Abstract
One of the most well-characterized tests for diagnosing neurocysticercosis (NCC) is the enzyme-linked immunoelectrotransfer blot (EITB) assay developed at the CDC, which uses lentil lectin-bound glycoproteins (LLGP) extracted from Taenia solium cysticerci. Although the test is very reliable, the purification process for the LLGP antigens has been difficult to transfer to other laboratories because of the need for expensive equipment and technical expertise. To develop a simpler assay, we previously purified and cloned the diagnostic glycoproteins in the LLGP fraction. In this study, we evaluated three representative recombinant or synthetic antigens from the LLGP fraction, individually and in different combinations, using an immunoblot assay (recombinant EITB). Using a panel of 249 confirmed NCC-positive and 401 negative blood serum samples, the sensitivity of the recombinant EITB assay was determined to be 99% and the specificity was 99% for diagnosing NCC. We also tested a panel of 239 confirmed NCC-positive serum samples in Lima, Peru, and found similar results. Overall, our data show that the performance characteristics of the recombinant EITB assay are comparable to those of the LLGP-EITB assay. This new recombinant- and synthetic antigen-based assay is sustainable and can be easily transferred to other laboratories in the United States and throughout the world.
INTRODUCTION
The diagnosis of neurocysticercosis (NCC), a condition caused by the larvae Taenia solium, is reliably established using results from both imaging and serological tests. The guidelines proposed for making a diagnosis of presumptive NCC (1, 2) define cases as definitive, probable, or possible based on computed tomography (CT) or magnetic resonance imaging (MRI) findings, exposure risk, and serological results. These guidelines specifically define the lentil lectin-bound glycoprotein enzyme-linked immunoelectrotransfer blot assay (LLGP-EITB) as the serological reference standard for diagnosing NCC (3).
The LLGP-EITB assay is an immunoblot method that detects antibodies to one or more of seven lentil lectin-bound glycoproteins, which are present in the soluble fraction of an extract of T. solium cysts. In patients with multiple enhancing intracranial lesions, the LLGP-EITB assay is 100% specific and 95% sensitive using blood serum or cerebrospinal fluid (CSF) samples (3–6). The original study that described and evaluated the LLGP-EITB assay was performed using serum samples from biopsy-proven cases of NCC, most with multiple lesions, as detected by skeletal radiographs, and reported a sensitivity of 98% and a specificity of 100% (3). Continued monitoring of the test performance compared with clinical findings using newer imaging techniques, such as CT and MRI, revealed that the sensitivity of the assay was lower, between 50 to 80%, in cases with a single lesion or calcified cysts (6–10). The specificity of the LLGP-EITB assay has been remarkable at essentially 100%, with only rare anecdotal reports of false-positive results (11–13).
Although the LLGP-EITB is an excellent test, the purification process for LLGP from cysts collected from naturally infected pigs has been difficult to standardize, and the polyacrylamide gel system used for the LLGP-EITB assay has been difficult to establish in other laboratories. Greater availability of simple and reliable diagnostics for NCC is anticipated through the use of recombinant or synthetic protein antigens. Therefore, we systematically purified and cloned the diagnostic glycoproteins in the LLGP fraction (14–16). We found that the seven diagnostic proteins comprise three distinct antigenic protein families: the gp50, gp24, and 8-kDa families. We chose representative recombinant or synthetic forms from each of the three antigen families and incorporated these into an immunoblot test (recombinant EITB) for use in the clinical diagnosis of NCC.
MATERIALS AND METHODS
Chemicals and reagents.
All reagents were reagent grade or better, and unless otherwise noted, they were obtained from Mallinckrodt (St. Louis, MO). The horseradish peroxidase-labeled goat anti-human IgG secondary antibody was prepared at the CDC (17).
Blood serum samples. (i) Sera for assay optimization.
A blood serum pool constructed by pooling 5 serum samples from human cases with confirmed cysticercosis was used both for optimizing the assay and as a positive control for the assay. This pool contained antibodies that reacted with all seven of the diagnostic proteins in the LLGP-EITB assay. A serum sample from a human case of alveolar echinococcosis (Echinococcus multilocularis) was used as a heterologous infection serum control. A negative serum pool was constructed from 5 serum samples from healthy U.S. residents with no reported history of international travel.
(ii) Serum panels for determinations of diagnostic sensitivity and specificity.
