Abstract
Accurate detection of vancomycin-resistant enterococci (VRE) is essential in preventing transmission in health care settings. Chromogenic media are widely used for screening VRE because of fast turnaround times (TAT) and high sensitivity. We report an outbreak of Enterococcus faecium bearing vanA yet susceptible to vancomycin (vancomycin-variable Enterococcus [VVE]). Between October 2009 to March 2011, clinical and screening specimens (n = 14,747) were screened for VRE using VRE-selective medium and/or PCR. VVE isolates were genotyped to determine relatedness. Plasmids from these isolates were characterized by sequencing. Overall, 52 VVE isolates were identified, comprising 15% of all VRE isolates identified. Isolates demonstrated growth on Brilliance VRE agar (Oxoid) at 24 h of incubation but did not grow on brain heart infusion agar with 6 μg/ml vancomycin (Oxoid) or bile esculin azide agar with 6 μg/ml vancomycin (Oxoid) and were susceptible to vancomycin. Genotyping of 20 randomly selected VVE isolates revealed that 15/20 were identical, while 5 were highly related. PCR of the VVE transposon confirmed the presence of vanHAXY gene cluster; however, vanS (sensor) and vanR (regulator) genes were absent. The outbreak was controlled through routine infection control measures. We report an emergence of a fit strain of E. faecium containing vanA yet susceptible to vancomycin. Whether this new strain represents VRE has yet to be determined; however, unique testing procedures are required for reliable identification of VVE.
INTRODUCTION
Vancomycin-resistant enterococcus (VRE) colonization and infection are increasingly common in both hospital and outpatient settings. Since the initial discovery of VRE during the 1980s in the United Kingdom and France, it has spread to many countries throughout the world (1, 2). In Canada, there has been a small but steady increase in rates of detection of VRE from 0.37 case per 1,000 patient admissions in 1999 to 1.32 in 2005 (3). VRE infection rates increased from 0.02 to 0.05 case per 1,000 admitted patients. Six different types of glycopeptide resistance (vanA, vanB, vanC, vanD, vanE, and vanG) have been described, of which the vanA- and vanB-mediated types are clinically significant in enterococci. Typically, vanA-mediated vancomycin resistance is found in Enterococcus faecium. VRE resistance in vanA-bearing isolates is mediated by the group of genes vanR, vanS, vanH, vanA, and vanX, which are usually carried on the Tn1546 transposon. The expression of these genes leads to replacement of the C-terminal d-Ala residue with d-Lac during cell wall synthesis, thus modifying the vancomycin-binding target. The transposon is often contained on plasmids, making it easy to transfer among enterococcal strains. Historically, there has been little sequence variation in this gene cluster (4).
Various studies have shown that infections caused by VRE lead to longer hospital stays, higher mortality, and significantly higher cost than infections caused by vancomycin-susceptible enterococci (5). Therefore, accurate and timely detection of VRE is imperative in preventing hospital outbreaks and providing effective antibiotic therapy in those with infections. Several methods have been developed over the years to detect and identify VRE in patient specimens. Selective chromogenic agars are commonly used in hospital microbiology laboratories, since this type of screening requires minimal specialized equipment and minimum interpretation. PCR testing has recently gained popularity given its high sensitivity and timely results; most assays detect the presence of vanA and vanB genes. The presence of vanA or vanB genes has been correlated with vancomycin resistance via traditional susceptibility methods. In this paper, we describe the identification and control of an institutional outbreak of a vancomycin-susceptible E. faecium strain containing the wild-type vanA gene. Because of the discrepant observations of vancomycin sensitivity and growth on VRE-selective medium, these organisms were termed vancomycin-variable enterococci (VVE).
MATERIALS AND METHODS
Outbreak.
Routine VRE screening among newly admitted patients according to established risk factors has been in place for many years at our institution (6). In addition, monthly point prevalence surveys have been carried out on all inpatient wards since 2000. Microbiology testing for VRE includes a combination of chromogenic agar and PCR. Since 1999, an in-house PCR assay targeting vanA, vanB, and ddl has been implemented to confirm VRE. PCR testing is carried out on all isolates demonstrating growth of enterococci on chromogenic agar. Of note, PCR testing on isolates recovered from sterile sites is performed regardless of vancomycin susceptibility testing.
