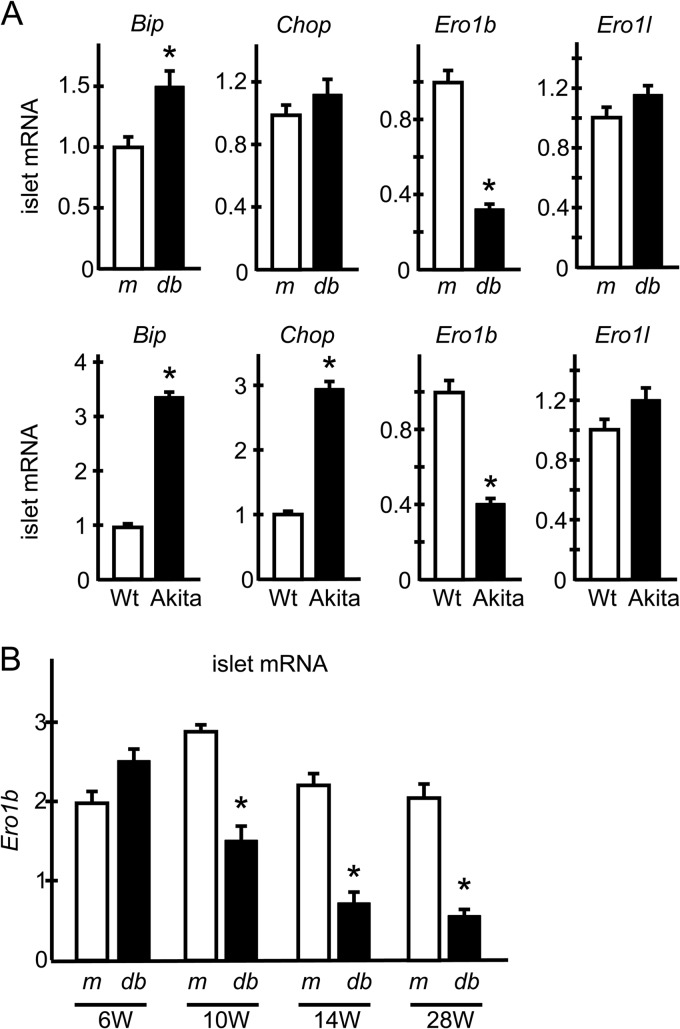
To investigate the roles of ERO1β in the pathogenesis of diabetes, we first examined ERO1β mRNA expression in the islets of diabetic db/db mice. As widely accepted, the expression of UPR genes such as Bip and Chop tended to be upregulated in db/db islets, most likely reflecting the increased ER stress in the β cells. In contrast, the expression of Ero1b was paradoxically lower than that in control misty/misty mouse islets (Fig. 1A, upper panels). The expression levels of Ero1l, which encodes the other isoform of ERO1 protein, ERO1α, were relatively similar in db/db and control misty/misty mouse islets (Fig. 1A, upper panels). We next investigated the islets of Akita mice as another diabetic model mouse that harbors a C96Y mutation in the insulin-2 gene resulting in misfolded proinsulin accumulation and progressive β cell loss due to ER stress-induced apoptosis (7, 31). Again, the expression of Ero1b was paradoxically decreased despite the robust upregulation of other typical UPR genes (Fig. 1A, lower panels). Moreover, the reduction of Ero1b in db/db islets occurred in an age-dependent manner, which was consistent with the time course of diabetes progression, with its expression being maintained, or tending to be higher, at early ages (Fig. 1B). These results highlight the special nature of ERO1β among UPR genes, namely, its lack of any upregulation under ER-stressed conditions. These data prompted us to hypothesize that ERO1β overexpression in β cells would benefit the β cells and rescue the glucose intolerance seen under pathological conditions such as those experienced during HFD feeding.
FIG 1.
Ero1b expression in the islets of diabetic model mice. (A) Ero1b expression in the islets of db/db and Akita mice. Pancreatic islets were isolated from db/db (db) and misty/misty (m) mice at 14 weeks of age or from Akita and control C57BL/6 mice at 7 weeks of age. Total mRNA was extracted and subjected to RT-PCR analysis of the genes indicated. n = 4 to 6; *, P < 0.05. (B) Pancreatic islets were isolated from db/db (db) or misty/misty (m) mice at the indicted weeks of age. Total mRNA was extracted and subjected to RT-PCR analysis of Ero1b expression. n = 4 to 6; *, P < 0.05. The data shown are means ± the standard errors of the means.
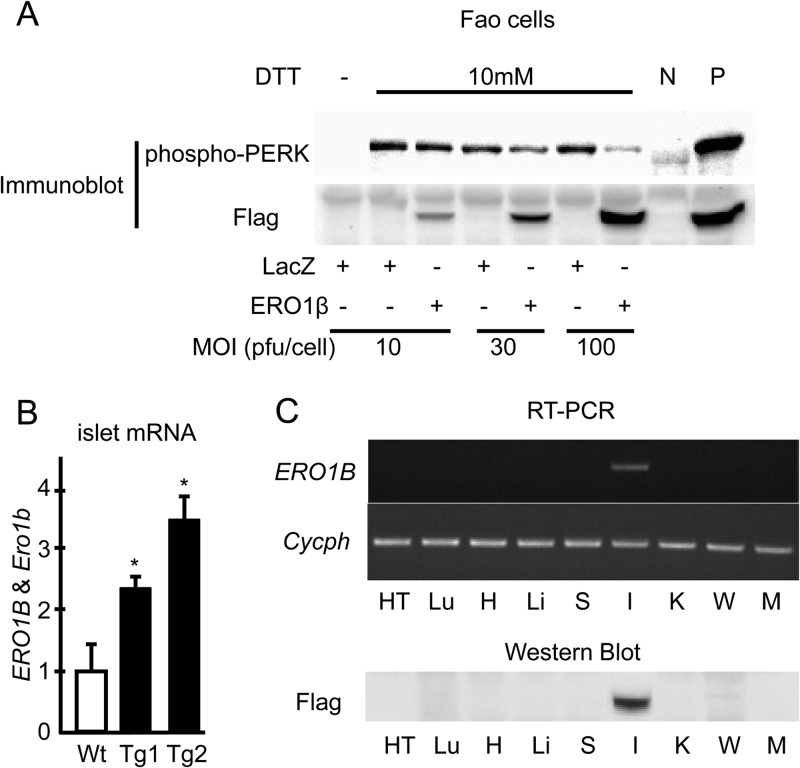
First we overexpressed Flag-tagged human ERO1β with adenovirus in Fao rat hepatoma cells. The Flag tag was added at the C terminus of the construct so that the tag would not interfere with the signal sequence at the N terminus of ERO1β (32). Overexpression of ERO1β in Fao cells ameliorated the dithiothreitol (DTT)-induced UPR response in a dose-dependent manner, as revealed by reduced pancreatic ER kinase (PERK) phosphorylation during DTT treatment (Fig. 2A), suggesting not only that human ERO1β was functionally valid as a redox regulator in rodent cells but also that ERO1β overexpression could counteract the reducing effects of DTT in Fao cells. Thus, we created a mouse line overexpressing Flag-tagged human ERO1β specifically in β cells under the control of a rat insulin promoter (hERO1βTg mice). We obtained two lines (Tg1 and Tg2) with different but similar levels of overexpression of the ERO1B gene, the mRNA expression levels of which were quantified by an RT-PCR analysis designed to amplify the mRNA region common to human ERO1B and mouse Ero1b (Fig. 2B). In the following experiments we essentially used the Tg1 line (referred to as Tg in this report) to characterize our overexpression model, while key experiments were also repeated with the Tg2 line. The expression of human ERO1β in these Tg mice, which was determined by measuring Flag expression, was detected specifically in pancreatic islets (Fig. 2C). These mice were born normally and showed no obvious abnormalities in their appearance.