A total of 249 serum specimens were collected by the Instituto Nacional de Ciencias Neurologicas, Lima, Peru, from patients presenting with clinical symptoms of NCC, and these were provided to the CDC for the initial evaluation studies. A definitive diagnosis of NCC was confirmed by CT or MRI brain imaging (1, 2). Blood serum samples from the NCC cases were sorted into three categories based on the imaging data from each patient: sera from patients with ≥2 viable cysts (n = 107) (these included sera from patients with multiple viable cysts or a racemose cyst), patients with a single viable cyst (n = 52), and patients with calcified and/or degenerated cysts (n = 90). Cases with a single lesion and that were seronegative were still considered to be confirmed NCC cases. Cases classified as calcified included patients with single or multiple calcified cysts, and those categorized as degenerated cysts had one or two degenerated cysts. All serum samples were collected in compliance with protocols approved by the ethics review boards of all participating institutions, with specific permission for the future use of stored samples.
A total of 401 serum samples were used to assess specificity (Table 1). A panel of 191 serum samples was assembled from healthy residents from the United States (n = 167) or Egypt (n = 24). The donors from Egypt were tested for the presence of intestinal parasites by stool examination, and all were negative. In addition, a panel of serum samples collected from patients with heterologous infections (n = 210) was used. All of these serum samples were collected in regions where the transmission of cysticercosis/taeniasis does not occur, except for the serum samples from persons with Taenia saginata taeniasis, which were collected in Peru. Differentiation of T. solium and T. saginata was made after examining the proglottids or by PCR (18–20).
TABLE 1.
Diagnostic specificity of LLGP-EITB and recombinant EITB
| Serum classification and related data | n | No. (%) of LLGP-EITB reactive samples | No. (%) of recombinant EITB reactive samples |
|---|---|---|---|
| Normal human sera origin | |||
| American nontravelers | 167 | 0 | 2 (1)a |
| Egyptian urbanites | 24 | 0 | 1 (1)a |
| Sera from patients diagnosed with other infections | |||
| Non-T. solium taeniasis | 14 | 0 | 0 |
| T. saginata taeniasis | 20 | 4 (20) | 0 |
| Ascariasis | 2 | 0 | 0 |
| Hydatid echinococcosis | 27 | 8 (30) | 0 |
| Alveolar echinococcosis | 1 | 0 | 0 |
| Filariasis | 7 | 0 | 0 |
| Amebiasis | 11 | 0 | 2 (18)a |
| Fascioliasis | 2 | 0 | 0 |
| Soil-transmitted helminths | 11 | 0 | 0 |
| Giardiasis | 1 | 0 | 0 |
| Heterophyiasis | 8 | 0 | 0 |
| Hymenolepiasis | 10 | 2 (20) | 2 (20)b |
| Hepatitis | 26 | 0 | 0 |
| Malaria | 8 | 0 | 0 |
| Paragonimiasis | 4 | 0 | 0 |
| Schistosomiasis | 54 | 0 | 5 (9)a |
| Clonorchiasis | 4 | 0 | 0 |
| Total | 401 | 14 (3) | 12 (3) |
Positive against rGP50 only.
Positive against TsRS1 only.
Another unique set of serum samples from NCC patients was tested in Lima, Peru. The 239 defined serum samples were collected from 119 patients with ≥2 viable cysts, 84 patients with a single viable cyst, and 36 patients with other stages of NCC.
Recombinant and synthetic T. solium protein antigens.
Recombinant GP50 (rGP50) was expressed in Sf21/Sf9 cells, and the hydrophilic extracellular domain of T24, rT24H, was expressed in the Tni cell line, using baculovirus expression systems, as described previously (15, 16). TsRS1, a 66-amino acid peptide and a member of the 8-kDa antigen family (21), was chemically synthesized (sTsRS1) (AnaSpec, San Jose, CA).
SDS-PAGE and EITB.
Proteins were electrophoretically separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose (catalog no. 10 541 103, 0.2 μm pore size; Whatman Protran BA83), as described previously (22). The blots were cut into 2.5-mm strips and stored in phosphate-buffered saline (PBS)-0.1% NaN3 at 4°C prior to use. The serum samples were tested, and specific antibodies were detected as described previously (3, 22). The blot strips and the initial experiments to establish the optimal concentration of each recombinant protein/synthetic peptide were prepared using 5 to 22.5% polyacrylamide gels that were made in our lab at the CDC (22). To see if the proteins/peptide could be separated adequately using commercially prepared gels, we used 15% and 4 to 22% gradient gels from Bio-Rad (Hercules, CA).
