Abstract
Tigecycline is one of the few remaining therapeutic options for extensively drug-resistant (XDR) Gram-negative bacilli (GNB). MICs of tigecycline to Acinetobacter baumannii have been reported to be elevated when determined by the Etest compared to determinations by the broth microdilution (BMD) method. The study aim was to compare the susceptibility of GNB to tigecycline by four different testing methods. GNB were collected from six health care systems (25 hospitals) in southeast Michigan from January 2010 to September 2011. Tigecycline MICs among A. baumannii, carbapenem-resistant Enterobacteriaceae (CRE), extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, and susceptible Enterobacteriaceae isolates were determined by Etest, BMD, Vitek-2, and MicroScan. Nonsusceptibility was categorized as a tigecycline MIC of ≥4 μg/ml for both A. baumannii and Enterobacteriaceae. The study included 4,427 isolates: 2,065 ESBL-producing Enterobacteriaceae, 1,105 A. baumannii, 888 susceptible Enterobacteriaceae, and 369 CRE isolates. Tigecycline nonsusceptibility among A. baumannii isolates was significantly more common as determined by Etest compared to that determined by BMD (odds ratio [OR], 10.3; P < 0.001), MicroScan (OR, 12.4; P < 0.001), or Vitek-2 (OR, 9.4; P < 0.001). These differences were not evident with the other pathogens. Tigecycline MICs varied greatly according to the in vitro testing methods among A. baumannii isolates. Etest should probably not be used by laboratories for tigecycline MIC testing of A. baumannii isolates, since MICs are significantly elevated with Etest compared to those determined by the three other methods.
INTRODUCTION
Infections due to multidrug-resistant (MDR) and extensively drug-resistant (XDR) Gram-negative bacillus (GNB) pathogens, such as extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, Acinetobacter baumannii, and carbapenem-resistant Enterobacteriaceae (CRE), continue to increase worldwide and pose significant management challenges due to the lack of available therapeutic alternatives (1–4). These infections are associated with devastating outcomes, and they place a huge burden on and threaten the general population (1, 2, 5–8). Tigecycline is one of the few available agents that possess antimicrobial activity against these resistant pathogens (9–14). Tigecycline has a relatively safe therapeutic profile compared to that of other agents that often have activity against these pathogens (e.g., polymyxins and aminoglycosides) (3, 15). However, tigecycline has limitations as a therapeutic agent. It achieves low serum levels, and data pertaining to the effective treatment of invasive infections with tigecycline are lacking (1–3, 13). Recent meta-analyses and reports from the U.S. Food and Drug Administration have detailed an increased mortality risk among subjects receiving tigecycline in clinical trials (13, 14, 16). Due to the lack of options, tigecycline is still frequently used for the treatment of clinical infections due to A. baumannii and CRE, often with similar and, in some instances, favorable clinical efficacy compared to that of other available therapeutic options (3).
In the past years, several in vitro studies reported a worrisome trend of elevated MICs to tigecycline among A. baumannii (17), ESBL producers (18), and CRE (19). For A. baumannii, elevated resistance was reported even in locales where the drug had not yet been introduced into clinical use (17). The MIC determinations in these reports were obtained by using Etest strips for in vitro susceptibility testing (17). When in vitro analyses compared MICs obtained using the Etest with those obtained using the broth microdilution (BMD) method, major discordances were reported. Pillar and colleagues (20) reported comparisons between Etest and BMD for in vitro susceptibility testing results among 227 U.S. A. baumannii isolates and 902 Klebsiella pneumoniae and Escherichia coli isolates. The differences in results obtained by the different methods were mostly evident among the A. baumannii isolates. Results obtained via Etest resulted in a ≥4-fold increase in MIC in 29% of the isolates. Among the 43% of A. baumannii strains considered resistant by Etest, none had an MIC in the resistant range when tested by BMD (20). In a study by Casal and colleagues (21), the MICs for A. baumannii isolates (n = 148) determined by Etest were completely discordant compared to those determined by BMD. Among the isolates with an MIC of ≥2 mg/liter determined by Etest, values were at least 2 dilutions lower when determined by BMD. It was hypothesized that high concentrations of manganese in Mueller-Hinton agar plates might have been responsible for the increased MICs of tigecycline to A. baumannii isolates determined by Etest (22).
