Abstract
We determined the capsular polysaccharide (CPS) type of 600 group B Streptococcus (GBS) (also known as Streptococcus agalactiae) strains recovered from patients with invasive infections in the greater Toronto area, Canada, between 2009 and 2012. GBS strains of CPS type III were the most prevalent among infants (44% in those with early-onset disease, 75% in those with late-onset disease), while type V strains were most frequently isolated from adult patients (26% in patients ≥19 years old). We next investigated the presence in our collection of GBS strains belonging to the hypervirulent multilocus sequence typing clonal complex 17 (CC17). We used a PCR test described as specific for the detection of CC17 strains, which targets the gene encoding the major virulence factor HvgA. We identified 91 hvgA-positive strains; of these, 88 were CPS type III, 2 were CPS type IV, and 1 was CPS type V. Using whole-genome sequencing, we showed that the two hvgA-positive CPS type IV strains are CC17 strains which underwent capsular switching. However, sequence analysis revealed that the hvgA-positive CPS type V strain does not belong to CC17 but instead is a bona fide CC1 strain which acquired hvgA, probably by recombination from a CC17 donor. Our findings underline the importance of recombination in GBS pathogenesis and caution against the use of single-gene-based PCR tests to detect CC17 GBS strains.
INTRODUCTION
Group B Streptococcus (GBS) (also known as Streptococcus agalactiae) is the causative agent of neonatal meningitis and pneumonia and a leading cause of neonatal sepsis (1). In infants, GBS infection presents as two distinct clinical syndromes: early-onset disease (EOD) characterized by onset of infection during the first week of life (0 to 6 days) and late-onset disease (LOD) characterized by onset of symptoms at up to 3 months of age (age 7 to 89 days). The use of intrapartum antibiotic prophylaxis for pregnant women at risk of GBS infection has been successful at reducing the incidence of EOD (1) but not the incidence of LOD (2, 3). It has been shown that up to 10% of neonatal GBS infections are lethal, and 25 to 35% of surviving infants with meningitis experience permanent neurological sequelae (4). Although disease risk is highest during the first 3 months of life, GBS also causes significant morbidity among older children as well as adults, especially in the immunocompromised and the elderly (5).
Typing information is important to understand the epidemiology of GBS infections. The capsular polysaccharide (CPS) is an important virulence factor and the basis of GBS serotyping. Ten CPS types have been described to date (Ia, Ib, and II-IX). All genes required for CPS synthesis are found in a single cps locus, which contains a highly variable serotype-determining region (cpsG-cpsK) flanked by other CPS biosynthesis genes which are conserved among the different serotypes (6). PCR typing schemes have been developed which target the serotype-determining region of the cps locus and permit the prediction of GBS serotypes. A substantial proportion of EOD and the majority of LOD in neonates are associated with CPS type III, while, among adults, CPS types V, Ia, III, and Ib are all recognized as significant causes of invasive disease (7–14).
GBS isolates can also be typed using multilocus sequence typing (MLST). Strains belonging to clonal complex (CC)17 GBS have been characterized as hypervirulent and appear to have an enhanced ability to invade the central nervous system of neonates (15–18). Studies have suggested that a large majority of neonatal invasive disease (and almost all cases of meningitis) are caused by this hypervirulent clone (3, 17, 19, 20). CC17 GBS strains have been described as a genetically homogenous group and, until recently, all described CC17 strains have been found to be CPS type III (7). In vivo experiments have shown that HvgA, a surface-anchored adhesin, enables persistent colonization by CC17 GBS strains and contributes to meningitis in neonates (21). This surface adhesin is thought to be specific for CC17 strains and thus PCR amplification of hvgA has been used to assign GBS strains to the CC17 (17).
In this study, we determined the serotype distribution of 600 GBS strains isolated from patients of all ages with invasive disease in the greater Toronto area, Canada. We also investigated the presence of hypervirulent CC17 GBS strains in our collection using hvgA PCR. By using whole-genome sequencing, we discovered capsular switching to CPS type IV in CC17 strains as well as acquisition of the major virulence factor-encoding gene hvgA by non-CC17 strains.
MATERIALS AND METHODS
Bacterial strains.