Data analysis.
The agreement between the LLGP-EITB and recombinant EITB assay results was determined using Cohen's kappa statistic (23). We used the following scale to interpret the kappa values: <0, less-than-chance agreement; 0.01 to 0.20, slight agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, substantial agreement; and 0.81 to 0.99, almost-perfect agreement (24).
RESULTS
An initial set of experiments was conducted to determine the optimal concentration of each individual antigen for the recombinant EITB assay. Each of the protein antigens, rGP50, rT24H, and sTsRS1, was diluted serially and analyzed separately using SDS-PAGE and immunoblot analysis. The blots were incubated with the pooled cysticercosis serum, the echinococcosis serum, or the pooled normal serum. The optimal concentration of each antigen was determined visually by choosing the concentration that gave the greatest specific reactivity, as observed by comparing the reactivity with the cysticercosis serum to reactivity with the echinococcosis serum and the pooled normal serum. The optimal concentrations of the antigens were determined to be 0.2 ng/mm for rGP50, 2.5 ng/mm for rT24H, and 10 ng/mm for sTsRS1 (data not shown).
The 3 antigens were mixed together at their respective final optimal concentrations and separated using SDS-PAGE. The pooled recombinant proteins, rGP50 and rT24H, and the synthetic peptide, sTSRS1, were separated on in-house 5 to 22.5% polyacrylamide gels (Fig. 1). The three antigens were successfully resolved when the mixture of recombinant proteins and synthetic peptides was separated using commercially prepared polyacrylamide gels (Fig. 2).
FIG 1.
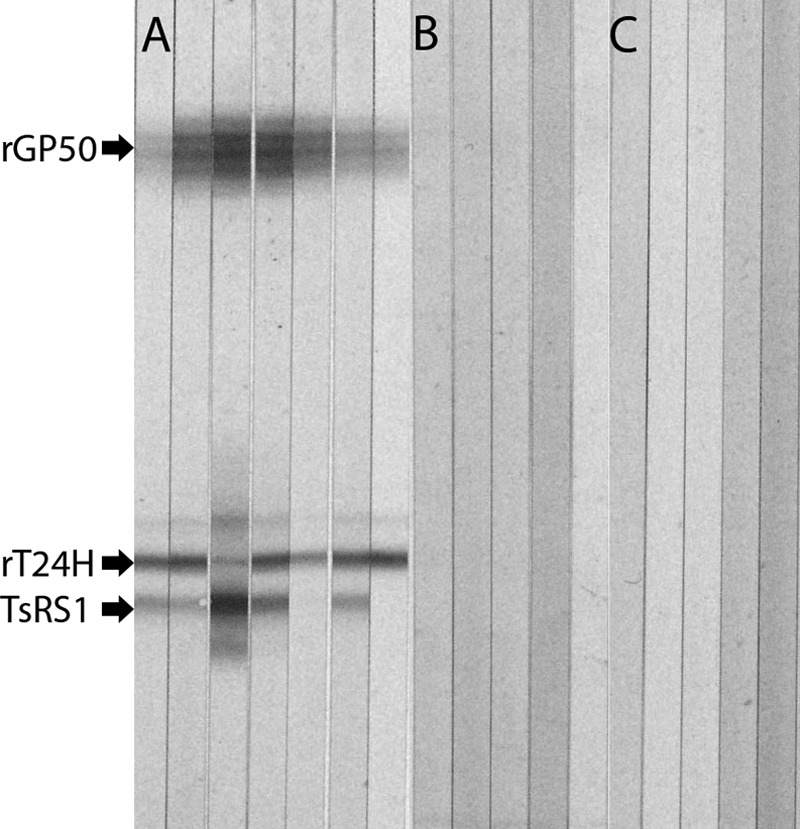
Recombinant protein immunoblot analysis. rGP50, rT24H, and TsRS1 were separated using SDS-PAGE with the CDC polyacrylamide gel system. Strips were incubated with sera from patients with NCC (A), patients with other heterologous parasitic infections (B), or healthy Americans (C).
FIG 2.