FIG 2.
Human ERO1B overexpression in Fao cells and hERO1βTg mouse islets. (A) Adenoviral overexpression of human ERO1B in Fao cells. Fao cells were infected with adenovirus encoding human ERO1β or the control LacZ at the indicated MOIs. The cells were incubated with 10 mM DTT for 0.5 h. Total cell lysates were prepared and subjected to immunoblotting with anti-phospho-PERK or anti-Flag antibody. N, negative control; P, positive input. (B) Ero1b and ERO1B expression in the islets of hERO1βTg mice. Islets were isolated from hERO1βTg (Tg1 and Tg2) or control Wt mice at 14 weeks of age. Total mRNA was extracted from the islets and subjected to RT-PCR analysis, which detects mouse Ero1b and human ERO1B, as described in Materials and Methods. n = 6 to 9; *, P < 0.05. The data shown are means ± the standard errors of the means. (C) Distribution of the transgene ERO1B in the tissues of hERO1βTg mice. Each tissue type was removed from hERO1βTg mice at 9 weeks of age. Total mRNA was extracted from the tissues, 0.2 μg of which was subjected to RT and subsequent PCR analysis amplifying Flag-tagged human ERO1B cDNA or cyclophilin (upper panel). Lysate of protein from each tissue type was prepared and subjected to immunoblotting with Flag antibody at 10 μg/lane (lower panel). Tissue types: HT, hypothalamus; Lu, lung; H, heart; Li, liver; S, spleen; I, islet; K, kidney; W, epididymal white adipose tissue; M, skeletal muscle.
hERO1βTg mice showed impaired glucose tolerance with reduced insulin secretion.
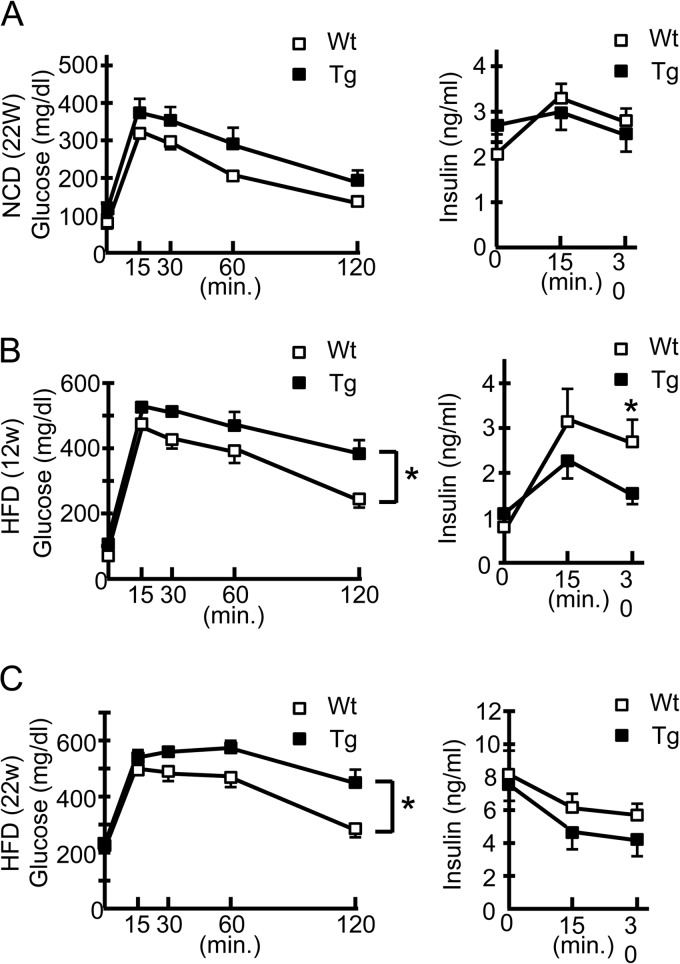
To explore the effects of ERO1β overexpression in β cells under physiological as well as pathological conditions, we fed hERO1βTg mice with a normal chow diet (NCD) or an HFD and examined their metabolic phenotypes. hERO1βTg mice showed a body weight gain similar to that of control wild-type (Wt) mice during both NCD and HFD feeding (data not shown). Unexpectedly, hERO1βTg mice showed impaired glucose tolerance in the GTT compared to findings for the control Wt mice, with a statistically significant difference only under the HFD feeding condition (Fig. 3A to C, left panels). The exacerbated glucose intolerance seen in the HFD-fed hERO1βTg mice was due to their lower insulin secretion than that of the Wt control mice (Fig. 3B and C, right panels). No difference in insulin sensitivity between hERO1βTg and Wt control mice was detectable in an insulin tolerance test (data not shown). To confirm that ERO1β overexpression does not benefit β cells, we also created hERO1βTg mice with a db/db background (Wt/Leprdb or Tg/Leprdb mice) by crossing Tg2 line mice with C57BLKS-Leprdb heterozygotes. We then tested their glucose tolerance with a GTT, in which we observed no improvement or worsening of the glucose levels in Tg/Leprdb mice compared with those of Wt/Leprdb mice, where the blood glucose level had already reached >500 mg/dl after a half-dose glucose challenge (data not shown).
FIG 3.
Metabolic phenotypes of hERO1βTg mice. Shown are blood glucose levels (right panels) and serum insulin concentrations (left panels) after the intraperitoneal injection of glucose. hERO1βTg (Tg) or Wt control mice were intraperitoneally injected with glucose at 1 g/kg body weight during NCD feeding at 22 weeks of age (A), during HFD feeding at 12 weeks of age (B), or during HFD feeding at 22 weeks of age (C). Blood samples were collected at the indicated time points and subjected to glucose and insulin measurements. n = 10; *, P < 0.05. The data shown are means ± the standard errors of the means.
hERO1βTg islets showed an impaired GSIS response with reduced insulin content.