Six hundred GBS strains were included in this study (see Table S1 in the supplemental material). The strains were collected between 2009 and 2012 by the Toronto Invasive Bacterial Diseases Network, a population-based surveillance system for invasive bacterial diseases in Metropolitan Toronto and the Peel region, Ontario, Canada (total population under surveillance estimated at 5.5 million in 2011, with an estimated annual number of live births of 58,000). The laboratory-based surveillance involves all hospitals (n = 28) providing care to and all laboratories processing sterile site cultures (n = 25) from residents of the population area; laboratory personnel submit all GBS isolates from sterile sites to the central study laboratory. The collection used here represents all available GBS isolated by the surveillance program during the time period and includes samples isolated from blood, cerebrospinal fluid, tissue, and other normally sterile sites (Table 1). Strains were cultured on Columbia agar plates containing 5% sheep blood and grown at 37°C with 5% CO2. Liquid cultures were grown in Todd-Hewitt broth supplemented with 0.2% yeast extract. All isolates were confirmed to be GBS by standard methodology. In addition, PCR amplification of a 234-bp region of the monocopy regulatory gene dltR, which is specific to GBS (22), was used to confirm species identification. Primers (dltRS and dltRAS) (Table 2) and amplification conditions were those previously described (17). DNA was prepared from overnight GBS cultures using the QIAamp DNA minikit (Qiagen, Toronto, ON, Canada) following the manufacturer's protocol for Gram-positive organisms.
TABLE 1.
Distribution of GBS by isolate source and age of disease onseta
| Culture source | No. of GBS isolates from: |
|||||
|---|---|---|---|---|---|---|
| Neonates |
Children | Adults | Older adults | Total | ||
| EOD | LOD | |||||
| Blood | 31 | 49 | 13 | 140 | 277 | 510 |
| Cerebrospinal fluid | 1 | 8 | 1 | 1 | 0 | 11 |
| Synovial fluid | 0 | 0 | 1 | 15 | 12 | 28 |
| Soft tissue | 2 | 1 | 1 | 5 | 9 | 18 |
| Bone | 0 | 0 | 0 | 3 | 0 | 3 |
| Otherb | 0 | 1 | 0 | 19 | 10 | 30 |
EOD, birth to 6 days; LOD, 7 to 89 days; children, 90 days to 18 years; adults, 19 to 59 years; older adults, ≥60 years.
Pleural fluid, peritoneal fluid, and other normally sterile body sites.
TABLE 2.
Oligonucleotide primers used in this study
| Primer name | Sequence (5′ to 3′) | Gene target(s) | Amplicon size(s) (bp) | Reference |
|---|---|---|---|---|
| dltRS | TTGACAGGTCTCTATGATTTAGTC | dltR | 234 | 17 |
| dltRAS | GTCTGGTTCTCAGCCTAATTC | dltR | 17 | |
| Ia-F | GGTCAGACTGGATTAATGGTATGC | cps1aH | 521 and 1,826 | 25 |
| Ia-R | GTAGAAATAGCCTATATACGTTGAATGC | cps1aH | 25 | |
| Ib-F | TAAACGAGAATGGAATATCACAAACC | cps1bJ | 770 | 25 |
| Ib-R | GAATTAACTTCAATCCCTAAACAATATCG | cps1bK | 25 | |
| II-F | GCTTCAGTAAGTATTGTAAGACGATAG | cps2K | 397 | 25 |
| II-R | TTCTCTAGGAAATCAAATAATTCTATAGGG | cps2K | 25 | |
| III-F | TCCGTACTACAACAGACTCATCC | cps1a/2/3I | 1,826 | 25 |
| III-R | AGTAACCGTCCATACATTCTATAAGC | cps1a/2/3J | 25 | |
| IV-F | GGTGGTAATCCTAAGAGTGAACTGT | cps4N | 578 | 25 |
| IV-R | CCTCCCCAATTTCGTCCATAATGGT | cps4N | 25 | |
| V-F | GAGGCCAATCAGTTGCACGTAA | cps5O | 701 | 25 |
| V-R | AACCTTCTCCTTCACACTAATCCT | cps5O | 25 | |
| VI-F | GGACTTGAGATGGCAGAAGGTGAA | cps6I | 487 | 25 |
| VI-R | CTGTCGGACTATCCTGATGAATCTC | cps6I | 25 | |
| VII-F | CCTGGAGAGAACAATGTCCAGAT | cps7M | 371 | 25 |
| VII-R | GCTGGTCGTGATTTCTACACA | cps7M | 25 | |
| VIII-F | AGGTCAACCACTATATAGCGA | cps8J | 282 | 25 |
| VIII-R | TCTTCAAATTCCGCTGACTT | cps8J | 25 | |
| cpsI-IA-6-7-F | GAATTGATAACTTTTGTGGATTGCGATGA | cpsI | 179 | 26 |
| cpsI-7-R | TGTCGCTTCCACACTGAGTGTTGA | cpsI | 26 | |
| cpsI-7-9-F | CTGTAATTGGAGGAATGTGGATCG | cpsI | 229 | 26 |
| cpsI-9-R | AATCATCTTCATAATTTATCTCCCATT | cpsI | 26 | |
| ST-17S | ATACAAATTCTGCTGACTACCG | hvgA | 210 | 17 |
| ST-17AS | TTAAATCCTTCCTGACCATTCC | hvgA | 17 |
CPS typing.
