Abstract
Coxiella burnetii causes Q fever, a zoonosis, which has acute and chronic manifestations. From 2007 to 2010, the Netherlands experienced a large Q fever outbreak, which has offered a unique opportunity to analyze chronic Q fever cases. In an observational cohort study, baseline characteristics and clinical characteristics, as well as mortality, of patients with proven, probable, or possible chronic Q fever in the Netherlands, were analyzed. In total, 284 chronic Q fever patients were identified, of which 151 (53.7%) had proven, 64 (22.5%) probable, and 69 (24.3%) possible chronic Q fever. Among proven and probable chronic Q fever patients, vascular infection focus (56.7%) was more prevalent than endocarditis (34.9%). An acute Q fever episode was recalled by 27.0% of the patients. The all-cause mortality rate was 19.1%, while the chronic Q fever-related mortality rate was 13.0%, with mortality rates of 9.3% among endocarditis patients and 18% among patients with a vascular focus of infection. Increasing age (P = 0.004 and 0.010), proven chronic Q fever (P = 0.020 and 0.002), vascular chronic Q fever (P = 0.024 and 0.005), acute presentation with chronic Q fever (P = 0.002 and P < 0.001), and surgical treatment of chronic Q fever (P = 0.025 and P < 0.001) were significantly associated with all-cause mortality and chronic Q fever-related mortality, respectively.
INTRODUCTION
Q fever is a zoonosis caused by the bacterium Coxiella burnetii, which has its main reservoir in small ruminants. Outbreaks of the disease occur worldwide (1). After primary infection, 50 to 60% of patients remain asymptomatic, while others develop symptomatic acute Q fever, a flu-like illness, which is mostly self-limiting (1–3). Between 2007 and 2010, a large outbreak of acute Q fever was observed in the Netherlands with >4,000 reported cases (4). These figures are probably rather conservative, as seroprevalence studies show that at least >40,000 people were infected by C. burnetii (5).
From previous observations, it is know that chronic Q fever can develop in approximately 1 to 5% of C. burnetii-infected patients and mostly manifests itself within the first year after infection, but the disease can also present itself several years later (1, 6). The most common manifestations are endocarditis, which according to the literature accounts for approximately 75% of all chronic Q fever cases, and vascular chronic Q fever, consisting of infections of vascular prostheses and aortic aneurysms (1, 7–9). Less common manifestations are osteomyelitis, pericarditis, and hepatitis (8). Important risk factors are heart valve pathology, especially valve prostheses, vascular prostheses, and aneurysms (9–12). Immunosuppression also seems to be associated with an elevated risk, as well as older age, pregnancy, and (mild) renal insufficiency (3, 11, 12). Before severe complications occur, most patients are asymptomatic or report only nonspecific symptoms such as low-grade fever, night sweats, and weight loss (1, 6, 9, 13). In case of endocarditis, findings on echocardiography are often nonspecific or absent (13). An early diagnosis of chronic Q fever has major clinical implications, as chronic Q fever causes high morbidity and a mortality rate of up to 60% when left untreated (1, 13). Long-term antibiotic treatment, consisting of doxycycline and hydroxychloroquine, and resection of infected vascular and valvular tissue are thought to improve prognosis (9, 13, 14).
Unfortunately, diagnosing chronic Q fever has proven to be challenging. A positive PCR or culture of C. burnetii in blood or tissue, in the absence of a serologic profile for acute Q fever, is considered diagnostic for chronic Q fever. However, PCR on blood has a limited sensitivity of only 50 to 60% (15–17). Therefore, serological analysis has an essential role in the diagnosis of chronic Q fever. C. burnetii exhibits antigenic variation when it is cultured in cells, as the virulent organisms producing phase I antigen shift to avirulent organisms producing phase II antigen. Chronic Q fever is characterized by persistent high titers of IgG antibodies against C. burnetii phase I antigens (phase I IgG) (3). A phase I IgG cutoff titer of 800, based on an in-house-developed immunofluorescence assay (IFA), as well as a cutoff titer of 1,024 based on a commercially available IFA (Focus Diagnostics) have been used as standards for the serological diagnosis of chronic Q fever (15, 18–20). Recent studies show that serology alone, in the absence of PCR positivity, is not sufficient for the diagnosis of chronic Q fever and should therefore be combined with clinical data (21). The Dutch consensus group for the diagnosis of Q fever proposed to categorize patients into proven, probable, or possible chronic Q fever. This classification ranks the probability of having chronic Q fever based on PCR, serology, clinical parameters, imaging studies, and pathology (Table 1) (15).
TABLE 1.
Dutch consensus guidelines on chronic Q fever diagnosisa
| Diagnosis | Characteristicsb |
|---|---|
| Proven chronic Q fever | Positive Coxiella burnetii PCR in blood or tissuec OR an IFA titer of ≥800 or 1,024 for C. burnetii phase I IgGd with either definite endocarditis according to the modified Duke criteria (19) or proven large vessel or prosthetic infection by imaging studies (FDG-PET [18], CT, MRI, or AUS) |
| Probable chronic Q fever | IFA titer of ≥800 or 1,024 for C. burnetii phase I IgGc AND valvulopathy not meeting the major criteria of the modified Duke criteria (19) OR known aneurysm and/or vascular or cardiac valve prosthesis without signs of infection by imaging studies (TEE/TTE, FDG-PET [18], CT, MRI, or AUS) OR suspected osteomyelitis, pericarditis, or hepatitis as manifestations of chronic Q fever OR pregnancy OR symptoms and signs of chronic infection such as fever, weight loss, night sweats, hepatosplenomegaly, and persistent raised ESR and CRP OR granulomatous tissue inflammation as proven by histological examination OR immunocompromised state |
| Possible chronic Q fever | IFA titer of ≥800 or 1,024 for C. burnetii phase I IgGd without manifestations meeting the criteria for proven or probable chronic Q fever |
See reference 15.