It is important that MICs for the “last-line” agents such as tigecycline be accurately determined. The issues pertaining to accurate susceptibility testing for tigecycline are especially urgent because many institutions use Etest to determine tigecycline MICs, as not all automated susceptibility-testing methods have tigecycline on their panels/cards. We therefore designed this multicenter study in order to compare the MIC results determined by four different susceptibility-testing methodologies for tigecycline to A. baumannii isolates, as well as to a variety of other GNB.
MATERIALS AND METHODS
A multicenter prospective investigation was conducted in southeastern Michigan between 1 January 2010 and 31 August 2011. Six health care systems participated, i.e., Detroit Medical Center (DMC), Henry Ford hospitals network, St. Joseph Mercy Hospital, St. John Providence Health System, University of Michigan Health System, and Oakwood Healthcare institutions. A total of 25 hospitals within these health systems participated. The Department of Infection Prevention, Epidemiology, and Antimicrobial Stewardship at the DMC served as the central coordinating unit for all data and organisms, storage, testing, and analyses. The study was approved separately in each health care system by the local institutional review boards, and the data were deidentified prior to transfer to the coordinating unit.
Unique clinical isolates of A. baumannii, K. pneumoniae, and E. coli (both ESBL and non-ESBL producing) and of CRE (K. pneumoniae, E. coli, and Enterobacter species) were prospectively collected. CRE were defined as any of the aforementioned bacteria that were resistant to ≥1 carbapenem or were carbapenemase producers (determined by the modified Hodge test, conducted in accordance with the 2009 Clinical and Laboratory Standards Institute [CLSI] criteria [23]). ESBL production was determined by the automated system and confirmed with the double disk diffusion test (23). The centers were asked to collect all resistant isolates (i.e., A. baumannii, ESBL-producing Enterobacteriaceae, and CRE) but were limited to 150 susceptible isolates per health system (collected randomly). For the purposes of this analysis, susceptible Enterobacteriaceae were those that were susceptible to third-generation cephalosporins and lacked detectable ESBL production. Isolates were tested for susceptibility to tigecycline by four different methods: MicroScan (Siemens, Germany), Etest (bioMérieux, France), Vitek-2 (bioMérieux, France), and BMD. All susceptibility testing was conducted at the DMC, except for Vitek-2 testing, which was conducted at the Henry Ford Medical Center. All Etest analyses were performed using the BBL brand of Mueller-Hinton agar previously determined to have a lower manganese content than that of other brands (22). All susceptibility testing and interpretations were conducted according to the 2009 CLSI criteria and recommendations (23). There are no CLSI Enterobacteriaceae MIC breakpoints for tigecycline (23), so the FDA breakpoints for susceptible (MIC ≤ 2 mg/liter), intermediate (4 mg/liter), and resistant (MIC ≥ 8 mg/liter) were used to categorize tigecycline susceptibility (Enterobacteriaceae strains with an MIC of ≥4 mg/liter were considered to be nonsusceptible). No MIC breakpoints exist for tigecycline to Acinetobacter spp. The common practice at the participating centers at the time of the study was to use the same FDA breakpoints that were set for Enterobacteriaceae for A. baumannii as well (i.e., an isolate with an MIC of ≥4 mg/liter was considered nonsusceptible).
All statistical analyses were performed using IBM-SPSS 20 (Chicago, IL, USA). Throughout the text, the percentages that are displayed are the valid percentages, which exclude missing data from the denominator. Bivariate analyses were performed using Fisher's exact test or the chi-square test for categorical variables and the independent samples t test, or the Mann-Whitney U test for continuous variables. All P values were two sided. We used several definitions in order to display the correlations between Etest and the other susceptibility-testing methods (20): (i) essential agreement was determined if the Etest MICs were either identical to or 1 doubling dilution from the MIC of the test to which Etest was being compared, (ii) a minor error was determined if the isolate was interpreted by Etest as intermediate (MIC = 4 mg/liter) but was either susceptible (MIC ≤ 2 mg/liter) or resistant (MIC ≥ 8 mg/liter) by the test to which Etest was being compared, (iii) a major error was determined when the isolate was interpreted as false resistant (nonsusceptible) by Etest (MIC ≥ 4 mg/liter), and (iv) a very major error was determined when the isolate was interpreted as falsely susceptible (MIC ≤ 2 mg/liter) by Etest.