Serological determination of the CPS types was performed by latex agglutination (SSI Diagnostica; Statens Serum Institute, Copenhagen, Denmark) as previously described (23). CPS types were also determined by PCR amplification of CPS type-specific regions of the cps locus as recommended by Yao et al. (24). Briefly, we used two previously described multiplex assays which allow for discrimination of all GBS CPS types, with the exception of CPS types VII and IX (25). These two CPS types were next resolved using primer pairs cpsI-Ia-6-7-F and cpsI-7-R and cpsI-7-9-F and cpsI-9-R, which amplify target regions specific to CPS types VII and IX, respectively (26). Primers are listed in Table 2. PCR conditions were as described previously (25, 26), with the exception that we used KOD Hot Start DNA polymerase (EMD Millipore, Billerica, MA). Expected sizes of the different CPS PCR amplicons are provided in Table 2.
Amplification of hvgA.
A discrete 210-bp genetic region encoding the S10 domain of the surface protein HvgA was amplified by PCR using previously described primers ST-17S and ST-17AS (Table 2) and amplification conditions (17). This genetic region has been described as being present in CC17 GBS strains only (17).
Whole-genome sequencing and bioinformatics analysis.
Genomic libraries were prepared using Nextera XT kits (Illumina, San Diego, CA) and sequenced as paired-end reads in an Illumina MiSeq instrument (Illumina). Parsing of the multiplexed sequencing reads, and removal of barcode information was done using MiSeq onboard software. Given that there are no closed genomes available for GBS strains of MLST sequence type (ST) 17 and ST1, to facilitate data analysis we first built reference pseudochromosomes for strains of these two STs using the unfinished genome sequences of strains GB00112 (ST17, CPS type III, GenBank accession number NZ_AKXO00000000) (27) and CJB111 (ST1, CPS type V, GenBank accession number NZ_AAJQ00000000) (28). Briefly, for each strain, we ordered all contigs against the complete genome sequence of strain NEM316 (GenBank accession number NC_004368) (29) using Mauve (30), then concatenated the ordered contigs using the sequence NNNNNCATTCCATTCATTAATTAATTAATGAATGAATGNNNNN as a separator. Illumina short reads were aligned to the reference pseudochromosomes using the Mosaik assembler (https://code.google.com/p/mosaik-aligner/). Polymorphisms were called against the reference pseudochromosomes using a variant ascertainment algorithm (VAAL) (31). The A5 pipeline was used for de novo assembly of newly sequenced GBS strains (32). Contigs >100 nucleotides in length were then used to search the NCBI nonredundant database using BLAST (33). Genome visualizations were created using the BLAST Ring Image Generator (BRIG) (34) and edited using Adobe Illustrator. MLST STs were determined directly from the Illumina short reads using SRST2 (https://github.com/katholt/srst2) (35).
RESULTS AND DISCUSSION
Serotype distribution of invasive GBS.
Our collection comprised 600 GBS isolates collected from patients with invasive infections between 2009 and 2012 in the greater Toronto area (average incidence of 0.36 per 10,000). The vast majority of the strains were isolated from blood (510 isolates, 85%). Twenty-eight GBS isolates (4.7%) were isolated from synovial fluid, 18 (3%) from soft tissue infections, 11 (1.8%) from cerebrospinal fluid, and 3 (0.5%) from bone. Thirty strains were isolated from other normally sterile sites, including pleural fluid (Table 1). In total, 93 GBS isolates (15.5%) were associated with neonatal infection: 34 (5.7%) from infants with EOD (average incidence of 0.19 per 1,000 live births) and 59 (9.8%) from infants with LOD. Sixteen GBS isolates (2.7%) were collected from children 3 months to 18 years old, while 183 (30.5%) GBS isolates were cultured from adults 19 to 59 years old. The remaining 308 GBS strains (51.3%) originated from older adults, defined here as adults 60 years of age and older (Fig. 1).
FIG 1.
Distribution of the different GBS serogroups by age. CPS type III GBS strains predominated among cases of neonatal disease (EOD, early-onset disease [patients 0 to 6 days old]; LOD, late-onset disease [patients 7 to 89 days old]) and were particularly prevalent (74.6%) in LOD. Type V GBS was the CPS type most frequently associated with invasive GBS disease in older adult patients (defined as those older than 60 years). Types III, Ia, and V were the most common among adult patients 19 to 59 years old. The child group includes patients 90 days to 18 years old. CPS types VI, VII, VIII, and IX are grouped under the designation “Other” in the pie charts.
Overall, CPS types III, V, and Ia were the most predominant. Together, these CPS types accounted for 70.3% of the GBS isolates (Fig. 1). CPS types II, Ib, and IV comprised another 26.5% of the isolates. CPS types VI to IX were found in low numbers. Seven isolates were nontypeable by serological methods. We also determined the CPS type of the GBS strains by PCR (25, 26). Overall, we observed a very good correlation between serology and PCR (see Table S2 in the supplemental material). Interestingly, discrepancies between methods were all found in strains identified by serology as being CPS type V (13/135, 9.6%). These discrepancies are being further investigated in our laboratory. PCR-based CPS typing also permitted the assignment of strains nontypeable by serology to CPS types Ia, V, and VII (see Table S2).
