Abstract
The recently launched Liaison XL Murex HIV Ab/Ag assay (DiaSorin S.p.A) uses chemiluminescence immunoassay technology for the combined qualitative determination of p24 antigen of HIV-1 and specific antibodies to both HIV-1 and HIV-2. We studied 571 serum samples from those submitted to our laboratory for HIV screening. The samples were divided into 3 subsets: subset A, 365 samples collected prospectively during 1 week; subset B, 158 samples from confirmed HIV-positive patients; and subset C, 48 samples with a positive screening result but a negative or indeterminate confirmatory test result. Our standard screening/confirmatory algorithm was used as a reference. In subset A (prospective), 5 samples were positive and 360 negative by the standard procedure. Liaison XL Murex HIV Ab/Ag correctly identified all 5 positive samples (100%) and 357 negative samples (99.2%). In subset B (confirmed positive), all 158 positive samples were in total agreement in both procedures. In subset C (screen positive only), Liaison XL Murex HIV Ab/Ag yielded accurate results in 42 out of 48 samples (87.5%). Global sensitivity and specificity for Liaison XL Murex HIV Ab/Ag (all subsets included) were 98.3% and 98.5%, respectively. Considering only nonselected prospective samples and confirmed positive samples (subsets A and B), the corresponding sensitivity and specificity values were 100% and 99.2%, respectively. The new fully automated HIV screening test showed high sensitivity and specificity compared to our standard algorithm. Its added advantage of being able to detect HIV-1 and HIV-2 antibodies and p24 antigen separately could prove useful in the diagnosis of early infections.
INTRODUCTION
Identification of human immunodeficiency virus (HIV) infection early in the seroconversion window period is essential for optimal patient management and significant reduction in the transmission rate (1).
Over the last few decades, considerable efforts have been made to narrow the window period for detection of HIV (2). Although antibody (Ab) tests (third-generation assays) have been developed to reduce this period, they are not able to identify patients with acute HIV infection who have not yet produced specific antibodies.
Fourth-generation HIV tests are designed to detect both HIV antibodies and the p24 antigen (Ag) in a single assay run. Due to their ability to detect HIV p24 Ag, these tests can detect HIV infection prior to seroconversion (3). The sensitivity of fourth-generation assays for the detection of early HIV infection has been proven in several prior studies (4–9).
Liaison XL Murex HIV Ab/Ag (DiaSorin S.p.A., Italy) is a fully automated fourth-generation screening test based on chemiluminescence immunoassay technology that performs complete sample processing. An advantage over previous fourth-generation assays is that it detects and reports separate values for HIV antigens and antibodies, thus enabling better interpretation of results. The performance of this new assay for the screening of HIV infection has not been extensively evaluated (10, 11).
The aim of the present study was to evaluate the fourth-generation Liaison XL Murex HIV Ab/Ag assay in a panel of well-characterized serum specimens.
MATERIALS AND METHODS
Standard algorithm.
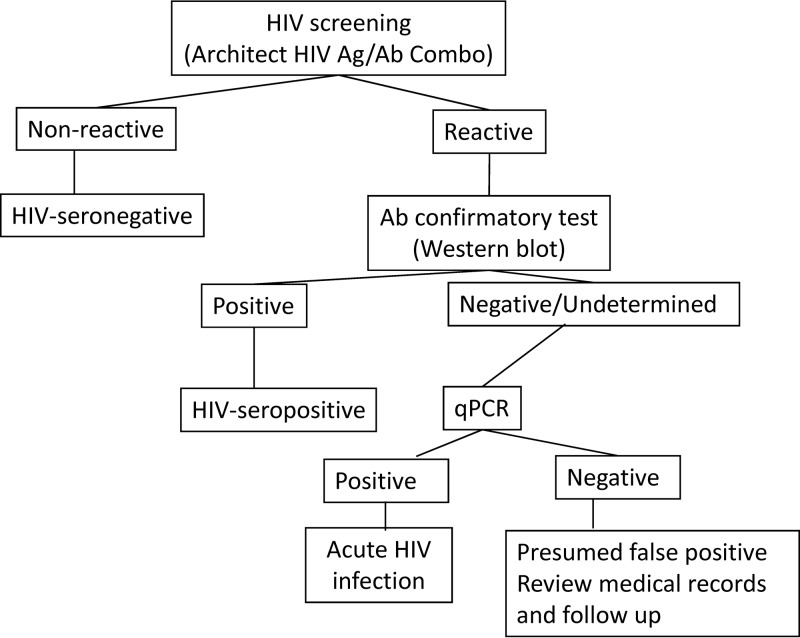
The standard diagnostic algorithm is represented in Fig. 1. Samples were considered to be HIV seropositive if they were reactive by the Architect HIV Ag/Ab Combo system (Abbott, Wiesbaden, Germany) and by a Western blot assay (WB) (New LabBlot; Bio-Rad) or nucleic acid amplification test (NAAT) (HIV Versant kPCR; Siemens). Specimens with negative or indeterminate WB and positive NAAT results indicated acute HIV infection. Specimens with negative Architect HIV Ag/Ab Combo results were identified as HIV seronegative.
