Abstract
The worldwide dissemination of Enterobacteriaceae producing AmpC β-lactamases and carbapenemases makes difficult the phenotypic detection of extended-spectrum β-lactamases (ESBLs), as they may be masked by these additional enzymes. A modification of the CLSI ESBL confirmatory test was developed and evaluated in a comparative study for its ability to successfully detect ESBLs among Enterobacteriaceae producing various carbapenemases (Klebsiella pneumoniae carbapenemase [KPC], VIM, NDM, and OXA-48) and plasmidic or derepressed AmpCs. The modified CLSI ESBL confirmatory test was performed with cefotaxime and ceftazidime disks with and without clavulanate, on which both boronic acid (BA) and EDTA were dispensed. A total of 162 genotypically confirmed ESBL-positive Enterobacteriaceae isolates (83 carbapenemase/ESBL producers, 25 AmpC/ESBL producers, and 54 ESBL-only producers) were examined. For comparison, 139 genotypically confirmed ESBL-negative Enterobacteriaceae isolates (94 of them possessed carbapenemases and 20 possessed AmpCs) were also tested. The standard CLSI ESBL confirmatory test was positive for 106 of the 162 ESBL producers (sensitivity, 65.4%) and showed false-positive results for 4 of the 139 non-ESBL producers (specificity, 97.1%). The modified CLSI ESBL confirmatory test detected 158 of 162 ESBL producers (sensitivity, 97.5%) and showed no false-positive results for non-ESBL producers (specificity, 100%). The findings of the study demonstrate that the modified CLSI ESBL confirmatory test using antibiotic disks containing both BA and EDTA accurately detects ESBLs in Enterobacteriaceae regardless of the coexistence of additional β-lactam resistance mechanisms.
INTRODUCTION
Extended-spectrum β-lactamases (ESBLs) are mostly plasmid-mediated β-lactamases that efficiently hydrolyze oxyimino-cephalosporins and monobactams, yet are inhibited by β-lactamase inhibitors (1). They were first detected in Enterobacteriaceae, and nowadays various groups of ESBLs are produced by these microorganisms, the most common being CTX-M and SHV enzyme types (1, 2). ESBLs are increasingly reported worldwide and have been linked to successful enterobacterial clones possessing great epidemic potential (1, 3). Plasmids coding for ESBLs may also carry additional β-lactamase genes as well as genes conferring resistance to other antimicrobial classes (2–4). This can limit the chemotherapeutic options for ESBL-producing pathogens and facilitate the inter- and intraspecies dissemination of ESBLs (3). Therefore, phenotypic detection of ESBLs among Enterobacteriaceae species is important for epidemiological purposes as well as for limiting the spread of resistance mechanisms.
The Clinical and Laboratory Standards Institute (CLSI) recommends a phenotypic confirmatory combined-disk test for ESBL production in Enterobacteriaceae. It consists of measuring the growth-inhibitory zones around both cefotaxime (CTX) and ceftazidime (CAZ) disks with or without clavulanate (CA) for Klebsiella pneumoniae, Klebsiella oxytoca, Escherichia coli, and Proteus mirabilis (5). Different combined-disk and double-disk synergy tests based on the synergy of CA with various expanded-spectrum cephalosporins and aztreonam have also been proposed (3, 6–9). In addition, a biochemical test based on the in vitro detection of cefotaxime hydrolysis that is inhibited by tazobactam was recently proposed for the rapid detection of ESBLs in Enterobacteriaceae (10). Moreover, boronic acid (BA) compounds, well-known reversible inhibitors of AmpCs and Klebsiella pneumoniae carbapenemases (KPCs) (11–15), in combination with CA have been employed to unmask the underlying ESBLs among AmpC- or KPC-possessing Enterobacteriaceae (16–18). ESBLs, however, may also coexist with other β-lactamase types, such as metallo-β-lactamases (MBLs) (e.g., NDM, VIM, and IMP) or both MBLs and KPCs (1, 19, 20), which may also interfere with the interpretation of ESBL detection methods, since they also hydrolyze extended-spectrum β-lactams.
There is a need, therefore, for an alternative method that can accurately detect ESBLs in Enterobacteriaceae, regardless of a possible coexistence of additional mechanisms of resistance to β-lactams. EDTA is a chelating agent that inhibits the enzymatic activity of MBLs, while BA inhibits the enzymatic activity of both AmpCs and KPCs (11, 19, 20, 21). We have also previously shown that the growth-inhibitory zone diameter around a meropenem (MER) disk with simultaneous addition of BA and EDTA is 5 mm or greater of the growth-inhibitory zone diameter around the disk containing MER alone when a KPC, an MBL, or both KPC and MBL are coexisting in a clinical isolate (19, 22). In this context, we further modified the CLSI ESBL confirmatory test by the simultaneous addition of both BA and EDTA on the antibiotic disks containing CTX and CAZ with or without CA and tested its sensitivity and specificity among Enterobacteriaceae producing additional β-lactamases (plasmid-mediated AmpCs or various carbapenemase types) or overproducing cephalosporinases.
MATERIALS AND METHODS
Clinical isolates and antimicrobial susceptibility testing.
A total of 301 nonrepetitive (one per patient) clinical isolates of Enterobacteriaceae were included in the study. The criteria for selection were devised to include a considerable number of bacteria producing various potent β-lactamases. They were recovered during 2007 to 2013 from nine tertiary care Greek hospitals. The collection consisted of K. pneumoniae (n = 174), E. coli (n = 42), P. mirabilis (n = 21), Enterobacter aerogenes (n = 24), Enterobacter cloacae (n = 17), Serratia marcescens (n = 9), Providencia stuartii (n = 9), and K. oxytoca (n = 5). A sum of 162 of these isolates were genotypically confirmed ESBL positive and the remaining 139 were genotypically confirmed ESBL negative. The presence of the ESBL gene was determined by using previously described oligonucleotide primers and cycling conditions (23). The identification of all isolates was confirmed by using the API20E system (bioMérieux, Marcy l'Etoile, France). Detailed susceptibility analysis was carried out by the agar dilution method following the recent CLSI guidelines and interpretative criteria (5).