We found a lower proportion of CPS type Ia (14.7%) and higher proportions of CPS types Ib (14.7%) and III (44.1%) in strains isolated from EOD cases than have been reported in North America during the past 2 decades (5, 36, 37). However, there was a significantly greater proportion of CPS type III in LOD (74.6%) than in EOD (Fisher's exact test, P < 0.01), higher than in a previously reported comprehensive study in the United States (5) and comparable to the values observed in a pan-European study (38). On the other hand, there was a significantly higher association of CPS type Ib strains with EOD than with LOD (Fisher's exact test, P < 0.05). We also found proportionally more CPS type V strains in EOD (14.7%) than in LOD (5.1%), although the differences were not significant (Fisher's exact test, P > 0.05). We did not observe significant differences between EOD and LOD for GBS isolates of other CPS types (Fisher's exact test, P > 0.05).
CPS type III and Ia strains predominated among the adult (19 to 59 years) group (Fig. 1). There were significant differences in the proportions of these two CPS types between the adult and older-adult (≥60-year-old) groups (Fisher's exact test, P < 0.05). Conversely, significantly more GBS strains of CPS types V and Ib were found in the older adult group (Fisher's exact test, P < 0.05). Strains of CPS type V were the most prevalent among older adults, accounting for 29% of the cases (Fig. 1). The CPS type distribution reported here is similar to that observed previously in Australasia (14) and countries such as Spain (39), Sweden (40), and the United States (5). In these previous reports, CPS type V was also the most prevalent. However, a Portuguese study recently found that CPS type Ia was responsible for the majority of invasive infections in all age groups (41).
Genomic rearrangements result in acquisition of the major virulence factor hvgA by non-CC17 GBS.
GBS strains belonging to CC17 are considered hypervirulent and appear to be strongly capable of invading the central nervous system of neonates (3, 19). To begin to understand the contribution of hypervirulent CC17 strains to the burden of GBS invasive disease in the greater Toronto area, we determined the number of CC17 isolates and their distributions among the different age groups. To screen our collection, we used a PCR assay which targets hvgA, the gene encoding the major virulence factor HvgA (21). This PCR assay has previously been shown to specifically detect CC17 GBS strains (17) and has been described as a less expensive and less time-consuming alternative to MLST. Using this surrogate MLST approach, we identified 91 hvgA-positive (putatively CC17) strains in our collection. The majority (n = 47) of these hvgA-positive strains were from neonatal samples, particularly LOD, but we also found 38 hvgA-positive isolates among adults and elderly patients (Fig. 2). The vast majority of hvgA-positive strains were CPS type III. However, two hvgA-positive organisms were CPS type IV, and one strain was found to be CPS type V (Fig. 2).
FIG 2.

Distribution of hvgA-positive GBS isolates identified in this study among the different age groups (as defined in the Fig. 1 legend). The CPS types of the isolates are indicated. The vast majority of hvgA-positive isolates were CPS type III (yellow). In the adult age group, of the 18 hvgA-positive strains, there was one CPS type IV (6%, indicated in blue) and one CPS type V (6%, indicted in red). In the older adult group, of 20 hvgA-positive strains, there was one type IV (5%).
To our knowledge, no CC17 strains of CPS type V have been described previously. However, capsular switching from serotype III to IV within CC17 has been reported recently and found to be the result of recombination involving the entire cps locus (7). Therefore, we hypothesized that the hvgA-positive CPS type V GBS strain (isolate NGBS375) had undergone a similar recombinatorial event involving cps locus switching. To test this hypothesis, we sequenced the genomes of all hvgA-positive strains in the collection, including NGBS375, using Illumina MiSeq technology. When we derived MLST typing data for this strain directly from the short reads using SRST2, we discovered that while all other strains clustered in CC17, strain NGBS375 belonged to sequence type 297 (ST297), an ST included in CC1 that is distantly related to CC17 (see Table S3 in the supplemental material). Further examination of genome sequences revealed that strain NGBS375 and the previously sequenced CPS type V CC1 strain BAA-23 (28) were highly homologous across the majority of their genomes, including the cps locus (Fig. 3). However, homology between NGBS375 and BAA-23 was lower in a genome region of approximately 300 kb, including gene glnA used in the GBS MLST schema. More thorough analysis identified high homology at the DNA level across the 300-kb region between NGBS375 and the previously sequenced CC17 strain GB00112 (27) (Fig. 3). Whole-genome data thus strongly support the hypothesis that NGB375 is a bona fide CC1, CPS type V strain which acquired a large genome region, including the major virulence factor hvgA, from a yet unknown CC17 donor. Although further experimentation is required to determine the actual mechanism by which NGBS375 acquired these CC17 sequences, our results are not surprising, since conjugal transfer of unusually large parts of the genome has long been documented in GBS (42). Based on our data, we caution against using the hvgA PCR as sole means to identify CC17 GBS. In addition, we note that acquisition of this virulence factor by GBS strains of other genomic backgrounds might result in strains with increased virulence potential. Indeed, hvgA has been shown to be a crucial contributor to CC17 hypervirulence (21).
