Abstract
Here we report the first human case of an outer ear canal infection with a free-living nematode of the genus Rhabditis. Otomicroscopy revealed viable worms in the outer ear canal of a patient suffering from chronic otorrhea and hearing loss. The nematode was identified by microscopy and internal transcribed spacer (ITS)-PCR.
CASE REPORT
A 37-year-old male presented with purulent otorrhea from both ears for 8 weeks in a tertiary care center. Additionally, he reported of mild hearing loss since the beginning of symptomatic disease. Both otorrhea and hearing loss were more pronounced for the right ear, on which the patient had undergone mastoidectomy 3 years earlier due to chronic otitis media. The patient reported no other underlying disease or recent travel history, owned a pet dog as well as a pet cat, and had been working in an industrial agriculture company. Macroscopic inspection and visualization by otomicroscopy of the outer ear canal confirmed otorrhea and revealed tympanostomy tubes and a retraction of the tympanic membranes on both sides. The tuning fork test according to Weber lateralized left, the tuning fork test according to Rinne was bilaterally negative, and no spontaneous nystagmus as a sign of disturbance of the vestibular system was detected. Pure-tone audiometry revealed a conductive hearing loss on both sides in combination with a mild sensorineural hearing loss of 20 dB hearing level (HL) in the middle frequencies on the right side. No other local or systemic signs of infection such as fever or enlarged cervical lymph nodes were detected.
Because a chronic bacterial otitis media infection was suspected, local antibiotic ear drops containing ciprofloxacin (3 mg per ml) were prescribed. Bacterial swab cultures from the outer ear canal obtained prior to antibiotic therapy detected bacteria of the normal skin flora (coagulase-negative staphylococci, nonhemolytic streptococci, and Corynebacterium spp.) as well as Alcaligenes faecalis, none of which are typically associated with otitis media. At that time, surgery was considered in the event of persistent clinical symptoms to exclude cholesteatoma. Cholesteatoma represents a benign proliferation of keratinizing squamous epithelium, which can lead to local tissue destruction and secondary chronic infection.
In a follow-up physical examination 4 weeks later, no regression of clinical symptoms was noted. Surprisingly, otomicroscopy of both outer ear canals under 25-fold magnification revealed a large number of viable, moving worm-like organisms (Fig. 1, arrow). Both external ear canals were rinsed with saline solution, and the lavage fluid was submitted for further parasitological examination. The patient was treated with repeated topical application of ethanol (dequaliniumchlorid [0.04 g], glycerin anhydricum, ethanol [90%] aa ad 20 g) twice a day. No blood tests for leukocytosis or eosinophilia were performed due to the absence of clinical symptoms of a general infection. Microscopy of the unstained lavage fluid revealed high numbers of viable, 500-to-1,500-μm long nematode-like larvae morphologically similar to human-pathogenic nematode species (Fig. 2A and B). Lavage material and serum was sent for PCR-based species identification and anti-nematode antibodies, respectively. Amplification of the ribosomal internal transcribed spacer (ITS) 2 region was performed by PCR as recently described (1). The amplified DNA was subsequently sequenced, and a 874-nucleotide fragment was analyzed by BLAST search (blast.ncbi.nlm.nih.gov), revealing 100% sequence homology to free-living nematodes of the genus Rhabditis (R. blumi [accession number DQ121436]; 100% query coverage). The levels of homology to the nearest sequence matches were 87% and 84% for Butlerius sp. and Pristionchus sp., respectively, two nematode genera, with only 32% and 30% query coverage. Serology detected low IgG-antibody titers against Ascaris lumbricoides (13 U/ml [normal value < 10 U/ml]) using an in-house enzyme-linked immunosorbent assay (ELISA) with A. lumbricoides crude antigen extract and negative results for Dirofilaria immitis, Strongyloides stercoralis, and Toxocara spp. To reveal the patient's immunological response to the identified parasite, formalin-fixed Rhabditis sp. worm larvae from ear canal lavage fluid were overlaid with 1:10 diluted patient serum and incubated for 30 min. at 37°C. Slides were washed in phosphate-buffered saline (PBS; 0.05%)–Tween 20 and incubated in 1:400-diluted goat anti-total human immunoglobulin (bioMérieux; fluoline H catalog no. 75603)-supplemented 1:10,000-diluted Evans Blue (bioMérieux; catalog no. 75491) for another 30 min at 37°C. Clearly, the patient's serum contained antibodies directed against Rhabditis nematode larvae whereas control serum obtained from a healthy blood donor remained negative (Fig. 2C). Stool samples from the patient contained no worm eggs or larvae.