In October 2009, PCR testing on an E. faecium isolate from a urine specimen confirmed the presence of vanA. The isolate grew on Brilliance agar but not bile esculin agar with azide. Vitek 2 reported this strain as vancomycin susceptible, and no growth was demonstrated on brain heart infusion agar with 6 mg/liter vancomycin (Oxoid) or bile esculin azide agar with 6 mg/liter vancomycin (Oxoid). Given the discordant findings of vancomycin sensitivity by an automated susceptibility system despite growth on Brilliance agar and the presence of the vanA gene, these isolates were conservatively documented as VRE. From 2009 onward, all isolates with this genotype/phenotype were recorded. A subset of these isolates was sent to the Public Health Ontario Laboratory (PHOL) for further testing and characterization. In addition, true VRE isolates were also identified and confirmed in samples from hospitalized patients.
Molecular characterization of VVE isolates.
At PHOL, the presence of vanA was confirmed using an in-house PCR assay adopted from published studies (7–10). In addition, PCR testing was also done to detect presence of vanB, vanC, vanD, vanE, and vanG genes. Molecular characterization of transposon Tn1546-like elements was characterized using previously published overlapping primers set (Fig. 1) (11). Isolates were screened for presence of insertion sequences (IS) IS1542, IS1216, IS1251, and IS1476 in intergenic regions by second sets of primers (12, 13). Briefly, PCR amplification was performed using Qiagen PCR master mix according to the manufacturer's recommendations. The PCR cycle was as follows: 1 cycle of 3 min at 94°C, 30 cycles at 94°C for 1 min, 54°C for 1 min, and 72°C for 2 min, followed by a final 10-min extension at 72°C. Following visualization of the PCR products using 1.0% agarose gel electrophoresis, the PCR products were sequenced using the BigDye terminator cycle sequencing kit v3.1 (Applied Biosystems, Foster City, CA, USA) and an ABI 3730XL analyzer.
FIG 1.
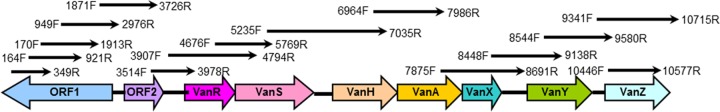
Genetic map and PCR amplification position of Tn1546. A schematic diagram of overlapping primers used to amplify and detect the transposon is shown.
For the extraction of plasmid DNA, a single colony from each isolate was used to inoculate 5 ml broth medium overnight at 37°C. Bacterial cells were lysed and plasmid DNA was purified using the QIAprep spin miniprep kit (Qiagen, Valencia, CA) as per the manufacturer's instructions. Purified plasmid DNA was prepared for sequencing using Roche's 454 GS FLX Titanium sequencing platform. Briefly, DNA was sheared into fragments of 400 to 600 bp, and DNA library fragments were immobilized onto streptavidin-coated beads, via the biotin moiety of one of the adaptors. The emulsion-based amplification (emPCR) of DNA was used to amplify entire library fragments. The prepared samples were then sequenced using Roche's 454 GS FLX system. Sequencing reads were assembled de novo using the 454 Newbler assembler (14). Major contigs were subjected to a BLAST search in NCBI to obtain the highest homology to published plasmid data.
New primers were designed, and Sanger sequencing using ABI BigDye 3.1 technology (Applied Biosystems, Germany) was performed to close the gaps. The Vector NTI software package (Invitrogen, CA, USA) was used for manipulation, assembly, and annotation of the DNA sequence. The annotation was then manually refined using GenBank BLAST outputs (15). IS elements were identified by using the IS Finder database. The G+C content of the plasmid was identified using the GC calculator (http://www.sciencebuddies.org/science-fair-projects/project_ideas/Genom_GC_Calculator.shtml).
PFGE and MLST.