FIG 3.
Whole-genome analysis indicates acquisition of CC17 sequences, including the major virulence factor hvgA, by strain NGBS375. Polymorphisms, TBLASTX comparisons, and coverage data for strain NGBS375 are plotted against the schematic pseudochromosome map of reference ST1 GBS strain BAA-23 (A). Note that NGBS375 presents high TBLASTX homology over the majority of the ST1 genome, including the type V cps locus, and that very few polymorphisms are identified by VAAL. However, homology decreases below 98% and an overabundance of polymorphisms is found over a region of approximately 300 kb in the ST1 genome. Results are inverted when TBLASTX homology and polymorphisms are plotted against the reference ST17 strain GB00112 (B). High homology and very low number of polymorphisms identified by VAAL are now observed for the same ∼300-kb region, suggesting it was acquired by NGBS375 from a CC17 donor, while low homology and overabundance of polymorphisms are observed for the rest of the ST17 genome. Reference genome landmarks, such as pilus islands, srtA, nusG, secE, and first and last genes of the cps locus (cpsA and neuA), are provided in blue for both pseudochromosome maps, while genes used for MLST typing are in light blue. Putative mobile genetic elements are represented in green. Gene hvgA is depicted in red in the ST17 strain. Its bibA homolog is depicted in pink in the ST1 strain. The innermost circle shows the contig numbers of the unfinished genome drafts used to generate the pseudochromosome maps (see Materials and Methods).
Capsular switching to CPS type IV among CC17 strains.
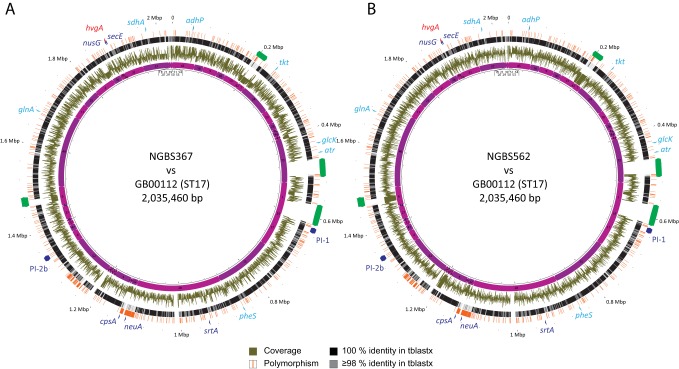
Strains of CC17 GBS have shown rapid global dissemination and their clonal expansion has been associated with the increase in neonatal morbidity due to GBS after 1960 (20). Until recently, CC17 was described as a homogenous cluster of CPS type III isolates. However, as mentioned above, there is recent evidence of capsular switching from CPS type III to type IV in this lineage due to the exchange of a 35.5-kb DNA fragment containing the entire cps operon (7). In that previous report, CC17 GBS strains with a CPS type IV were found to be ST291 by MLST. Consistently, MLST typing derived from the short-read whole-genome sequence data revealed that the two hvgA-positive CPS type IV strains in our collection (strains NGBS367 and NGBS562) were also ST291 (see Table S3 in the supplemental material). Further, whole-genome sequencing analysis strongly suggested that the two strains likely acquired a type IV cps locus by recombination (Fig. 4). The GBS capsule is the main target of antibody-mediated killing (43) and changes in the capsular locus are spurred by selection pressure due to development of host immunity to certain serotypes. Capsular switching may thus become problematic for current efforts in GBS vaccine development, which do not include CPS type IV in their formulation (44), by providing vaccine escape recombinants, as has been seen in the pneumococcus vaccine (45).
FIG 4.
Whole-genome analysis suggest recombination as the driver of capsular switching in two CC17 strains. Polymorphisms, TBLASTX comparison and coverage data for strains NGBS367 (A) and NGBS562 (B) are plotted against reference ST17 strain GB00112. High homology and very low number of polymorphism called by VAAL are observed across most of the genome, with the notable exception of the cps locus, suggesting acquisition of the entire locus from an unknown CPS type IV donor. Reference genome landmarks such as pilus islands, srtA, nusG, secE, and first and last genes of the cps locus (cpsA and neuA) are provided in blue while genes used for GBS MLST typing are in light blue. Putative mobile genetic elements are represented in green. Gene hvgA is depicted in red. The innermost circle shows the contig numbers of the unfinished genome drafts used to generate the pseudochromosome maps (see Materials and Methods).