Molecular testing for β-lactamase genes.
β-Lactamase genes were amplified in single PCRs using a panel of primers for detection of all types of ESBL (SHV, TEM, CTX-M, GES. and PER), carbapenemase (KPC, SME, VIM, NDM, IMP, and OXA-48), and plasmidic AmpC genes (23–27). Among Enterobacter aerogenes and Enterobacter cloacae isolates, total RNA from logarithmic-phase-grown cultures was extracted with TRI reagent (Ambion, Austin, TX), and reverse transcription (RT) of 1 μg of total RNA was performed with the ThermoScript RT-PCR system (Invitrogen, Carlsbad, CA). Derepressed AmpC-hyperproducing E. aerogenes and E. cloacae isolates were identified with quantitative real-time PCR using the Quanti Test SYBR green (Qiagen, Hilden, Germany) and primers described previously (28, 29). As positive controls, we used previously characterized isolates from our collection carrying all types of tested β-lactamases. The PCR products were subjected to direct sequencing. PCR products were purified using ExoSAP-IT reagent (USB Corporation, Cleveland, OH, USA) and used as the templates for sequencing on both strands with an ABI Prism 377 sequencer (Applied Biosystems, Foster City, CA).
Phenotypic methods to detect ESBLs, AmpCs, and carbapenemases.
ESBL production was initially tested with the CLSI confirmatory test using both CTX (30 mg) and CAZ (30 mg) disks alone and in combination with CA (10 mg) (Becton, Dickinson, Sparks, MD). The test was considered positive when an increase in the growth-inhibitory zone around either the CTX or the CAZ disk with CA was 5 mm or greater of the diameter around the disk containing CTX or CAZ alone (5).
For detecting and differentiating the production of MBL, KPC, or both MBL and KPC carbapenemases, a phenotypic method was applied using disks of MER (10 μg) alone and with 400 μg of phenylboronic acid or 292 μg of EDTA or both 400 μg of phenylboronic acid and 292 μg of EDTA (19). Phenotypic detection of AmpC production was carried out by using disks of cefotetan without and with BA (11) and Etest strips (bioMérieux), which contain cefotetan without or with cloxacillin.
Phenotypic detection of ESBLs using the modified CLSI ESBL confirmatory test.
The modification of the CLSI ESBL confirmatory test was performed employing disks of CTX and CAZ with or without CA, on which both BA and EDTA were dispensed. The stock of BA solution was prepared by dissolving phenylboronic acid (benzeneboronic acid) at a concentration of 40 mg/ml (11, 30). From this solution, 10 μl (containing 400 μg of BA) was dispensed onto commercially available antibiotic disks containing CTX (30 μg) or CAZ (30 μg) with or without CA (10 μg). Additionally, 10 μl of 0.1 M EDTA (containing 292 μg of EDTA) was dispensed onto the same antibiotic disks. The test was performed by inoculating a Mueller-Hinton agar plate with a sample of the tested strain. The agar plates were incubated at 37¼ C for 18 h. Similar to the standard CLSI ESBL confirmatory test, an augmentation of ≥5 mm in the growth-inhibitory zone diameter of either CTX-CA or CAZ-CA in combination with BA and EDTA (CTX-CA-BA-EDTA and CAZ-CA-BA-EDTA, respectively) compared with the zone diameter of CTX or CAZ disks containing BA and EDTA (CTX-BA-EDTA and CAZ-BA-EDTA, respectively) was considered a positive result for ESBL production. It should be noted that the concentration of BA and EDTA employed in the present study did not show any detectable effect on bacterial growth.
Sensitivity and specificity.
The performance of the phenotypic tests for the detection of ESBLs among Enterobacteriaceae producing various β-lactamases was evaluated using PCR along with DNA sequencing as the gold standard. For each test, the sensitivity was calculated from the number of ESBL-possessing organisms that were correctly determined, while the specificity was calculated from the number of non-ESBL-possessing organisms that were correctly determined.
RESULTS
Species distribution and β-lactamase content.
The species distributions of the studied isolates and their β-lactamases are presented in Table 1. Phenotypic and molecular testing revealed that among the 162 ESBL-positive isolates, 83 possessed carbapenemases (35 KPC-2 producers, 22 VIM-1 producers, 10 KPC-2/VIM-1 producers, 8 NDM-1 producers, and 8 OXA-48 producers), 19 possessed plasmid-mediated AmpCs (15 belonged to the clusters MOX-1, MOX-2, CMY-1, and CMY-8 to CMY-11 and 4 belonged to the clusters LAT-1 to LAT-4, CMY-2 to CMY-7, and BIL-1), 6 hyperproduced chromosomal AmpCs, while the remaining 54 possessed only ESBLs (6 of them were ertapenem resistant due to porin deficiency) (31). Among the 139 ESBL-negative isolates, 94 possessed carbapenemases (32 KPC-2 producers, 33 VIM-1 producers, 21 KPC-2/VIM-1 producers, 5 NDM-1 producers, and 3 OXA-48 producers), 15 possessed plasmid-mediated AmpCs (12 belonged to the clusters LAT-1 to LAT-4, CMY-2 to CMY-7, and BIL-1; 2 belonged to the clusters MOX-1, MOX-2, CMY-1, and CMY-8 to CMY-11; and 1 produced DHA-1), and 5 hyperproduced chromosomal AmpCs, while the remaining 25 did not contain any expanded-spectrum β-lactamase (ESBL, AmpC, or carbapenemase).
TABLE 1.