IFA, immunofluorescence assay; FDG-PET, fluorodeoxyglucose positron emission tomography; CT, computed tomography; MRI, magnetic resonance imaging; AUS, abdominal ultrasound; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
In the absence of acute infection.
Cutoff is dependent on the IFA technique used (developed in-house or a commercial IFA technique, respectively).
To gain more insight into the chronic consequences and long-term prognosis of infection with C. burnetii after the recent outbreak in the Netherlands, the initiative called the Dutch National Chronic Q Fever Database was started. This is a joint effort of multiple hospitals of the Q fever-afflicted areas to monitor and assess all chronic Q fever cases in the Netherlands. Five years after the start of the Q fever epidemic, we present data generated from this initiative, which is one of the largest cohorts of chronic Q fever patients in the world, with special emphasis on chronic Q fever mortality.
MATERIALS AND METHODS
Dutch National Chronic Q Fever Database.
All Dutch hospitals that treat chronic Q fever patients were actively approached to include in the database chronic Q fever patients who were detected since the start of the Dutch Q fever epidemic in 2007. Most of the hospitals were situated in the epidemic region, the southwest of the Netherlands, in which the majority (>95%) of the patients were treated. The other patients were treated in numerous hospitals situated in regions where the epidemic did not occur. Design of the database and the use of the collected information for analysis and scientific publications were approved by the Medical Research Ethics Committee of the University Medical Centre Utrecht. In the current study, we used information about patients included in this database until the end of May 2012.
Patients.
Patients were included in the database if they were 18 years old or older and they fulfilled the criteria of proven, probable, or possible chronic Q fever according to the recent Dutch consensus guideline (Table 1) (15). In this guideline, the proven chronic Q fever group represents patients with the most definite form of chronic Q fever, in contrast to the possible chronic Q fever group, which represents patients with serology indicating chronic Q fever but without other manifestations of chronic disease. For the analysis of mortality of chronic Q fever patients, we chose to include only the patients with proven and probable chronic Q fever, as most likely a certain number of possible chronic Q fever patients do not have actual chronic Q fever. For deceased patients, we assessed the cause of death and whether mortality was due to chronic Q fever. Some of these patients died of diseases not related to chronic Q fever (e.g., malignancies) or it could not be determined whether death was due or related to chronic Q fever (e.g., malaise, sepsis). Death association with chronic Q fever was defined by one researcher (L.M.K.) as definitely (caused by vascular complications, endocarditis), probably (caused by heart failure in case of suspected endocarditis, gastrointestinal bleeding in case of suspected arteriointestinal fistula, clinical deterioration in case of antibiotic refusal, or severe side effects of medication), possibly (coinciding with severe comorbidities or unknown cause of mortality) or not associated (other cause of mortality, such as cancer).
Data collection and storage.
Data on patient characteristics, imaging results, laboratory results, antibiotic therapy, and outcome were collected from patient records provided by the hospitals that participated in the database. All data were stored and processed anonymously in SPSS version 18 (SPSS, Inc., Chicago, IL).
Microbiological analysis.
In the Netherlands, routine microbiological work-up for the diagnosis of chronic Q fever consists of serology, using an indirect fluorescent-antibody assay (IFA) (Focus Diagnostics, Inc., Cypress, CA) and PCR for C. burnetii DNA on plasma or serum and, if available, tissue. Titration of antibody levels was carried out at the different hospital sites with dilutions according to a binary scale and a detection cutoff titer of 32. Some hospitals performed complement binding reactions (CBR), also referred to as complement fixation (CF) tests, for the diagnosis and follow-up of chronic Q fever. However, in all but three patients CBR results were at least, at one time point, confirmed with IFA. PCR for C. burnetii DNA was performed using an in-house assay as previously described (22). An input volume of 500 μl plasma or serum was used for DNA isolation.
Statistical methods.
We used descriptive statistics to present data on baseline and clinical characteristics. In univariate analyses, patient characteristics were related to mortality using the log-rank test. Analyses were conducted separately for all-cause mortality and chronic Q fever-related mortality, the latter defined as death definitely or probably caused by chronic Q fever. Risk factors that were significantly and mostly associated with mortality in univariate analysis were additionally evaluated using multivariable Cox regression analysis. Due to multicollinearity and the limited number of deceased subjects, we could not include all risk factors simultaneously. The multivariable analyses were also conducted for all-cause mortality and chronic Q fever-related mortality separately in two models. Given the limited number of deceased subjects, only univariate evaluation was used for variables that applied to only part of the population (e.g., characteristics of acute Q fever episodes and surgical treatment). Differences were considered significant at P values of ≤0.05.