FIG 1.
Standard algorithm used for the screening and confirmation of HIV infection (gold standard). qPCR, quantitative PCR.
Clinical specimens.
Three subsets were used to evaluate the Liaison XL Murex HIV Ab/Ag assay, as follows: subset A, 365 routine serum samples collected prospectively during 1 week; subset B, 158 confirmed HIV-positive samples; and subset C, 48 archived samples with positive HIV screening (Architect HIV Ag/Ab Combo) and negative/undetermined WB results.
Fourth-generation assay.
Liaison XL Murex HIV Ab/Ag uses chemiluminescence immunoassay technology for the combined qualitative determination of HIV-1 p24 antigen and specific antibodies to both HIV-1 (group M and group O) and HIV-2 in human serum or plasma samples. Specimens with signal-to-cutoff (S/CO) ratios of ≥1 are considered reactive for HIV p24 antigen or HIV antibodies. The system generates 2 different results—one for p24 antigen and the other for antibody detection in each sample—and a combined final result. The test was performed following the manufacturer's instructions.
Statistical analysis.
Sensitivity and specificity values were calculated with a 95% confidence interval following an exact binomial distribution. The data were analyzed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
RESULTS
A total of 571 serum samples from 567 patients were tested using Liaison XL Murex HIV Ab/Ag. Table 1 shows the results of the Liaison XL Murex HIV Ab/Ag assay compared with the standard algorithm for all 3 subsets.
TABLE 1.
Detailed results for all samples assayed and all techniques
| Subset | No. of samples | Resulta |
||||||
|---|---|---|---|---|---|---|---|---|
| Standard algorithm |
New fourth-generation assay |
|||||||
| Architect | WB | NAAT | Final | Ab | Ag | Final | ||
| A | 357 | Negative | NA | NA | Negative | Negative | Negative | True negative |
| 3 | Negative | Negative | <37 | Negative | Positive | Negative | False positive | |
| 2 | Positive | Positive | NA | Positive | Positive | Negative | True positive | |
| 2 | Positive | Positive | NA | Positive | Positive | Positive | True positive | |
| 1b | Positive | UD | Detectable | Positive | Positive | Positive | True positive | |
| B | 145 | Positive | Positive | NA | Positive | Positive | Negative | True positive |
| 13 | Positive | Positive | NA | Positive | Positive | Positive | True positive | |
| C | 23c | Positive | Negative | <37 | Negative | Negative | Negative | True negative |
| 4c | Positive | UD | <37 | Negative | Negative | Negative | True negative | |
| 2c | Positive | Negative | <37 | Negative | Positive | Negative | False positive | |
| 1c | Positive | UD | <37 | Negative | Positive | Negative | False positive | |
| 4b | Positive | UD | Detectable | Positive | Positive | Negative | True positive | |
| 2b | Positive | Negative | Detectable | Positive | Positive | Positive | True positive | |
| 2b | Positive | Negative | Detectable | Positive | Negative | Positive | True positive | |
| 1b | Positive | UD | Detectable | Positive | Positive | Positive | True positive | |
| 1b | Positive | UD | Detectable | Positive | Negative | Positive | True positive | |
| 3d | Positive | Negative | <37 | Positive | Negative | Negative | False negative | |
| 3d | Positive | Negative | <37 | Positive | Positive | Negative | True positive | |
| 1d | Positive | UD | <37 | Positive | Positive | Negative | True positive | |
| 1e | Positive | Negativef | <37 | Positive | Positive | Negative | True positive | |
NA, not applicable; UD, undetermined.