Distribution of expanded-spectrum β-lactamase genes among ESBL-producing (n = 162) and non-ESBL producing (n = 139) isolates used for the evaluation of the modified CLSI ESBL confirmatory test
| Strain group and genotype(s) | No. of isolates |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Klebsiella pneumoniae (n = 174) | Klebsiella oxytoca (n = 5) | Escherichia coli (n = 42) | Enterobacter aerogenes (n = 24) | Enterobacter cloacae (n = 17) | Proteus mirabilis (n = 21) | Providencia stuartii (n = 9) | Serratia marcescens (n = 9) | Total (n = 301) | |
| ESBL-producing isolates (n = 162)a | |||||||||
| blaCTX-M-3 | 6 | 5 | 1 | 4 | 16 | ||||
| blaCTX-M-15 | 5 | 1 | 5 | 11 | |||||
| blaCTX-M-15 + porin deficient | 6 | 6 | |||||||
| blaCTX-M-32 | 1 | 1 | |||||||
| blaSHV-5 | 6 | 5 | 3 | 14 | |||||
| blaGES-7 | 2 | 2 | |||||||
| blaSHV-5 + blaCTX-M-3 | 4 | 4 | |||||||
| blaCMY-1-like + blaSHV-5 | 13 | 2 | 15 | ||||||
| blaCMY-2-like + blaCTX-M-15 | 2 | 1 | 3 | ||||||
| blaCMY-2-like + blaSHV-5+blaCTX-M-15 | 1 | 1 | |||||||
| blaSHV-5 + AmpC hyperproducers | 3 | 3 | 6 | ||||||
| blaKPC-2 + blaCTX-M-15 | 4 | 4 | |||||||
| blaKPC-2 + blaSHV-12 | 24 | 1 | 1 | 26 | |||||
| blaKPC-2 + blaSHV-12 + blaCTX-M-15 | 3 | 3 | |||||||
| blaKPC-2 + blaSHV-5 | 2 | 2 | |||||||
| blaKPC-2 + blaVIM-1 + blaSHV-5 | 9 | 9 | |||||||
| blaKPC-2 + blaVIM-1 + blaCTX-M-15 | 1 | 1 | |||||||
| blaVIM-1 + blaCTX-M-3 | 3 | 3 | |||||||
| blaVIM-1 + blaCTX-M-15 | 4 | 4 | |||||||
| blaVIM-1 + blaSHV-5 | 8 | 3 | 4 | 15 | |||||
| blaNDM-1 + blaCTX-M-15 | 8 | 8 | |||||||
| blaOXA-48 + blaCTX-M-15 | 8 | 8 | |||||||
| Subtotal | 111 | 2 | 26 | 12 | 3 | 4 | 4 | 162 | |
| Non-ESBL- producing isolates (n = 139)b | |||||||||
| Non-expanded-spectrum β-lactamase producers | 4 | 2 | 4 | 2 | 3 | 4 | 3 | 3 | 25 |
| blaCMY-1-like | 2 | 2 | |||||||
| blaCMY-2-like | 1 | 5 | 6 | 12 | |||||
| blaDHA-1 | 1 | 1 | |||||||
| AmpC hyperproducers | 2 | 3 | 5 | ||||||
| blaKPC-2 | 18 | 2 | 6 | 6 | 32 | ||||
| blaKPC-2 + blaVIM-1 | 21 | 21 | |||||||
| blaVIM-1 | 11 | 3 | 2 | 8 | 7 | 2 | 33 | ||
| blaNDM-1 | 5 | 5 | |||||||
| blaOXA-48 | 3 | 3 | |||||||
| Subtotal | 63 | 3 | 16 | 12 | 14 | 17 | 5 | 9 | 139 |
The blaTEM-1 gene was detected in 104 of the ESBL-producing isolates.
The blaTEM-1 gene was detected in 76 of the non-ESBL-producing isolates.
PCR and sequencing analyses showed that among the 162 ESBL-positive isolates, 87 (53.7%) harbored SHV-type ESBLs (61 SHV-5 and 26 SHV-12), 65 (41.1%) harbored CTX-M-type ESBLs (45 CTX-M-15, 19 CTX-M-3, and 1 CTX-M-32), 8 (4.9%) harbored both SHV and CTX-M ESBLs (4 SHV-5 plus CTX-M-3, 3 SHV-12 plus CTX-M-15, and 1 SHV-5 plus CTX-M-15), and 2 (1.2%) harbored GES-7 ESBL (Table 1). Moreover, 104 (64.2%) of the ESBL producers and 76 (54.7%) of the non-ESBL producers harbored the broad-spectrum TEM-1 β-lactamase.
Antimicrobial susceptibilities.
The susceptibility data for ESBL-producing and non-ESBL-producing isolates of the study are summarized in Table 2. Aztreonam MIC50s, MIC90s, ranges of MICs, and resistance rates were considerably higher among ESBL producers than among non-ESBL producers. Nevertheless, regarding the remaining β-lactam antibiotics, the above parameters did not differ considerably among ESBL and non-ESBL producers, due to the presence of additional β-lactamases.
TABLE 2.
