Abstract
A urinary tract coinfection, caused by Encephalitozoon cuniculi genotype II and Enterocytozoon bieneusi genotype D, was identified in an HIV-seronegative renal transplant recipient kept under lifelong immunosuppression. To our knowledge, this is the first report describing concurrent infection with these two microsporidia species in organ transplant recipients.
CASE REPORT
A 39-year-old male patient with a history of arterial hypertension, hepatitis B, type 1 diabetes mellitus, and renal failure, caused by diabetic nephropathy diagnosed in 2001, was subjected to peritoneal dialysis starting in January 2003, but due to recurrent peritonitis the patient started hemodialysis the year after. The patient underwent renal transplantation in March 2005 at the age of 31 years. The postoperative period was complicated by delayed graft function (the patient required hemodialysis for 10 days). The initial immunosuppressive regimen consisted of prednisone (20 mg/day), mycophenolate mofetil (MMF; 2.0 g/day), and tacrolimus (16 mg/day). An acute rejection episode (12.7 months after transplantation) was treated with three 0.5-g methylprednisolone pulses. Due to the poor tolerability of MMF causing diarrhea despite the use of low doses, mycophenolate sodium (MPS; 1,080 mg/day) was included starting in February 2006.
The patient was admitted to the nephrology unit in April 2013 because of fever, diarrhea, nausea, and a decrease in urine output. Immunosuppressive therapy before admission consisted of prednisone (7.5 mg/day), MPS (720 mg/day), and tacrolimus (1.5 mg/day). Laboratory examinations showed increased levels of serum creatinine (2.1 mg/dl) and C-reactive protein (CRP; 21.7 mg/liter), slightly elevated aspartate aminotransferase (71 U/liter), and gamma-glutamyl transferase (78 U/liter). Apart from Candida albicans colonies observed in stool samples, the tests for bacteria, viruses, and Clostridium difficile toxin were negative. The therapy of diarrhea included fluid infusions, ciprofloxacin intravenously (800 mg/day), and nystatin (1,500,000 U/day). MPS was withheld for 2 days, and then the dose was reduced to 360 mg daily. The patient was discharged afebrile, without clinical symptoms, with improvement in renal function (serum creatinine level of 1.94 mg/dl) and normalized CRP concentration (4.78 mg/liter).
During admission, both stool and urine specimens were examined for the presence of microsporidia by microscopic and molecular methods. Microscopic analysis of Uvitex 2B and modified trichrome-stained smears (1) showed the presence of microsporidia spores in urine sediment (Fig. 1). Genus-specific nested-PCR protocols amplifying the internal transcribed spacer (ITS) region of Encephalitozoon spp. and Enterocytozoon bieneusi (2, 3) with subsequent genotyping revealed the presence of Encephalitozoon cuniculi genotype II and E. bieneusi genotype D in urine sediment. Stool examination was negative for microsporidia.
FIG 1.
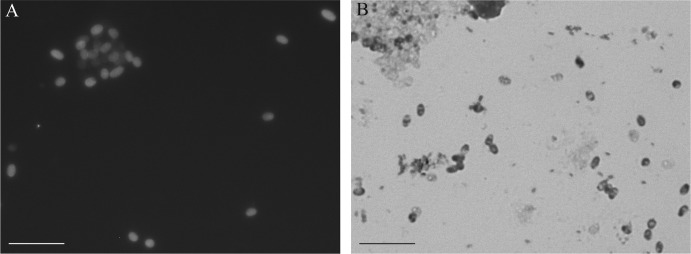
Microsporidial spores detected in urine sediments after Uvitex 2B (A) and modified trichrome staining (B). Scale bars, 5 μm.
During the ambulatory control in August 2013, the patient was in good general condition, without symptoms of gastrointestinal or urinary tract infection. Serum creatinine level was 1.94 mg/dl. Control molecular examination of urine sediment showed the presence of E. bieneusi genotype D but not E. cuniculi genotype II DNA. Molecular examination of stool showed no microsporidial DNA. However, serological examination (4) revealed specific anti-E. cuniculi antibodies. Because of the inability to culture E. bieneusi and the lack of a serologic assay, serum was not tested for specific antibodies against this species.
Microsporidia are obligate, intracellular, opportunistic parasites infecting a wide range of invertebrate and vertebrate hosts, including humans. Of 14 species belonging to 8 genera known to infect humans, Enterocytozoon bieneusi, Encephalitozoon intestinalis, E. cuniculi, and Encephalitozoon hellem are the most common ones. Interest in this group of parasites increased with the AIDS pandemic onset in 1985, after microsporidiosis was recognized as a cause of life-threatening disease in HIV-infected patients (5). Recently, an increasing number of microsporidiosis cases in immunocompromised individuals other than HIV-infected patients, such as bone marrow and solid organ transplant recipients, has been reported (6). In addition, recent studies have shown high prevalence of unapparent microsporidial infection within the human population, including in otherwise healthy immunocompetent individuals (7).