Acute infection.
Architect false-positive result.
Maternal antibodies.
HIV-2 infection.
Our routine confirmatory test detects only antibodies against HIV-1 (New LabBlot HIV-1); this sample was confirmed when assayed with a Western blot assay specific for HIV-2 (New LabBlot HIV-2).
Subset A (prospective).
Of the 365 nonselected prospective samples, 5 were HIV positive (1 from a patient with acute infection) and 360 were HIV negative by the standard algorithm. Liaison XL Murex HIV Ab/Ag correctly identified all 5 positive samples (100%). As for the negative samples, the new system correctly identified 357 of 360 samples (99.2%). Three samples classified as negative by the reference method had a positive antibody result and were considered false-positive results. The results of WB and NAAT were also negative for these samples (Table 1).
Subset B (confirmed positive).
Liaison XL Murex HIV Ab/Ag correctly identified all 158 archived positive samples (100%). p24 antigen was detected in 13 samples (Table 1).
Subset C (screen positive only).
Samples included in subset C were Architect HIV Ag/Ab Combo positive and WB negative (n = 36) or undetermined (n = 12).
Thirty samples were HIV negative by the standard algorithm. All these samples were considered false positive in the screening technique (Architect HIV Ag/Ab Combo). With the Liaison XL Murex HIV Ab/Ag, 27 samples were true negatives and 3 were false positives.
Eighteen samples were HIV positive by the standard algorithm. The screening technique correctly identified all the positive samples. Considering additional clinical data, these samples were classified as follows. (i) Ten samples were from patients in the seroconversion window. Liaison XL Murex HIV Ab/Ag yielded positive results in all the samples. Both antigen and antibody were positive in 3 samples, 4 samples were reactive only to antibodies, and 3 samples had only a positive p24 antigen result. (ii) Seven samples corresponded to newborns from seropositive mothers. Although all 7 children were clearly non-HIV infected, their samples were considered positive by the standard algorithm because they still carried maternal antibodies. Liaison XL Murex HIV Ab/Ag was reactive in only 4 of the 7 samples. (iii) One sample from a patient infected by an HIV-2 strain was clearly positive by Liaison XL Murex HIV Ab/Ag.
In summary, Liaison XL Murex HIV Ab/Ag yielded accurate results in 42 of 48 samples in this subset (30 HIV negative and 18 HIV -positive; agreement, 87.5%) (Table 1).
Sensitivity and specificity analysis.
When all the samples were analyzed, the sensitivity and specificity of the Liaison XL Murex HIV Ab/Ag assay were 98.3 and 98.5% (subsets A, B, and C) (Table 2).
TABLE 2.
Comparison of Liaison XL Murex HIV Ab/Ag with the gold standard approach (Architect plus Western blotting) in all the samples assayed (subsets A, B, and C)
| Liaison XL Murex HIV Ab/Ag result | Standard algorithm resulta |
|
|---|---|---|
| Positive | Negative | |
| Positive | 178 | 6 |
| Negative | 3 | 384 |
| Total | 181 | 390 |
Sensitivity (95% confidence interval [CI]), 98.3% (94.8% to 99.5%); specificity (95% CI), 98.5% (96.5% to 99.4%).
When only subsets A and B were analyzed (samples collected prospectively and confirmed positive samples), the sensitivity and specificity were 100% and 99.2% (Table 3).
TABLE 3.