VVE isolates were genotyped by pulsed-field gel electrophoresis (PFGE) using an established procedure (16). The banding pattern was analyzed using Bio-Numerics software and interpreted according to the criteria of Tenover et al. (17). In addition, these isolates were characterized by multilocus sequence typing (MLST) (18). Allelic numbers and profiles were determined using the online E. faecium MLST database (http://efaecium.mlst.net).
Nucleotide sequence accession number.
The entire DNA sequence of plasmid pF856 has been deposited in the NCBI GenBank database under accession number JQ663598.
RESULTS
Outbreak characterization.
An outbreak of VRE was first declared in October 2009 based on more than 3 cases of health care-acquired colonization over a 7-day period in a single 36-bed unit. Subsequently, monthly rates remained above baseline between October 2009 and December 2010, constituting an institutional outbreak. Between October 2009 and March 2011, VRE was identified in samples from 285 patients. Of these, VVE isolates were recovered from 44 (15%) patients. Thirty-six percent (16/44) of patients were identified during admission screening tests. Eight additional VVE isolates were from samples collected from patients at a separate local hospital. As such, 52 total cases were found. The average age of patients with VVE isolates was 72 years, and 54% were male. Eighty-five percent (44/52) of isolates were from screening swabs. A chart review of the six clinical samples recovered at our institution revealed that three specimens were from urine, two from skin wounds and one from a tongue swab. None of the 52 patients developed clinical infections requiring either vancomycin or VRE-targeted antibiotic therapy. Nosocomial cases of VVE were scattered throughout the hospital and were not clearly linked to a single ward or point source of exposure.
All VVE isolates grew on Brilliance agar but typically demonstrated weak or patchy growth. According to Vitek 2, all VVE isolates demonstrated a vancomycin MIC of ≤1. The Vitek 2 susceptibility profile for other antibiotics revealed ampicillin resistance (100%), ciprofloxacin resistance (100%), tetracycline resistance (98%), and streptomycin resistance (96%). At the reference laboratory, susceptibility testing using the reference agar dilution method was performed on a subset (n = 30) of isolates. All of these isolates displayed vancomycin MICs of 1 mg/liter (Table 1).
TABLE 1.
Microbiological characteristics of VVE isolates (n = 30) from the outbreaka
| Antibiotic | MIC (mg/liter) |
|---|---|
| Penicillin | ≥128 |
| Ampicillin | ≥16 |
| Erythromycin | ≥8 |
| Clindamycin | ≥4 |
| Tetracycline | ≥16 |
| Ciprofloxacin | ≥4 |
| Linezolid | ≤2 |
| Vancomycin | 1 |
All isolates belonged to sequence type 18.
Outbreak management.
An outbreak of VRE was declared between August 2009 and December 2010. Multiple approaches were used to curtail the outbreak. As per usual, all VRE-infected individuals, including patients colonized or infected with VVE isolates, were subjected to contact precautions, which included grouping of patients with the same conditions when private rooms were not available; enhanced infection prevention and control (IPAC) measures were undertaken, including the use of personal protective equipment, strategies to increase adherence to hand hygiene recommendations, and daily double terminal cleaning of all rooms according to the Ontario Provincial Infectious Diseases Advisory Committee (PIDAC) guidelines (19). All VRE contacts had screening swabs performed on days 1, 5, and 7 after exposure. As of July 2010, contacts of all VRE- and VVE-positive patients were subjected to contact precautions pending the results of screening. New alcohol sanitizers were placed on wards. Auditing and numerous in-services were performed to increase hand hygiene practices. Improved environmental cleaning was accomplished with use of standardized checklists, auditing, hiring of new cleaning staff, and education led by a physician for housekeeping staff regarding cleaning of frequently touched surfaces. Lastly, in October 2010, Virox-based cleaning was replaced with a hypochlorite-based product, Process Cleaning Solution (PCS) 1000. The outbreak was declared over in December 2010 when the rates of nosocomial VRE returned to baseline levels and a 2 week period elapsed without any new nosocomial cases. This success has been maintained, with no sustained outbreaks since that time.
Molecular characterization of VVE.