RESULTS
In total, 4,427 patients' unique Gram-negative isolates were collected, including 2,065 ESBL-producing Enterobacteriaceae, 1,105 strains of A. baumannii, 888 strains of susceptible Enterobacteriaceae, and 369 strains of CRE. Tables 1 and 2 display the tigecycline susceptibilities and MICs determined by the four different testing methodologies.
TABLE 1.
Susceptibilities to tigecycline among Gram-negative isolates from unique patients as determined by various testing methodologiesa
| Organism (n) | Susceptibility of isolates (no. [%]), determined by: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Etest |
BMDb |
MicroScan |
Vitek-2 |
|||||||||
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | |
| A. baumannii (1,105) | 436 (40.3) | 515 (47.6) | 132 (12.2) | 956 (88) | 109 (10) | 21 (1.9) | 1,009 (93.2) | 56 (5.2) | 18 (1.7) | 643 (63.9) | 311 (31.1) | 50 (5) |
| ESBL-producing Enterobacteriaceaec (2,065) | 1,979 (96.4) | 66 (3.3) | 8 (0.3) | 2,008 (98.1) | 33 (1.6) | 5 (0.2) | 2,024 (98.7) | 25 (1.2) | 2 (0.1) | 1,781 (96.3) | 38 (2) | 32 (1.8) |
| CREd (369) | 291 (79.3) | 70 (19) | 6 (1.7) | 336 (91.6) | 23 (6.3) | 8 (2.1) | 331 (90.7) | 29 (7.9) | 5 (1.4) | 264 (78.8) | 29 (8.7) | 42 (12.6) |
| Susceptible Enterobacteriaceae (888) | 844 (96.8) | 24 (2.7) | 4 (0.4) | 855 (98.2) | 11 (1.3) | 5 (0.6) | 854 (98.8) | 10 (1.2) | 0 | 665 (97.5) | 4 (0.6) | 13 (1.9) |
n = 4,427.
BMD, broth microdilution.
ESBL, extended-spectrum β-lactamase.
CRE, carbapenem-resistant Enterobacteriaceae.
TABLE 2.
MICs to tigecycline among patient unique Gram-negatives determined by various testing methodologiesa
| Organism | Etest |
BMDb |
MicroScan |
Vitek-2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | |
| A. baumannii (n = 1,105) | 0.12–48 | 3 | 8 | 0.06–16 | 1 | 4 | <1–8 | <1 | 2 | 0.25–16 | 2 | 4 |
| ESBL-producing Enterobacteriaceaec (n = 2,065) | 0.03–24 | 0.5 | 1.5 | 0.03–16 | 0.25 | 1 | <1–8 | <1 | <1 | 0.25–16 | <0.5 | 1 |
| CREd (n = 369) | 0.19–32 | 2 | 3 | 0.12–32 | 1 | 2 | <1–8 | <1 | <1 | 0.25–32 | 2 | 2 |
| Susceptible Enterobacteriaceae (n = 888) | 0.09–12 | <0.5 | 1.5 | 0.05–8 | 0.25 | 1 | <1–4 | <1 | <1 | <0.25–16 | 0.25 | 1 |
n = 4,427.
BMD, broth microdilution.
ESBL, extended-spectrum β-lactamase.
CRE, carbapenem-resistant Enterobacteriaceae.
Nonsusceptibility among A. baumannii isolates was significantly more common when determined by Etest than when determined with BMD (59.8% versus 11.9%; odds ratio [OR], 10.3; P < 0.001), MicroScan (59.8% versus 6.9%; OR, 12.4; P < 0.001), or Vitek-2 (59.8% versus 36.1%; OR, 9.4, P < 0.001); the median MICs were significantly elevated when testing was conducted using Etest (P < 0.001 for all comparisons). The rate of nonsusceptibility measured by Vitek-2 among A. baumannii isolates was also significantly elevated compared to those measured by BMD (36.1% versus 11.9%; OR, 8.7; P < 0.001) and MicroScan (36.1% versus 6.9%; OR, 10.6; P < 0.001).