Resolution of the recombinant and synthetics antigens using commercially available polyacrylamide gels. rGP50, rT24H, and TsRS1 were applied to 2 commercially available polyacrylamide gels and then transferred to nitrocellulose. The proteins were visualized using a pool of cysticercosis sera for immunoblot analysis. Lane 1, 15% polyacrylamide gel; lane 2, 4 to 20% polyacrylamide gel.
The sensitivity of the recombinant EITB assay for diagnosing NCC was assessed at two sites using two different serum batteries, one at the CDC in Atlanta, GA, USA, and one at the Instituto Nacional de Ciencias Neurologicas, Lima, Peru. When the data from both sites are considered, the combination of all three antigens proved to be more sensitive than any single antigen alone for detecting cases of NCC with ≥2 viable cysts (Table 2); however, the sensitivity was excellent (>95%) for all combinations of antigens and except for TSRS1 alone, which had a sensitivity for detecting NCC cases of 85% or 75%, depending on the serum battery examined. When the sensitivity of testing for the individual antigens was evaluated, rT24H performed better than rGP50, which performed better than TSRS1 (rT24H > rGP50 > TSRS1). Similar patterns of reactivity occurred for sera from NCC patients with a single viable cyst or degenerate or calcified cysts only (Tables 2 and 3).
TABLE 2.
Sensitivities and specificities of the recombinant EITB test performed at two sites (CDC, Atlanta, GA, and Instituto Nacional de Ciencias Neurologicas, Lima, Peru)a
| Antigen(s) | Testing site | Sensitivity (%) (95% CI) for detecting cases with: |
Specificity (%) (95% CI) | |
|---|---|---|---|---|
| ≥2 cysts | 1 cyst | |||
| LLGP | Lima | 100 (100) | 79 (70–88) | ND |
| Atlanta | 96 (93–100) | 52 (58–60) | 97 (95–98) | |
| rGP50 + rT24H + TsRS1 | Lima | 97 (94–100) | 65 (55–75) | 98 (96–100) |
| Atlanta | 99 (97–100) | 56 (40–67) | 98 (97–100) | |
| rGP50 + rT24H | Lima | 96 (93–99) | 65 (55–75) | 98 (96–100) |
| Atlanta | 99 (97–100) | 52 (39–65) | 99 (98–100) | |
| rGP50 + TsRS1 | Lima | 92 (88–96) | 44 (33–54) | 100 (100) |
| Atlanta | 97 (94–100) | 48 (35–61) | 98 (97–100) | |
| rT24H + TsRS1 | Lima | 96 (93–99) | 52 (41–62) | 98 (96–100) |
| Atlanta | 99 (97–100) | 46 (33–60) | 99 (98–100) | |
| rGP50 | Lima | 89 (84–94) | 44 (33–54) | 100 (100) |
| Atlanta | 96 (93–100) | 44 (31–58) | 99 (98–100) | |
| rT24H | Lima | 96 (93–99) | 52 (41–62) | 98 (96–100) |
| Atlanta | 99 (97–100) | 43 (29–56) | 100 (100) | |
| TsRS1 | Lima | 85 (80–91) | 39 (28–49) | 100 (100) |
| Atlanta | 75 (67–83) | 20 (10–31) | 99 (98–100) | |
CI, confidence interval; ND, not determined.
TABLE 3.
Sensitivities of the recombinant EITB test against samples with degenerated or calcified cyst(s) performed at the CDC
| Protein(s) | Sensitivity (%) (95% CI) for detecting cases with: |
|
|---|---|---|
| Degenerated cysts only | Calcified cysts only | |
| rGP50 + rT24H + TsRS1 | 83 (67–98) | 78 (68–87) |
| rGP50 + rT24H | 74 (56–92) | 78 (68–87) |
| rGP50 + TsRS1 | 74 (56–92) | 64 (53–74) |
| rT24H + TsRS1 | 70 (51–88) | 76 (67–86) |
| rGP50 | 65 (46–85) | 64 (53–74) |
| rT24H | 61 (41–81) | 73 (63–82) |
| TsRS1 | 35 (15–54) | 16 (8–24) |
The specificity of the recombinant EITB assay for diagnosing NCC was also evaluated using a panel of 401 serum samples (Table 1). The serum samples used for the specificity analysis were collected from healthy individuals (n = 191) or patients with heterologous infections (n = 210). The specificity of the recombinant EITB assay using the recombinant/synthetic antigens, either alone or in combination, was ≥98% (Table 2).