At the reference laboratory, 30 random VVE isolates were received for further characterization, including confirmation of the presence of the vanA gene. With the in-house PCR assay, all VVE isolates were confirmed as E. faecium containing vanA. These isolates were also tested for the presence of vanB, vanC, vanD, vanE, and vanG and were found to be negative. Comparison of vanA to the data available in NCBI's GenBank demonstrated significant homology (99 to 100%) to vanA isolated from different strains of E. faecium or E. faecalis. Amplification and sequencing of genes involved in vancomycin resistance using overlapping primers showed that all isolates carried Tn1546-like elements containing vanH, vanA, vanX, vanY, and vanZ genes. However, none of the isolates contained orf1, orf2, vanR, or vanS upstream of vanH, suggesting partial deletion of the Tn1546 element (Fig. 1). Instead of these genes, we found IS1251 upstream of vanH (Fig. 2). The translation of vanH, vanA, vanX, vanY, and vanZ sequences into amino acids revealed that all of these genes would produce functional proteins.
FIG 2.
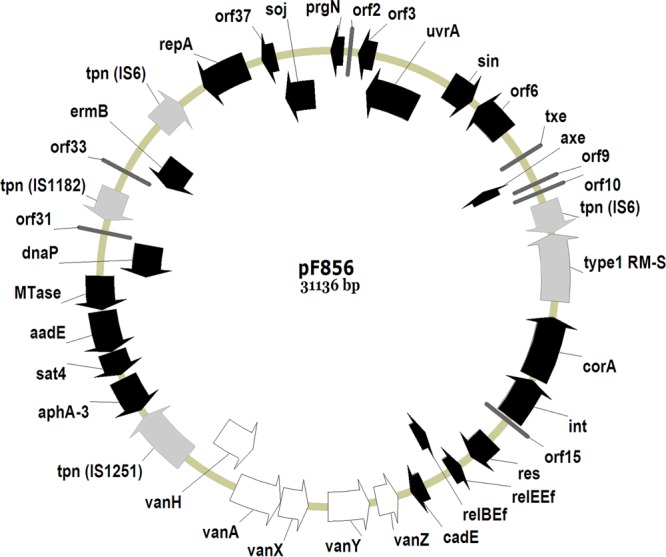
Genetic map of the full-length 31,136-bp plasmid, pF856. Coding regions are represented by arrows indicating the direction of transcription. Insertion sequence elements and type 1 RM-S are shown in gray, and Tn1546 transposon elements are in white. Putative ORFs are labeled according to the most significant homologue (pS177) in the public databases.
Structural analysis of the plasmid.
To better understand the structure of Tn1546 in these isolates, we extracted and sequenced the entire plasmid using Roche 454 GS FLX. The next-generation sequences showed that these strains contained multiple plasmids. For the purpose of this study, the plasmid containing Tn1546 was fully assembled. The entire nucleotide sequence of isolated plasmid pF856 was determined to be 31,136 bp, forming a circular plasmid with a G+C content of 34.9% (Fig. 2). Analysis of the plasmid sequence predicted 38 open reading frames (ORFs) with high similarity to genes encoding proteins of known functions. Sequence alignment showed that pF856 shared similarity with pS177 (accession no. HQ115078) at both the sequence and the gene levels. pS177 and pF856 shared 95% homology at the nucleotide level, with 29,607 bp of pF856 showing 99 to 100% identity with the plasmid pS177. The regions in common with pS177 included positions 1 to 5985, 6489 to 7299, 7300 to 11180, 28132 to 39032, 12859 to 19307, and 19344 to 20929 with homology of 99, 100, 99, 99, 99, and 99%, respectively.
Three types of IS elements were found within the plasmid genome, including IS6, IS1251, and IS1182. The most significant difference between pF856 and pS177 was the absence of a large DNA segment between aphA3 and vanH, including IS6, orf1, orf2, and the vanR and vanS elements of Tn1546. Interestingly, pF856 contained toxin-antitoxin (TA) systems comprising two ORFs, axe and txe, encoding a TA pair. The plasmid also contained the M subunit of the type I restriction-modification system (type I R-M). Type I R-M enzymes are multifunction, multisubunit enzymes consisting of three nonidentical subunits: R (for restriction), S (for DNA sequence specificity), and M (for methylation).