RESULTS
In total, 284 patients were included in the chronic Q fever database. Of these, 151 patients (53.7%) had proven chronic Q fever, 64 patients (22.5%) probable chronic Q fever, and 69 patients (24.3%) possible chronic Q fever. Baseline characteristics and details of chronic and acute Q fever episodes of these patients are shown in Table 2. Microbiology results of all chronic Q fever patients are presented in Table 3.
TABLE 2.
Baseline and clinical characteristics of chronic Q fever cases in the Netherlands
| Characteristica | All chronic Q fever (n = 284) | Proven chronic Q fever (n = 151) | Probable chronic Q fever (n = 64) | Possible chronic Q fever (n = 69) |
|---|---|---|---|---|
| Male (n [%]) | 204 (71.6) | 115 (76) | 48 (75) | 41 (59) |
| Age (mean [SD]) (yr) | 64.9 (14.1) | 68.6 (12) | 65.2 (15) | 56.6 (15) |
| Focus of infectionb (n [%]) | ||||
| Endocarditis | 75 (26.4) | 49 (32) | 26 (41) | |
| Infection of valve prosthesis | 38 (13.3) | 31 (21) | 7 (11) | |
| Vascular infection | 122 (42.0) | 104 (69) | 18 (28) | |
| Infection of vascular prosthesis | 57 (20.1) | 45 (30) | 12 (19) | |
| Other focus (n [%]) | 6 (2.1) | 2 (1)i | 4 (76)l | |
| No focus (n [%]) | 92 (33.1) | 7 (5)j | 16 (25)m | 69 (100) |
| Known acute Q fever episode (n [%]) | 106 (37.3) | 28 (19)k | 30 (47) | 48 (70) |
| Adequate treatmentc,d | 81 (76) | 19 (68) | 24 (80) | 38 (79) |
| Echocardiogram (TEE or TTE) performedd | 21 (20) | 8 (29) | 3 (10) | 10 (21) |
| Imaging of abdominal aorta by AUSd | 14 (13) | 5 (18) | 4 (13) | 5 (10) |
| Risk factor for chronic Q feverd | 34 (32) | 17 (61) | 15 (50) | 2 (4) |
| Chronic Q fever prophylaxisd,e | 4 (4) | 4 (14) | ||
| Risk factors for chronic Q fever (n [%]) | 152 (53.5) | 107 (71) | 42 (66) | 3 (4) |
| History of valvulopathy | 68 (23.9) | 48 (32) | 19 (30) | 1 (1) |
| Valve prosthesis or plasty | 45 (15.8) | 36 (24) | 9 (14) | |
| History of aneurysm | 28 (9.9) | 22 (15) | 6 (9) | |
| History of vascular prosthesis | 61 (21.5) | 49 (32) | 12 (19) | |
| Immunosuppressionf | 19 (6.7) | 11 (7) | 8 (13) | |
| Pregnancy during acute episode | 5 (1.8) | 1 (1) | 2 (3) | 2 (3) |
| Surgical treatment due to Q fever (n [%]) | 65 (22.9) | 64 (42) | 1 (2) | |
| Valve surgeryg | 13 (4.6) | 12 (8) | 1 (2) | |
| Vascular surgeryg | 53 (19.0) | 54 (36) | ||
| Deceasedh (n [%]) | 45 (15.8) | 35 (23) | 6 (9) | 4 (6) |
| No. of deceased patients with endocarditis/total no. of patients with endocarditis (%) | 12/75 (16) | 10/49 (20) | 2/24 (8) | NAn |
| No. of deceased patients with vascular infection/total no. of patients with vascular infection (%) | 28/122 (23) | 26/104 (25) | 2/18 (11) | NA |
| Definitely or probably deceased due to chronic Q fever (n [%]) | 28 (9.8) | 27 (18) | 1 (2) |
TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; AUS, abdominal ultrasound.
In 11 proven chronic Q fever patients, imaging studies revealed that the focus of infection could be both on the heart valves and vascular structures.
Proven effective antibiotic treatment regime for at least 10 days.
Percentages estimated for all chronic cases with an acute Q fever episode only.
Doxycycline and hydroxychloroquine for at least 6 months.
Prednisone cumulative dose >750 mg, tumor necrosis factor alpha (TNF-α)-blocker usage, methotrexate usage, mycophenolate mofetil usage, splenectomy, hematologic malignancies.
Two patients underwent both vascular and heart valve surgery, one patient underwent replacement of thoracic aneurysm and aortic valve and one patient underwent replacement of aortic valve and vascular surgery of an aneurysm of the left arteria iliaca.
In two deceased patients with proven chronic Q fever, the focus of infection could have been both on heart valves and vascular structures.
One patient with pericarditis and one patient with an infected placenta (confirmed by PCR).
All had positive PCR results for blood samples; six patients had cardiovascular risk factors, and two were immunocompromised.
Data missing in seven patients.
One patient with pericarditis, two patients with chronic serologic profiles during pregnancy, and one patient with spondylodiscitis.
Six patients with clinical signs of systemic infection, 10 patients with immunocompromised status.
NA, not applicable.
TABLE 3.