We compared reactivity in the recombinant EITB assay to that of the currently accepted standard for laboratory diagnosis, the LLGP-EITB assay (Tables 2 and 4). The concordance between the recombinant EITB and the LLGP-EITB assay results was almost perfect (kappa = 0.89). Most (10/12) of the nonconcordant results occurred because of reactions with the rGP50 antigen only. Two serum samples from Hymenolepis infections generated false-positive results in both the recombinant EITB and the LLGP-EITB assays and reacted with native 8-kDa-protein antigens and TSRS1.
TABLE 4.
Comparison of test sensitivities using the LLGP-EITB and the recombinant EITB, performed at the CDC and the Instituto Nacional de Ciencias Neurologicas
| Assay, reactivity | Sensitivity (%) (95% CI) for detecting cases with: |
|||
|---|---|---|---|---|
| ≥2 viable cysts |
1 viable cyst |
|||
| Atlanta | Lima | Atlanta | Lima | |
| LLGP-EITB, any band | 96 (92–99) | 100 (100) | 52 (58–60) | 79 (70–88) |
| Recombinant EITB, any band | 99 (98–100) | 98 (96–100) | 56 (47–65) | 65 (55–75) |
| LLGP-EITB, native gp42 + native gp24 | 95 (91–99) | 100 (100) | 52 (43–61) | 71 (61–80) |
| Recombinant EITB, rT24H only | 99 (98–100) | 96 (93–99) | 44 (35–53) | 64 (53–74) |
We also compared the reactivity of the rT24H antigen with native gp42 and gp24. Similar levels of reactivity were seen using sera from cases with ≥2 viable cysts with those of the recombinant and the native proteins (Table 4). The native gp24 and gp42 proteins were more sensitive for detecting single-cyst infections. The kappa statistic, measuring the concordance between the recombinant EITB assay and reactivity to the native gp42 plus gp24, was 0.93, demonstrating an almost-perfect degree of concordance.
DISCUSSION
This study demonstrates that recombinant proteins can replace the native LLGP antigens in the diagnosis of NCC with comparable sensitivity and specificity when used in an immunoblot assay format. The sensitivity of the recombinant EITB assay was similar to that of the native LLGP-EITB assay when all 3 antigens were used in combination and when rT24H was used in any combination or alone. The specificity of the new recombinant EITB assay was also similar to that of the LLGP-EITB assay. Since its introduction >30 years ago, the specificity of the LLGP-EITB assay has remained exquisite, very near 100%, although there have been a few anecdotal reports of false-positive results mainly associated with reactivity to the native gp50 protein alone (11–13). In one study, only 2 of 13 patients with antibodies to native gp50 only were associated with a clinical diagnosis of NCC; seven cases had other final diagnoses (12), suggesting that reactivity to gp50 alone should be interpreted with caution. Similarly, in this study, the inclusion of rGP50 increased sensitivity for detecting patients with single lesions on CT; however, the overall specificity of the assay was reduced when rGP50 was included. In this study, cross-reactivity was seen in patients with schistosomiasis. This may represent cross-reactivity or undetected exposure to T. solium in these patients. This observation might have implications in regions where the two diseases are endemic.
Instead of using one of the other 8-kDa peptides, sTSRS1 was specifically selected for use in this study based on data from previous studies. When sTSRS1 was used in an immunoblot assay format, sTSRS1 detected 96% of the NCC cases with ≥2 viable cysts (25). In another study that evaluated the performance of several 8-kDa proteins in a multiantigen print immunoassay (MAPIA), sTSRS1 showed a sensitivity of 81% (26). Typically, members of the 8-kDa-protein family have been the least sensitive antigens when these peptides were compared to other LLGP-derived recombinant proteins, with sensitivities ranging from 77% in the MAPIA to 97% using an immunoblot test (25–29). It is unclear why the 8-kDa peptides performed differently in an earlier study using a similar immunoblot test (25). Different serum panels were used in the two comparisons, but no obvious differences in the sera were found upon investigation. In this study, the addition of the sTsRS1 peptide did not improve the sensitivity or specificity of the recombinant EITB assay for the diagnosis of NCC in clinically ill patients.