PFGE and MLST.
Twenty VVE isolates were randomly selected for PFGE and MLST testing. Of these, 15 were found to be identical using the criteria of Tenover et al. (Fig. 3) (17). The remaining five isolates were classified as closely related. The MLST profile showed that these isolates were identical to each other and belonged to sequence type 18 (ST18).
FIG 3.
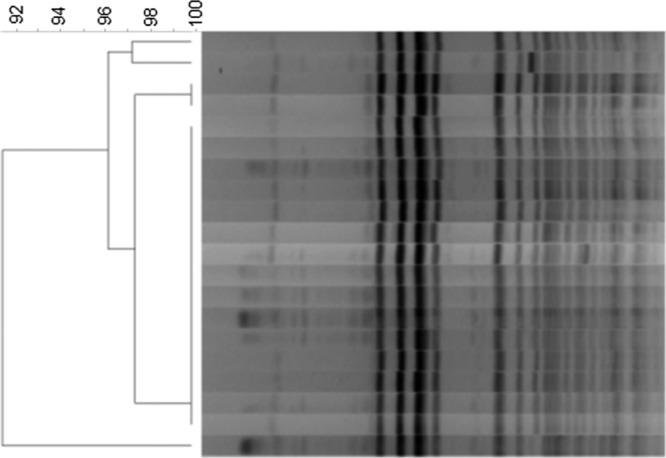
Pulse-field gel electrophoresis (PFGE) patterns of VVE strains. Chromosomal DNA was digested with SmaI.
DISCUSSION
The data show that within a VRE outbreak among hospitalized patients, a novel genotype/phenotype (vanA positive-vancomycin susceptible E. faecium or VVE) occurred. Outbreak investigation, including PFGE and MLST revealed a single strain circulating among hospitalized patients. These isolates grew on Brilliance agar chromogenic medium, which was surprising, as this selective medium was specifically developed to detect enterococci that are resistant to vancomycin. At this point, it is unclear what constituents in Brilliance agar allow growth, while other selective media do not.
Molecular analysis of genes associated with vancomycin resistance showed that vanA, vanH, vanX, vanY, and vanZ were present in these isolates with presumed functional proteins if expressed. However, these isolates did not contain vanR or vanS genes, which are essential to the expression of the vanHAX gene cluster. vanS acts as a glycopeptide sensor, whereas vanR transcriptionally regulates vanHAX gene cluster expression (20). In the presence of vancomycin, the VanR protein is phosphorylated by the VanS protein to induce transcription of the vanHAX gene cluster, resulting in a vancomycin resistance phenotype (20). In the absence of vanR and vanS genes, these isolates may not express vanH, vanA, or vanX and may remain vancomycin susceptible. However, in a previous study, a partial deletion of vanS in two E. faecium isolates resulted in one isolate being susceptible to vancomycin, whereas the other was resistant to vancomycin (21). Therefore, expression of vancomycin resistance may not be completely dependent on the presence of functional vanR and vanS genes. Sequencing of the whole plasmid containing Tn1546 revealed that this plasmid contained txe and axe. The genes encoding the Axe-Txe system were initially identified on pRUM (a multidrug resistance plasmid) in E. faecium (22, 23). The exact role of the Axe-Txe system is not fully elucidated, though it has been suggested that it is involved in maintaining plasmid in dividing cells. The presence of axe-txe genes on plasmids in these isolates may allow strains containing these plasmids to survive and disseminate in the environment as well as among patients.
The discovery of these isolates in hospital settings represents unique challenges to infection control practices and the diagnosis and management of infections caused by these isolates. Our data clearly show that these isolates are stable and able to be transmitted among hospitalized patients. Therefore, until it is known whether infections due to such isolates respond to vancomycin therapy and whether they can develop resistance when exposed to vancomycin, hospitals may wish to manage them as VRE infections from both a clinical and infection control perspective.
