ABSTRACT
Rice ragged stunt virus (RRSV), an oryzavirus in the family Reoviridae, is transmitted by the brown planthopper, Nilaparvata lugens, in a persistent-propagative manner. Here, we established a continuous cell line of brown planthopper to investigate the mechanism underlying the formation of the viroplasm, the putative site for viral replication and assembly, during infection of RRSV in its insect vector cells. Within 24 h of viral infection of cultured cells, the viroplasm had formed and contained the viral nonstructural proteins Pns6 and Pns10, known to be constituents of viroplasm. Core capsid protein P3, core particles, and newly synthesized viral RNAs were accumulated inside the viroplasm, while outer capsid protein P8 and virions were accumulated at the periphery of the viroplasm, confirming that the viroplasm induced by RRSV infection was the site for viral replication and assembly. Pns10 formed viroplasm-like inclusions in the absence of viral infection, suggesting that the viroplasm matrix was largely composed of Pns10. Pns6 was recruited in the viroplasm by direct interaction with Pns10. Core capsid protein P3 was recruited to the viroplasm through specific association with Pns6. Knockdown of Pns6 and Pns10 expression using RNA interference inhibited viroplasm formation, virion assembly, viral protein expression, and viral double-stranded RNA synthesis. Thus, the present study shows that both Pns6 and Pns10 of RRSV play important roles in the early stages of viral life cycle in its insect vector cells, by recruiting or retaining components necessary for viral replication and assembly.
IMPORTANCE The brown planthopper, a commonly distributed pest of rice in Asia, is the host of numerous insect endosymbionts, and the major vector of two rice viruses (RRSV and rice grassy stunt virus). For the first time, we successfully established the continuous cell line of brown planthopper. The unique uniformity of brown planthopper cells in the monolayer can support a consistent, synchronous infection by endosymbionts or viral pathogens, improving our understanding of molecular insect-microbe interactions.
INTRODUCTION
Viruses in the Reoviridae family replicate and assemble within cytoplasmic viral inclusions called viroplasms or viral factories (1). Plant reoviruses, which comprise the genera Phytoreovirus, Fijivirus, and Oryzavirus in the family Reoviridae, are transmitted propagatively by cicadellid leafhoppers or planthoppers (1). Plant reoviruses are icosahedral, double-layered particles with 10 or 12 double-stranded RNA (dsRNA) segments (1). The genesis and maturation of viroplasms of phytoreoviruses and fijiviruses in their insect vectors are beginning to be understood due to the development of culture systems for insect vector cells (2–7).
Continuous cell cultures derived from rice green leafhopper, Nephotettix cincticeps provide a good system for studying the replication cycles of rice dwarf virus (RDV), a phytoreovirus, in its vector cells, including viral entry, replication, and spread (3, 5–7). Viroplasms, globular electron-dense inclusion bodies, are formed within 6 h postinfection (hpi) of RDV in leafhopper cultured cells (7). Nonstructural protein Pns12 of RDV is essential for the formation of viroplasm matrix and the nucleation of viral assembly complexes for the production of viral progeny virions (7). Recently, we established the continuous cell cultures derived from white-backed planthopper, Sogatella furcifera, to investigate the genesis and maturation of viroplasms induced by southern rice black streaked dwarf virus (SRBSDV), a fijivirus, in its insect vector cells (2, 4). The viroplasm of SRBSDV consists of a granular region formed by viral nonstructural proteins P6 and P9-1 for the accumulation of viral RNAs and a filamentous region formed by viral nonstructural proteins P5 and P6 for the assembly of progeny virions (2, 4). Currently, the mechanisms responsible for the genesis and maturation of the viroplasm induced by oryzaviruses remain largely unknown due to the lack of continuous cultured cells derived from their insect vectors.
Rice ragged stunt virus (RRSV), an oryzavirus, is transmitted by the brown planthopper (BPH), Nilaparvata lugens, in a persistent-propagative manner and has spread throughout southern China (8–10). RRSV virions are icosahedral, double-layered particles with 65 to 70 nm in diameter (11). The genome of RRSV encodes at least seven putative structural proteins (P1, P2, P3, P4A, P5, P8B, and P9) and three nonstructural proteins (Pns6, Pns7, and Pns10) (11–17). The core particles of RRSV contain core capsid protein P3, RNA-dependent RNA polymerase P4A protein, and 10 segments of dsRNAs (11, 12, 15, 18). The major outer capsid protein P8 and spike protein P9 are added onto the core particles to assemble double-layered particles of RRSV (12, 19). A continuous cell culture derived from BPH would enable us to define the process of progeny virion assembly during early infection by RRSV.