FIG 1.
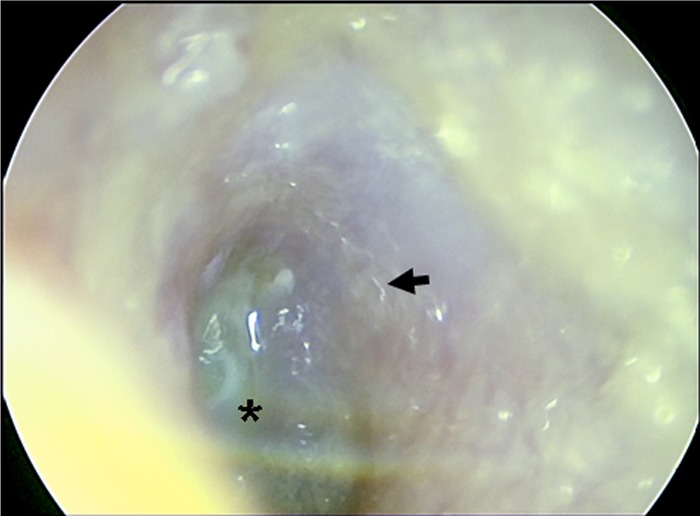
Otomicrosocopy of the external auditory canal. An endoscopic view into the left outer ear canal onto the tympanic membrane with the central umbo (*) and the atypical cone of light in the upper half is shown. Larvae can be discerned as undulatory light reflections (arrow).
FIG 2.
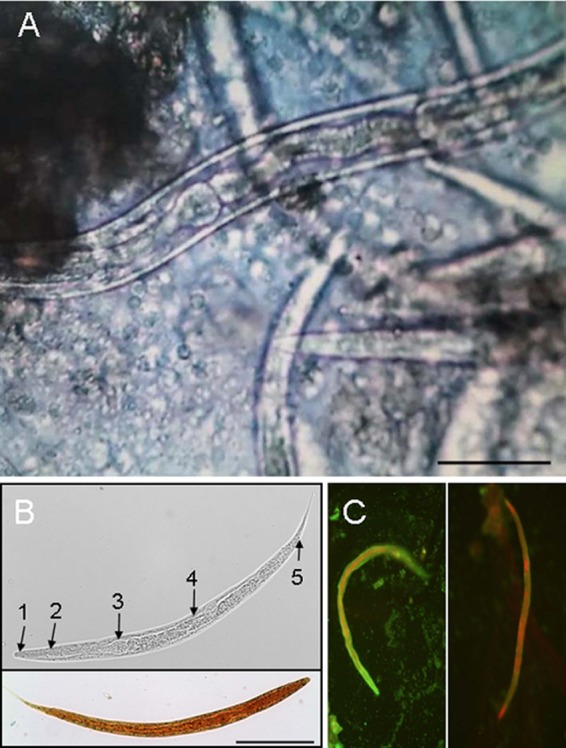
Microscopic visualization of Rhabditis larvae in ear canal lavage fluid. (A) Unstained microscopy of the ear canal lavage fluid. Bar, 50 μm. (B) Unstained (upper panel) and iodine-stained (lower panel) male larvae. Bar, 100 μm. Upper panel: 1, stoma/buccal cavity; 2, esophagus; 3, esophageal bulb; 4, intestine; 5, anus. (C) Indirect immunofluorescence staining of larvae in the lavage fluid using the patient's serum (left panel [diluted 1:10]) or serum obtained from a healthy blood donor (right panel [diluted 1:10]).
A follow-up examination 3 weeks later revealed no signs of purulent infection of the external ear canal on either side, and no viable nematode larvae were identified in ear canal lavage fluid. Cone beam computed tomography demonstrated the signs of chronic bilateral mastoiditis and a postmastoidectomy status on the right side (Fig. 3). Regular follow-up examinations during subsequent 3 months did not reveal any sign of recurrent disease.
FIG 3.