Results of microbiology tests (PCR and IFAa) of all proven, probable, and possible chronic Q fever patients
| Result | All chronic Q fever (n = 284) (n [%]) | Proven chronic Q fever (n = 151)b (n [%]) | Probable chronic Q fever (n = 64)d (n [%]) | Possible chronic Q fever (n = 69) (n [%]) |
|---|---|---|---|---|
| PCR positive in blood only | 60 (21.1) | 60 (40) | ||
| PCR positive in tissue only | 25 (8.8) | 25 (17) | ||
| PCR positive in blood and tissue | 35 (12.3) | 35 (23) | ||
| Phase I IgG titer | ||||
| 512 | 2 (0.7) | 2c (1) | ||
| 1,024 | 35 (12.3) | 6 (4) | 13 (20) | 16 (23) |
| 2,048 | 61 (21.5) | 24 (16) | 13 (20) | 24 (35) |
| 4,096 | 62 (21.8) | 28 (19) | 17 (27) | 17 (25) |
| 8,192 | 36 (12.7) | 17 (11) | 10 (16) | 9 (13) |
| >8,192 | 79 (27.8) | 66 (44) | 10 (6) | 3 (4) |
IFA, immunofluorescence assay.
For two patients, no titration for a titer above 4,096 was performed; for three patients, no reliable IFA titer results were obtained due to blood transfusion or fluid supplementation; for three patients, no IFA titer was performed (PCR results in blood and/or tissue were positive), but complement binding reaction (CBR) was performed (PCR results in blood and/or tissue were positive).
One patient with positive PCR for blood, one patient with positive PCR for vascular tissue.
In one patient no titration of serology above 4,096 was performed.
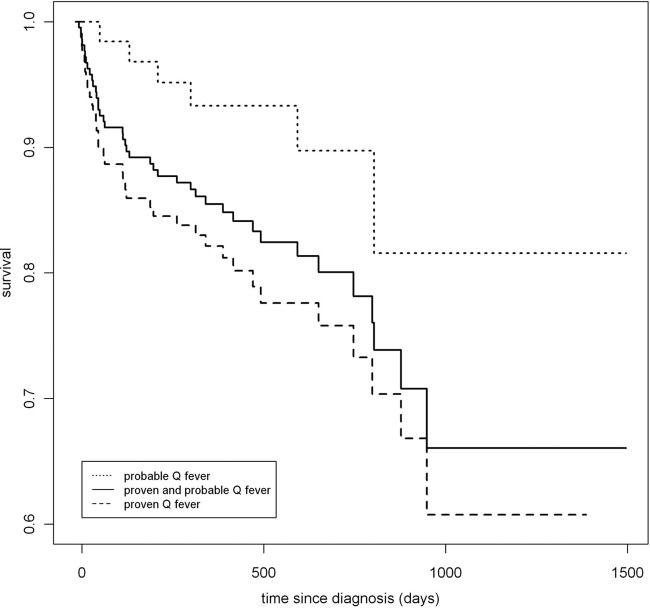
The overall mortality rate among chronic Q fever patients was 15.8%, of which 23.2% comprised proven cases, 9.4% comprised probable cases, and 5.8% comprised possible cases. Of the 215 patients with proven or probable chronic Q fever, 41 (19.1%) died during a median follow-up of 14.3 months (interquartile range 25 to 75% [10.1 to 23.1 months]). Mortality definitely or probably associated with chronic Q fever among patients with proven or probable chronic Q fever was 13.0% (28/215 patients), with a 9.3% mortality rate among endocarditis patients, and 18.0% in patients with a vascular focus of infection. The causes of mortality of the proven and probable chronic Q fever cases are shown in Fig. 1. Figure 2 presents a Kaplan-Meier survival curve based on the multivariable model. Of the deceased patients with chronic Q fever, 50% died upon presentation or within the first 4 months after diagnosis.
FIG 1.
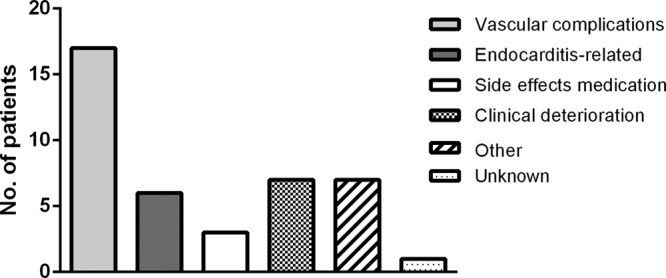
Causes of death of deceased proven and probable chronic Q fever patients. Other causes of death were malignancies, renal failure, and sepsis due to another pathogen which was unrelated to endocarditis or vascular infection.
FIG 2.
Kaplan-Meier survival curve for proven and probable chronic Q fever patients.
In Table 4, clinical characteristics of all proven and probable chronic Q fever patients who died (n = 41) are compared with those of patients who did not die (n = 174). The group of deceased patients was analyzed as an all-cause mortality group (n = 41) and as a group with chronic Q fever-related mortality (n = 28). Increasing age (P = 0.004 and 0.010), proven chronic Q fever (P = 0.020 and 0.002), vascular infection as the focus of chronic Q fever (P = 0.024 and 0.005), acute presentation with chronic Q fever (P = 0.002 and P < 0.001), and surgical treatment of chronic Q fever (P = 0.025 and P < 0.001) were significantly associated with all-cause mortality and chronic Q fever-related mortality, respectively. Analysis of only those patients who underwent surgical intervention demonstrated that emergency surgical treatment in particular was associated with mortality (P = 0.007 for all-cause mortality and 0.017 for chronic Q fever-related mortality).