Native cyst-derived GP39-42 and GP24 are among the more immunodominant antigens in the LLGP fraction, generating antibodies in approximately 95% of the NCC cases with more than one viable cyst (3, 30). Studies have demonstrated that GP39-42 is a dimeric form of GP24 (16), suggesting that a large percentage of NCC cases would be detected using a recombinant form of GP24. In this study, the rT24H antigen alone had a sensitivity of 99% and a specificity of 100% for NCC cases with more than one viable cyst, which was comparable to those of the LLGP-EITB assay using native proteins. In multiple assay formats, rT24H alone has performed as well or better than the combination of antigens (16, 26, 28). Based on the data from this and other studies using the recombinant LLGP-derived proteins, we propose that rT24H can be used alone and should be an option for laboratories and manufacturers to consider, since its use alone may simplify the interpretation of results and reduce the cost of the assay. Although rT24H can be used alone for most applications, particularly diagnostics, it may be useful to include a representative antigen from all three antigen families in certain situations, such as epidemiological studies, to stratify parasite loads in pigs (31, 32).
The recombinant EITB assay has not improved the sensitivity for diagnosing NCC cases with single cysts. Significant improvement in the sensitivity for single-cyst cases may not be possible. Virtually all serological tests for NCC have reduced sensitivity in this scenario, and we hypothesize that this is a result of a lower infection burden and thus lower antigenic stimulation, which results in a limited antibody response. The only data that suggest that the detection of cases with single cysts can be improved are those in a preliminary report by Handali et al. (33), which utilized these same recombinant proteins in a multiplex fluorescent bead-based assay. In that work, the sensitivity for the detection of single-lesion cases of NCC was 92%.
In conclusion, the recombinant EITB assay using recombinant- and synthetic-derived proteins based on the native LLGP antigens represents an improvement in laboratory diagnostics for NCC and other assay formats, such as MAPIA and immunochromatographic test (ICT), and might also be explored for clinical diagnosis (26, 29). The dependence on cyst-derived antigens is eliminated, thereby lowering the cost and eliminating the lot-to-lot variability associated with native protein antigens. Furthermore, the analysis of the results is simplified due to less background and the absence of cross-reacting proteins that are sometimes present in native parasite extracts (3). Perhaps equally important, the ability to use commercially manufactured polyacrylamide gels allows for its implementation in most reference laboratories and bodes well for the commercialization of the method for clinical and epidemiological use or the incorporation of the recombinant proteins into other formats.
ACKNOWLEDGMENTS
This work was supported in part by the Fogarty International Center/NIH (training grant D43 TW001140), the Bill and Melinda Gates Foundation (grant 23981), and the Epilepsy Program, National Center for Chronic Disease Prevention and Health Promotion, CDC. H.H.G. is supported by a Wellcome Trust Senior International Research Fellowship in public health and tropical medicine. The funders had no role in the study design, data collection, analysis, or interpretation, in writing the manuscript, or in the decision to submit the article for publication.
Footnotes
Published ahead of print 19 February 2014
REFERENCES
- 1.Del Brutto OH, Rajshekhar V, White AC, Jr, Tsang VC, Nash TE, Takayanagui OM, Schantz PM, Evans CA, Flisser A, Correa D, Botero D, Allan JC, Sarti E, Gonzalez AE, Gilman RH, García HH. 2001. Proposed diagnostic criteria for neurocysticercosis. Neurology 57:177–183. 10.1212/WNL.57.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Brutto OH, Wadia NH, Dumas M, Cruz M, Tsang VC, Schantz PM. 1996. Proposal of diagnostic criteria for human cysticercosis and neurocysticercosis. J. Neurol. Sci. 142:1–6 [DOI] [PubMed] [Google Scholar]