Both Encephalitozoon spp. and Enterocytozoon bieneusi are ubiquitous pathogens primarily infecting enterocytes of their host; hence, the common clinical manifestations are chronic diarrhea with weight loss, fatigue, and fever. In immunocompromised individuals, chronic diarrhea may lead to malabsorption and wasting (6, 8). While E. bieneusi infection is considered to occur mainly in the gastrointestinal tract and biliary tree, species of the genus Encephalitozoon have been shown to disseminate, causing central nervous system, ocular, respiratory, and urinary tract infections (5, 6, 8, 9). Because of various susceptibilities of these pathogens for drugs, infections caused by Encephalitozoon spp. and E. bieneusi require different medical treatment: albendazole and fumagillin, respectively (5).
In the present study, we detected for the first time microsporidial infection of the urinary tract with both E. cuniculi genotype II and E. bieneusi genotype D in a renal transplant recipient. While Encephalitozoon cuniculi or E. intestinalis was found during disseminated microsporidiosis in urine samples in renal transplant recipients (10, 11), the concurrent presence of E. cuniculi and E. bieneusi in the urinary tract has been previously demonstrated only in immunocompetent humans under long-term observation without any clinical signs (7). Enterocytozoon bieneusi spores were also detected in the urine sample of an HIV-seronegative patient (12). In addition, disseminated infection caused by E. cuniculi accompanied by an intestinal coinfection with E. bieneusi was found in an HIV-positive patient (13). Unlike in the study by Sak et al. (7), we detected microsporidia only in urine sediments but not in stool samples.
Although organ transplant recipients represent a group of patients under the highest risk of opportunistic pathogen infection because of their immune system deficiency as a result of lifelong immunosuppression, the actual source of infection remains speculative. Microsporidia are released into the environment through infected individuals; they exist in water, soil, and food and may be infective to subsequent generations of hosts (5, 6). Diagnosis of microsporidial infections is not actively sought, and the lack of information has generated a circular pattern of ignorance: with no information concerning the prevalence, these parasites are not recognized as a problem (14). However, since organ donors are not routinely tested for microsporidial infection, the organ transplant recipient could become infected via a microsporidium-positive graft. Furthermore, due to decreased recipient immunity, microsporidia could reactivate (9). The infection could also be responsible for decreased function of graft or cause pathologies without an identified causative agent, such as in the patient in our study. Although the dehydration due to diarrhea might be the probable cause of the worsening graft function in our patient, microsporidial infection could at least partially strengthen the renal failure.
In the present study, the immunosuppressive treatment dose was diminished, implying that symptom recovery could be associated with the improvement of immune function. It is in accordance with results obtained in E. bieneusi-infected transplant recipients, where reduction of immunosuppressive treatment by MMF led to recovery from microsporidial infection (15). A similar effect was reported in cases of HIV-infected patients, who cleared the microsporidiosis after immune reconstitution in the absence of specific antiparasitic treatment (16). The same results were obtained experimentally using murine model hosts (9). Taking into account that only partially effective chemotherapy against Encephalitozoon spp. or E. bieneusi with serious side effects can be utilized (17), reducing the level of immunosuppression seems to be the most efficient treatment strategy.
Our findings highlight the necessity of consideration of microsporidia infection in both renal transplant donors and recipients, especially in the case of unspecified renal infections. Moreover, determination of microsporidia species is very important due to their different treatment. Therefore, treatment of microsporidiosis caused by coinfection may impose more difficulties.
ACKNOWLEDGMENTS
We are grateful to Maria Boratyńska from the Department and Clinic of Nephrology and Transplantation Medicine, Wroclaw Medical University, for her willingness to cooperate.
This study was supported by a grant of the National Science Centre, Poland (DEC-2012/05/D/NZ6/00615). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This study is approved by the Human Research Ethics Committee of Wroclaw Medical University according to agreement no. KB-549/2012.
We declare that we have no relevant financial interests.