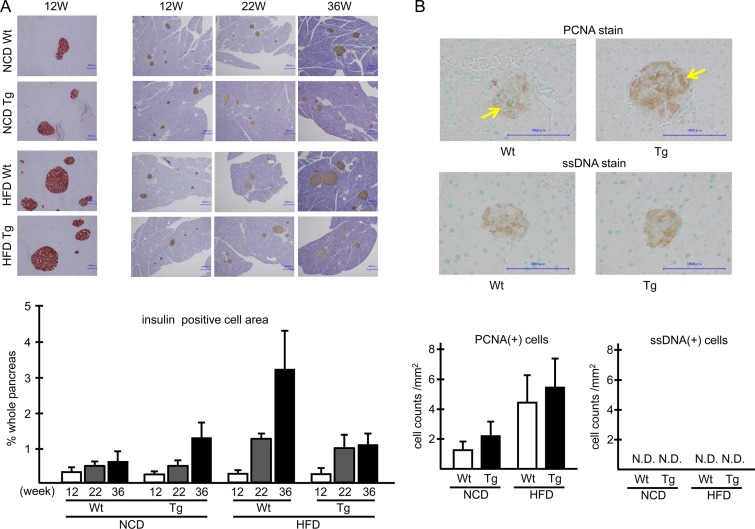
To explore the mechanisms whereby ERO1β overexpression led to reduced insulin secretion in glucose challenge tests during HFD feeding, we first examined the morphology of hERO1βTg islets by light microscopy. Microscopic analyses of hERO1βTg mouse islets showed no morphological changes detectable by insulin and glucagon staining (Fig. 4A, upper left panels). Insulin staining showed that the insulin-positive areas of hERO1βTg and control mouse islets were similar under both of the feeding conditions at 12 and 22 weeks, the time points when the glucose intolerance phenotype was already observed in hERO1βTg mice, whereas there was a nonsignificant reduction of the insulin-positive areas of HFD-fed hERO1βTg mouse islets only at 36 weeks of age (Fig. 4A, upper right and lower panels). Single-stranded DNA (ssDNA) staining and proliferating cell nuclear antigen (PCNA) staining showed no evidence of accelerated apoptosis or cell proliferation in the islets under any of the conditions (Fig. 4B). These data indicated that the exacerbated glucose intolerance in hERO1βTg mice was not associated with β cell mass reduction.
FIG 4.
Histological analysis of islets of hERO1βTg mice. (A) Representative images of islets of hERO1βTg mice. Pancreas sections of hERO1βTg (Tg) or Wt control mice under NCD or HFD feeding conditions at 12 weeks of age were stained with insulin (red) or glucagon (dark red) antibody (left panels). Pancreas sections of the mice at the indicated weeks (W) of age were stained with insulin antibody (brown) (right side), and β cell areas determined as insulin-positive areas by staining were quantified (bottom). The occupancy of pancreatic β cells in the whole pancreas was determined as described in Materials and Methods. n = 6. Bars, 100.0 μm (left column) and 300.0 μm (right three columns). (B) Cell proliferation and apoptosis markers in hERO1β islets. Pancreas sections of hERO1βTg (Tg) or Wt control mice under NCD or HFD feeding conditions at 12 weeks of age were stained with insulin (brown) and PCNA or ssDNA (dark purple) antibody (representative images are shown at the top). The arrows indicate PCNA-positive cells. The PCNA- or ssDNA-positive cells were counted. Bars, 100.0 μm. Cell counts normalized to the insulin-positive area are shown at the bottom. n = 6. The data shown are means ± the standard errors of the means. N.D., not detected.
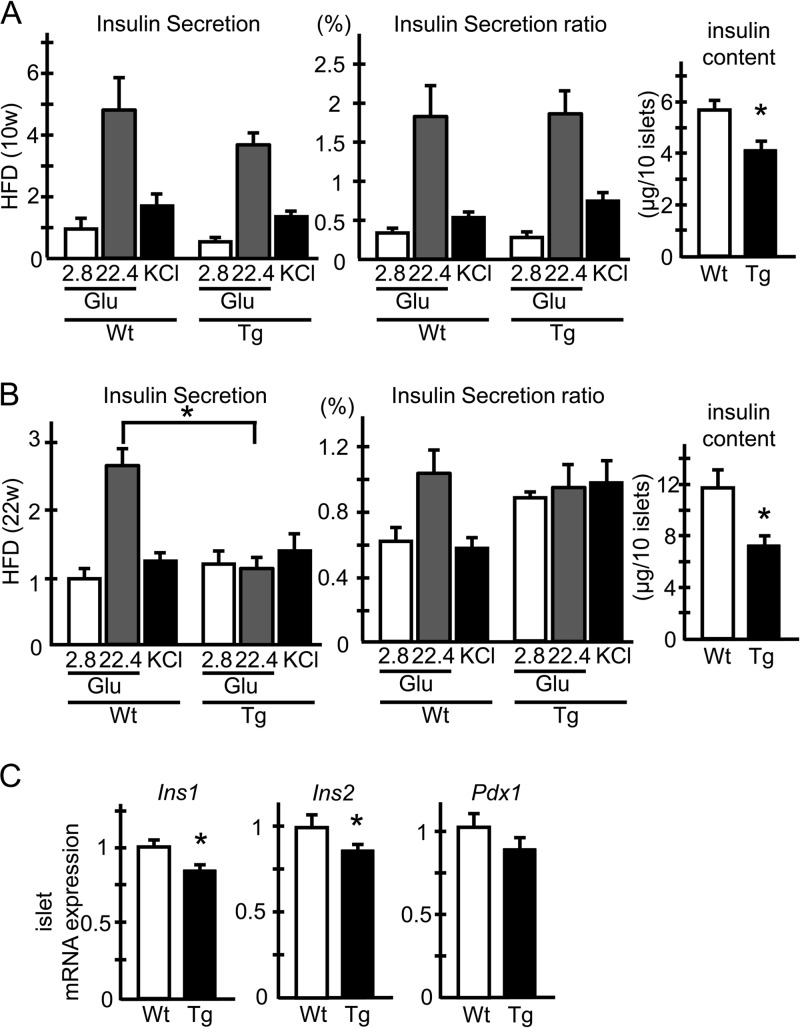
We next investigated the GSIS response of islets isolated from HFD-fed hERO1βTg mice. Islets isolated from HFD-fed hERO1βTg mice showed a weaker GSIS response than those from HFD-fed control mice, and the difference was more pronounced and reached statistical significance after longer HFD feeding (Fig. 5A and B, left panels). The weaker GSIS responses in Tg islets were due to reduced insulin contents in the islets (Fig. 5A and B, right panels), as insulin secretion did not differ between hERO1βTg and control Wt islets when normalized to their insulin contents (Fig. 5A and B, middle panels). The analyses of mRNA expression in Tg islets revealed a marginal reduction in Ins1 and Ins2 expression by about 15%, the degree of which was, however, relatively small compared to the reduction in the insulin contents of Tg islets (Fig. 5C). Collectively, these data suggested that HFD-fed hERO1βTg mice showed exacerbated glucose intolerance, which was attributed to the reduced islet insulin contents with the possible involvement of posttranscriptional mechanisms.
FIG 5.
Static-incubation study of islets from hERO1βTg mice. (A and B) GSIS of hERO1βTg islets. Islets were freshly isolated from 10-week-old (A) or 22-week-old (B) hERO1βTg (Tg) or control Wt mice fed an HFD from 7 weeks of age. The islets were incubated for 30 min in KRB buffer containing 2.8 mM glucose (Glu), 22.4 mM glucose, or 50 mM KCl, respectively, and the media were collected. The insulin concentrations in the incubation media were measured with an insulin radioimmunoassay kit. Insulin secretion is displayed as a ratio normalized to the basal secretion of Wt mice (left panels). Insulin secretion was determined as the ratio of secreted insulin to the insulin content of the islets (middle panels). n = 6 to 18; 6 islets for each condition. For measurements of the insulin contents of islets, insulin was extracted from the islets by overnight incubation with acid ethanol and measured with an insulin radioimmunoassay kit (right panels). n = 18 to 54; 6 islets for each condition; *, P < 0.05. (C) mRNA expression of insulin-related genes in hERO1βTg islets. Pancreatic islets were isolated from 10-week-old Tg or control Wt mice fed an HFD from 7 weeks of age. Total mRNA was extracted and subjected to RT-PCR analysis of Ins1, Ins2, and Pdx1 mRNA expression. n = 6 to 8; *, P < 0.05. The data shown are means ± the standard errors of the means.
