Abstract
Classical scrapie is one of the transmissible spongiform encephalopathies (TSEs), a group of fatal infectious diseases that affect the central nervous system (CNS). Classical scrapie can transmit laterally from ewe to lamb perinatally or between adult animals. Here we report detection of infectivity in tissues of an unborn fetus, providing evidence that in utero transmission of classical scrapie is also possible.
TEXT
Transmissible spongiform encephalopathies (TSEs) are chronic, fatal neurodegenerative diseases affecting animals and humans. The causative agent of TSEs is considered to be PrPSc, a pathogenic isoform of a normal host protein (PrPC). However, detectable PrPSc may not always be associated directly with infectivity and proof of infectivity is considered to be the most reliable method for determining the true infectious potential of a tissue (1–3).
Classical scrapie (c-scrapie) is a TSE affecting small ruminants. In addition to the central nervous system, various peripheral tissues and natural body secretions can harbor PrPSc and infectivity (4, 5). However, susceptibility to c-scrapie and peripheral distribution of the agent are strongly influenced by the PrP amino acid sequence, and in sheep, valine at codon 136 confers the greatest susceptibility to disease and widespread distribution of prions in peripheral tissues (6, 7).
Environmental contamination is believed to be a major contributor to lateral transmission of c-scrapie, with oral ingestion being a major route of entry of the agent into the host (5–7). Although c-scrapie can be transmitted laterally and susceptible adult animals can be infected if they are exposed to the agent, the disease can also be transmitted maternally via ingestion of the agent either directly (consumption of colostrum or milk) or indirectly (close contact with the placenta) (8–11). Here, we provide evidence that prenatal exposure to the agent can also occur, and lead to infection in utero, by demonstrating infectivity in fetal tissues from a dam infected with c-scrapie.
All sheep in this study were bred and reared in the Animal Health and Veterinary Laboratories Agency (AHVLA) Ripley flock, which was created from sheep purchased from commercial flocks with a high prevalence of scrapie and managed, using methods such as genotype selection, to maximize TSE prevalence. In other respects, commercial farming practices were applied in order to study the disease in conditions as close as possible to its natural environment. All animal procedures were carried out in accordance with the Animals (Scientific Procedures) Act 1986 under Home Office licence numbers 70/5780 (ruminant experimentation) and 70/6310 (mouse bioassays) and were approved by the local ethics committee of AHVLA.
One VRQ/VRQ ewe was allowed to mate naturally with a VRQ/VRQ ram. Another two VRQ/VRQ ewes were inseminated artificially with semen from a VRQ/VRQ ram. Details of the ewes and the resulting fetuses are presented in Tables 1 and 2, respectively. All three ewes were euthanized at the fourth month of gestation by an overdose of sodium pentobarbital administered intravenously followed by exsanguination. At this point, one ewe had already been exhibiting clinical signs of c-scrapie, while the other two were asymptomatic. Postmortem, the gravid uterus was removed aseptically and processed separately from the remainder of the carcass. Placentome, amniotic fluid, allantoic fluid, umbilical cord, fetal mesenteric lymph node (MLN), fetal spleen, and fetal liver were each sampled using new, disposable sterile instruments to prevent cross-contamination between tissues. Brain samples from the ewes were immersed in formal saline and processed routinely for histology and immunohistochemistry (IHC) with the anti-PrP monoclonal antibody R145. Fetal tissues were also fixed and immunolabeled with R145 diluted 1/500.
TABLE 1.
Details for ewes used in the study
| Ewe ID no. | Conception method | Age (mo) | Neurological signs | Scrapie diagnosis |
|---|---|---|---|---|
| PG 1657/05 | Artificial insemination | 21 | No | Positive |
| PG 1671/05 | Artificial insemination | 21 | No | Positive |
| PG1672/05 | Natural mating | 30 | Yes | Positive |
TABLE 2.
Details for fetuses used in the study
| Ewe ID no. | Fetus ID no. | Gestational stage (mo) | PrPSc detection in fetus |
|---|---|---|---|
| PG1657/05 | PG1658/05 | 4 | Positive |
| PG1671/05 | PG1673/05 | 4 | Negative |
| PG1672/05 | PG1674/05a | 4 | Negative |
| PG1675/05a | 4 | Negative |
Twin fetus.