The assembly of progeny core and virions of RRSV is believed to occur within the viroplasms, globular electron-dense inclusion bodies, induced by RRSV infection in host plants or insect vectors (9, 20). Our previous study indicates that the nonstructural protein Pns10 of RRSV plays an essential role in viroplasm formation and viral replication (20). Formation of the viroplasm for the assembly of progeny virions is essential for the establishment of a persistent infection of RRSV in its BPH vector (20). Whether other nonstructural proteins are involved in the genesis and maturation of the viroplasm induced by RRSV infection, however, is still unknown. The nonstructural protein Pns7 forms filament-like structures in the absence of viral infection and has not been detected in RRSV-infected BPH vectors by Western blotting assay (20; unpublished data). Our previous study shows that the nonstructural protein Pns10 can recruit Pns6 into the viroplasm-like structures formed by Pns10 during coexpression of these two proteins in cells of the nonhost insect Spodoptera frugiperda (Sf9) (20), suggesting that Pns6 might be one of the components of viroplasm matrix induced by RRSV infection.
In the present study, we developed a continuous culture system derived from BPH to trace the early infection process of RRSV. Our results indicated that the nonstructural proteins Pns6 and Pns10 of RRSV played important roles in the formation of the initial viroplasm matrix and that Pns6 could recruit or retain components necessary for viral replication and assembly.
MATERIALS AND METHODS
Establishment of continuous cell culture derived from BPH for RRSV infection.
The continuous cell culture derived from BPH was established by adapting the protocols for similar systems for the white-backed planthopper and small brown planthopper, as described by Ma et al. (21) and Mao et al. (4). The embryos at the blastokinetic stage, with red eye spots on the BPH eggs on day 8 after oviposition, proved suitable for primary cell culture. The eggs were sterilized with 70% ethanol for 5 min and washed with Tyrode's solution. The embryonic fragments were dissected from the eggs and then incubated with 0.25% trypsin in Tyrode's solution (pH 6.7) for 30 min at room temperature. The embryonic tissues were transferred to a centrifuge tube and centrifuged at 250 × g for 3 min. The pellet was resuspended in Kimura's insect medium (3) and transferred to a culture flask. The culture was incubated at 25°C and the medium was changed at intervals of 7 to 10 days. Epithelium-like cells grew out from the explants of embryonic tissue to form a monolayer of primary culture cells by 16 days. The primary culture reached almost confluence in the culture flask within 60 days. The cells were then passed to culture flasks for further subculturing. The intervals of subculturing were gradually shortened, from monthly intervals to 12- to 16-day intervals after passage 20. After 27 passages, a continuous cell culture derived from BPH was established and used for viral infection.
Fresh RRSV inoculum for infecting BPH cells was prepared from infected plants, essentially as described previously (22). Synchronous infection of cultured cells of BPH by RRSV was developed as described by Kimura (22).
Baculovirus expression of P3, Pns6 and Pns10 of RRSV.
Baculovirus expression of P3, Pns6, or Pns10 was performed according to the manufacturer's instructions (Invitrogen). Briefly, recombinant baculovirus vectors containing P3, Pns6, or Pns10 were introduced into DH10Bac for transposition into the bacmid. The recombinant bacmids were transfected into Sf9 cell via Cellfectin reagent (Invitrogen). Sf9 cells infected with recombinant bacmids were processed for immunofluorescence microscopy.
Immunofluorescence microscopy.
Rabbit polyclonal antisera against structural proteins P3 and P8 and nonstructural proteins Pns6 and Pns10 of RRSV were prepared as described previously (2). IgG isolated from polyclonal antisera was directly conjugated to fluorescein isothiocyanate (FITC), rhodamine, or Alexa Fluor 647 carboxylic acid according to the instructions of the user manual (Invitrogen). BPH cells infected with RRSV or Sf9 cells infected with recombinant bacmids on coverslips were fixed for 30 min in 4% paraformaldehyde, permeabilized for 5 min in 0.1% Triton X-100, and then processed for immunofluorescence microscopy, as described previously (2, 23). Cells on coverslips were incubated with a 50-fold-diluted solution of the directly conjugated IgG. Samples were then imaged by a Leica TCS SP5 confocal microscope, as described previously (2, 23).