ERO1β overexpression caused ER stress in pancreatic β cells.
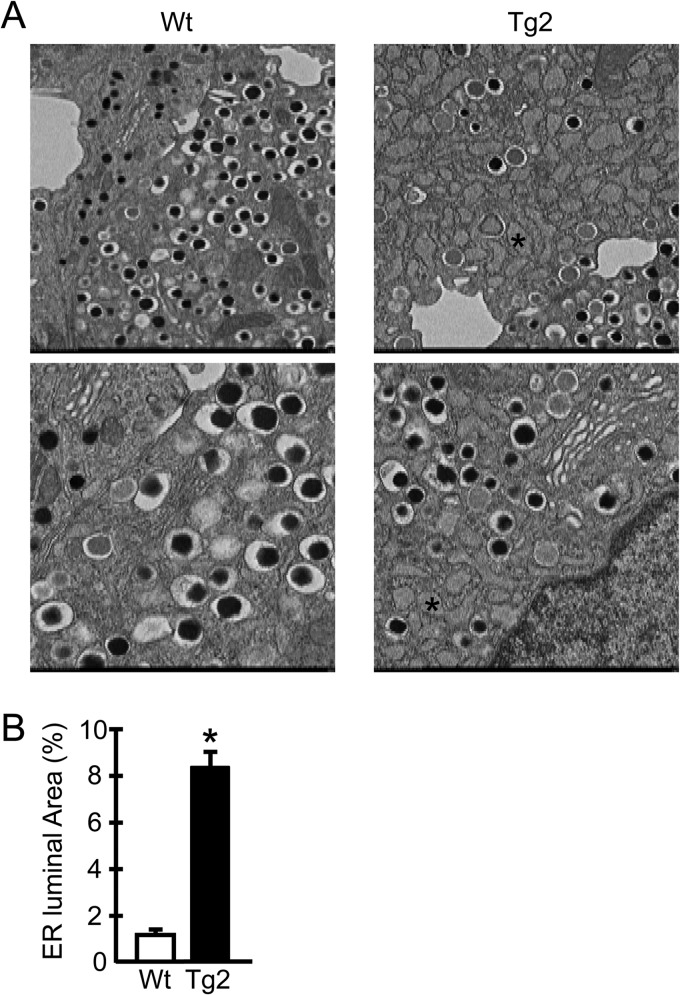
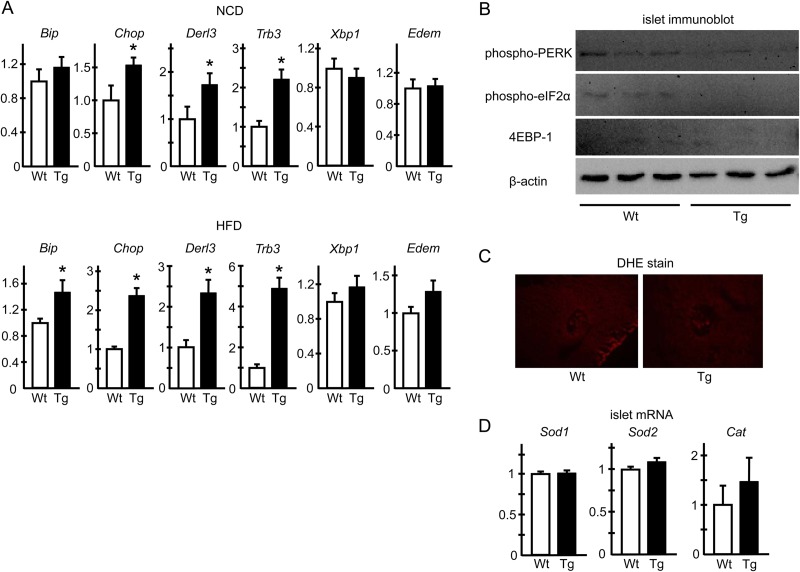
To further characterize the phenotypes of ERO1β-overexpressing β cells, we investigated the morphology of hERO1βTg β cells in detail with an electron microscope. Electron microscopic analyses revealed severely enlarged ER lumens in the β cells of hERO1βTg mice (Fig. 6A and B), showing a sharp contrast to the scarce changes observed in the light microscopic analyses. The ER dilation of hERO1βTg β cells was already observed under NCD-fed conditions, the magnitude of which did not change further under HFD-fed conditions (data not shown). No apparent changes were detected in the organelles other than the ER, such as the Golgi apparatus or insulin-containing granules, with regard to their numbers or their morphology. As previous reports have indicated, cells under ER stress or with compromised ER homeostasis often show ER enlargement (31, 33, 34), suggesting that the β cells of hERO1βTg mice were also subjected to ER stress. In fact, the analyses of mRNA expression showed upregulation of the expression of multiple UPR genes in Tg islets, including Bip, Chop, Derl3, and Trb3 (Fig. 7A). Upregulation of UPR genes was again observed in the islets of NCD-fed hERO1βTg mice, the degree of which tended to be higher in the islets of HFD-fed Tg mice. These data collectively indicated that ERO1β overexpression caused ER stress in β cells. Interestingly, however, phosphorylation of PERK and the α subunit of eukaryotic transcription initiation factor 2 (eIF2) or upregulation of 4E binding protein 1 (4E-BP1) was not evident in hERO1βTg islets (Fig. 7B).
FIG 6.
Electron microscopic analysis of pancreatic β cells of hERO1βTg mice. Representative electron micrographs (A) and quantifications of ER luminal areas (B) of pancreatic β cells of hERO1βTg (Tg2) or Wt control mice during NCD feeding at 12 weeks of age are shown. The asterisks indicate the markedly dilated ER lumens. ER lumen areas were quantified as described in Materials and Methods.
FIG 7.
ER stress and oxidative stress markers in hERO1βTg islets. (A) UPR gene mRNA expression in hERO1βTg islets. Pancreatic islets were isolated from 12-week-old hERO1βTg (Tg) or control Wt mice fed either an NCD or an HFD from 7 weeks of age. Total mRNA was extracted and subjected to RT-PCR analysis of the genes as indicated. n = 7 or 8; *, P < 0.05. (B) UPR signaling pathways in hERO1β islets. Pancreatic islets were freshly isolated from 12-week-old hERO1βTg (Tg) or control Wt mice fed an NCD. Total cell lysates were prepared from the islets, and the same amounts of protein were loaded and subjected to immunoblotting with anti-phospho-PERK, anti-phospho-eIF2α, or anti-4E-BP1 antibody. The same membrane was reblotted with anti-β-actin antibody. Representative images of immunoblotting of hERO1β islets are shown. (C and D) Oxidative stress in hERO1β islets. (C) Representative images of DHE staining of islets of hERO1βTg mice. Pancreas sections of hERO1βTg (Tg) or Wt control mice during NCD feeding at 12 weeks of age were stained with DHE. (D) Antioxidant pathway gene mRNA expression in hERO1βTg islets. Pancreatic islets were isolated from 12-week-old hERO1βTg (Tg) or control Wt mice fed an HFD from 7 weeks of age. Total mRNA was extracted and subjected to RT-PCR analysis of Sod1, Sod2, and Cat mRNA expression (n = 6 to 8). The data shown are means ± the standard errors of the means.