Together with our findings of acquisition of hvgA by CC1 GBS strain NGBS375, demonstration of capsular switching in NGBS367 and NGBS562 highlights the important role played by recombination in the acquisition of virulence factors by the different GBS genotypes, and is in agreement with previous reports on the contribution of horizontal gene exchange of extensive regions of DNA among isolates of this important pathogen (42).
Supplementary Material
ACKNOWLEDGMENTS
We thank Sylvia Pong-Porter and Agron Plevneshi (Mount Sinai Hospital) for their help with strain handling and strain metadata. We are indebted to Alex Marchand-Austin and to Vanessa Gray Allen and Sandra Zittermann (Public Health Ontario) for help with statistical analysis and for generously sharing their laboratory equipment, respectively. This study used the GBS MLST database, developed by K. Jolley, curated by P. Glaser and colleagues, and funded by the Wellcome Trust.
Funding for this study was provided by Public Health Ontario.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 19 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.03554-13.
REFERENCES
- 1.Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, Heath PT. 2012. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet 379:547–556. 10.1016/S0140-6736(11)61651-6 [DOI] [PubMed] [Google Scholar]
- 2.Moore MR, Schrag SJ, Schuchat A. 2003. Effects of intrapartum antimicrobial prophylaxis for prevention of group-B-streptococcal disease on the incidence and ecology of early-onset neonatal sepsis. Lancet Infect. Dis. 3:201–213. 10.1016/S1473-3099(03)00577-2 [DOI] [PubMed] [Google Scholar]
- 3.Poyart C, Reglier-Poupet H, Tazi A, Billoet A, Dmytruk N, Bidet P, Bingen E, Raymond J, Trieu-Cuot P. 2008. Invasive group B streptococcal infections in infants, France. Emerg. Infect. Dis. 14:1647–1649. 10.3201/eid1410.080185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards MS, Baker CJ. 2001. Group B streptococcal infections, p 1091–1156 In Remington JS, Klein JO. (ed), Infectious diseases of the fetus and newborn infant, 5th ed. Saunders, Philadelphia, PA [Google Scholar]
- 5.Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ, Active Bacterial Core surveillance/Emerging Infections Program Network 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299:2056–2065. 10.1001/jama.299.17.2056 [DOI] [PubMed] [Google Scholar]
- 6.Cieslewicz MJ, Chaffin D, Glusman G, Kasper D, Madan A, Rodrigues S, Fahey J, Wessels MR, Rubens CE. 2005. Structural and genetic diversity of group B Streptococcus capsular polysaccharides. Infect. Immun. 73:3096–3103. 10.1128/IAI.73.5.3096-3103.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellais S, Six A, Fouet A, Longo M, Dmytruk N, Glaser P, Trieu-Cuot P, Poyart C. 2012. Capsular switching in group B Streptococcus CC17 hypervirulent clone: a future challenge for polysaccharide vaccine development. J. Infect. Dis. 206:1745–1752. 10.1093/infdis/jis605 [DOI] [PubMed] [Google Scholar]
- 8.Hauge M, Jespersgaard C, Poulsen K, Kilian M. 1996. Population structure of Streptococcus agalactiae reveals an association between specific evolutionary lineages and putative virulence factors but not disease. Infect. Immun. 64:919–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Azavedo JC, McGavin M, Duncan C, Low DE, McGeer A. 2001. Prevalence and mechanisms of macrolide resistance in invasive and noninvasive group B Streptococcus isolates from Ontario, Canada. Antimicrob. Agents Chemother. 45:3504–3508. 10.1128/AAC.45.12.3504-3508.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kothari NJ, Morin CA, Glennen A, Jackson D, Harper J, Schrag SJ, Lynfield R. 2009. Invasive group B streptococcal disease in the elderly, Minnesota, USA, 2003–2007. Emerg. Infect. Dis. 15:1279–1281. 10.3201/eid1508.081381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambertsen L, Ekelund K, Skovsted IC, Liboriussen A, Slotved HC. 2010. Characterisation of invasive group B streptococci from adults in Denmark 1999 to 2004. Eur. J. Clin. Microbiol. Infect. Dis. 29:1071–1077. 10.1007/s10096-010-0941-z [DOI] [PubMed] [Google Scholar]