Footnotes
Published ahead of print 12 February 2014
REFERENCES
- 1.Didier ES, Orenstein JM, Aldras A, Bertucci D, Rogers LB, Janney FA. 1995. Comparison of three staining methods for detecting microsporidia in fluids. J. Clin. Microbiol. 33:3138–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katzwinkel-Wladarsch S, Lieb M, Helse W, Löscher T, Rinder H. 1996. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop. Med. Int. Health 1:373–378. 10.1046/j.1365-3156.1996.d01-51.x [DOI] [PubMed] [Google Scholar]
- 3.Buckholt MA, Lee JH, Tzipori S. 2002. Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl. Environ. Microbiol. 68:2595–2599. 10.1128/AEM.68.5.2595-2599.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagnerova P, Sak B, Květoňová D, Maršálek M, Langrová I, Kváč M. 2013. Humoral immune response and spreading of Encephalitozoon cuniculi infection in experimentally infected ponies. Vet. Parasitol. 197:1–6. 10.1016/j.vetpar.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 5.Didier ES, Weiss LM. 2006. Microsporidiosis: current status. Curr. Opin. Infect. Dis. 19:485–492. 10.1097/01.qco.0000244055.46382.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franzen C, Müller A. 2001. Microsporidiosis: human diseases and diagnosis. Microbes Infect. 3:389–400. 10.1016/S1286-4579(01)01395-8 [DOI] [PubMed] [Google Scholar]
- 7.Sak B, Kváč M, Kučerová Z, Květoňová D, Saková K. 2011. Latent microsporidial infection in immunocompetent individuals—a longitudinal study. PLoS Negl. Trop. Dis. 5:e1162. 10.1371/journal.pntd.0001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matos O, Lobo ML, Xiao L. 2012. Epidemiology of Enterocytozoon bieneusi infection in humans. J. Parasitol. Res. 10.1155/2012/981424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotková M, Sak B, Květoňová D, Kváč M. 2013. Latent microsporidiosis caused by Encephalitozoon cuniculi in immunocompetent hosts: a murine model demonstrating the ineffectiveness of the immune system and treatment with albendazole. PLoS One 8:e60941. 10.1371/journal.pone.0060941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talabani H, Sarfati C, Pillebout E, van Gool T, Derouin F, Menotti J. 2010. Disseminated infection with a new genovar of Encephalitozoon cuniculi in a renal transplant recipient. J. Clin. Microbiol. 48:2651–2653. 10.1128/JCM.02539-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagpal A, Pritt BS, Lorenz EC, Amer H, Nasr SH, Cornell LD, Iqbal S, Wilhelm MP. 2013. Disseminated microsporidiosis in a renal transplant recipient: case report and review of the literature. Transpl. Infect. Dis. 10.1111/tid.12119 [DOI] [PubMed] [Google Scholar]
- 12.Abreu-Acosta N, Lorenzo-Morales J, Leal-Guio Y, Coronado-Alvarez N, Foronda P, Alcoba-Florez J, Izquierdo F, Batista-Díaz N, Del Aguila C, Valladares B. 2005. Enterocytozoon bieneusi (microsporidia) in clinical samples from immunocompetent individuals in Tenerife, Canary Islands, Spain. Trans. R. Soc. Trop. Med. Hyg. 99:848–855. 10.1016/j.trstmh.2005.05.010 [DOI] [PubMed] [Google Scholar]
- 13.Weitzel T, Wolff M, Dabanch J, Levy I, Schmetz C, Visvesvara GS, Sobottka I. 2001. Dual microsporidial infection with Encephalitozoon cuniculi and Enterocytozoon bieneusi in an HIV-positive patient. Infection 29:237–239. 10.1007/s15010-001-1164-0 [DOI] [PubMed] [Google Scholar]
- 14.Kučerová Z, Sokolova OI, Demyanov AV, Kváč M, Sak B, Květonová D, Secor WE. 2011. Microsporidiosis and cryptosporidiosis in HIV/AIDS patients in St. Petersburg, Russia: serological identification of microsporidia and Cryptosporidium parvum in sera samples from HIV/AIDS patients. AIDS Res. Hum. Retroviruses 27:13–15. 10.1089/aid.2010.0132 [DOI] [PubMed] [Google Scholar]
- 15.Galván AL, Sánchez AM, Valentín MA, Henriques-Gil N, Izquierdo F, Fenoy S, del Aguila C. 2011. First cases of microsporidiosis in transplant recipients in Spain and review of the literature. J. Clin. Microbiol. 49:1301–1306. 10.1128/JCM.01833-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr A, Marriott D, Field A, Vasak E, Cooper DA. 1998. Treatment of HIV-1-associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet 351:256–261. 10.1016/S0140-6736(97)07529-6 [DOI] [PubMed] [Google Scholar]
- 17.Audemard A, Le Bellec ML, Carluer L, Dargère S, Verdon R, Castrale C, Lobbedez T, Hurault de Ligny B. 2012. Fumagillin-induced aseptic meningoencephalitis in a kidney transplant recipient with microsporidiosis. Transpl. Infect. Dis. 14:E147–E149. 10.1111/tid.12010 [DOI] [PubMed] [Google Scholar]