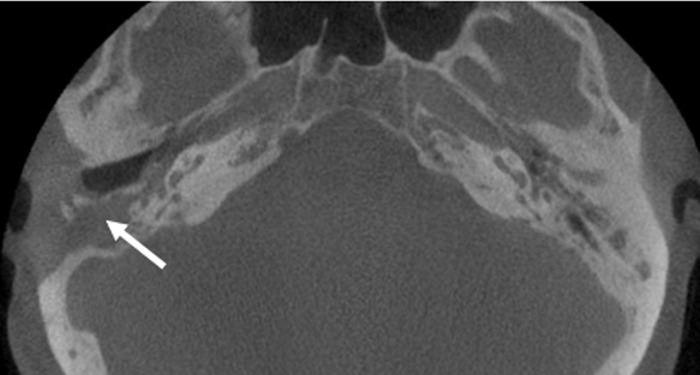
Cone beam computed tomography of the patient's temporal bones. Signs of chronic bilateral mastoiditis as well as status postmastoidectomy on the right side (arrow) can be seen.
The family Rhabditiae encompasses small free-living, saprophytic nematodes, including the well-studied model organism Caenorhabditis elegans, that live in soil and organic debris. Adult male worms have an average length of 1.2 mm; female worms have an average length of 1.5 mm. They possess a buccal cavity and a rhabditiform esophagus. Female worms show two ovaries and can be viviparous or oviparous. The life cycle of most Rhabditis species is completed after several days, and the nematode has occasionally been associated with human disease. Rhabditis larvae have been isolated from human stool samples (2, 3, 4, 5, 6, 7, 8), urine samples (9, 10, 11, 12), and vaginal swabs (4). The clinical relevance of the presence of the nematodes, however, has remained obscure in most cases, and efforts to confirm the causative role of Rhabditis sp., e.g., by immunological tests or larger association studies or posttreatment analyses, have not been published. The presence of Rhabditis sp. in the diagnostic material might therefore have been the result of environmental contamination. Meamar et al. (8) isolated R. axei from two imprisoned AIDS patients with watery diarrhea, but no follow-up analysis was performed, and the causative role was not established. Ahn (5) reported the detection of Rhabditis sp. in stool samples of rural schoolchildren. Colonization, however, was temporary, and no association with any clinical symptoms was reported. Also, isolation from urine samples was not associated with any particular symptom or disease (9). To date, no luminal infection of the outer ear canal with nematodes has been reported in humans. However, Rhabditis is well known to cause external otitis in cows, mainly in older animals, during both the rainy season and the dry season in tropical areas in Brazil and many African countries such as Zimbabwe or Tanzania (13).
The present case, to the best of our knowledge, represents the first report of a human ear canal infection with Rhabditis sp. Infection was associated with acute conductive hearing loss and purulent otorrhea and confirmed by the presence of anti-Rhabditis antibodies in the patient's serum. The differential diagnosis consisted of a broad range of bacterial and fungal infectious agents and cholesteatoma (14, 15, 16). The concomitant finding of A. feacalis in swab cultures of the ear canal was regarded as an accidental finding. It might, however, hint at a fecal route of contamination. However, the origin of the Rhabditis sp. infection has remained unclear. The patient reported neither travel to areas of bovine external otitis endemicity nor intentional or unintentional occupational or leisure contact with animal feces. Also, the patient denied the application of any type of product to his ears or treatment with environmental material which might have been a possible source of infection. His pet dog and pet cat were screened by routine veterinary microbiology but found negative for parasitic diseases. As nematode larvae from different genera are morphologically very similar, molecular identification of such organisms provides a potent diagnostic tool. The weakly positive Ascaris serology results may reflect cross-reacting anti-Rhabditis antibodies; this result, however, may also have originated from a past true ascariasis. The recommended treatment for Rhabditis infection in animals includes broad-spectrum anthelminthic agents such as albendazole and ivermectin. An additional treatment option of local, noninvasive infection consists of the local instillation of concentrated alcohol, which was effective in the treatment of the presented patient.
Taking the data together, we present the first human case of outer ear Rhabditis sp. infection. Although the incidence of parasitic infections of the ear in humans is certainly low, our report indicates that careful inspection of the ear canal by otomicroscopy and microscopic analysis of lavage fluid provides valuable diagnostic information. Molecular diagnostic tools also help to identify uncommon pathogens.