TABLE 4.
Characteristics of deceased and nondeceased patients with proven and probable chronic Q fever
| Characteristic | Nondeceased (n = 174) | Deceased (n = 41) | Pe | Definitely or probably deceased due to chronic Q fever (n = 28) | Pe |
|---|---|---|---|---|---|
| Male (n [%]) | 133 (76) | 30 (73) | 0.67 | 21 (75) | 0.83 |
| Age (mean [SD]) (yr) | 66.5 (13) | 72.1 (12) | 0.004 | 72.5 (13) | 0.010 |
| Risk factor for chronic Q fever (n [%]) | 119 (68) | 30 (73) | 0.40 | 20 (71) | 0.62 |
| Proven chronic Q fever (n [%]) | 116 (67) | 35 (85) | 0.020 | 27 (96) | 0.002 |
| Endocarditis (n [%]) | 63 (36) | 12 (29) | 0.28 | 7 (25) | 0.21 |
| Valve prosthesis or plasty | 31 (18) | 7 (17) | 0.92 | 4 (14) | 0.70 |
| Vascular infection (n [%]) | 94 (54) | 28 (68) | 0.024 | 22 (79) | 0.005 |
| Vascular prosthesis | 50 (29) | 7 (17) | 0.36 | 4 (14) | 0.25 |
| Other focus (n [%]) | 6 (3) | 0 | 0.19 | 0 | 0.28 |
| No focus (n [%]) | 20 (11) | 3 (10) | 0.31 | 0 | 0.05 |
| Acute presentation with chronic Q fever (n [%]) | 35 (20) | 18 (44) | 0.002 | 16 (57) | <0.001 |
| Acute Q fever episode (n [%]) | 45 (26) | 13 (32) | 0.77 | 8 (29) | 0.66 |
| Adequately treated with antibioticsa,b | 34 (76) | 9 (69) | 0.91 | 4 (50) | 0.27 |
| Risk factor for chronic Q feverb | 22 (50) | 10 (77) | 0.076 | 6 (75) | 0.18 |
| Prophylactic therapyb,c | 2 (9) | 1 (10) | 0.79 | 0 | 0.47 |
| Adequate antibiotic treatment of chronic Q fever (n [%]) | 145 (83) | 35 (85) | 0.96 | 23 (82) | 0.69 |
| Surgical treatment of chronic Q fever (n [%]) | 49 (28) | 19 (46) | 0.025 | 17 (61) | <0.001 |
| Emergency surgical treatmentd | 21 (43) | 15 (79) | 0.006 | 13 (76) | 0.013 |
Proven effective antibiotic treatment regime for at least 10 days.
Percentages and P values estimated on all chronic cases with an acute Q fever episode only.
Doxycycline and hydroxychloroquine for at least 6 months.
Percentages and P values estimated on all chronic cases who underwent surgical treatment only.
P values estimated with log-rank test.
In multivariable Cox regression analysis, age (hazard ratio [HR] 1.57; 95% confidence interval [CI] 1.13 to 2.19 per 10-year increase of age) and acute presentation with chronic Q fever (HR, 1.96; 95% CI, 1.02 to 3.78) were independent predictors of all-cause mortality (Table 5). Age was also an independent predictor of chronic Q fever related-mortality (HR, 1.59; 95% CI, 1.14 to 2.21 per 10-year increase of age).
TABLE 5.
Adjusted risk factors for mortality among patients with proven and probable chronic Q fever
| Multivariable model with risk factors includeda | All-cause mortality (HR [95% CI])b | Mortality definitely or probably due to chronic Q fever (HR [95% CI]) |
|---|---|---|
| Model 1 | ||
| Age in 10 yr (continuous) | 1.57 (1.13–2.19) | 1.68 (1.09–2.59) |
| Proven chronic Q fever | 1.63 (0.62–4.26) | 5.65 (0.71–44.82) |
| Vascular infection | 1.48 (0.73–2.99) | 1.81(0.71–4.59) |
| Acute presentation with chronic Q fever | 1.96 (1.02–3.78) | 2.75 (1.27–5.95) |
| Model 2 | ||
| Age in 10 yr (continuous) | 1.59 (1.14–2.21) | 1.71 (1.11–2.62) |
| Proven Q fever | 1.84 (0.71–4.76) | 6.66 (0.84–52.59) |
| Vascular infection | 1.34 (0.63–2.85) | 1.46 (0.53–3.99) |
| Surgical treatment of chronic Q fever | 1.51 (0.75–3.04) | 2.09 (0.89–4.89) |
Two multivariable models were constructed because of multicollinearity between acute presentation with chronic Q fever and surgical treatment of chronic Q fever. Model 1 includes acute presentation with chronic Q fever and model 2 surgical treatment of chronic Q fever.
HR, hazard ratio; 95% CI, 95% confidence interval.