Antimicrobial susceptibilities to β-lactam antibiotics for the 162 ESBL-producing isolates and 139 non-ESBL-producing isolates
| Strain group and antimicrobial | MIC values (μg/ml) |
% of isolates resistant | ||
|---|---|---|---|---|
| Range | MIC50 | MIC90 | ||
| ESBL-producing isolates (n = 162) | ||||
| Aztreonam | 8 to >256 | 256 | >256 | 80.2 |
| Cefepime | 1 to 128 | 32 | 128 | 71.6 |
| Cefoxitin | 1 to 256 | 32 | 256 | 64.8 |
| Cefotaxime | 1 to >128 | 64 | >128 | 91.4 |
| Ceftazidime | 2 to >256 | 128 | >256 | 86.4 |
| Ertapenem | 0.125 to 128 | 16 | 64 | 56.8 |
| Imipenem | 0.250 to 128 | 2 | 32 | 48.8 |
| Meropenem | 0.125 to 64 | 4 | 32 | 51.9 |
| Piperacillin-tazobactam | 2 to >256 | 128 | >256 | 67.3 |
| Non-ESBL-producing isolates (n = 139) | ||||
| Aztreonam | 1 to >256 | 4 | 256 | 40.3 |
| Cefepime | 0.5 to 128 | 32 | 64 | 59.7 |
| Cefoxitin | 0.5 to 256 | 64 | 256 | 79.1 |
| Cefotaxime | 0.250 to 128 | 64 | 128 | 76.9 |
| Ceftazidime | 0.5 to 256 | 128 | 256 | 78.4 |
| Ertapenem | 0.125 to 128 | 32 | 128 | 67.6 |
| Imipenem | 0.250 to 64 | 16 | 64 | 64.7 |
| Meropenem | 0.125 to 64 | 16 | 32 | 63.3 |
| Piperacillin-tazobactam | 2 to >256 | 128 | >256 | 76.9 |
Comparative phenotypic testing for ESBLs.
Table 3 summarizes results of the phenotypic tests for ESBL production and their performance characteristics for the 162 genotypically ESBL-positive and the 139 genotypically ESBL-negative clinical isolates.
TABLE 3.
Phenotypic detection of ESBLs among genotypically ESBL-positive (n = 162) and ESBL-negative (n = 139) clinical isolates using the CLSI ESBL confirmatory test and the modified CLSI ESBL confirmatory test
| ESBL screening method | No. (%) of isolates confirmed by PCR to have the indicated phenotype | Test performance (%)a |
||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | |||
| KPC- and ESBL-producing isolates (n = 35) | KPC- and non-ESBL-producing isolates (n = 32) | |||||
| CLSI ESBL confirmatory testb | 23 (65.7) | 0(0) | 65.7 | 100 | 100 | 72.7 |
| Modified CLSI ESBL confirmatory testc | 35 (100) | 0(0) | 100 | 100 | 100 | 100 |
| MBL- and ESBL-producing isolates (n = 30) | MBL- and non-ESBL-producing isolates (n = 38) | |||||
| CLSI ESBL confirmatory testb | 4 (13.3) | 2 (0) | 13.3 | 94.7 | 66.7 | 60 |
| Modified CLSI ESBL confirmatory testc | 29 (96.7) | 0 (0) | 96.7 | 100 | 100 | 97.4 |
| KPC-, MBL-, and ESBL-producing isolates (n = 10) | KPC-, MBL-, and non-ESBL-producing isolates (n = 21) | |||||
| CLSI ESBL confirmatory testb | 0 (0) | 0 (0) | 0 | 100 | 0 | 67.7 |
| Modified CLSI ESBL confirmatory testc | 9 (90) | 0 (0) | 90 | 100 | 100 | 95.5 |
| AmpC- and ESBL-producing isolates (n = 25) | AmpC- and non-ESBL-producing isolates (n = 20) | |||||
| CLSI ESBL confirmatory testb | 18 (72) | 2 (0) | 72 | 90 | 90 | 72 |
| Modified CLSI ESBL confirmatory testc | 25 (100) | 0 (0) | 100 | 100 | 100 | 100 |
| OXA-48- and ESBL-producing isolates (n = 8) | OXA-48- and non-ESBL-producing isolates (n = 3) | |||||
| CLSI ESBL confirmatory testb | 8 (100) | 0 (0) | 100 | 100 | 100 | 100 |
| Modified CLSI ESBL confirmatory testc | 8 (100) | 0 (0) | 100 | 100 | 100 | 100 |
| Only ESBL-producing isolates (n = 54) | Non-ESBL-producing isolates (n = 25) | |||||
| CLSI ESBL confirmatory testb | 53 (98.1) | 0 (0) | 98.1 | 100 | 100 | 96.2 |
| Modified CLSI ESBL confirmatory testc | 52 (96.3) | 0 (0) | 96.3 | 100 | 100 | 92.6 |
| Total ESBL-producing isolates (n = 162) | Total non-ESBL-producing isolates (n = 139) | |||||
| CLSI ESBL confirmatory testb | 106 (65.4) | 4(0) | 65.4 | 97.1 | 96.4 | 70.7 |
| Modified CLSI ESBL confirmatory testc | 158 (97.5) | 0(0) | 97.5 | 100 | 100 | 97.2 |
PPV, positive predictive value; NPV, negative predictive value.
CTX-CA versus CTX and/or CAZ-CA versus CAZ.
CTX-CA-BA-EDTA versus CTX-BA-EDTA and/or CAZ-CA-BA-EDTA versus CAZ-BA-EDTA.
(i) CLSI ESBL confirmatory test.
By employing the standard CLSI ESBL confirmatory test, we found that 106 of the 162 ESBL PCR-positive isolates had a ≥5-mm increase in the growth-inhibitory zone diameter around either CTX-CA or CAZ-CA and were considered phenotypically positive for ESBL production (sensitivity, 65.4%). In more detail, 52 (32.1%) of the 162 isolates had a ≥5-mm increase in the growth-inhibitory zone diameter around both CTX-CA and CAZ-CA, 43 (26.5%) isolates had a ≥5-mm increase in the growth-inhibitory zone diameter only around CAZ-CA, and 11 (6.8%) isolates had a ≥5-mm increase in the growth-inhibitory zone diameter only around CTX-CA (Fig. 1). It is of note that the test detected ESBLs only in 4 (13.3%) of the 30 MBL/ESBL producers and in none of the 10 KPC/MBL/ESBL producers. The test was negative for all but 4 of the 139 non-ESBL-producing isolates. In these isolates (2 MBL producers and 2 AmpC producers) the test showed a ≥5-mm increase in the zone diameter around either the CTX-CA or CAZ-CA disks (specificity, 97.1%) (Table 3).
FIG 1.