Comparison of Liaison XL Murex HIV Ab/Ag with the gold standard approach (Architect plus Western blotting) only in samples from subsets A and B (prospective, nonselected, and confirmed HIV-positive samples)
| Liaison XL Murex HIV Ab/Ag result | Standard algorithm resulta |
|
|---|---|---|
| Positive | Negative | |
| Positive | 163 | 3 |
| Negative | 0 | 357 |
| Total | 163 | 360 |
Sensitivity (95% CI), 100% (97.76% to 100%); specificity (95% CI), 99.2% (98.7% to 100%).
DISCUSSION
The present study shows that the Liaison XL Murex HIV Ab/Ag assay is highly sensitive and specific for the detection of HIV infection. It yielded very few false-positive and false-negative results and correctly identified all specimens classified as acute HIV infection by the standard algorithm.
Fourth-generation HIV assays have been widely used for screening. Although their performance has varied (8, 12–14), the assays have demonstrated higher sensitivity than earlier assays by reducing the window period (1, 4, 7–9, 12, 14, 15).
In the present study, Liaison XL Murex HIV Ab/Ag was highly sensitive (100%) in both routine serum samples and confirmed positive samples (98.3% when samples from subset C were included) compared with the standard algorithm. Salmona et al. (16) recently evaluated the BioPlex 220 assay, which also separately detects antibody and antigen. The sensitivity of the BioPlex 2200 was also 100%.
In the literature, specificity values for fourth-generation assays range between 97.6% and 99.8% (16, 17). Several studies have suggested lower specificity of fourth-generation assays because of the combination of 2 different test principles (3, 12, 18). This lack of specificity results in the need to perform additional tests to rule out false-positive results. In our study, we recorded 6 false-positive results: 3 in samples from subset A (prospective) and 3 in samples from subset C (screen positive only). The specificity of Liaison XL Murex HIV Ab/Ag was 99.2% in the prospective and confirmed positive populations (98.5% when samples from subset C were included), i.e., similar to that reported by Salmona et al. (99.5%) (16).
Since subset C is a very specific subset that also includes false-positive samples with the reference screening method, we decided to perform sensitivity and specificity analysis with and without the subset.
Although comparing Liaison XL Murex HIV Ab/Ag with Architect HIV Ag/Ab Combo was not a goal of the present study, we observed more false-positive results with Architect HIV Ag/Ab Combo than with Liaison XL Murex HIV Ab/Ag. Therefore, we suggest that fewer WB/NAAT would be required to rule out false-positive screening results if Liaison XL Murex HIV Ab/Ag was used as the screening assay.
False-negative results were obtained in 3 specimens from noninfected newborns whose mothers were seropositive. Since these newborns were presumably very close to antibody clearance, we suggest that this lack of sensitivity was due to the low antibody levels present in these specimens. In other clinical situations where antibody levels are low, such as acute infection, false-negative results should not be reported, because the assay also detects p24 antigen with high sensitivity.
The detection of acute HIV infection is crucial for the prompt initiation of antiretroviral therapy. In addition, several studies suggest that expansion of the viral reservoir can be reduced by initiating early and adapted antiretroviral treatment (19–21). In the present study, Liaison XL Murex HIV Ab/Ag showed excellent sensitivity for the detection of acute HIV infection by detecting HIV p24 antigen in 7 of the 11 samples classified by the standard algorithm as indicative of acute HIV infection.
Liaison XL Murex HIV Ab/Ag has the added advantage of being able to detect and report separate antibody and antigen results, thus providing more information about a patient's infection. For example, in subset B, which corresponded to confirmed positive samples, only 13 samples (8%) showed detectable p24 antigenemia. Lack of antigen detection provides valuable information on the effectiveness of antiretroviral treatment in the patient's virological control. We were able to obtain information about acute infection by detecting HIV p24 antigen without antibody detection, as was the case in 3 patients in subset C.
Liaison XL Murex HIV Ab/Ag identifies patients infected by HIV-1 or HIV-2. Since the prevalence of HIV type 2 is low in Spain (22), the use of a HIV-1 WB assay as the sole confirmatory test is common. Consequently, HIV-2-infected individuals can be misclassified as HIV-1 positive because of the cross-reactivity between HIV-2 antibody and envelope glycoproteins of HIV-1 (16). In the present study, 1 specimen was considered non-HIV infected by the standard algorithm because the WB was nonreactive; the HIV-2 WB assay was performed based on the patient's epidemiological history. This misclassification could have been avoided by using an assay to discriminate between HIV-1 and HIV-2.