DISCUSSION
Until the end of May 2012, 284 patients with proven, probable, or possible chronic Q fever were identified in the Dutch National Chronic Q Fever Database. Of all chronic Q fever cases, 108 patients (38.0%) recalled an acute Q fever episode, although among proven and probable cases, only 58 patients (27.0%) had a known acute Q fever episode. During the Q fever epidemic, over 4,000 cases of acute Q fever were reported (4). Therefore, the risk for chronic Q fever development after a symptomatic acute Q fever episode is approximately 2.5% if all chronic Q fever cases are taken into account, and 1.5% if limited only to proven and probable chronic Q fever cases. These figures are in line with the previously reported rate of 1 to 5% (1, 6). However, based on seroprevalence studies, estimates of the total number of C. burnetii infections in the Dutch outbreak are up to 10-fold higher than the number of reported cases (5, 23). Extrapolating these data, the number of identified chronic Q fever patients in the Netherlands does not reach this rate of 1 to 5% of C. burnetii infections by far (1). This could indicate that the majority of chronic Q fever cases are still undetected, although this is less likely as it is more than 2 years after the end of the Q fever epidemic and it has been shown that most chronic Q fever cases manifest themselves in the first year after infection (1, 6). It could also indicate that in the Dutch outbreak the percentage of C. burnetii-infected patients who eventually developed chronic Q fever was lower than previously reported.
The majority of chronic Q fever patients did not recall an acute Q fever episode, an exception being patients with possible chronic Q fever (69.6%), who were mostly detected in follow-up programs after a recognized acute Q fever episode. This could be due to a low detection rate of acute Q fever cases, or to overlap with other febrile diseases, especially in the beginning of the epidemic. It could also indicate that asymptomatic primary infections also bring about the risk for development of chronic Q fever, maybe even more so than symptomatic infections. Therefore, early detection of chronic Q fever might not be accomplished only by follow-up programs of recognized acute Q fever cases; screening of high-risk groups after an acute Q fever outbreak should also be prioritized.
Notably, among the proven and probable chronic Q fever patients in the Netherlands, we found more patients with a vascular focus of infection (56.7%) than patients with endocarditis (35.3%). This is in contrast with earlier reports from France, where endocarditis predominates (1, 7). It might be that this disparity is caused by strain-specific differences, leading to distinct clinical presentations (24). Another explanation might be increased awareness in the Netherlands of this previously relatively unacknowledged manifestation of chronic Q fever. In 11 patients, imaging studies revealed that the focus of infection could be both on the heart valves and vascular structures, which was confirmed in one deceased patient at autopsy, where both the aortic valve as well as an aneurysm were found to be C. burnetii DNA positive by PCR. We found nine proven chronic Q fever patients with a positive PCR result for blood samples who did not have a clear vascular infection or endocarditis. One of these patients had signs of a pericarditis, while another patient had serologic results consistent with chronic Q fever during pregnancy and a positive C. burnetii PCR of placental tissue. In seven patients, no infection focus was found, despite thorough work-ups with echocardiography and fluorodeoxyglucose positron emission tomography-computed tomography. Six of these patients had risk factors for cardiovascular disease and two were immunocompromised (one patient with acute lymphatic leukemia and one patient on long-term prednisone therapy).
Recognized risk factors for chronic Q fever development were present in the majority of patients (53.5% of all cases, 69.3% in proven and probable cases), except for patients with possible chronic Q fever infection (4.3%), in whom risk factors consisted of minor valvulopathies only. In a significant number of proven and probable chronic Q fever patients, risk factors were not recognized before presentation. More than half of the patients with proven vascular chronic Q fever had no history of vascular anomalies beforehand. These patients presented at the hospital with aneurysms, for which in various instances emergency vascular surgery was needed. Although the role of echocardiography after an acute Q fever episode remains controversial and requires further evaluation (13, 25, 26), screening for an aortic aneurysm with abdominal ultrasound at the time of acute Q fever presentation could result in detection at an early stage of patients at risk for vascular chronic Q fever. Therefore, we advise to screen for aortic aneurysms in case of acute Q fever, especially when risk factors for vascular disease are present, like diabetes mellitus, hypertension, smoking, dyslipidemia, or positive family history for vascular disease. Moreover, active case finding in a Q fever epidemic area could be initiated by screening for Q fever in high-risk groups, including patients with aneurysms, vascular prostheses, or heart valve prostheses (12). In our current analysis, minor heart valve lesions (for example, grade I aortic valve insufficiency) were also included as risk factors for chronic Q fever, although the influence of minor valvulopathies on chronic Q fever development is debated (13, 24, 25). This could have led to overestimation of the amount of risk factors in all chronic Q fever cases.