Immunoelectron microscopy.
BPH cells infected with RRSV or Sf9 cells on coverslips were fixed in 2% paraformaldehyde plus 2% glutaraldehyde, and then processed for immunoelectron microscopy, as described previously (2, 4, 23). Cell sections were treated with antibodies to P3, P8, Pns6, and Pns10 of RRSV and then with anti-rabbit IgG conjugated to 15-nm gold particles (Sigma). Samples were observed with a Hitachi H-7650 electron microscope as described previously (2, 4, 23).
Immunofluorescence detection of newly synthesized viral RNAs.
Infection of BPH cells by RRSV was allowed to proceed for 28 or 56 h, at which time cells were treated for 1 h with 10 μg of actinomycin D (Sigma)/ml to inhibit cellular RNA polymerase II (24). Cells were then transfected with 10 mM BrUTP (Sigma) via Cellfectin reagent and incubated for an additional 1 h before fixation and immunofluorescence microscopy. BrUTP-labeled viral RNA was immunostained with anti-BrdU from mouse (Sigma), followed by anti-mouse IgG conjugated to FITC (Sigma).
Yeast two-hybrid assay.
The yeast two-hybrid assay for detecting interactions among P3, Pns6, and Pns10 of RRSV was performed according to the instructions in the DUALmembrane starter kit user manual (Dualsystems Biotech). The prey and bait vectors containing P3, Pns6, or Pns10 were cotransformed into yeast strain NMY51 using a yeast transformation kit (Dualsystems Biotech). Independent positive transformants were selected and grown in SD/−Leu/−Trp/−His/−Ade liquid medium at 30°C for 4 days.
Effect of synthesized dsRNAs from Pns6 and Pns10 genes of RRSV on viroplasm formation and viral infection in the continuous cell cultures of BPH.
A DNA fragment spanning a 1,000-bp segment of Pns6 gene or a 793-bp segment of Pns10 gene of RRSV was used for dsRNA synthesis according to the protocol of a T7 RiboMAX Express RNAi system kit (Promega), as described previously (20). BPH cells on coverslips were transfected with 0.5 μg of dsRNAs/μl from the Pns6 gene (dsPns6), from the Pns10 gene (dsPns10), or from the green fluorescence protein (GFP)-encoding gene (dsGFP) via Cellfectin reagent (Invitrogen), as reported by Chen et al. (23). After 24 h, the cells were inoculated with RRSV and grown further in culture medium. The cells were fixed 30 or 84 hpi and processed for immunofluorescence microscopy. Alternatively, at 84 hpi, total proteins were extracted from infected cells and further analyzed by immunoblotting with antibodies against P3, P8, Pns6, and Pns10, respectively. Insect actin was detected with actin-specific antibodies (Sigma) as a control. Furthermore, viral dsRNAs were isolated from BPH cells and then separated in 10% polyacrylamide gels, as described previously (25).
RESULTS
Establishment of continuous cell cultures derived from BPH.
Primary cell cultures of BPH, originally established from the embryonic fragments dissected from BPH eggs that had been oviposited 8 days earlier, were maintained at 25°C in Kimura's insect medium. After 27 passages of subculturing at 12- to 16-day intervals, the monolayer of the epithelium-like cells, approximately 45 to 65 μm in diameter, was established (Fig. 1). About 15% of frozen BPH cells after 1 year of storage in liquid nitrogen were recovered by the procedure described previously (3; data not shown). This is the first report of the establishment of a continuous BPH cell line. These vector cells in a monolayer (VCM) were used to investigate early infection of RRSV in its insect vector.
FIG 1.

Monolayer of established continuous cell culture derived from BPH after 27 passages of subculturing. Bar, 30 μm.
Viral nonstructural proteins Pns6 and Pns10 colocalized in viroplasm matrix during viral infection.