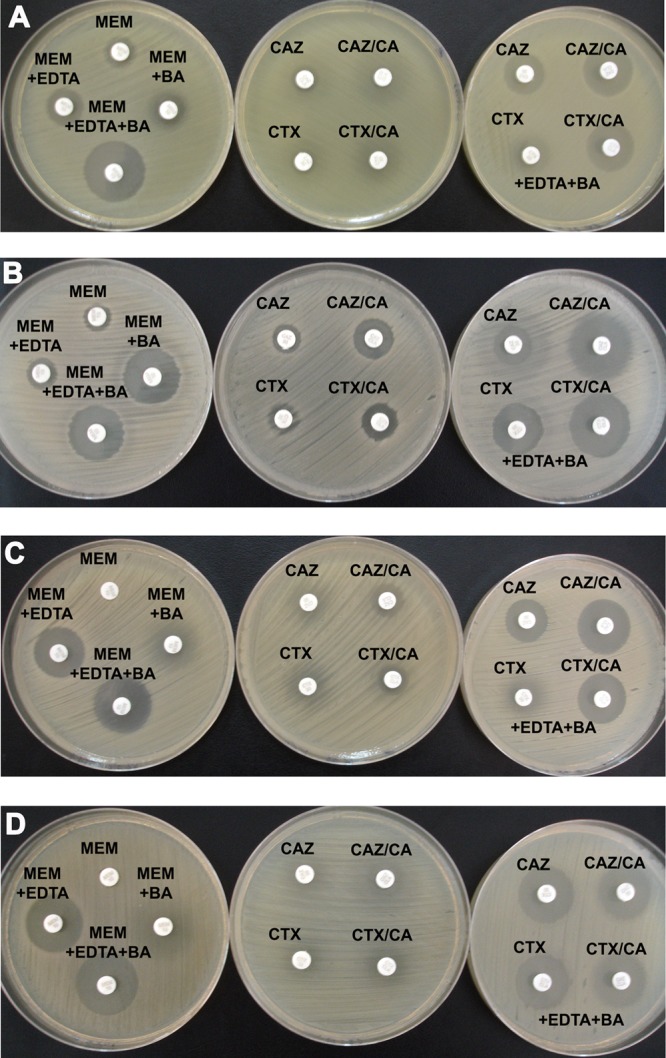
Representative results of the CLSI ESBL confirmatory test (second column) and the proposed modification (third column) using antibiotic disks containing EDTA and BA for representative isolates producing KPC, VIM, and ESBL (A), KPC and ESBL (B), VIM and ESBL (C), and VIM (D) β-lactamases. The first column represents results from phenotypic testing for the detection and differentiation of carbapenemases in Enterobacteriaceae using disks of meropenem (MEM) without and with EDTA, BA, or EDTA plus BA (19).
(ii) Modified CLSI ESBL confirmatory test with CA, BA, and EDTA.
By employing the modified CLSI ESBL confirmatory test, we found that 158 of the 162 ESBL PCR-positive isolates showed a ≥5-mm increase in the growth-inhibitory zone diameter around either CTX-CA-BA-EDTA or CAZ-CA-BA-EDTA (sensitivity, 97.5%). In more detail, 106 (65.4%) of the 162 ESBL PCR-positive isolates showed a ≥5-mm increase in the growth-inhibitory zone diameter around both CTX-CA-BA-EDTA and CAZ-CA-BA-EDTA, 35 (21.6%) isolates showed a ≥5-mm increase in the growth-inhibitory zone diameter only around CAZ-CA-BA-EDTA, and 17 (10.5%) isolates showed a ≥5-mm increase in the growth-inhibitory zone diameter only around CTX-CA-BA-EDTA (Fig. 1). In contrast to the CLSI ESBL confirmatory test, the modified test detected ESBLs among all KPC/ESBL or AmpC/ESBL producers, as well as among 29 (96.7%) of the 30 MBL/ESBL producers and 9 (90%) of the 10 KPC/VIM/ESBL producers. Using the modified test, we found that none of the 132 non-ESBL-producing isolates showed a ≥5-mm increase in the zone diameter around either the CTX-CA-BA-EDTA or the CAZ-CA-BA-EDTA disks (specificity, 100%) (Table 3).
(iii) Increases in the inhibition zone diameters using the two phenotypic tests for ESBL detection.
Among genotypically ESBL-positive isolates, the modified CLSI ESBL confirmatory test in comparison with the CLSI ESBL confirmatory test showed higher increases in the inhibition zone diameters of disks containing either CAZ or CTX. This was more obvious among ESBL-positive isolates possessing carbapenemases (KPC, VIM, NDM, and KPC/VIM) or AmpCs (Table 4). Moreover, using the modified test, the average increases in the inhibition zone diameters among ESBL producers harboring KPC, KPC/VIM, or AmpC were higher for the CAZ-CA-BA-EDTA disk than for the CTX-CA-BA-EDTA disk (Table 4), since the majority of these isolates carried SHV-type ESBLs.
TABLE 4.