In contrast, among Enterobacteriaceae isolates (ESBL-producing Enterobacteriaceae, CRE, or susceptible isolates), the discordances and variations in MIC testing between Etest and the BMD-based methods (BMD and MicroScan) were not as notable. For some Enterobacteriaceae, the MICs determined by Etest were actually lower than the MICs determined by the other methods (Tables 1 and 2).
Additional significant variations were evident among the CRE isolates, as the rates of resistance and the MICs of tigecycline were significantly higher when the MICs were measured by Vitek-2 than when they were measured by BMD (21.3% versus 7.8%, respectively, were resistant; OR, 16.7; P < 0.001), MicroScan (21.3% versus 9.1%, respectively, were resistant; OR, 178.8; P < 0.001), and Etest (21.3% versus 7.8%, respectively, were resistant; OR, 21.6; P < 0.001). There were multiple other minor variations among the various testing methodologies, but none reached statistical significance.
Figure 1a displays comparative MICs determined by Etest and BMD for A. baumannii isolates. Figure 1b displays the variations between the MICs determined by Vitek-2 and BMD. For both comparisons, the discordances between the methods were particularly evident in the higher-MIC range, as determined by Etest or Vitek-2. Figure 1c displays the variations between the MICs determined by MicroScan and BMD. Variations in the MICs were insignificantly discordant for MicroScan and BMD.
FIG 1.
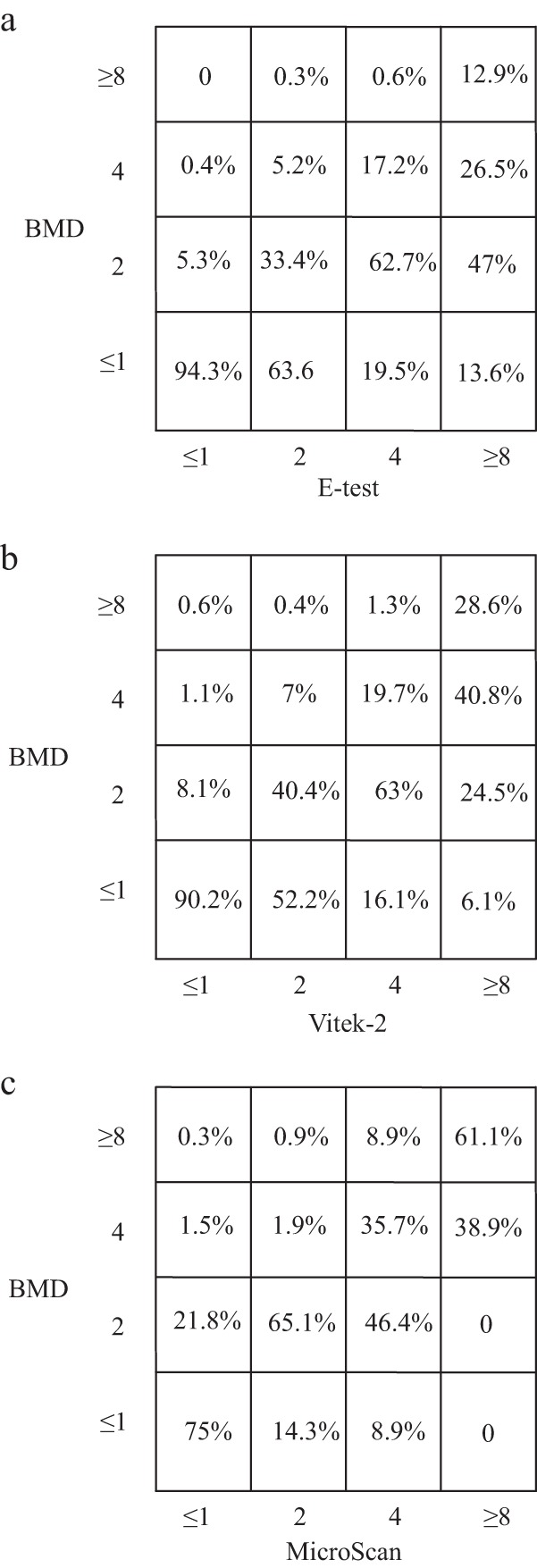
Variations in the MICs of tigecycline among Acinetobacter baumannii isolates as determined by three methodologies, Etest versus BMD (a), Vitek-2 versus BMD (b), and MicroScan versus BMD (c).