Overall mortality in chronic Q fever patients was 15.8%. Mortality was highest in patients with proven chronic Q fever (23.2%) compared to patients with probable chronic Q fever (9.4%) and possible chronic Q fever (5.8%). As possible chronic Q fever cases most likely represent, at least in part, patients who do not have actual chronic Q fever, we omitted them from the analysis of chronic Q fever mortality. In total, we identified 215 patients with proven or probable chronic Q fever (among whom all-cause mortality was 19.1%) during a median follow-up of 14.3 months (interquartile range, 25 to 75% [10.1 to 23.1 months]). When only patients with chronic Q fever-related mortality were analyzed, the mortality rate among patients with proven and probable chronic Q fever was 13.0%, with a 9.3% mortality rate among endocarditis patients and 18.0% in vascular chronic Q fever cases. These figures confirm the results of previous studies showing differences in mortality rates of both disease entities (9, 13, 14). Most patients died due to vascular complications of vascular chronic Q fever. Mortality was highest in the first months after the diagnosis of chronic Q fever. There were no significant differences in antibiotic treatment among deceased and nondeceased patients. Although all patients were offered treatment, only 83% received adequate antibiotic treatment, as some patients refused therapy, while others died before treatment could be initiated. As illustrated in Fig. 1, two patients probably died due to severe side effects of the medication, consisting of kidney insufficiency and gastrointestinal side effects. Not unexpectedly, in multivariable analysis, older age was significantly associated with death in both the all-cause mortality and chronic Q fever-related mortality groups. Moreover, acute presentations with chronic Q fever (e.g., symptomatic aneurysm or severe endocarditis) were significantly associated with all-cause mortality. Although in univariate analysis proven chronic Q fever, surgical treatment of chronic Q fever, and vascular focus of infection were also significantly associated with all-cause and Q fever-related mortality, this was not confirmed in multivariable analysis. However, the number of deceased patients was relatively small for the performance of reliable multivariable analysis. Moreover, there were correlations between the included variables. Analysis of the data showed that the elevated mortality risk in cases of surgical intervention was caused by the necessity of emergency surgical procedures for patients who presented with acute complications of chronic Q fever, especially in case of vascular infections, and to a lesser extent endocarditis.
In conclusion, the data of the Dutch National Chronic Q Fever Database demonstrate that vascular chronic Q fever is a more common manifestation than Q fever endocarditis in the Netherlands. Most patients with chronic Q fever did not recall an episode of acute Q fever. Chronic Q fever is a severe infection with an overall high mortality rate. In particular, older patients with vascular infections who present with acute (vascular) complications needing urgent surgical intervention are at risk of death due to chronic Q fever. Measures to reduce mortality and morbidity of chronic Q fever should be directed at early case finding. This can be achieved through the screening of high-risk groups in case of a Q fever epidemic and surveillance for risk factors of chronic Q fever.
ACKNOWLEDGMENTS
We thank all the participants in the Dutch National Chronic Q fever Database in the Netherlands: Tom Sprong (Canisius-Wilhelmina Hospital, Nijmegen), Cornelis A. R. Groot (Bernhoven Hospital, Uden), Yvonne Soethoudt (Elkerliek Hospital, Helmond), Sybrandus N. Blank (Maxima Medical Center, Eindhoven/Veldhoven), Marjolijn J. H. Pronk (Catharina Hospital, Eindhoven), Gijs J. Limonard (Diakonessenhuis, Utrecht), Steven F. Thijssen (Diakonessenhuis, Utrecht), Bart J. Vlaminckx (St. Antonius Hospital, Nieuwegein), Frans Stals (Atrium Medical Centre, Heerlen), Bas J. M. van Kraaij (Atrium Medical Centre, Heerlen), Frederika Dijkstra (Centre for Infectious Disease Control, Bilthoven), Clemens Richter (Rijnstate Hospital, Arnhem), E. H. Gisolf (Rijnstate Hospital, Arnhem), Rik Heijligenberg (Gelderse Vallei Hospital, Ede), Ries Schouten (Gelderse Vallei Hospital, Ede), Karin Schurink (Erasmus University Medical Centre, Rotterdam), and Leo G. Visser (Leiden University Medical Centre, Leiden).
We thank Olivier Dams for proofreading our manuscript.
Footnotes
Published ahead of print 5 March 2014
REFERENCES
- 1.Maurin M, Raoult D. 1999. Q fever. Clin. Microbiol. Rev. 12:518–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kampschreur LM, Wegdam-Blans MC, Thijsen SF, Groot CA, Schneeberger PM, Hollander AA, Schijen JH, Arents NL, Oosterheert JJ, Wever PC. 2010. Acute Q fever related in-hospital mortality in the Netherlands. Neth. J. Med. 68:408–413 http://www.njmonline.nl/getpdf.php?t=a&id=10000657 [PubMed] [Google Scholar]
- 3.Raoult D, Marrie T, Mege J. 2005. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 5:219–226. 10.1016/S1473-3099(05)70052-9 [DOI] [PubMed] [Google Scholar]
- 4.van der Hoek W, Dijkstra F, Schimmer B, Schneeberger PM, Vellema P, Wijkmans C, ter Schegget R, Hackert V, van Duynhoven Y. 2010. Q fever in the Netherlands: an update on the epidemiology and control measures. Euro Surveill. 15:pii=19520 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19520 [PubMed] [Google Scholar]