- 3.Tsang VC, Brand JA, Boyer AE. 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J. Infect. Dis. 159:50–59. 10.1093/infdis/159.1.50 [DOI] [PubMed] [Google Scholar]
- 4.García HH, Del Brutto OH. 2000. Taenia solium cysticercosis. Infect. Dis. Clin. North Am. 14:97–119. 10.1016/S0891-5520(05)70220-8 [DOI] [PubMed] [Google Scholar]
- 5.White AC., Jr 2000. Neurocysticercosis: updates on epidemiology, pathogenesis, diagnosis, and management. Annu. Rev. Med. 51:187–206. 10.1146/annurev.med.51.1.187 [DOI] [PubMed] [Google Scholar]
- 6.Wilson M, Bryan RT, Fried JA, Ware DA, Schantz PM, Pilcher JB, Tsang VC. 1991. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J. Infect. Dis. 164:1007–1009. 10.1093/infdis/164.5.1007 [DOI] [PubMed] [Google Scholar]
- 7.Rawlings D, Ferriero DM, Messing RO. 1989. Early CT reevaluation after empiric praziquantel therapy in neurocysticercosis. Neurology 39:739–741. 10.1212/WNL.39.5.739 [DOI] [PubMed] [Google Scholar]
- 8.Singh G. 1997. Neurocysticercosos in South-Central America and the Indian subcontinent. A comparative evaluation. Arq. Neuropsiquiatr. 55:349–356. 10.1590/S0004-282X1997000300001 [DOI] [PubMed] [Google Scholar]
- 9.Rajshekhar V, Oommen A. 2001. Utility of the cysticercus immunoblot in a patient with an atypical solitary cerebral cysticercus granuloma. Neurol. India 49:75–77 [PubMed] [Google Scholar]
- 10.Garcia HH, Herrera G, Gilman RH, Tsang VC, Pilcher JB, Diaz JF, Candy EJ, Miranda E, Naranjo J. 1994. Discrepancies between cerebral computed tomography and western blot in the diagnosis of neurocysticercosis. The Cysticercosis Working Group in Peru (Clinical Studies Coordination Board). Am. J. Trop. Med. Hyg. 50:152–157 [DOI] [PubMed] [Google Scholar]
- 11.Kojic EM, White AC., Jr 2003. A positive enzyme-linked immunoelectrotransfer blot assay result for a patient without evidence of cysticercosis. Clin. Infect. Dis. 36:e7–e9. 10.1086/344445 [DOI] [PubMed] [Google Scholar]
- 12.Furrows SJ, McCroddan J, Bligh WJ, Chiodini P. 2006. Lack of specificity of a single positive 50-kDa band in the electroimmunotransfer blot (EITB) assay for cysticercosis. Clin. Microbiol. Infect. 12:459–462. 10.1111/j.1469-0691.2006.01381.x [DOI] [PubMed] [Google Scholar]
- 13.Ong S, Talan DA, Moran GJ, Mower W, Newdow M, Tsang VCW, Pinner RW, EMERGEncy ID NET Study Group 2002. Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerg. Infect. Dis. 8:608–613. 10.3201/eid0806.010377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene RM, Hancock K, Wilkins PP, Tsang VC. 2000. Taenia solium: molecular cloning and serologic evaluation of 14- and 18-kDa related, diagnostic antigens. J. Parasitol. 86:1001–1007. 10.1645/0022-3395(2000)086[1001:TSMCAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 15.Hancock K, Pattabhi S, Greene RM, Yushak ML, Williams F, Khan A, Priest JW, Levine MZ, Tsang VC. 2004. Characterization and cloning of GP50, a Taenia solium antigen diagnostic for cysticercosis. Mol. Biochem. Parasitol. 133:115–124. 10.1016/j.molbiopara.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 16.Hancock K, Pattabhi S, Whitfield FW, Yushak ML, Lane WS, Garcia HH, Gonzalez AE, Gilman RH, Tsang VC. 2006. Characterization and cloning of T24, a Taenia solium antigen diagnostic for cysticercosis. Mol. Biochem. Parasitol. 147:109–117. 10.1016/j.molbiopara.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 17.Tsang VC, Greene RM, Pilcher JB. 1995. Optimization of the covalent conjugating procedure (NaIO4) of horseradish peroxidase to antibodies for use in enzyme-linked immunosorbent assay. J. Immunoassay 16:395–418. 10.1080/15321819508013570 [DOI] [PubMed] [Google Scholar]
- 18.Jeri C, Gilman RH, Lescano AG, Mayta H, Ramirez ME, Gonzalez AE, Nazerali R, Garcia HH. 2004. Species identification after treatment for human taeniasis. Lancet 363:949–950. 10.1016/S0140-6736(04)15791-7 [DOI] [PubMed] [Google Scholar]