The present study is subject to a series of limitations. First, few patients had acute HIV infection. Second, we did not assess the sensitivity of the assay in different viral subtypes. Third, we did not test seroconversion subsets to establish a more accurate comparison of window periods.
In conclusion, this study shows that the new Liaison XL Murex HIV Ab/Ag assay is highly sensitive and specific in the diagnosis of HIV infection. In addition, it identifies patients with acute HIV infection, thus enabling rapid initiation of highly active antiretroviral therapy (HAART).
ACKNOWLEDGMENTS
We are grateful to Thomas O'Boyle for proofreading the manuscript.
Paula Lopez Roa holds a Río Hortega contract (CM11/00142) from the Instituto de Salud Carlos III-FIS.
Footnotes
Published ahead of print 26 February 2014
REFERENCES
- 1.Hoffman CJ, Gallant JE. 2007. When and how to use tipranavir and darunavir. AIDS Read 17:194–198, 201 [PubMed] [Google Scholar]
- 2.Weber B. 2006. Screening of HIV infection: role of molecular and immunological assays. Expert Rev. Mol. Diagn. 6:399–411. 10.1586/14737159.6.3.399 [DOI] [PubMed] [Google Scholar]
- 3.Weber B, Fall EH, Berger A, Doerr HW. 1998. Reduction of diagnostic window by new fourth-generation human immunodeficiency virus screening assays. J. Clin. Microbiol. 36:2235–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber B, Berger A, Rabenau H, Doerr HW. 2002. Evaluation of a new combined antigen and antibody human immunodeficiency virus screening assay, VIDAS HIV DUO Ultra. J. Clin. Microbiol. 40:1420–1426. 10.1128/JCM.40.4.1420-1426.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber B, Orazi B, Raineri A, Thorstensson R, Burgisser P, Muhlbacher A, Areal C, Eiras A, Villaescusa R, Camacho R, Diogo I, Roth HJ, Zahn I, Bartel J, Bossi V, Piro F, Atamasirikul K, Permpikul P, Webber L, Singh S. 2006. Multicenter evaluation of a new 4th generation HIV screening assay Elecsys HIV combi. Clin. Lab. 52:463–473 [PubMed] [Google Scholar]
- 6.Muhlbacher A, Schennach H, van Helden J, Hebell T, Pantaleo G, Burgisser P, Cellerai C, Permpikul P, Rodriguez MI, Eiras A, Alborino F, Cunningham P, Axelsson M, Andersson S, Wetlitzky O, Kaiser C, Moller P, de Sousa G. 2013. Performance evaluation of a new fourth-generation HIV combination antigen-antibody assay. Med. Microbiol. Immunol. 202:77–86. 10.1007/s00430-012-0250-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pumarola T, Freeman J, Saxton E, Dillon P, Bal T, van Helden J. 2010. Performance evaluation of the ADVIA Centaur((R)) HIV Ag/Ab Combo assay. J. Virol. Methods 170:16–20. 10.1016/j.jviromet.2010.08.012 [DOI] [PubMed] [Google Scholar]
- 8.Kwon JA, Yoon SY, Lee CK, Lim CS, Lee KN, Sung HJ, Brennan CA, Devare SG. 2006. Performance evaluation of three automated human immunodeficiency virus antigen-antibody combination immunoassays. J. Virol. Methods 133:20–26. 10.1016/j.jviromet.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 9.Malm K, von Sydow M, Andersson S. 2009. Performance of three automated fourth-generation combined HIV antigen/antibody assays in large-scale screening of blood donors and clinical samples. Transfus. Med. 19:78–88. 10.1111/j.1365-3148.2009.00907.x [DOI] [PubMed] [Google Scholar]