Next we investigated whether reactive oxygen species (ROS) could contribute to the β cell dysfunction of hERO1βTg mice. As previously described, in the relay of oxidative equivalents among EROs, PDIs, and client proteins during oxidative protein folding, the final acceptor of the electron is molecular oxygen; thus, ERO-mediated oxidative protein folding could lead to ROS production (35). However, we did not observe any evidence of ROS accumulation in hERO1βTg islets, as revealed by DHE staining of hERO1βTg islets (Fig. 7C). In addition, the mRNA expression of genes involved in the antioxidant pathway, such as Sod1, Sod2, and Cat, was unaltered in hERO1βTg islets compared to that in control Wt islets (Fig. 7D). These results suggested the absence of ROS overproduction in hERO1βTg islets.
ERO1β overexpression caused impaired insulin secretion with ER stress in MIN6 cells.
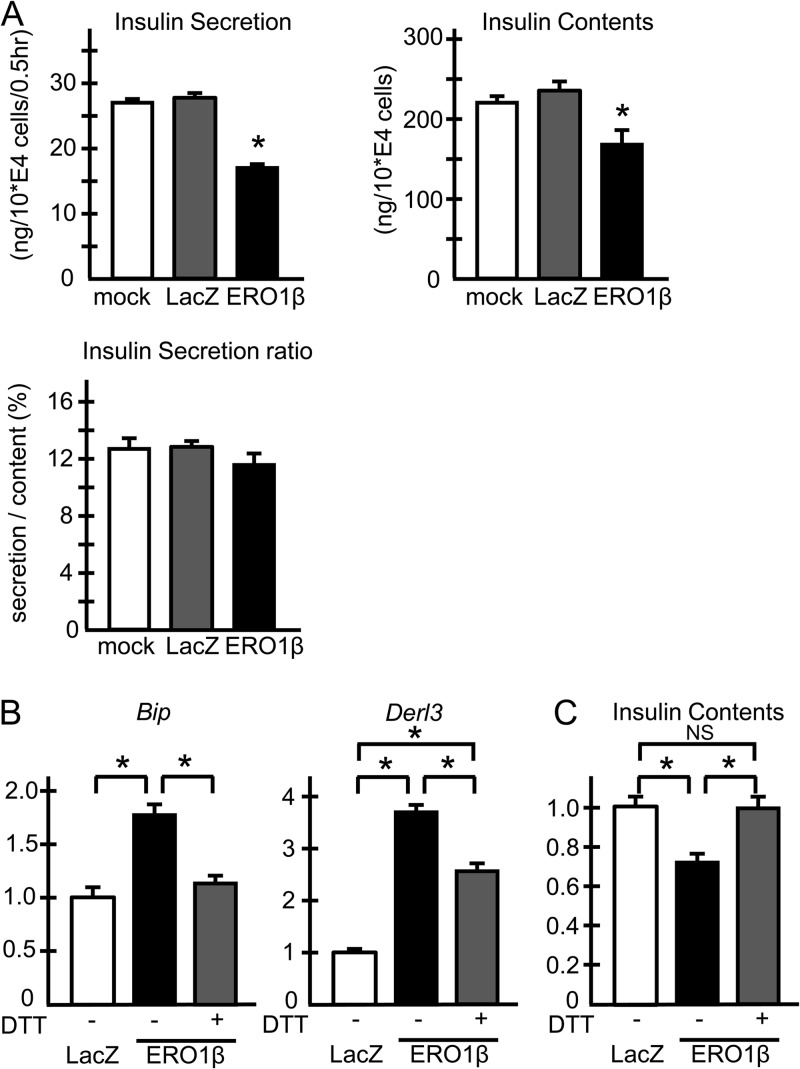
To investigate whether ERO1β overexpression in cultured cells could lead to phenotypes similar to those in islets, we next overexpressed human ERO1β with adenovirus in MIN6 β cells. The amount of insulin secreted under the high-glucose condition was significantly lower in ERO1β-overexpressing MIN6 cells, while the insulin secretion ratio, normalized to the insulin content, did not decrease with ERO1β overexpression (Fig. 8A). These results indicated that the reduced insulin secretion under ERO1β overexpression was due to reduced insulin contents in MIN6 β cells, essentially mimicking the phenotypes of hERO1βTg islets. The mRNA analyses showed that ERO1β overexpression caused UPR gene upregulation (Fig. 8B), while mRNA expression of antioxidant pathway genes such as Sod1, Sod2, and Cat was unaltered (data not shown), suggesting that ERO1β overexpression led to ER stress in MIN6 cells without collateral ROS overproduction, again showing characteristics similar to those in hERO1βTg islets. Importantly, mild DTT treatment paradoxically led to attenuation of UPR gene upregulation (Fig. 8B, gray bars), which was associated with restored insulin contents under ERO1β overexpression (Fig. 8C). These data suggested the possibility that ERO1β overexpression caused ER stress by shifting ER redox states toward overly oxidizing conditions, which was countersuppressed by the reducing effect of the mild DTT treatment.
FIG 8.
Adenoviral overexpression of ERO1B in MIN6 cells. (A) Insulin secretion rate of MIN6 cells with human ERO1B overexpression under high-glucose conditions. MIN6 cells infected with adenovirus as indicated were incubated with KRB buffer with 2.8 mM glucose for 60 min at 37°C, and then the medium was changed to KRB buffer with 22.4 mM glucose for a further 30 min of incubation. The medium was then subjected to insulin concentration measurement (left upper panel). Insulin was extracted from the cells by overnight incubation with acid ethanol at −20°C for the measurement of insulin content (right upper panel). Insulin concentrations were measured with an insulin radioimmunoassay kit. Insulin secretion was determined as the ratio of secreted insulin to the insulin content of the cells (lower panel). Representative results of two independent experiments are shown. n = 4; *, P < 0.05. (B) UPR gene expression of MIN6 cells with human ERO1B overexpression. MIN6 cells infected with the adenovirus indicated were incubated for 4 h with or without 0.5 mM DTT. Total mRNA was extracted from the cells and subjected to RT-PCR analysis of Bip and Derl3. n = 4; *, P < 0.05. (C) Restored insulin contents via mild DTT treatment under ERO1B overexpression. MIN6 cells infected with adenovirus as indicated were incubated for 12 h with or without 0.1 mM DTT. Insulin was extracted from the cells by overnight incubation with acid ethanol at −20°C and subjected to insulin measurement. n = 9; *, P < 0.05. The data shown are means ± the standard errors of the means.
Insulin maturation was not compromised in hERO1βTg β cells.