- 12.Murayama SY, Seki C, Sakata H, Sunaoshi K, Nakayama E, Iwata S, Sunakawa K, Ubukata K, Invasive Streptococcal Disease Working Group 2009. Capsular type and antibiotic resistance in Streptococcus agalactiae isolates from patients, ranging from newborns to the elderly, with invasive infections. Antimicrob. Agents Chemother. 53:2650–2653. 10.1128/AAC.01716-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persson E, Berg S, Bevanger L, Bergh K, Valso-Lyng R, Trollfors B. 2008. Characterisation of invasive group B streptococci based on investigation of surface proteins and genes encoding surface proteins. Clin. Microbiol. Infect. 14:66–73. 10.1111/j.1469-0691.2007.01877.x [DOI] [PubMed] [Google Scholar]
- 14.Zhao Z, Kong F, Zeng X, Gidding HF, Morgan J, Gilbert GL. 2008. Distribution of genotypes and antibiotic resistance genes among invasive Streptococcus agalactiae (group B streptococcus) isolates from Australasian patients belonging to different age groups. Clin. Microbiol. Infect. 14:260–267. 10.1111/j.1469-0691.2007.01914.x [DOI] [PubMed] [Google Scholar]
- 15.Musser JM, Mattingly SJ, Quentin R, Goudeau A, Selander RK. 1989. Identification of a high-virulence clone of type III Streptococcus agalactiae (group B Streptococcus) causing invasive neonatal disease. Proc. Natl. Acad. Sci. U. S. A. 86:4731–4735. 10.1073/pnas.86.12.4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones N, Oliver KA, Barry J, Harding RM, Bisharat N, Spratt BG, Peto T, Crook DW, Oxford Group B Streptococcus Consortium 2006. Enhanced invasiveness of bovine-derived neonatal sequence type 17 group B Streptococcus is independent of capsular serotype. Clin. Infect. Dis. 42:915–924. 10.1086/500324 [DOI] [PubMed] [Google Scholar]
- 17.Lamy MC, Dramsi S, Billoet A, Reglier-Poupet H, Tazi A, Raymond J, Guerin F, Couve E, Kunst F, Glaser P, Trieu-Cuot P, Poyart C. 2006. Rapid detection of the “highly virulent” group B Streptococcus ST-17 clone. Microbes Infect. 8:1714–1722. 10.1016/j.micinf.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 18.Bisharat N, Crook DW, Leigh J, Harding RM, Ward PN, Coffey TJ, Maiden MC, Peto T, Jones N. 2004. Hyperinvasive neonatal group B Streptococcus has arisen from a bovine ancestor. J. Clin. Microbiol. 42:2161–2167. 10.1128/JCM.42.5.2161-2167.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning SD, Springman AC, Lehotzky E, Lewis MA, Whittam TS, Davies HD. 2009. Multilocus sequence types associated with neonatal group B streptococcal sepsis and meningitis in Canada. J. Clin. Microbiol. 47:1143–1148. 10.1128/JCM.01424-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen UB, Poulsen K, Ghezzo C, Margarit I, Kilian M. 2010. Emergence and global dissemination of host-specific Streptococcus agalactiae clones. mBio 1:pii:e00178-10. 10.1128/mBio.00178-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tazi A, Disson O, Bellais S, Bouaboud A, Dmytruk N, Dramsi S, Mistou MY, Khun H, Mechler C, Tardieux I, Trieu-Cuot P, Lecuit M, Poyart C. 2010. The surface protein HvgA mediates group B Streptococcus hypervirulence and meningeal tropism in neonates. J. Exp. Med. 207:2313–2322. 10.1084/jem.20092594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poyart C, Lamy MC, Boumaila C, Fiedler F, Trieu-Cuot P. 2001. Regulation of d-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 183:6324–6334. 10.1128/JB.183.21.6324-6334.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slotved HC, Elliott J, Thompson T, Konradsen HB. 2003. Latex assay for serotyping of group B Streptococcus isolates. J. Clin. Microbiol. 41:4445–4447. 10.1128/JCM.41.9.4445-4447.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao K, Poulsen K, Maione D, Rinaudo CD, Baldassarri L, Telford JL, Sorensen UB, Kilian M. 2013. Capsular gene typing of Streptococcus agalactiae compared to serotyping by latex agglutination. J. Clin. Microbiol. 51:503–507. 10.1128/JCM.02417-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poyart C, Tazi A, Reglier-Poupet H, Billoet A, Tavares N, Raymond J, Trieu-Cuot P. 2007. Multiplex PCR assay for rapid and accurate capsular typing of group B Streptococci. J. Clin. Microbiol. 45:1985–1988. 10.1128/JCM.00159-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imperi M, Pataracchia M, Alfarone G, Baldassarri L, Orefici G, Creti R. 2010. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J. Microbiol. Methods 80:212–214. 10.1016/j.mimet.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 27.Singh P, Springman AC, Davies HD, Manning SD. 2012. Whole-genome shotgun sequencing of a colonizing multilocus sequence type 17 Streptococcus agalactiae strain. J. Bacteriol. 194:6005. 