Average increases in the inhibition zone diameters of CAZ-CA versus those of CAZ and of CTX-CA versus those of CTX by the CLSI ESBL confirmatory test and average increases in the inhibition zone diameters of CAZ-CA-BA-EDTA versus those of CAZ-BA-EDTA and of CTX-CA-BA-EDTA versus those of CTX-BA-EDTA by the modified CLSI ESBL confirmatory test
| Strain group (genotypes) | Average increases (mm) in inhibition zone diameter with: |
|||
|---|---|---|---|---|
| CLSI ESBL confirmatory test |
Modified CLSI ESBL confirmatory test |
|||
| CAZ-CA/CAZ | CTX-CA/CTX | CAZ-CA-BA-EDTA/CAZ-BA-EDTA | CTX-CA-BA-EDTA/CTX-BA-EDTA | |
| KPC/ESBL producers (28 SHV type, 4 CTX-M type, 3 SHV + CTX-M type) | 3.9 | 3.3 | 7.3 | 5.8 |
| VIM/ESBL producers (15 SHV type, 7 CTX-M type) | 3.8 | 1.0 | 8.6 | 8.3 |
| NDM/ESBL producer (8 CTX-M type) | 3.1 | 2.2 | 8.3 | 10.2 |
| KPC/VIM/ESBL producers (9 SHV type, 1 CTX-M type) | 2.6 | 1.3 | 7.4 | 4.0 |
| AmpC/ESBL producers (21 SHV type, 3 CTX-M type, 1 SHV + CTX-M type) | 8.9 | 3.7 | 9.3 | 5.8 |
| OXA-48/ESBL producers (8 CTX-M type) | 5.8 | 6.9 | 6.5 | 6.4 |
| Only ESBL producers (14 SHV type, 34 CTX-M type, 2 IBC type, 4 SHV + CTX-M type) | 8.9 | 12.0 | 8.5 | 10.9 |
DISCUSSION
ESBLs have emerged gradually during the last decades in species of Enterobacteriaceae and their prevalences reach alarming rates (1, 3, 32). Infections caused by such pathogens often limit therapeutic options and cause treatment failures (3, 32, 33). Thus, in order to successfully detect and treat infections due to ESBL-producing Enterobacteriaceae, the CLSI has recommended a phenotypic confirmatory test for ESBL production. This test can accurately detect ESBLs among enterobacterial species when no other potent β-lactamases are coproduced (2, 5, 6, 17).
However, this confirmatory method needs to be adjusted, as multiple mechanisms of resistance to β-lactam antibiotics may be present in a single ESBL-producing isolate (1, 6, 34, 35). Hence, the coexistence of ESBLs with derepressed chromosomal cephalosporinases, plasmid-mediated AmpCs, and carbapenemases may complicate their phenotypic detection (6, 7, 16–18). The expression of the latter β-lactamases can mask the presence of ESBLs, so that, in terms of phenotypic screening, the prevalences of ESBLs may be underestimated (8, 11, 17, 18). Thus, although the detection of ESBLs among Enterobacteriaceae producing other potent β-lactamases is overlooked more often than the detection of ESBLs among Enterobacteriaceae producing only ESBLs, a number of alternative phenotypic tests have been proposed to improve detection of ESBLs among strains with derepressed chromosomal AmpCs (6–9), plasmid-mediated AmpCs (8, 9, 16, 17), or KPCs (18). It should also be mentioned that plasmid-mediated AmpC and carbapenemase genes are characterized by high mobility and may be cotransferred with ESBLs among various species (1, 20, 32, 33, 34, 35). Moreover, ESBL genes are often cotransferred with plasmid-mediated fluoroquinolone and aminoglycoside resistance genes, thus contributing to the dissemination of multidrug resistance mechanisms (2, 4, 33). Therefore, an accurate method for the phenotypic detection of ESBLs among Enterobacteriaceae irrespective of the presence of other β-lactamases is essential in order to successfully address surveillance studies as well as for infection control issues (16, 36). The accurate detection of ESBLs might also guide therapeutic options for infections caused by multidrug-resistant pathogens possessing AmpCs, OXA-48, or MBLs; absence of ESBLs among such pathogens may allow the use of cefepime, oxyimino-cephalosporins, and aztreonam, respectively (25, 32, 36, 37).
In the present study, the standard CLSI ESBL confirmatory test was found unable to detect the vast majority of ESBL producers when MBLs, or KPCs and MBLs, were coproduced. Also, it was negative in several isolates coproducing ESBLs with KPCs, plasmid-mediated AmpCs, or derepressed chromosomal cephalosporinases. It should be noted, however, that the test sufficiently detected ESBLs among OXA-48-possessing isolates, since OXA-48 derivatives only weakly hydrolyze cephalosporins (25, 37). Also, as expected, among the set of isolates producing only ESBL, the conventional test adequately detected ESBL production.
Furthermore, in order to improve the sensitivity of the CLSI ESBL confirmatory test, we evaluated a modification of this test using antibiotic disks containing both BA and EDTA, well-known inhibitors of KPCs/AmpCs and MBLs, respectively. The design of our modified method was based on the hypothesis that the inhibitory activity of BA and EDTA will enlarge differences in zone diameters between CTX and CTX-CA disks as well as between CAZ and CAZ-CA disks in the phenotypic detection of ESBLs among Enterobacteriaceae expressing various carbapenemases or AmpC β-lactamases.
The modified method was found accurate for detecting ESBLs not only among Enterobacteriaceae producing various potent β-lactamase genes but also among those producing only ESBLs. It identified almost all genotypically ESBL-positive isolates and did not give false-positive results for any of the ESBL-negative isolates. In contrast to the CLSI ESBL confirmatory test, the modified test detected ESBLs among all KPC, NDM, and AmpC producers, as well as the vast majority of VIM and KPC/VIM producers. Moreover, the modified test detected almost all of the study isolates producing ESBL only, including those that exhibited ertapenem resistance due to porin deficiency. It is also of note that although the CLSI ESBL confirmatory test showed false-positive results among four non-ESBL-producing isolates, the modified test was negative among all non-ESBL producers of the study. Accordingly, previous surveys have shown that the CLSI ESBL confirmatory test may give a few false-positive results among non-ESBL-, AmpC-producing Enterobacteriaceae, while a modification of the test using BA did not give any false-positive results in this bacterial population (16, 38). In addition, among isolates coproducing AmpCs or carbapenems, the proposed modified test provided considerably higher increases in the inhibitory zone diameters around disks containing CAZ or CTX, allowing an easy and straightforward interpretation of the phenotypic test.
In conclusion, the modification of the CLSI ESBL confirmatory test was found to accurately detect ESBL-producing Enterobacteriaceae regardless of the underlying β-lactam resistance mechanism. It is understandable that molecular assays may provide accurate results in the identification of ESBL genes (3), but their accessibility is often limited; nevertheless, they are expensive. The proposed phenotypic method is a highly sensitive and specific method that can be easily performed and interpreted in <24 h without requiring the previous knowledge of the presence of other β-lactamases.