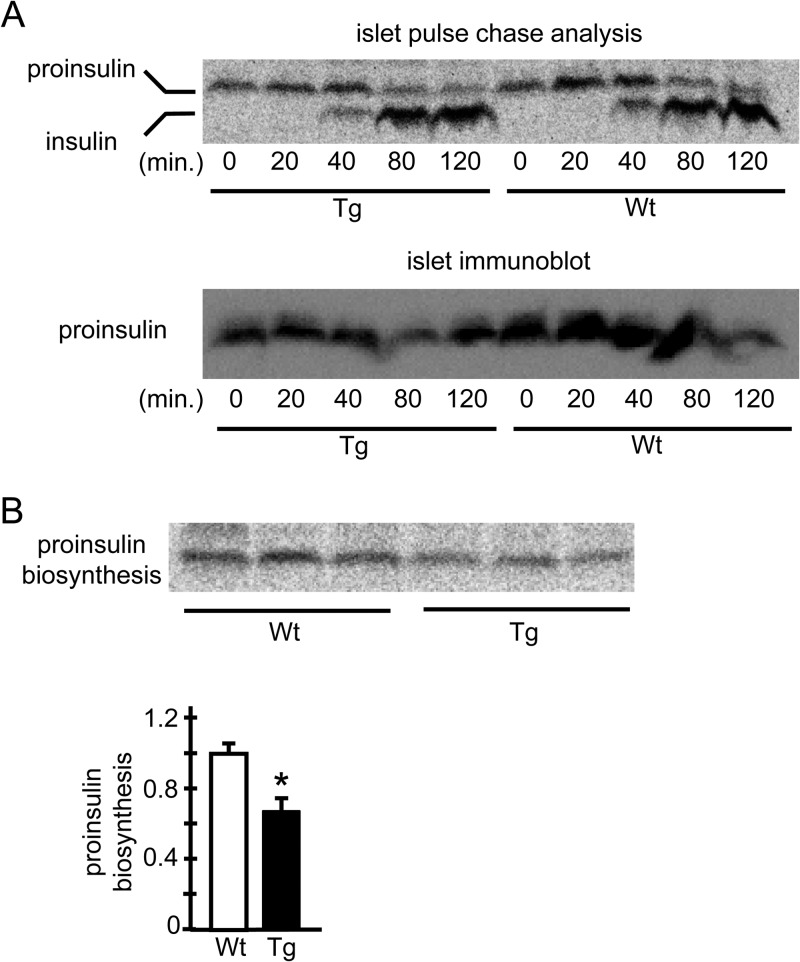
How did the overexpression of ERO1β cause ER stress in β cells? Generally, ER stress can result from an accumulation of misfolded proteins, which is due to either accelerated misfolding of client proteins or impaired removal of irreparably misfolded proteins from the ER lumens by a mechanism called ER-associated degradation (ERAD). Recent studies have pointed out that the reduction of protein disulfides is required for the dislocation and degradation of misfolded proteins targeted for ERAD (36, 37). To directly address these issues, we conducted a pulse-chase analysis with hERO1βTg islets and investigated proinsulin processing and insulin maturation with an antibody detecting proinsulin and insulin with equal efficiency. The pulse-chase analyses showed that there was no delay in the appearance of processed insulin, which was reflected in the band shift downward (Fig. 9A, upper panel), suggesting that the proinsulin maturation with the C-peptide cleavage occurred in hERO1βTg β cells as smoothly as that in the control Wt β cells. Additionally, no delay in the disappearance of proinsulin was observed, as reflected in the similarly remaining upper bands in Tg and Wt islets until the end of the chase period. The decrease in insulin content in the islets of hERO1βTg mice was confirmed in the proinsulin immunoblot assay, as detected by anti-C-peptide immunoblotting of the same membrane (Fig. 9A, lower panel). In fact, the amount of newly synthesized proinsulin, which was investigated by collecting islets just after 30 min of metabolic “pulse” labeling, was lower in the islets of hERO1βTg mice than in control Wt mouse islets (Fig. 9B). These data collectively suggested the possibility that the decrease in insulin contents in the islets of hERO1βTg mice could be accounted for by reduced protein synthesis, while the conversion of proinsulin to insulin occurred normally in the hERO1βTg β cells, and that the misfolded proinsulin, if it existed, could be removed from the ER with similar efficiency in Tg β cells compared to its clearance from control Wt β cells.
FIG 9.
Analysis of insulin synthesis in hERO1βTg islets by pulse-chase experiments. Pancreatic islets were isolated from 12-week-old hERO1βTg (Tg) or control Wt mice. On the day after isolation, the islets were subjected to pulse-labeling with [35S]methionine-cysteine for 30 min. (A) Subsequently, the islets were incubated in radiolabel-free medium for the indicated periods. The lysates were immunoprecipitated with anti-insulin/proinsulin antibody. Immunoprecipitated proteins were resolved by Tricine-SDS-PAGE and detected by autoradiography (top). The same membrane was subjected to immunoblotting for proinsulin with a C-peptide antibody (bottom). (B) The islets were directly collected. The lysates were immunoprecipitated with anti-insulin/proinsulin antibody. (Top) Immunoprecipitated proteins were resolved by Tricine-SDS PAGE and detected by autoradiography. (Bottom) Quantification (n = 3; *, P < 0.05). The data are means ± the standard errors of the means.
DISCUSSION
Here we report for the first time the phenotypes of mice with ERO1β overexpression specifically in pancreatic β cells. While it has been well documented that EROs play critical roles in ER protein folding or in ER homeostasis (12, 14, 15), their roles in the pathogenesis of diseases such as diabetes mellitus have remained obscure, with only one report showing that the disruption of ERO1β expression compromised oxidative folding of insulin and thus led to glucose intolerance in mice (23).
In the first place, we observed a special feature of ERO1β among other UPR genes, which showed a paradoxical decrease in its expression in the islets of db/db and Akita mice despite the evidence of increased ER stress. Considering that the β cell mass itself is decreased in these model mice and that ERO1β is specifically expressed in β cells, it would be reasonable to assume that the observed reductions in ERO1β expression could be partly accounted for by the reduction in β cell mass itself. Nevertheless, the reduction of ERO1β showed a striking contrast to findings for other UPR genes like Bip, the mRNA upregulation of which in response to ER stress is due to its induction exclusively within β cells (31), indicating that there occurred either a reduction or, more precisely, an inadequate upregulation of ERO1β expression in the stressed β cells in these models.
EROs are essentially double-bladed molecules; they are necessary proteins for the cells to facilitate disulfide protein folding but at the same time could be toxic to the cells by imposing oxidative stress, as EROs produce ROS as by-products when they couple the oxidizing power to molecular oxygen during disulfide bond formation (11). In Saccharomyces cerevisiae, cell death under ER stress is attributed partly to ROS production resulting from ERO1 upregulation (35), while Perk−/− cells, in which protein synthesis is not properly attenuated under ER stress, accumulate ROS, leading to apoptosis (33), which is ameliorated by ERO1 abrogation (38).
Interestingly, ERO1β overexpression did not lead to ROS accumulation in the β cells in our model, nor was upregulation of antioxidant pathways observed. In contrast, we observed evidence of severe ER stress induced by ERO1β overexpression. However, despite the upregulation of proapoptotic genes such as Chop or Trb3, as well as the severe dilation of the ER lumen of hERO1βTg β cells, which is generally regarded as indicative of unfolded protein accumulation and ER stress (31, 33, 34), hERO1βTg islets did not show evidence of ER stress-induced β cell death; thus, the glucose intolerance of hERO1βTg mice was mild and became obvious only after an HFD load. This lack of apoptosis could simply be explained as a consequence of successful compensations achieved through the strongly invoked UPRs, possibly via the downregulation of insulin synthesis, leading to a sort of balanced and maintained status within the ER.