ACKNOWLEDGMENTS
We are indebted to Tanja Trompisch, Hanover, and Birgit Muntau, Hamburg, for excellent technical assistance.
Footnotes
Published ahead of print 5 March 2014
REFERENCES
- 1.Oliveros R, Cutillas C, De Rojas M, Arias P. 2000. Characterization of four species of Trichuris (Nematoda: Enoplida) by their second internal transcribed spacer ribosomal DNA sequence. Parasitol. Res. 86:1008–1013. 10.1007/PL00008519 [DOI] [PubMed] [Google Scholar]
- 2.Joyeux C, Baer JC. 1942. Sur en ver probablement pseudoparasite. Mars. Med. 2:676–690 [Google Scholar]
- 3.Shinohara T. 1960. Studies on Rhabditis (Nematoda, Rhabditiae). 1. Rhabditis spp. obtained from human faeces. J. Kurme Med. Assoc. 23:2777–2819 [Google Scholar]
- 4.Faust EC, Rusell PF. 1964. Craig and Faust's clinical parasitology. Klimpton, London, England [Google Scholar]
- 5.Ahn YK, Chung PR, Lee KT. 1985. Rhabditis sp. infected cases in rural school children. Kisaengchunghak Chapchi 23:1–6 (In Korean.) [DOI] [PubMed] [Google Scholar]
- 6.Campos DM, Araújo JL, Vieira MC, Damasceno F, Barbosa AP. 2002. A case of parasitism by Rhabditis sp in a child from Goiânia, Goiás, Brazil. Rev. Soc. Bras. Med. Trop. 35:519–522 (In Portuguese.) [PubMed] [Google Scholar]
- 7.Ye LP, Zhu CG, Zhang JN. 2002. Two cases of Rhaditis axei infections in human disgestive system. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 14:187 (In Chinese.) [Google Scholar]
- 8.Meamar AR, Kia EB, Zahabiun F, Jafari-Mehr A, Moghadam A, Sadjjadi SM. 2007. The occurrence of severe infections with Rhabditis axei in AIDS patients in Iran. J. Helminthol. 81:351–352. 10.1017/S0022149X07792301 [DOI] [PubMed] [Google Scholar]
- 9.Goldsmid JM. 1967. Rhabditis (Rhabditella) axei in the urine of an African in Rhodesia. J. Helminthol. 41:305–308. 10.1017/S0022149X00021842 [DOI] [PubMed] [Google Scholar]
- 10.Hara M, Nakazato H, Araki T, Iwata S. 1974. A case of urinary tract infection by a nematode larva (Rhabditis sp.). Kisechugaku Zasshi 23:48. (In Japanese.) [Google Scholar]
- 11.He YX, Jiang H. 1985. 3 human cases of urinary tract infection with Rhabditis. Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 3:206–208 (In Chinese.) [PubMed] [Google Scholar]
- 12.Eldridge B. 1993. Human urinary tract infection caused by Rhabditis sp. Publ. Health Lab. Serv. Microbiol. Dig. 10:202–203 [Google Scholar]
- 13.Duarte ER, Melo MM, Hamdan JS. 2001. Epidemiological aspects of bovine parasitic otitis caused by Rhabditis spp. and/or Raillietia spp. in the state of Minas Gerais, Brazil. Vet. Parasitol. 101:45–52. 10.1016/S0304-4017(01)00492-7 [DOI] [PubMed] [Google Scholar]
- 14.Teschner M, Hinz K, Stöver T, Lenarz T, Becker H. 2009. Diffusion-weighted MRI in the diagnosis of cholesteatomas. ORL J. Otorhinolaryngol. Relat. Spec. 71:99–104. 10.1159/000194662 [DOI] [PubMed] [Google Scholar]
- 15.Teschner M, Kramer S, Donnerstag F, Länger F, Lenarz T, Schwab B. 2008. Tuberculous Otitis media - a rare differential diagnosis in Germany. Laryngorhinootologie 87:503–506. 10.1055/s-2007-995511 [DOI] [PubMed] [Google Scholar]
- 16.Biermann E. 1997. Pneumocystis carinii otitis. Laryngorhinootologie 76:745–748. 10.1055/s-2007-997518 [DOI] [PubMed] [Google Scholar]