10.1128/JB.01378-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, Angiuoli SV, Crabtree J, Jones AL, Durkin AS, Deboy RT, Davidsen TM, Mora M, Scarselli M, Margarit y Ros I, Peterson JD, Hauser CR, Sundaram JP, Nelson WC, Madupu R, Brinkac LM, Dodson RJ, Rosovitz MJ, Sullivan SA, Daugherty SC, Haft DH, Selengut J, Gwinn ML, Zhou L, Zafar N, Khouri H, Radune D, Dimitrov G, Watkins K, O'Connor KJ, Smith S, Utterback TR, White O, Rubens CE, Grandi G, Madoff LC, Kasper DL, Telford JL, Wessels MR, Rappuoli R, Fraser CM. 2005. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome.” Proc. Natl. Acad. Sci. U. S. A. 102:13950–13955. 10.1073/pnas.0506758102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glaser P, Rusniok C, Buchrieser C, Chevalier F, Frangeul L, Msadek T, Zouine M, Couve E, Lalioui L, Poyart C, Trieu-Cuot P, Kunst F. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 45:1499–1513. 10.1046/j.1365-2958.2002.03126.x [DOI] [PubMed] [Google Scholar]
- 30.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nusbaum C, Ohsumi TK, Gomez J, Aquadro J, Victor TC, Warren RM, Hung DT, Birren BW, Lander ES, Jaffe DB. 2009. Sensitive, specific polymorphism discovery in bacteria using massively parallel sequencing. Nat. Methods 6:67–69. 10.1038/nmeth.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tritt A, Eisen JA, Facciotti MT, Darling AE. 2012. An integrated pipeline for de novo assembly of microbial genomes. PLoS One 7:e42304. 10.1371/journal.pone.0042304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. 10.1186/1471-2164-12-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inouye M, Conway TC, Zobel J, Holt KE. 2012. Short read sequence typing (SRST): multilocus sequence types from short reads. BMC Genomics 13:338. 10.1186/1471-2164-13-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaleznik DF, Rench MA, Hillier S, Krohn MA, Platt R, Lee ML, Flores AE, Ferrieri P, Baker CJ. 2000. Invasive disease due to group B Streptococcus in pregnant women and neonates from diverse population groups. Clin. Infect. Dis. 30:276–281. 10.1086/313665 [DOI] [PubMed] [Google Scholar]
- 37.Davies HD, Raj S, Adair C, Robinson J, McGeer A. 2001. Population-based active surveillance for neonatal group B streptococcal infections in Alberta, Canada: implications for vaccine formulation. Pediatr. Infect. Dis. J. 20:879–884. 10.1097/00006454-200109000-00011 [DOI] [PubMed] [Google Scholar]
- 38.Melin P, Efstratiou A. 2013. Group B streptococcal epidemiology and vaccine needs in developed countries. Vaccine 31(Suppl 4):D31–D42. 10.1016/j.vaccine.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 39.Bolaños M, Hernández A, Santana O-E, Molina J, Martín-Sánchez AM. 2005. Distribution of Streptococcus agalactiae serotypes in samples from nonpregnant adults. Clin. Microbiol. Newsl. 27:151–153. 10.1016/j.clinmicnews.2005.09.004 [DOI] [Google Scholar]
- 40.Persson E, Berg S, Trollfors B, Larsson P, Ek E, Backhaus E, Claesson BEB, Jonsson L, Rådberg G, Ripa T, Johansson S. 2004. Serotypes and clinical manifestations of invasive group B streptococcal infections in western Sweden 1998–2001. Clin. Microbiol. Infec. 10:791–796. 10.1111/j.1469-0691.2004.00931.x [DOI] [PubMed] [Google Scholar]
- 41.Martins ER, Melo-Cristino J, Ramirez M, Portuguese Group for the Study of Streptococcal Infections 2012. Dominance of serotype Ia among group B Streptococci causing invasive infections in nonpregnant adults in Portugal. J. Clin. Microbiol. 50:1219–1227. 10.1128/JCM.05488-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brochet M, Rusniok C, Couve E, Dramsi S, Poyart C, Trieu-Cuot P, Kunst F, Glaser P. 2008. Shaping a bacterial genome by large chromosomal replacements, the evolutionary history of Streptococcus agalactiae. Proc. Natl. Acad. Sci. U. S. A. 105:15961–15966. 10.1073/pnas.0803654105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards MS, Baker CJ, Kasper DL. 1979. Opsonic specificity of human antibody to the type III polysaccharide of group B Streptococcus. J. Infect. Dis. 140:1004–1008. 10.1093/infdis/140.6.1004 [DOI] [PubMed] [Google Scholar]
- 44.Madhi SA, Dangor Z, Heath PT, Schrag S, Izu A, Sobanjo-ter Meulen A, Dull PM. 2013. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine 31(Suppl 4):D52–D57. 10.1016/j.vaccine.2013.02.029 [DOI] [PubMed] [Google Scholar]
- 45.Brueggemann AB, Pai R, Crook DW, Beall B. 2007. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 3:e168. 10.1371/journal.ppat.0030168 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.