Samples for bioassay were kept frozen at −80°C until they were homogenized in normal saline (10%, wt/vol) to produce inocula. All inocula were tested microbiologically and were treated with antibiotics if required. After microbiological clearance, inocula were refrozen at −80°C until they were used to challenge transgenic tg338 mice, which overexpress an ovine PrP VRQ transgene on a murine PrP null background (12). Each mouse received 20 μl of homogenate intracerebrally and 100 μl intraperitoneally. Mice were monitored for clinical signs of neurological disease and were euthanized when they reached terminal TSE disease. Mice showing ill health unrelated to TSEs were also euthanized on welfare grounds and were recorded as intercurrent deaths. Postmortem, irrespective of clinical status, the brain of each mouse was removed and fixed in buffered formalin. Each sample was processed, sectioned, and stained with hematoxylin and eosin (H&E) and immunolabeled using the polyclonal antibody Rb486. The presence of vacuoles in H&E sections and immunolabeling for PrPSc were recorded.
All three ewes were diagnosed as positive for c-scrapie by demonstration of vacuolation and PrPSc detection in the obex (Fig. 1). PrPSc was also detectable in the placentomes of all four fetuses (one ewe was carrying twins). No PrPSc deposits were detected in the umbilical cord and the tissues (mesenteric lymph node, spleen, liver) of the four fetuses. For three of the four fetuses, the bioassay results were in agreement with the IHC observations. However, tg338 mice challenged with umbilical cord or mesenteric lymph node from a single fetus did succumb to TSE despite a negative IHC result in the respective tissues (Table 3).
FIG 1.
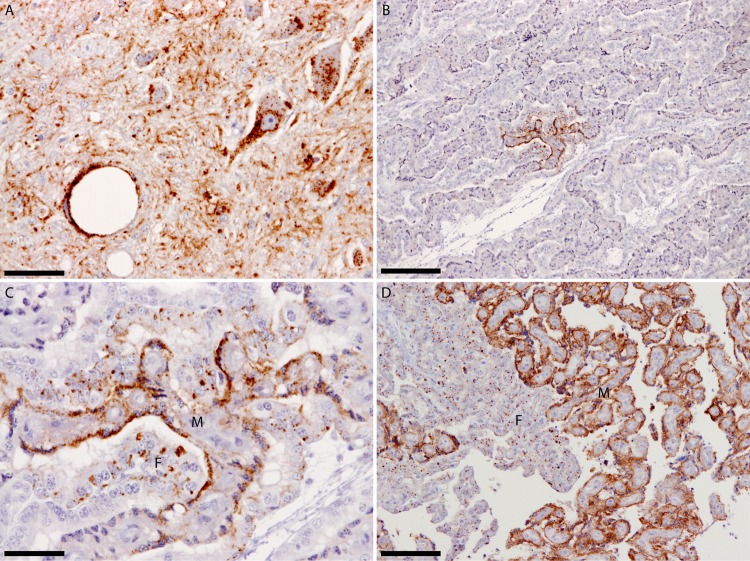
(A) Dorsal motor nucleus of the vagus nerve from ewe PG1657/05 showing widespread PrPSc deposits. (B) Placentome from fetus PG1658/05 at the 4th month of pregnancy showing restricted PrPSc deposits. (C) Greater magnification of the PrPSc-positive area shown in panel B to demonstrate that both maternal and fetal opposing units are affected and to illustrate the different PrPSc deposition patterns between maternal and fetal units. These different PrPSc deposition patterns most likely reflect the different physiological roles of maternal and fetal units, as both units were of the same PrP genotype. (D) For comparison, a placentome collected near term demonstrates the widespread PrPSc dissemination at the end of the 5th month of gestation. All sections were labeled with the monoclonal antibody R145. The maternal unit is denoted by the letter M, the fetal unit by the letter F. The scale bars in panels A and C represent 50 μm, in panels B and D, 200 μm.
TABLE 3.
Summary of bioassay data from fetal tissues
| Tissue | Values for mice inoculated with fetal tissuesa: |
|||
|---|---|---|---|---|
| PG1658/05 | PG1673/05 | PG1674/05 | PG1675/05 | |
| Placentomeb | 18/19 (16; 543 ± 17) | 19/20 (15; 545 ± 18) | 18/19 (15; 470 ± 27) | 20/20 (17; 500 ± 20) |
| Umbilical Cordc | 13/18 (13; 530 ± 18) | NDd | 0/20 | 0/20 |
| Mesenteric LNc | 3/10 (3; 630 ± 46) | 0/10 | 0/5 | 0/10 |
| Spleenc | 0/20 | 0/20 | 0/20 | 0/20 |
| Liverc | 0/20 | 0/19 | 0/20 | 0/19 |
| Allantoic fluid | 0/20 | 0/20 | 0/20 | 0/20 |
| Amniotic fluid | 0/20 | 0/20 | 0/19 | 0/20 |
Values indicate numbers of TSE-positive mice/mice analyzed. TSE-positive mice include clinically positive animals and mice which died intercurrently and were subsequently diagnosed as TSE positive by immunohistochemistry. The number of clinically positive mice and the incubation period in days (mean ± standard error of the mean [SEM]) are indicated in parentheses. Mice were monitored for 850 days.