The exact mechanisms whereby ERO1β overexpression caused ER stress in β cells were uncertain. The ER stress caused by ERO1β overexpression seems to be due to the oxidizing actions of ERO1β, instead of being a nonspecific artifact, as DTT treatments reversed UPR gene upregulation, as well as reduced insulin contents by ERO1β overexpression in MIN6 β cells. One possible mechanism is that inappropriately high oxidizing conditions in the ER created by ERO1β overexpression resulted in aberrant disulfide formation within client proteins and thus led to the accumulation of misfolded proteins. Another possibility is that ERO1β overexpression caused ER stress by impairing the ERAD system. Given that the reduction of protein disulfides is required within misfolded proteins before they are dislocated and successfully degraded (36, 37), ERO1β overexpression could have hampered the reduction of misfolded proteins and thereby interfered with the ERAD system. Although we could not demonstrate delayed insulin maturation or compromised ERAD in the pulse-chase analysis, these two possibilities are still not excluded as causes of the increased ER stress in our model. Importantly, one of the most upregulated UPR genes under ERO1β overexpression was Derl3, which is essential to the machinery of the ERAD system (39). Therefore, it is tempting to suspect that ERAD functions were enhanced in our ERO1β overexpression model as a compensatory response by which healthy insulin handling was, if impaired by EROβ overexpression, successfully restored.
ERO1β overexpression led to impaired glucose tolerance due to impaired insulin secretion. Insulin staining in mice showed that there was a tendency toward reduction of the insulin-positive areas of hERO1βTg islets only at 36 weeks under HFD feeding, which did not reach statistical significance because of their large variations. At earlier time points of HFD feeding, ERO1β overexpression did not lead to changes in the insulin-positive areas, when impairment of insulin secretion was already observed upon a glucose challenge, indicating that the mechanism of impaired insulin secretion could be explained not by the changes in β cell mass but only by the altered functions of β cells. A GSIS study with isolated islets suggested that the functional impairment of ERO1β Tg islets was associated with a reduction of islet insulin contents. We observed no consistent changes in basal insulin secretion under low-glucose status in our experimental settings, including GTT of mice or GSIS of islets or MIN6 cells. Although the reason for this is unclear, there might be a specific mechanism whereby ERO1β overexpression preferentially affected the insulin granules responsible for phase 1 and 2 insulin release or, more plausibly, with the relatively small decrease in the insulin contents in any of our models, it might be due to a mere lack of enough sensitivity to detect the difference in the basal states. In fact, previous models with a more pronounced decrease in islet insulin contents do not consistently show a decrease in basal insulin secretion at low glucose concentrations (40, 41).
There could be more than one mechanism whereby ERO1β overexpression caused the reduction of islet insulin contents. Ins1 and Ins2, as well as Pdx1, gene expression was significantly downregulated, while the magnitude of the reduction was relatively smaller than the magnitude of the insulin content reduction. Considering that ERO1β overexpression led to ER stress and that one of the fundamental ER stress responses is to downregulate protein synthesis (42), it is tempting to speculate that global repression of protein synthesis is taking place as well. In fact, the pulse-chase analysis showed a significant decrease in insulin biosynthesis in ERO1βTg islets, the magnitude of which was greater than the decrease in Ins1 and Ins2 mRNA levels and comparable to the decrease in insulin contents. However, the exact mechanism of insulin synthesis suppression, as well as the mechanism of decreased Ins1 and Ins2 mRNA levels, during ERO1β overexpression is unclear and remains to be further investigated and clarified.
So, how is ERO1β involved in the pathogenesis of diabetes mellitus? Here we have shown that ERO1β expression gradually decreases with age in the islets of db/db mice in parallel with the progression of glucose intolerance and that ERO1β expression was also decreased in the islets of Akita mice. The reductions in ERO1β expression are in a sharp contrast to the expression of other UPR genes, which were all upregulated in the islets of these model mice possibly because of the increased ER stress. These results indicate that ERO1β has a special place among the UPR components in the islets of diabetic model mice and that ERO1β regulation during diabetes progression is subject to mechanisms distinct from those of UPR, which are currently unknown and need to be clarified by further research. Given that ERO1β suppression leads to decreased insulin content and increased susceptibility to ER stress in β cells (24), we speculate that the reduced expression of ERO1β, or its paradoxical response to ER stress during diabetes progression, could be associated with β cell dysfunction and the inability to synthesize adequate insulin to compensate for peripheral insulin resistance. However, as we have reported here, simply upregulating ERO1β in β cells would not benefit β cell homeostasis and, on the contrary, could worsen ER stress and lead to the suppression of insulin synthesis. Although there remains the possibility that the overexpressed levels of ERO1β in our Tg models are beyond the physiological range and pathologically damaged β cell homeostasis, these results nevertheless clearly illustrate the importance of the fine-tuning of ERO1β regulation required in the maintenance of ER homeostasis, the disturbance of which compromises β cell function for insulin synthesis and could contribute to the pathogenesis of diabetes mellitus.
ACKNOWLEDGMENTS
We thank F. Takahashi, Y. Sakuma, R. Honma, K. Narisawa, Y. Kanto, and R. Hoshino for their excellent technical assistance.
This work was supported by grant support from Astellas Pharma Inc. (to K.U.), a grant-in-aid for scientific research in priority areas (B) (to K.U.); a grant-in-aid for scientific research in priority areas (S) (to T.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and a grant for the Translational Systems Biology and Medicine Initiative (to T.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
None of us have any financial conflict of interest to declare in relation to this work.