Footnotes
Published ahead of print 26 February 2014
REFERENCES
- 1.Bush K, Fisher JF. 2011. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from Gram-negative bacteria. Annu. Rev. Microbiol. 65:455–478. 10.1146/annurev-micro-090110-102911 [DOI] [PubMed] [Google Scholar]
- 2.Pitout JD, Laupland KB. 2008. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8:159–166. 10.1016/S1473-3099(08)70041-0 [DOI] [PubMed] [Google Scholar]
- 3.Zahar JR, Lortholary O, Martin C, Potel G, Plesiat P, Nordmann P. 2009. Addressing the challenge of extended-spectrum β-lactamases. Curr. Opin. Investig. Drugs 10:172–180 [PubMed] [Google Scholar]
- 4.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 53:2227–2238. 10.1128/AAC.01707-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing. Twenty second informational supplement update. CLSI document M100-S22 U Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6.Drieux L, Brossier F, Sougakoff W, Jarlier V. 2008. Phenotypic detection of extended spectrum β-lactamase production in Enterobacteriaceae: review and bench guide. Clin. Microbiol. Infect. 14(Suppl 1):90–103. 10.1111/j.1469-0691.2007.01846.x [DOI] [PubMed] [Google Scholar]
- 7.Tzelepi E, Giakkoupi P, Sofianou D, Loukova V, Kemeroglou A, Tsakris A. 2000. Detection of extended-spectrum β-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 38:542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrec H, Drieux-Rouzet L, Golmard JL, Jarlier V, Robert J. 2011. Comparison of nine phenotypic methods for detection of extended-spectrum β-lactamase production by Enterobacteriaceae. J. Clin. Microbiol. 49:1048–1057. 10.1128/JCM.02130-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart JC, Diederen B, Al Naiemi N, Fluit A, Arents N, Thijsen S, Vlaminckx B, Mouton JW, Leverstein-van Hall M. 2011. Method for phenotypic detection of extended-spectrum β-lactamases in enterobacter species in the routine clinical setting. J. Clin. Microbiol. 49:2711–2713. 10.1128/JCM.00864-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordmann P, Dortet L, Poirel L. 2012. Rapid detection of extended-spectrum-β-lactamase-producing Enterobacteriaceae. J. Clin. Microbiol. 50:3016–3022. 10.1128/JCM.00859-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coudron PE. 2005. Inhibitor-based methods for detection of plasmid-mediated AmpC β-lactamases in Klebsiella spp., Escherichia coli, and Proteus mirabilis. J. Clin. Microbiol. 43:4163–4167. 10.1128/JCM.43.8.4163-4167.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi Y, Potoski BA, Adams-Haduch JM, Sidjabat HE, Pasculle AW, Paterson DL. 2008. Simple disk-based method for detection of Klebsiella pneumoniae carbapenemase-type β-lactamase by use of a boronic acid compound. J. Clin. Microbiol. 46:4083-4086. 10.1128/JCM.01408-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitout JD, Le PG, Moore KL, Church DL, Gregson DB. 2010. Detection of AmpC β-lactamases in Escherichia coli, Klebsiella spp., Salmonella spp. and Proteus mirabilis in a regional clinical microbiology laboratory. Clin. Microbiol. Infect. 16:165–170. 10.1111/j.1469-0691.2009.02756.x [DOI] [PubMed] [Google Scholar]
- 14.Tsakris A, Kristo I, Poulou A, Markou F, Ikonomidis A, Pournaras S. 2008. First occurrence of KPC-2-possessing Klebsiella pneumoniae in a Greek hospital and recommendation for detection with boronic acid disc tests. J. Antimicrob. Chemother. 62:1257–1260. 10.1093/jac/dkn364 [DOI] [PubMed] [Google Scholar]
- 15.Giske CG, Gezelius L, Samuelsen Ø, Warner M, Sundsfjord A, Woodford N. 2011. A sensitive and specific phenotypic assay for detection of metallo-β-lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin. Microbiol. Infect. 17:552–556. 10.1111/j.1469-0691.2010.03294.x [DOI] [PubMed] [Google Scholar]
- 16.Jeong SH, Song W, Park MJ, Kim JS, Kim HS, Bae IK, Lee KM. 2008. Boronic acid disk tests for identification of extended-spectrum β-lactamase production in clinical isolates of Enterobacteriaceae producing chromosomal AmpC β-lactamases. Int. J. Antimicrob. Agents 31:467–471. 10.1016/j.ijantimicag.2007.12.014 [DOI] [PubMed] [Google Scholar]
- 17.Song W, Bae IK, Lee YN, Lee CH, Lee SH, Jeong SH. 2007. Detection of extended-spectrum β-lactamases by using boronic acid as an AmpC β-lactamase inhibitor in clinical isolates of Klebsiella spp and Escherichia coli. J. Clin. Microbiol. 45:1180–1184. 10.1128/JCM.02322-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsakris A, Poulou A, Themeli-Digalaki K, Voulgari E, Pittaras T, Sofianou D, Pournaras S, Petropoulou D. 2009. Use of boronic acid disk tests to detect extended-spectrum β-lactamases in clinical isolates of KPC carbapenemase-possessing Enterobacteriaceae. J. Clin. Microbiol. 47:3420–3426. 10.1128/JCM.01314-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsakris A, Poulou A, Pournaras S, Voulgari E, Vrioni G, Themeli-Digalaki K, Petropoulou D, Sofianou D. 2010. A simple phenotypic method for the differentiation of metallo-β-lactamases and class A KPC carbapenemases in Enterobacteriaceae clinical isolates. J. Antimicrob. Chemother. 65:1664–1671. 10.1093/jac/dkq210 [DOI] [PubMed] [Google Scholar]