- 10.Krawczyk A, Hintze C, Ackermann J, Goitowski B, Trippler M, Gruner N, Neumann-Fraune M, Verheyen J, Fiedler M. 2014. Clinical performance of the novel DiaSorin LIAISON XL murex: HBsAg Quant, HCV-Ab, HIV-Ab/Ag assays. J. Clin. Virol. 59:44–49. 10.1016/j.jcv.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 11.Ly TD, Plantier JC, Leballais L, Gonzalo S, Lemee V, Laperche S. 2012. The variable sensitivity of HIV Ag/Ab combination assays in the detection of p24Ag according to genotype could compromise the diagnosis of early HIV infection. J. Clin. Virol. 55:121–127. 10.1016/j.jcv.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 12.Ly TD, Laperche S, Brennan C, Vallari A, Ebel A, Hunt J, Martin L, Daghfal D, Schochetman G, Devare S. 2004. Evaluation of the sensitivity and specificity of six HIV combined p24 antigen and antibody assays. J. Virol. Methods 122:185–194. 10.1016/j.jviromet.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 13.Ly TD, Ebel A, Faucher V, Fihman V, Laperche S. 2007. Could the new HIV combined p24 antigen and antibody assays replace p24 antigen specific assays? J. Virol. Methods 143:86–94. 10.1016/j.jviromet.2007.02.013 [DOI] [PubMed] [Google Scholar]
- 14.Pandori MW, Branson BM. 2010. 2010 HIV Diagnostics Conference. Expert Rev. Anti Infect. Ther. 8:631–633. 10.1586/eri.10.48 [DOI] [PubMed] [Google Scholar]
- 15.Bourlet T, Pretis C, Pillet S, Lesenechal M, Piche J, Pozzetto B. 2005. Comparative evaluation of the VIDAS HIV DUO Ultra assay for combined detection of HIV-1 antigen and antibodies to HIV. J. Virol. Methods 127:165–167. 10.1016/j.jviromet.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 16.Salmona M, Delarue S, Delaugerre C, Simon F, Maylin S. 2014. Clinical evaluation of BioPlex(R) 2200 HIV Ag-Ab: an automated screening method providing discrete detection of HIV-1 p24 antigen, HIV-1 antibody, and HIV-2 antibody. J. Clin. Microbiol. 52:103–107. 10.1128/JCM.02460-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brust S, Duttmann H, Feldner J, Gurtler L, Thorstensson R, Simon F. 2000. Shortening of the diagnostic window with a new combined HIV p24 antigen and anti-HIV-1/2/O screening test. J. Virol. Methods 90:153–165. 10.1016/S0166-0934(00)00229-9 [DOI] [PubMed] [Google Scholar]
- 18.Ly TD, Laperche S, Courouce AM. 2001. Early detection of human immunodeficiency virus infection using third- and fourth-generation screening assays. Eur. J. Clin. Microbiol. Infect. Dis. 20:104–110. 10.1007/s100960000430 [DOI] [PubMed] [Google Scholar]
- 19.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, Dewar R, Marovich M, van Griensven F, Sekaly R, Pinyakorn S, Phanuphak N, Trichavaroj R, Rutvisuttinunt W, Chomchey N, Paris R, Peel S, Valcour V, Maldarelli F, Chomont N, Michael N, Phanuphak P, Kim JH, RV254/SEARCH 010 Study Group 2012. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 7:e33948. 10.1371/journal.pone.0033948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, HPTN 052 Study Team 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 365:493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saez-Cirion A, Pancino G. 2013. HIV controllers: a genetically determined or inducible phenotype? Immunol. Rev. 254:281–294. 10.1111/imr.12076 [DOI] [PubMed] [Google Scholar]
- 22.Toro C, Rodes B, Aguilera A, Caballero E, Benito R, Bassani S, Rodriguez C, Tuset C, Ortiz de Lejarazu R, Eiros J, Garcia J, Calderon E, Capote FJ, Vallejo A, Gutierrez M, Soriano V, Grupo Espanol para el Estudio del VIHydH-III 2004. HIV-2 and HTLV-I/II infections in Spain. Enferm Infecc. Microbiol. Clin. 22:177–182 (In Spanish.) 10.1157/13058026 [DOI] [PubMed] [Google Scholar]