Tissues diagnosed as TSE positive when examined with immunohistochemistry.
Tissues diagnosed as TSE negative when examined with immunohistochemistry.
ND, not done.
Thirteen of 18 mice (72%) inoculated with umbilical cord from the affected fetus were TSE positive, but only 3 of 10 mice (30%) inoculated with MLN were positive by IHC (Table 3). These data are indicative of an ascending infection from the placenta to the fetus.
These data demonstrate in utero transmission of c-scrapie based on proof of infectivity and are in agreement with a report that identified PrPSc in fetal tissues after protein misfolding cyclic amplification (PMCA) (13). In our view, in utero transmission probably requires peripheral distribution of PrPSc and therefore it is a risk only in animals where peripheral PrPSc distribution, or at least TSE infectivity, can be demonstrated, particularly in the placenta. C-scrapie is an example of such a TSE, although peripheral distribution of the agent is a function of the PrP genotype (6, 7).
In this study, we used sheep with the genotype VRQ/VRQ, which are considered to be the most susceptible to c-scrapie and which show the most widespread and abundant PrPSc in peripheral tissues (7, 11, 14). All three dams were positive postmortem, but only one had reached clinical disease. Nevertheless, placentomes from all four fetuses were positive on bioassay, although infectivity was detected in tissues from only a single fetus. The other three fetuses, including the one that was recovered from the ewe that showed clinical signs, were negative. Possibly the infectivity levels in these fetuses or in the PrPSc-negative tissues from the affected fetus were below detection level. It is logical to assume that if the fetuses were allowed to develop further and be born a higher incidence of infectivity could have been detected. This assumption is further supported by the fact that the ewes were euthanized at the fourth month of gestation, when the distribution of PrPSc in the placentomes was restricted compared to that in full-term pregnancy (Fig. 1).
The possibility of in utero transmission has also been proposed for chronic wasting disease (CWD) in cervids, where accumulation of PrPSc in the peripheral tissues is a common finding (15). Similar peripheral accumulation of PrPSc in small ruminants affected experimentally with bovine spongiform encephalopathy (BSE) means that in these species the possibility of in utero BSE transmission cannot be dismissed (16, 17). Peripheral accumulation of PrPSc has also been demonstrated in variant Creutzfeldt-Jakob disease (CJD) cases and asymptomatic carriers have been detected (18–20), so theoretically, in utero transmission in humans cannot be excluded unequivocally although there are histological and physiological differences between human and ruminant placentae. However, with our current knowledge, it is not possible to conclude whether the human placenta confers better protection to the conceptus from TSEs or not.
The data presented here raise questions regarding the efficiency of current control measures for the eradication of animal TSEs, which have been based on the assumption that TSEs do not transmit in utero. They also indicate that the risk analysis approaches regarding TSEs with evident peripheral distribution of PrPSc may have to be reviewed, although more research is required to increase the amount of information available in order to quantify the risk.
ACKNOWLEDGMENTS
We thank colleagues in the Pathology Department for their skilled technical support. We also thank Ian Dexter and Emma Popescu and their teams for their technical expertise and support.
This project was supported by Defra project SE1856.