To identify hospital-associated resistant organisms, including methicillin-resistant Staphylococcus aureus (MRSA), VRE, and carbapenemase-producing Enterobacteriaceae (CPE), many laboratories are increasingly using molecular techniques, as they offer higher sensitivity and faster turnaround time than traditional culture methods. Generally, molecular techniques detect genes that are associated with resistance phenotypes. Among enterococcal species, the presence of vanA has been closely correlated with the vancomycin resistance phenotype. However, our data showed that the presence of vanA alone did not correlate with vancomycin resistance among these isolates, and thus, molecular testing on these isolates would have identified these isolates as VRE. Therefore, we recommend that laboratories that use molecular techniques to detect VRE isolates should confirm VRE organisms by susceptibility testing methods. Our data also showed that many of these isolates grew on Brilliance agar selective medium, emphasizing the need to confirm vancomycin resistance in enterococci growing on this medium using different methods.
It is unclear whether vancomycin can still be used to treat infection caused by VVE. There is always a possibility that vancomycin pressure may select for a promoter that may induce vanH, vanA, and vanX expression, resulting in vancomycin resistance. During the outbreak, patients identified with these isolates in clinical specimens were not treated with vancomycin, despite the fact that the isolates were susceptible according to in vitro testing. As such, the clinical utility of vancomycin in these cases remains unknown. However, as described in the accompanying paper (24), a recent observation in a 69-year-old patient colonized with VVE showed that subsequent vancomycin exposure resulted in development of vancomycin resistance, with a MIC of 256 μg/ml in vivo. Further research involving both in vitro and in vivo studies with animal models are needed to determine whether vancomycin exposure induces resistance in these strains. If resistance is induced, further studies will be needed to determine how these strains become vancomycin resistant in the absence of the vanR and vanS regulatory system.
In summary, we report the detection of vanA-positive vancomycin-susceptible E. faecium in both clinical and screening specimens among hospitalized patients. We demonstrate that a strain with this unique genotype/phenotype combination is able to be transmitted among hospitalized patients. Further studies are required to fully understand these isolates.
ACKNOWLEDGMENT
The funding for this work was from Public Health Ontario Laboratory.
Footnotes
Published ahead of print 12 February 2014
REFERENCES
- 1.Woodford N. 2001. Epidemiology of the genetic elements responsible for acquired glycopeptide resistance in enterococci. Microb. Drug Resist. 7:229–236. 10.1089/10766290152652774 [DOI] [PubMed] [Google Scholar]
- 2.Leclercq R, Derlot E, Duval J, Courvalin P. 1988. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 319:157–161. 10.1056/NEJM198807213190307 [DOI] [PubMed] [Google Scholar]
- 3.Ofner-Agostini M, Johnston BL, Simor AE, Embil J, Matlow A, Mulvey M, Ormiston D, Conly J, Canadian Nosocomial Infection Surveillance Program 2008. Vancomycin-resistant enterococci in Canada: results from the Canadian nosocomial infection surveillance program, 1999–2005. Infect. Control Hosp. Epidemiol. 29:271–274. 10.1086/528812 [DOI] [PubMed] [Google Scholar]
- 4.Arthur M, Courvalin P. 1993. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 37:1563–1571. 10.1128/AAC.37.8.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. 2002. Health and economic outcomes of vancomycin-resistant enterococci. Arch. Intern. Med. 162:2223–2228. 10.1001/archinte.162.19.2223 [DOI] [PubMed] [Google Scholar]
- 6.Provincial Infectious Diseases Advisory Committee. 2013. Screening, testing and surveillance for antibiotic-resistant organisms (AROs)in all health care settings. http://www.publichealthontario.ca/en/eRepository/PIDAC-IPC_Annex_A_Screening_Testing_Surveillance_AROs_2013.pdf Accessed 3 December 2013