- 5.Hogema BM, Slot E, Molier M, Schneeberger PM, Hermans MH, van Hannen EJ, van der Hoek W, Cuijpers HT, Zaaijer HL. 2012. Coxiella burnetii infection among blood donors during the 2009 Q-fever outbreak in the Netherlands. Transfusion 52:144–150. 10.1111/j.1537-2995.2011.03250.x [DOI] [PubMed] [Google Scholar]
- 6.Landais C, Fenollar F, Thuny F, Raoult D. 2007. From acute Q fever to endocarditis: serological follow-up strategy. Clin. Infect. Dis. 44:1337–1340. 10.1086/515401 [DOI] [PubMed] [Google Scholar]
- 7.Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier PE, Bernit E, Stein A, Nesri M, Harle JR, Weiller PJ. 2000. Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine 79:109–123 [DOI] [PubMed] [Google Scholar]
- 8.Frankel D, Richet H, Renvoise A, Raoult D. 2011. Q fever in France, 1985–2009. Emerg. Infect. Dis. 17:350–356. 10.3201/eid1703.100882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botelho-Nevers E, Fournier PE, Richet H, Fenollar F, Lepidi H, Foucault C, Branchereau A, Piquet P, Maurin M, Raoult D. 2007. Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. Eur. J. Clin. Microbiol. Infect. Dis. 26:635–640. 10.1007/s10096-007-0357-6 [DOI] [PubMed] [Google Scholar]
- 10.Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. 2001. Risks factors and prevention of Q fever endocarditis. Clin. Infect. Dis. 33:312–316. 10.1086/321889 [DOI] [PubMed] [Google Scholar]
- 11.Tissot-Dupont H, Vaillant V, Rey S, Raoult D. 2007. Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin. Infect. Dis. 44:232–237. 10.1086/510389 [DOI] [PubMed] [Google Scholar]
- 12.Kampschreur LM, Dekker S, Hagenaars JC, Lestrade PJ, Renders NH, de Jager-Leclercq MG, Hermans MH, Groot CA, Groenwold RH, Hoepelman AJ, Wever PC, Oosterheert JJ. 2012. Identification of risk factors for chronic Q fever, the Netherlands. Emerg. Infect. Dis. 18:563–570. 10.3201/eid1804.111478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Million M, Thuny F, Richet H, Raoult D. 2010. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect. Dis. 10:527–535. 10.1016/S1473-3099(10)70135-3 [DOI] [PubMed] [Google Scholar]
- 14.Wegdam-Blans MC, Vainas T, van Sambeek MR, Cuypers PW, Tjhie HT, van Straten AH, Teijink JA. 2011. Vascular complications of Q fever infections. Eur. J. Vasc. Endovasc. Surg. 42:384–392. 10.1016/j.ejvs.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 15.Wegdam-Blans MC, Kampschreur LM, Delsing CE, Bleekers-Rovers CP, Sprong T, van Kasteren ME, Notermans DW, Renders NH, Bijlmer HA, Lestrade PJ, Koopmans MP, Nabuurs-Franssen MH, Oosterheert JJ; Dutch Q fever Consensus Group. 2012. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J. Infect. 64:247–259. 10.1016/j.jinf.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 16.Fenollar F, Fournier PE, Raoult D. 2004. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J. Clin. Microbiol. 42:4919–4924. 10.1128/JCM.42.11.4919-4924.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musso D, Raoult D. 1995. Coxiella burnetii blood cultures from acute and chronic Q-fever patients. J. Clin. Microbiol. 33:3129–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupont HT, Thirion X, Raoult D. 1994. Q fever serology: cutoff determination for microimmunofluorescence. Clin. Diagn. Lab. Immunol. 1:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VF, Jr, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30:633–638. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 20.van der Hoek W, Versteeg B, Meekelenkamp JC, Renders NH, Leenders AC, Weers-Pothoff I, Hermans MH, Zaaijer HL, Wever PC, Schneeberger PM. 2011. Follow-up of 686 patients with acute Q fever and detection of chronic infection. Clin. Infect. Dis. 52:1431–1436. 10.1093/cid/cir234 [DOI] [PubMed] [Google Scholar]
- 21.Kampschreur LM, Oosterheert JJ, Koop AM, Wegdam-Blans MC, Delsing CE, Bleeker-Rover CP, MG de Jager-Leclercq Groot CA, Sprong T, Nabuurs-Franssen MH, Renders NH, van Kasteren ME, Soethoudt Y, Blank SN, Pronk MJ, Groenwold RH, Hoepelman AI, Wever PC. 2012. Microbiological challenges in the diagnosis of chronic Q fever. Clin. Vaccine Immunol. 19:787–790. 10.1128/CVI.05724-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeberger PM, Hermans MH, van Hannen EJ, Schellekens JJ, Leenders AC, Wever PC. 2010. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin. Vaccine Immunol. 17:286–290. 10.1128/CVI.00454-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kampschreur LM, Hagenaars JC, Wielders CC, Elsman P, Lestrade PJ, Koning OH, Oosterheert JJ, Renders NH, Wever PC. 2013. Screening for Coxiella burnetii seroprevalence in chronic Q fever high-risk groups reveals the magnitude of the Dutch Q fever outbreak. Epidemiol. Infect. 141:847–851. 10.1017/S0950268812001203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limonard GJ, Nabuurs-Franssen MH, Weers-Pothoff G, Wijkmans C, Besselink R, Horrevorts AM, Schneeberger PM, Groot CA. 2010. One-year follow-up of patients of the ongoing Dutch Q fever outbreak: clinical, serological and echocardiographic findings. Infection 38:471–477. 10.1007/s15010-010-0052-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limonard GJ, Nabuurs-Franssen MH, Dekhuijzen PN, Groot CA. 2011. Prevention of Q fever endocarditis. Lancet Infect. Dis. 11:82–83. 10.1016/S1473-3099(11)70016-0 [DOI] [PubMed] [Google Scholar]
- 26.Raoult D. 2012. Chronic Q fever: expert opinion versus literature analysis and consensus. J. Infect. 65:102–108. 10.1016/j.jinf.2012.04.006 [DOI] [PubMed] [Google Scholar]