- 19.Mayta H, Gilman RH, Prendergast E, Castillo JP, Tinoco YO, Garcia HH, Gonzalez AE, Sterling CR, Cysticercosis Working Group in Peru 2008. Nested PCR for specific diagnosis of Taenia solium taeniasis. J. Clin. Microbiol. 46:286–289. 10.1128/JCM.01172-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayta H, Talley A, Gilman RH, Jimenez J, Verastegui M, Ruiz M, Garcia HH, Gonzalez AE. 2000. Differentiating Taenia solium and Taenia saginata infections by simple hematoxylin-eosin staining and PCR-restriction enzyme analysis. J. Clin. Microbiol. 38:133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hancock K, Khan A, Williams FB, Yushak ML, Pattabhi S, Noh J, Tsang VC. 2003. Characterization of the 8-kilodalton antigens of Taenia solium metacestodes and evaluation of their use in an enzyme-linked immunosorbent assay for serodiagnosis. J. Clin. Microbiol. 41:2577–2586. 10.1128/JCM.41.6.2577-2586.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsang VC, Peralta JM, Simons AR. 1983. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 92:377–391. 10.1016/0076-6879(83)92032-3 [DOI] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. 1977. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33:363–374. 10.2307/2529786 [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 25.Scheel CM, Khan A, Hancock K, Garcia HH, Gonzalez AE, Gilman RH, Tsang VC, Cysticercosis Working Group in Peru 2005. Serodiagnosis of neurocysticercosis using synthetic 8-kD proteins: comparison of assay formats. Am. J. Trop. Med. Hyg. 73:771–776 [PubMed] [Google Scholar]
- 26.Handali S, Klarman M, Gaspard AN, Noh J, Lee YM, Rodriguez S, Gonzalez AE, Garcia HH, Gilman RH, Tsang VC, Wilkins PP. 2010. Multiantigen print immunoassay for comparison of diagnostic antigens for Taenia solium cysticercosis and taeniasis. Clin. Vaccine Immunol. 17:68–72. 10.1128/CVI.00339-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bueno EC, Scheel CM, Vaz AJ, Machado LR, Livramento JA, Takayanagui OM, Tsang VC, Hancock K. 2005. Application of synthetic 8-kD and recombinant GP50 antigens in the diagnosis of neurocysticercosis by enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 72:278–283 [PubMed] [Google Scholar]
- 28.Lee YM, Handali S, Hancock K, Pattabhi S, Kovalenko VA, Levin A, Rodriguez S, Lin S, Scheel CM, Gonzalez AE, Gilman RH, Garcia HH, Tsang VC. 2011. Serologic diagnosis of human Taenia solium cysticercosis by using recombinant and synthetic antigens in QuickELISA. Am. J. Trop. Med. Hyg. 84:587–593. 10.4269/ajtmh.2011.10-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Handali S, Klarman M, Gaspard AN, Dong XF, Laborde R, Noh J, Lee YM, Rodriguez S, Gonzalez AE, Garcia HH, Gilman RH, Tsang VC, Wilkins PP. 2010. Development and evaluation of a magnetic immunochromatographic test to detect Taenia solium, which causes taeniasis and neurocysticercosis in humans. Clin. Vaccine Immunol. 17:631–637. 10.1128/CVI.00511-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia HH, Del Brutto OH, Nash TE, White AC, Jr, Tsang VC, Gilman RH. 2005. New concepts in the diagnosis and management of neurocysticercosis (Taenia solium). Am. J. Trop. Med. Hyg. 72:3–9 [PubMed] [Google Scholar]
- 31.Jayashi CM, Gonzalez AE, Castillo Neyra R, Rodríguez S, García HH, Lightowlers MW, Cysticercosis Working Group in Peru 2013. Validity of the enzyme-linked immunoelectrotransfer blot (EITB) for naturally acquired porcine cysticercosis. Vet. Parasitol. 10.1016/j.vetpar.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Neal SE, Moyano LM, Ayvar V, Gonzalvez G, Diaz A, Rodriguez S, Wilkins PP, Tsang VCW, Gilman RH, Garcia HH, Gonzalez AE, Cysticercosis Working Group in Peru 2012. Geographic correlation between tapeworm carriers and heavily infected cysticercotic pigs. PLoS Negl. Trop. Dis. 6:e1953. 10.1371/journal.pntd.0001953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handali S, Moss D, McAuliffe IT, Noh J, Lee Y-M, Rodriguez S, Garcia HH, Gonzalez AE, Gilman RH, Tsang VCW, Wilkins PP. 2011. Development of a Luminex based assay for detection of antibodies against Taenia solium cysts and adult worms, p 19 60th Annu. Meet. Am. Soc. Trop. Med. Hyg. American Society of Tropical Medicine & Hygiene, Philadelphia, PA [Google Scholar]