Table 3 shows the various correlations among A. baumannii MICs determined by Etest versus those determined by the other methodologies. An essential agreement of only 55% was noted between Etest and BMD; compared to MicroScan, the essential agreement between the tests (Etest versus MicroScan) was <50%. Major errors were noted, again, between MICs as determined by Etest compared to the MICs determined by broth microdilution methodologies. There were only a few very major errors when the MICs for A. baumannii were determined by Etest. The rate of very major errors was 2.6% when Etest was compared to Vitek-2.
TABLE 3.
Correlation between Etest and other susceptibility testing methods for Acinetobacter baumannii, southeast Michigan, January 2010 to August 2011
| Tests compared | No. of isolates | % MIC variation (log2 dilutions)a |
Essential agreement (%)b | Error rate (%)c |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤−3 | −2 | −1 | 0 | 1 | 2 | ≥3 | Minor | Major | Very major | |||
| Etest vs BMDd | 1,080 | 0.4 | 2.1 | 3.9 | 14.2 | 36.9 | 34.4 | 8.1 | 55 | 32.9 | 48.1 | 0.3 |
| Etest vs MicroScan | 1,089 | 0.1 | 0 | 9 | 13.8 | 25.1 | 24.2 | 27.8 | 47.9 | 35.2 | 52.3 | 0 |
| Etest vs Vitek-2 | 979 | 1.3 | 1 | 4.2 | 30.1 | 39.9 | 16 | 7.5 | 74.2 | 21.3 | 27.1 | 2.6 |
A negative number indicates that the Etest MIC was lower than the MIC of the test to which it was being compared. A positive number indicates that the Etest MIC was higher than the MIC of the test to which it was being compared. Zero indicates that the Etest MIC and the MIC of the test to which it was being compared were equal.
Essential agreement is indicated by Etest MICs that are either identical or 1 doubling dilution from the MIC of the test to which Etest was being compared.
Minor error, isolate interpreted by Etest as intermediate (MIC = 4 mg/L), susceptible (MIC ≤ 2 mg/L), or resistant (MIC ≥ 8 mg/L) by the test to which Etest was being compared; major error, isolate interpreted as false resistant/nonsusceptible by Etest (MIC ≥ 4 mg/L); very major error, isolate interpreted as false susceptible (MIC ≤ 2 mg/L) by Etest. No Acinetobacter species MIC breakpoints for tigecycline exist. The common practice at the participating centers at the time of the study was to use the FDA breakpoints set for Enterobacteriaceae and A. baumannii.
BMD, broth microdilution.
DISCUSSION
This cohort consisting of nearly 4,500 Gram-negative isolates from unique patients, collected from 25 hospitals during a 20-month period, clearly demonstrates that discordances and elevated MICs of tigecycline determined by Etests are issues that are unique to the testing of A. baumannii isolates among the bacteria that were trailed. MICs also varied among Enterobacteriaceae isolates as a result of the susceptibility-testing methodology, but the Etest MIC results were not exceptionally higher. The reason for this unique phenomenon in A. baumannii has been reported before in small single-center studies (20, 21), but this report, conducted on a large multicenter cohort, confirms this finding and provides important new information. Previous reports have associated the variation in MIC results with different compositions of the Mueller-Hinton medium used for Etesting (particularly the manganese concentration) (22, 24). This study demonstrates that similar results are observed even when using testing plates with a low manganese concentration.
One plausible explanation for the high MICs reported via the Etest methodology might be that tigecycline heteroresistance is particularly common among A. baumannii isolates and that heteroresistant colonies are identified by Etest, which elevates the absolute MIC values (25). This theory, however, should be subjected to future advanced population analysis investigations.
Interestingly, there were also significant variations among the tigecycline MICs for A. baumannii isolates when the MICs were determined by Vitek-2 compared to those determined by BMD. The reason for this discordance is unclear, and it suggests that Vitek-2 might not be appropriate for determining tigecycline MICs for A. baumannii isolates. Clinicians who utilize Vitek-2 test results should be aware of this issue related to tigecycline susceptibility testing for A. baumannii isolates.