- 7.Elsayed S, Hamilton N, Boyd D, Mulvey M. 2001. Improved primer design for multiplex PCR analysis of vancomycin-resistant Enterococcus spp. J. Clin. Microbiol. 39:2367–2368. 10.1128/JCM.39.6.2367-2368.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd DA, Du T, Hizon R, Kaplen B, Murphy T, Tyler S, Brown S, Jamieson F, Weiss K, Mulvey MR. 2006. VanG-type vancomycin-resistant Enterococcus faecalis strains isolated in Canada. Antimicrob. Agents Chemother. 50:2217–2221. 10.1128/AAC.01541-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd DA, Kibsey P, Roscoe D, Mulvey MR. 2004. Enterococcus faecium N03-0072 carries a new VanD-type vancomycin resistance determinant: characterization of the VanD5 operon. J. Antimicrob. Chemother. 54:680–683. 10.1093/jac/dkh391 [DOI] [PubMed] [Google Scholar]
- 10.Boyd DA, Cabral T, Van Caeseele P, Wylie J, Mulvey MR. 2002. Molecular characterization of the vanE gene cluster in vancomycin-resistant Enterococcus faecalis N00-410 isolated in Canada. Antimicrob. Agents Chemother. 46:1977–1979. 10.1128/AAC.46.6.1977-1979.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huh JY, Lee WG, Lee K, Shin WS, Yoo JH. 2004. Distribution of insertion sequences associated with Tn1546-like elements among Enterococcus faecium isolates from patients in Korea. J. Clin. Microbiol. 42:1897–1902. 10.1128/JCM.42.5.1897-1902.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willems RJ, Top J, van den Braak N, van Belkum A, Mevius DJ, Hendriks G, van Santen-Verheuvel M, van Embden JD. 1999. Molecular diversity and evolutionary relationships of Tn1546-like elements in enterococci from humans and animals. Antimicrob. Agents Chemother. 43:483–491. 10.1093/jac/43.4.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WG, Huh JY, Cho SR, Lim YA. 2004. Reduction in glycopeptide resistance in vancomycin-resistant enterococci as a result of vanA cluster rearrangements. Antimicrob. Agents Chemother. 48:1379–1381. 10.1128/AAC.48.4.1379-1381.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380. 10.1038/nature03959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 16.Murray BE, Singh KV, Heath JD, Sharma BR, Weinstock GM. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homan WL, Tribe D, Poznanski S, Li M, Hogg G, Spalburg E, Van Embden JD, Willems RJ. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963–1971. 10.1128/JCM.40.6.1963-1971.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provincial Infectious Diseases Advisory Committee. 2012. Routine practices and additional precautions in all health care settings, 3rd ed. http://www.publichealthontario.ca/en/eRepository/RPAP_All_HealthCare_Settings_Eng2012.pdf Accessed 3 December 2013
- 20.Hong HJ, Hutchings MI, Buttner MJ, Biotechnology Biological Sciences Research Council UK 2008. Vancomycin resistance VanS/VanR two-component systems. Adv. Exp. Med. Biol. 631:200–213. 10.1007/978-0-387-78885-2_14 [DOI] [PubMed] [Google Scholar]
- 21.Choi HJ, Nam D, Peck KR, Song JH, Shin D, Ko KS. 2011. Loss of vancomycin resistance not completely dependent on the Tn1546 element in Enterococcus faecium isolates. Diagn. Microbiol. Infect. Dis. 69:105–110. 10.1016/j.diagmicrobio.2010.08.030 [DOI] [PubMed] [Google Scholar]
- 22.Grady R, Hayes F. 2003. Axe-Txe, a broad-spectrum proteic toxin-antitoxin system specified by a multidrug-resistant, clinical isolate of Enterococcus faecium. Mol. Microbiol. 47:1419–1432. 10.1046/j.1365-2958.2003.03387.x [DOI] [PubMed] [Google Scholar]
- 23.Halvorsen EM, Williams JJ, Bhimani AJ, Billings EA, Hergenrother PJ. 2011. Txe, an endoribonuclease of the enterococcal Axe-Txe toxin-antitoxin system, cleaves mRNA and inhibits protein synthesis. Microbiology 157:387–397. 10.1099/mic.0.045492-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coburn B, Low DE, Patel SN, Pouteanen SM, Shahinas D, Eshaghi A, Willey BM, McGeer A. 2014. Vancomycin-variable Enterococcus faecium: in vivo emergence of vancomycin resistance in a vancomycin-susceptible isolate. J. Clin. Microbiol. 52:1766–1767. 10.1128/JCM.03579-13 [DOI] [PMC free article] [PubMed] [Google Scholar]