- 20.Nordmann P, Gniadkowski M, Giske CG, Poirel L, Woodford N, Miriagou V, European Network on Carbapenemases 2012. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Infect. 18:432–438. 10.1111/j.1469-0691.2012.03815.x [DOI] [PubMed] [Google Scholar]
- 21.Franklin C, Liolios L, Peleg AY. 2006. Phenotypic detection of carbapenem-susceptible metallo-β-lactamase-producing gram-negative bacilli in the clinical laboratory. J. Clin. Microbiol. 44:3139–3144. 10.1128/JCM.00879-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pournaras S, Poulou A, Tsakris A. 2010. Inhibitor-based methods for the detection of KPC carbapenemase-producing Enterobacteriaceae in clinical practice by using boronic acid compounds. J. Antimicrob. Chemother. 65:1319–1321. 10.1093/jac/dkq124 [DOI] [PubMed] [Google Scholar]
- 23.Tzelepi E, Magana C, Platsouka E, Sofianou D, Paniara O, Legakis NJ, Vatopoulos AC, Tzouvelekis LS. 2003. Extended-spectrum β-lactamase types in Klebsiella pneumoniae and Escherichia coli in two Greek hospitals. Intern. J. Antimicrob. Agents 21:285–288. 10.1016/S0924-8579(02)00361-8 [DOI] [PubMed] [Google Scholar]
- 24.Smith Moland E, Hanson ND, Herrera VL, Black JA, Lockhart TJ, Hossain A, Johnson JA, Goering RV, Thomson KS. 2003. Plasmid-mediated, carbapenem-hydrolysing β-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J. Antimicrob. Chemother. 51:711–714. 10.1093/jac/dkg124 [DOI] [PubMed] [Google Scholar]
- 25.Poirel L, Héritier C, Tolün V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22. 10.1128/AAC.48.1.15-22.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agodi A, Voulgari E, Barchitta M, Politi L, Koumaki V, Spanakis N, Giaquinta L, Valenti G, Romeo MA, Tsakris A. 2011. Containment of an outbreak of KPC-3-producing Klebsiella pneumoniae in Italy. J. Clin. Microbiol. 49:3986–3989. 10.1128/JCM.01242-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Pérez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162. 10.1128/JCM.40.6.2153-2162.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preston KE, Radomski CC, Venezia RA. 2000. Nucleotide sequence of the chromosomal ampC gene of Enterobacter aerogenes. Antimicrob. Agents Chemother. 44:3158–3162. 10.1128/AAC.44.11.3158-3162.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnaud G, Labia R, Raskine L, Sanson-Le Pors MJ, Philippon A, Arlet G. 2001. Extension of resistance to cefepime and cefpirome associated to a six amino acid deletion in the H-10 helix of the cephalosporinase of an Enterobacter cloacae clinical isolate. FEMS Microbiol. Lett. 195:185–190. 10.1111/j.1574-6968.2001.tb10519.x [DOI] [PubMed] [Google Scholar]
- 30.Tsakris A, Kristo I, Poulou A, Themeli-Digalaki K, Ikonomidis A, Petropoulou D, Pournaras S, Sofianou D. 2009. Evaluation of boronic acid disk tests for differentiating KPC-possessing Klebsiella pneumoniae isolates in the clinical laboratory. J. Clin. Microbiol. 47:362–367. 10.1128/JCM.01922-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulou A, Voulgari E, Vrioni G, Koumaki V, Xidopoulos G, Chatzipantazi V, Markou F, Tsakris A. 2013. Outbreak caused by an ertapenem-resistant, CTX-M-15-producing Klebsiella pneumoniae sequence type 101 clone carrying an OmpK36 porin variant. J. Clin. Microbiol. 51:3176–3182. 10.1128/JCM.01244-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32..Rossolini GM, Mantengoli E, Docquier JD, Musmanno RA, Coratza G. 2007. Epidemiology of infections caused by multiresistant gram-negatives: ESBLs, MBLs, panresistant strains. New Microbiol. 30:332–339 [PubMed] [Google Scholar]
- 33.Livermore DM. 2009. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 64:29–36. 10.1093/jac/dkp255 [DOI] [PubMed] [Google Scholar]
- 34.Endimiani A, Hujer AM, Perez F, Bethel CR, Hujer KM, Kroeger J, Oethinger M, Paterson DL, Adams MD, Jacobs MR, Diekema DJ, Hall GS, Jenkins SG, Rice LB, Tenover FC, Bonomo RA. 2009. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J. Antimicrob. Chemother. 63:427–437. 10.1093/jac/dkn547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pournaras S, Poulou A, Voulgari E, Vrioni G, Kristo I, Tsakris A. 2010. Detection of the new metallo-β-lactamase VIM-19 along with KPC-2, CMY-2 and CTX-M-15 in Klebsiella pneumoniae. J. Antimicrob. Chemother. 65:1604–1607. 10.1093/jac/dkq190 [DOI] [PubMed] [Google Scholar]
- 36.Livermore DM, Andrews JM, Hawkey PM, Ho PL, Keness Y, Doi Y, Paterson D, Woodford N. 2012. Are susceptibility tests enough, or should laboratories still seek ESBLs and carbapenemases directly? J. Antimicrob. Chemother. 67:1569-1577. 10.1093/jac/dks088 [DOI] [PubMed] [Google Scholar]
- 37.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J. Antimicrob. Chemother. 67:1597–1606. 10.1093/jac/dks121 [DOI] [PubMed] [Google Scholar]
- 38.Robberts FJ, Kohner PC, Patel R. 2009. Unreliable extended-spectrum β-lactamase detection in the presence of plasmid-mediated AmpC in Escherichia coli clinical isolates. J. Clin. Microbiol. 47:358–361. 10.1128/JCM.01687-08 [DOI] [PMC free article] [PubMed] [Google Scholar]