The discordances between MICs determined by Etest and the other test methods are cause for concern, as many clinical microbiology laboratories utilize Etest for tigecycline testing of A. baumannii isolates. Future studies should analyze the impact of MICs of tigecycline determined by Etest on the outcomes of patients with infection due to A. baumannii who are treated with tigecycline. As tigecycline is often one of the only available agents available to treat some strains of A. baumannii, it is critically important for health care providers to be aware of the limitations and discordances associated with in vitro susceptibility testing performed using the Etest methodology.
ACKNOWLEDGMENTS
Keith S. Kaye is supported by the National Institute of Allergy and Infectious Diseases (NIAID), DMID protocol 10-0065. Marcus J. Zervos has received grant support from Merck, Cubist, and Forrest.
This study was partially funded by Pfizer Inc. The company had no access to or involvement in laboratory testing, data collection, or data interpretation and was not involved in the process of drafting the manuscript.
Keith S. Kaye and Jason M. Pogue serve as consultants and on the speakers' bureau of Pfizer, and Keith S. Kaye received grant support from Pfizer. Marcus J. Zervos has received grant support from Pfizer. Anurag N. Malani is a shareholder of Pfizer. The other authors have no potential conflicts of interest to declare in relation to the content of this article.
Footnotes
Published ahead of print 5 March 2014
REFERENCES
- 1.Gilad J, Carmeli Y. 2008. Treatment options for multidrug-resistant Acinetobacter species. Drugs 68:165–189. 10.2165/00003495-200868020-00003 [DOI] [PubMed] [Google Scholar]
- 2.Peleg AY, Hooper DC. 2010. Hospital-acquired infections due to Gram-negative bacteria. N. Engl. J. Med. 362:1804–1813. 10.1056/NEJMra0904124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ku K, Pogue JM, Moshos J, Bheemreddy S, Wang Y, Bhargava A, Campbell M, Khandker N, Lephart PR, Chopra T, Hayakawa K, Martin ET, Abreu-Lanfranco O, Dhar S, Kaye KS, Marchaim D. 2012. Retrospective evaluation of colistin versus tigecycline for the treatment of Acinetobacter baumannii and/or carbapenem-resistant Enterobacteriaceae infections. Am. J. Infect. Control 40:983–987. 10.1016/j.ajic.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 4.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18:268–281. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 5.Fishbain J, Peleg AY. 2010. Treatment of Acinetobacter infections. Clin. Infect. Dis. 51:79–84. 10.1086/653120 [DOI] [PubMed] [Google Scholar]
- 6.Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, Shalit I, Carmeli Y. 2011. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin. Infect. Dis. 52:848–855. 10.1093/cid/cir025 [DOI] [PubMed] [Google Scholar]
- 7.Marchaim D, Chopra T, Perez F, Hayakawa K, Lephart PR, Bheemreddy S, Blunden C, Hujer AM, Rudin S, Shango M, Campbell M, Varkey J, Slim J, Ahmad F, Patel D, Chen TY, Pogue JM, Salimnia H, Dhar S, Bonomo RA, Kaye KS. 2011. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect. Control Hosp. Epidemiol. 32:861–871. 10.1086/661597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchaim D, Gottesman T, Schwartz O, Korem M, Maor Y, Rahav G, Karplus R, Lazarovitch T, Braun E, Sprecher H, Lachish T, Wiener-Well Y, Alon D, Chowers M, Ciobotaro P, Bardenstein R, Paz A, Potasman I, Giladi M, Schechner V, Schwaber MJ, Klarfeld-Lidji S, Carmeli Y. 2010. National multicenter study of predictors and outcomes of bacteremia upon hospital admission caused by Enterobacteriaceae producing extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 54:5099–5104. 10.1128/AAC.00565-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. 2013. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob. Agents Chemother. 57:1756–1762. 10.1128/AAC.01232-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Y, Wang R, Liang B, Bai N, Liu Y. 2011. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob. Agents Chemother. 55:1162–1172. 10.1128/AAC.01402-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giamarellou H, Poulakou G. 2009. Multidrug-resistant Gram-negative infections: what are the treatment options? Drugs 69:1879–1901. 10.2165/11315690-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 12.Nicasio AM, Kuti JL, Nicolau DP. 2008. The current state of multidrug-resistant Gram-negative bacilli in North America. Pharmacotherapy 28:235–249. 10.1592/phco.28.2.235 [DOI] [PubMed] [Google Scholar]
- 13.Tasina E, Haidich AB, Kokkali S, Arvanitidou M. 2011. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect. Dis. 11:834–844. 10.1016/S1473-3099(11)70177-3 [DOI] [PubMed] [Google Scholar]
- 14.Yahav D, Lador A, Paul M, Leibovici L. 2011. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J. Antimicrob. Chemother. 66:1963–1971. 10.1093/jac/dkr242 [DOI] [PubMed] [Google Scholar]
- 15.Rello J. 2005. Pharmacokinetics, pharmacodynamics, safety and tolerability of tigecycline. J. Chemother. 17(Suppl 1):12–22. 10.1179/joc.2005.17.Supplement-1.12 [DOI] [PubMed] [Google Scholar]
- 16.FDA. 2010. Drug safety communication—increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. FDA, Washington, DC [Google Scholar]
- 17.Navon-Venezia S, Leavitt A, Carmeli Y. 2007. High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 59:772–774. 10.1093/jac/dkm018 [DOI] [PubMed] [Google Scholar]
- 18.Lu CT, Chuang YC, Sun W, Liu YC, Cheng YJ, Lu PL, Chen CM, Hsu GJ, Jang TN, Lee CM, Chiang PC, Shi ZY, Wang LS, Kung HC, Lin HC, Liao CH, Liu JW, Huang CH, Tsao SM, Hsueh PR. 2008. Nationwide surveillance in Taiwan of the in-vitro activity of tigecycline against clinical isolates of extended-spectrum beta-lactamase-producing Enterobacteriaceae. Int. J. Antimicrob. Agents 32(Suppl 3):S179–S183. 10.1016/S0924-8579(08)70024-4 [DOI] [PubMed] [Google Scholar]
- 19.Spanu T, De Angelis G, Cipriani M, Pedruzzi B, D'Inzeo T, Cataldo MA, Sganga G, Tacconelli E. 2012. In vivo emergence of tigecycline resistance in multidrug-resistant Klebsiella pneumoniae and Escherichia coli. Antimicrob. Agents Chemother. 56:4516–4518. 10.1128/AAC.00234-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pillar CM, Draghi DC, Dowzicky MJ, Sahm DF. 2008. In vitro activity of tigecycline against Gram-positive and Gram-negative pathogens as evaluated by broth microdilution and Etest. J. Clin. Microbiol. 46:2862–2867. 10.1128/JCM.00637-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casal M, Rodriguez F, Johnson B, Garduno E, Tubau F, de Lejarazu RO, Tenorio A, Gimenez MJ, Bartolome R, Garcia-Rey C, Aguilar L, Garcia-Escribano N. 2009. Influence of testing methodology on the tigecycline activity profile against presumably tigecycline-non-susceptible Acinetobacter spp. J. Antimicrob. Chemother. 64:69–72. 10.1093/jac/dkp169 [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Mazarrasa C, Mazarrasa O, Calvo J, del Arco A, Martinez-Martinez L. 2009. High concentrations of manganese in Mueller-Hinton agar increase MICs of tigecycline determined by Etest. J. Clin. Microbiol. 47:827–829. 10.1128/JCM.02464-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing. 19th informational supplement. Approved standard M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 24.Veenemans J, Mouton JW, Kluytmans JA, Donnely R, Verhulst C, van Keulen PH. 2012. Effect of manganese in test media on in vitro susceptibility of Enterobacteriaceae and Acinetobacter baumannii to tigecycline. J. Clin. Microbiol. 50:3077–3079. 10.1128/JCM.01485-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo-Ten-Foe JR, de Smet AM, Diederen BM, Kluytmans JA, van Keulen PH. 2007. Comparative evaluation of the Vitek 2, disk diffusion, Etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 51:3726–3730. 10.1128/AAC.01406-06 [DOI] [PMC free article] [PubMed] [Google Scholar]