Footnotes
Published ahead of print 27 January 2014
REFERENCES
- 1.Taniguchi A, Nakai Y, Fukushima M, Kawamura H, Imura H, Nagata I, Tokuyama K. 1992. Pathogenic factors responsible for glucose intolerance in patients with NIDDM. Diabetes 41:1540–1546 [DOI] [PubMed] [Google Scholar]
- 2.Rhodes CJ. 2005. Type 2 diabetes—a matter of β-cell life and death? Science 307:380–384. 10.1126/science.1104345 [DOI] [PubMed] [Google Scholar]
- 3.Eizirik DL, Cardozo AK, Cnop M. 2008. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29:42–61. 10.1210/er.2007-0015 [DOI] [PubMed] [Google Scholar]
- 4.Scheuner D, Kaufman RJ. 2008. The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr. Rev. 29:317–333. 10.1210/er.2007-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Lai E, Teodoro T, Volchuk A. 2009. GRP78, but not protein-disulfide isomerase, partially reverses hyperglycemia-induced inhibition of insulin synthesis and secretion in pancreatic β-cells. J. Biol. Chem. 284:5289–5298. 10.1074/jbc.M805477200 [DOI] [PubMed] [Google Scholar]
- 6.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. 2008. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 118:3378–3389. 10.1172/JCI34587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. 2002. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109:525–532. 10.1172/JCI14550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szegezdi E, Logue SE, Gorman AM, Samali A. 2006. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7:880–885. 10.1038/sj.embor.7400779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ron D, Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8:519–529. 10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- 10.Freedman RB, Hirst TR, Tuite MF. 1994. Protein disulphide isomerase: building bridges in protein folding. Trends Biochem. Sci. 19:331–336. 10.1016/0968-0004(94)90072-8 [DOI] [PubMed] [Google Scholar]
- 11.Tu BP, Weissman JS. 2004. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 164:341–346. 10.1083/jcb.200311055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sevier CS, Kaiser CA. 2008. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim. Biophys. Acta 1783:549–556. 10.1016/j.bbamcr.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 13.Zito E, Melo EP, Yang Y, Wahlander Å, Neubert TA, Ron D. 2010. Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol. Cell 40:787–797. 10.1016/j.molcel.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frand AR, Kaiser CA. 1998. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell 1:161–170. 10.1016/S1097-2765(00)80017-9 [DOI] [PubMed] [Google Scholar]
- 15.Pollard MG, Travers KJ, Weissman JS. 1998. Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell 1:171–182. 10.1016/S1097-2765(00)80018-0 [DOI] [PubMed] [Google Scholar]
- 16.Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R. 2000. Endoplasmic reticulum oxidoreductin 1-Lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J. Biol. Chem. 275:23685–23692. 10.1074/jbc.M003061200 [DOI] [PubMed] [Google Scholar]
- 17.Gess B, Hofbauer K-H, Wenger RH, Lohaus C, Meyer HE, Kurtz A. 2003. The cellular oxygen tension regulates expression of the endoplasmic oxidoreductase ERO1-Lα. Eur. J. Biochem. 270:2228–2235. 10.1046/j.1432-1033.2003.03590.x [DOI] [PubMed] [Google Scholar]
- 18.May D, Itin A, Gal O, Kalinski H, Feinstein E, Keshet E. 2005. Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene 24:1011–1020. 10.1038/sj.onc.1208325 [DOI] [PubMed] [Google Scholar]
- 19.Dias-Gunasekara S, Gubbens J, van Lith M, Dunne C, Williams JAG, Kataky R, Scoones D, Lapthorn A, Bulleid NJ, Benham AM. 2005. Tissue-specific expression and dimerization of the endoplasmic reticulum oxidoreductase Ero1{beta}. J. Biol. Chem. 280:33066–33075. 10.1074/jbc.M505023200 [DOI] [PubMed] [Google Scholar]
- 20.Van Lommel L, Janssens K, Quintens R, Tsukamoto K, Vander Mierde D, Lemaire K, Denef C, Jonas J-C, Martens G, Pipeleers D, Schuit FC. 2006. Probe-independent and direct quantification of insulin mRNA and growth hormone mRNA in enriched cell preparations. Diabetes 55:3214–3220. 10.2337/db06-0774 [DOI] [PubMed] [Google Scholar]
- 21.Schuit FC, Kiekens R, Pipeleers DG. 1991. Measuring the balance between insulin synthesis and insulin release. Biochem. Biophys. Res. Commun. 178:1182–1187. 10.1016/0006-291X(91)91017-7 [DOI] [PubMed] [Google Scholar]
- 22.Goodge KA, Hutton JC. 2000. Translational regulation of proinsulin biosynthesis and proinsulin conversion in the pancreaticβ-cell. Semin. Cell Dev. Biol. 11:235–242. 10.1006/scdb.2000.0172 [DOI] [PubMed] [Google Scholar]
- 23.Zito E, Chin K-T, Blais J, Harding HP, Ron D. 2010. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J. Cell Biol. 188:821–832. 10.1083/jcb.200911086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoo C, Yang J, Rajpal G, Wang Y, Liu J, Arvan P, Stoffers DA. 2011. Endoplasmic reticulum oxidoreductase-1-like β (ERO1lβ) regulates susceptibility to endoplasmic reticulum stress and is induced by insulin flux in β-cells. Endocrinology 152:2599–2608. 10.1210/en.2010-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. 2009. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem. Biophys. Res. Commun. 382:51–56. 10.1016/j.bbrc.2009.02.131 [DOI] [PubMed] [Google Scholar]
- 26.Ueki K, Yballe CM, Brachmann SM, Vicent D, Watt JM, Kahn CR, Cantley LC. 2002. Increased insulin sensitivity in mice lacking p85beta subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. U. S. A. 99:419–424. 10.1073/pnas.012581799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN. 2006. Total insulin and IGF-I resistance in pancreatic [beta] cells causes overt diabetes. Nat. Genet. 38:583–588. 10.1038/ng1787 [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. 1999. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96:329–339. 10.1016/S0092-8674(00)80546-2 [DOI] [PubMed] [Google Scholar]
- 29.Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E, Gotow T, Peters C, von Figura K, Mizushima N, Saftig P, Uchiyama Y. 2005. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease). Am. J. Pathol. 167:1713–1728. 10.1016/S0002-9440(10)61253-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szöcs K, Lassègue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. 2002. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler. Thromb. Vasc. Biol. 22:21–27. 10.1161/hq0102.102189 [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Takeuchi T, Tanaka S, Kubo S-K, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. 1999. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J. Clin. Invest. 103:27–37. 10.1172/JCI4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R. 2000. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 275:4827–4833. 10.1074/jbc.275.7.4827 [DOI] [PubMed] [Google Scholar]
- 33.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. 2001. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7:1153–1163. 10.1016/S1097-2765(01)00264-7 [DOI] [PubMed] [Google Scholar]
- 34.Francisco AB, Singh R, Li S, Vani AK, Yang L, Munroe RJ, Diaferia G, Cardano M, Biunno I, Qi L, Schimenti JC, Long Q. 2010. Deficiency of suppressor enhancer Lin12 1 Like (SEL1L) in mice leads to systemic endoplasmic reticulum stress and embryonic lethality. J. Biol. Chem. 285:13694–13703. 10.1074/jbc.M109.085340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes CM, Titus EA, Cooper AA. 2004. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell 15:767–776. 10.1016/j.molcel.2004.08.025 [DOI] [PubMed] [Google Scholar]
- 36.Fagioli C, Mezghrani A, Sitia R. 2001. Reduction of interchain disulfide bonds precedes the dislocation of Ig-μ chains from the endoplasmic reticulum to the cytosol for proteasomal degradation. J. Biol. Chem. 276:40962–40967. 10.1074/jbc.M107456200 [DOI] [PubMed] [Google Scholar]
- 37.Ushioda R, Hoseki J, Araki K, Jansen G, Thomas DY, Nagata K. 2008. ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science 321:569–572. 10.1126/science.1159293 [DOI] [PubMed] [Google Scholar]
- 38.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. 2003. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11:619–633. 10.1016/S1097-2765(03)00105-9 [DOI] [PubMed] [Google Scholar]
- 39.Oda Y, Okada T, Yoshida H, Kaufman RJ, Nagata K, Mori K. 2006. Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J. Cell Biol. 172:383–393. 10.1083/jcb.200507057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eizirik DL, Welsh M, Strandell E, Welsh N, Sandler S. 1990. Interleukin-1 beta depletes insulin messenger ribonucleic acid and increases the heat shock protein hsp70 in mouse pancreatic islets without impairing the glucose metabolism. Endocrinology 127:2290–2297. 10.1210/endo-127-5-2290 [DOI] [PubMed] [Google Scholar]
- 41.Marchetti P, Lupi R, Federici M, Marselli L, Masini M, Boggi U, Del Guerra S, Patane G, Piro S, Anello M, Bergamini E, Purrello F, Lauro R, Mosca F, Sesti G, Del Prato S. 2002. Insulin secretory function is impaired in isolated human islets carrying the Gly(972)→Arg IRS-1 polymorphism. Diabetes 51:1419–1424. 10.2337/diabetes.51.5.1419 [DOI] [PubMed] [Google Scholar]
- 42.Brostrom CO, Brostrom MA. 1998. Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol. 58:79–125 [DOI] [PubMed] [Google Scholar]