Footnotes
Published ahead of print 22 January 2014
REFERENCES
- 1.Piccardo P, Manson JC, King D, Ghetti B, Barron RM. 2007. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc. Natl. Acad. Sci. U. S. A. 104:4712–4717. 10.1073/pnas.0609241104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron RM, Campbell SL, King D, Bellon A, Chapman KE, Williamson RA, Manson JC. 2007. High titers of transmissible spongiform encephalopathy infectivity associated with extremely low levels of PrPSc in vivo. J. Biol. Chem. 282:35878–35886. 10.1074/jbc.M704329200 [DOI] [PubMed] [Google Scholar]
- 3.Dobie K, Barron R. 2013. Dissociation between transmissible spongiform encephalopathy (TSE) infectivity and proteinase K-resistant PrPSc levels in peripheral tissue from a murine transgenic model of TSE disease. J. Virol. 87:5895–5903. 10.1128/JVI.03469-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Keulen LJ, Bossers A, van Zijderveld F. 2008. TSE pathogenesis in cattle and sheep. Vet. Res. 39:24. 10.1051/vetres:2007061 [DOI] [PubMed] [Google Scholar]
- 5.Gough KC, Maddison BC. 2010. Prion transmission: prion excretion and occurrence in the environment. Prion 4:275–282. 10.4161/pri.4.4.13678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Keulen LJ, Vromans ME, van Zijderveld FG. 2002. Early and late pathogenesis of natural scrapie infection in sheep. APMIS 110:23–32. 10.1034/j.1600-0463.2002.100104.x [DOI] [PubMed] [Google Scholar]
- 7.Ersdal C, Ulvund MJ, Espenes A, Benestad SL, Sarradin P, Landsverk T. 2005. Mapping PrPSc propagation in experimental and natural scrapie in sheep with different PrP genotypes. Vet. Pathol. 42:258–274. 10.1354/vp.42-3-258 [DOI] [PubMed] [Google Scholar]
- 8.Ryder S, Dexter G, Bellworthy S, Tongue S. 2004. Demonstration of lateral transmission of scrapie between sheep kept under natural conditions using lymphoid tissue biopsy. Res. Vet. Sci. 76:211–217. 10.1016/j.rvsc.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 9.Konold T, Moore SJ, Bellworthy SJ, Simmons HA. 2008. Evidence of scrapie transmission via milk. BMC Vet. Res. 4:14. 10.1186/1746-6148-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konold T, Moore SJ, Bellworthy SJ, Terry LA, Thorne L, Ramsay A, Salguero FJ, Simmons MM, Simmons HA. 2013. Evidence of effective scrapie transmission via colostrum and milk in sheep. BMC Vet. Res. 9:99. 10.1186/1746-6148-9-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andréoletti O, Lacroux C, Chabert A, Monnereau L, Tabouret G, Lantier F, Berthon P, Eychenne F, Lafond-Benestad S, Elsen JM, Schelcher F. 2002. PrPSc accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J. Gen. Virol. 83:2607–2616 [DOI] [PubMed] [Google Scholar]
- 12.Vilotte JL, Soulier S, Essalmani R, Stinnakre MG, Vaiman D, Lepourry L, Da Silva JC, Besnard N, Dawson M, Buschmann A, Groschup M, Petit S, Madelaine MF, Rakatobe S, Le Dur A, Vilette D, Laude H. 2001. Markedly increased susceptibility to natural sheep scrapie of transgenic mice expressing ovine PrP. J. Virol. 75:5977–5984. 10.1128/JVI.75.13.5977-5984.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garza MC, Fernandez-Borges N, Bolea R, Badiola JJ, Castilla J, Monleon E. 2011. Detection of PrPres in genetically susceptible fetuses from sheep with natural scrapie. PLoS One 6:e27525. 10.1371/journal.pone.0027525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andréoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen JM, Lantier F. 2000. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115–3126 [DOI] [PubMed] [Google Scholar]
- 15.Nalls AV, McNulty E, Powers J, Seelig DM, Hoover C, Haley NJ, Hayes-Klug J, Anderson K, Stewart P, Goldmann W, Hoover EA, Mathiason CK. 2013. Mother to offspring transmission of chronic wasting disease in Reeves' muntjac deer. PLoS One 8:e71844. 10.1371/journal.pone.0071844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffrey M, Ryder S, Martin S, Hawkins SA, Terry L, Berthelin-Baker C, Bellworthy SJ. 2001. Oral inoculation of sheep with the agent of bovine spongiform encephalopathy (BSE). 1. Onset and distribution of disease-specific PrP accumulation in brain and viscera. J. Comp. Pathol. 124:280–289. 10.1053/jcpa.2001.0465 [DOI] [PubMed] [Google Scholar]
- 17.Bellworthy SJ, Hawkins SA, Green RB, Blamire I, Dexter G, Dexter I, Lockey R, Jeffrey M, Ryder S, Berthelin-Baker C, Simmons MM. 2005. Tissue distribution of bovine spongiform encephalopathy infectivity in Romney sheep up to the onset of clinical disease after oral challenge. Vet. Rec. 156:197–202 [DOI] [PubMed] [Google Scholar]
- 18.Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J. 2001. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 358:171–180. 10.1016/S0140-6736(01)05403-4 [DOI] [PubMed] [Google Scholar]
- 19.Hilton DA, Ghani AC, Conyers L, Edwards P, McCardle L, Ritchie D, Penney M, Hegazy D, Ironside JW. 2004. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J. Pathol. 203:733–739. 10.1002/path.1580 [DOI] [PubMed] [Google Scholar]
- 20.Gill ON, Spencer Y, Boyes L, Kelly C, Richard-Loendt A, Dabaghian R, Linehan J, Simmons M, Webb P, Bellerby P, Andrews N, Hilton DA, Ironside JW, Beck J, Poulter M, Mead S, Brandner S. 2013. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ 347:f5675. 10.1136/bmj.f5675 [DOI] [PMC free article] [PubMed] [Google Scholar]