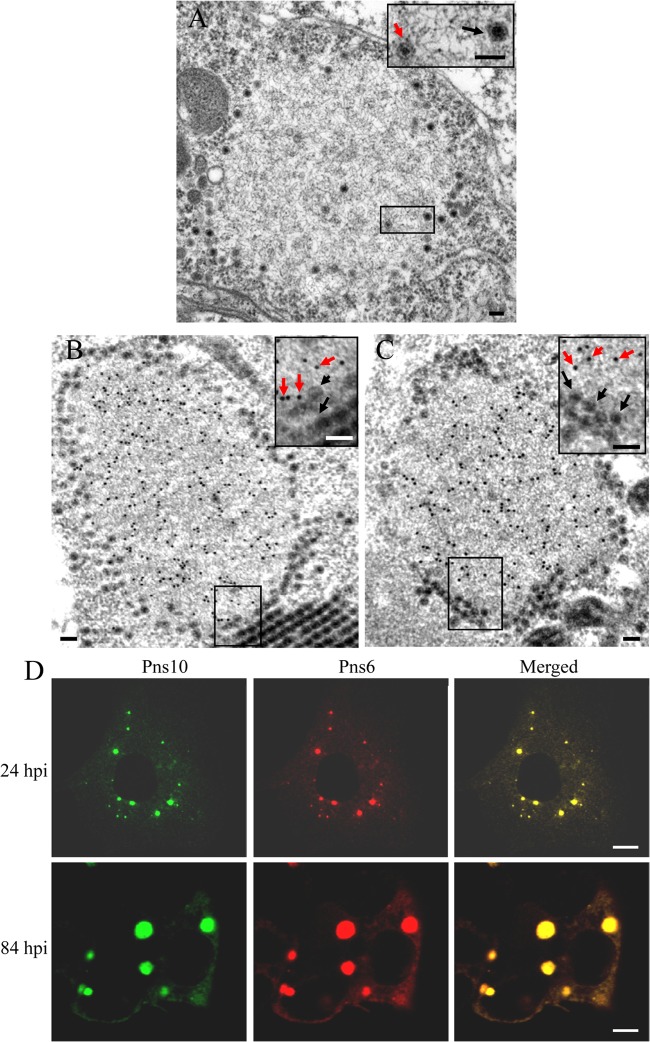
The persistent-propagative infection of RRSV in the bodies of BPH initiates the formation of the viroplasms for viral replication and the assembly of progeny virions (20). To determine the roles of nonstructural proteins Pns6 and Pns10 in the formation of the viroplasm matrix, we used immunoelectron microscopy to localize these two proteins in infected VCMs at 84 hpi. In ultrathin sections of RRSV-infected VCMs, electron-dense inclusions with core-like particles of ∼50 nm in diameter interspersed within or with double-layered particles of ∼70 nm in diameter distributed at the periphery of the matrix, namely, viroplasms (Fig. 2A). Our results showed that both Pns6 and Pns10 antibodies reacted specifically with the matrix of viroplasm, but they were not associated with virions (Fig. 2B and C).
FIG 2.
Viral nonstructural proteins Pns6 and Pns10 are the constituents of viroplasm matrix induced by RRSV infection in VCMs. (A) Morphogenesis of viroplasm. Inset is enlarged image of the boxed area. Black arrows: intact virions, red arrows: core-like particles. Bars, 100 nm. (B and C) Immunogold labeling of Pns6 (B) and Pns10 (C) in the viroplasm matrix at 84 hpi. VCMs were labeled for Pns6 and Pns10 with Pns6- and Pns10-specific antibodies in panels B and C, respectively, as primary antibodies, followed by anti-rabbit IgG conjugated to 15-nm gold particles as secondary antibodies. Insets show enlarged images of the boxed areas. Black arrows, virions; red arrows, gold particles. Bars, 100 nm. (D) Confocal immunofluorescence micrographs of the colocalization of Pns6 and Pns10 within the viroplasm. RRSV-infected VCMs were immunostained with Pns10-FITC and Pns6-rhodamine at 24 and 84 hpi. Bars, 5 μm.
To confirm our observations, we immunostained RRSV-infected VCMs with Pns10-specific IgG conjugated to FITC (Pns10-FITC) and Pns6-specific IgG conjugated to rhodamine (Pns6-rhodamine). As early as 24 hpi, Pns6 and Pns10 were colocalized in numerous, small viral inclusions throughout the cytoplasm of infected VCMs (Fig. 2D, 24 hpi). As infection proceeded, by 84 hpi, Pns6 and Pns10 continued to colocalize in viral inclusions as they grew in size but decreased in number (Fig. 2D, 84 hpi). These viral inclusions obviously corresponded to the viroplasm viewed with electron microscopy (Fig. 2B and C). Taken together, our results indicated that viral nonstructural proteins Pns6 and Pns10 were the major constituents of the matrix of viroplasm in virus-infected cells.
Progeny core particles assembled inside the viroplasm matrix, while progeny virions assembled at the periphery of the matrix.
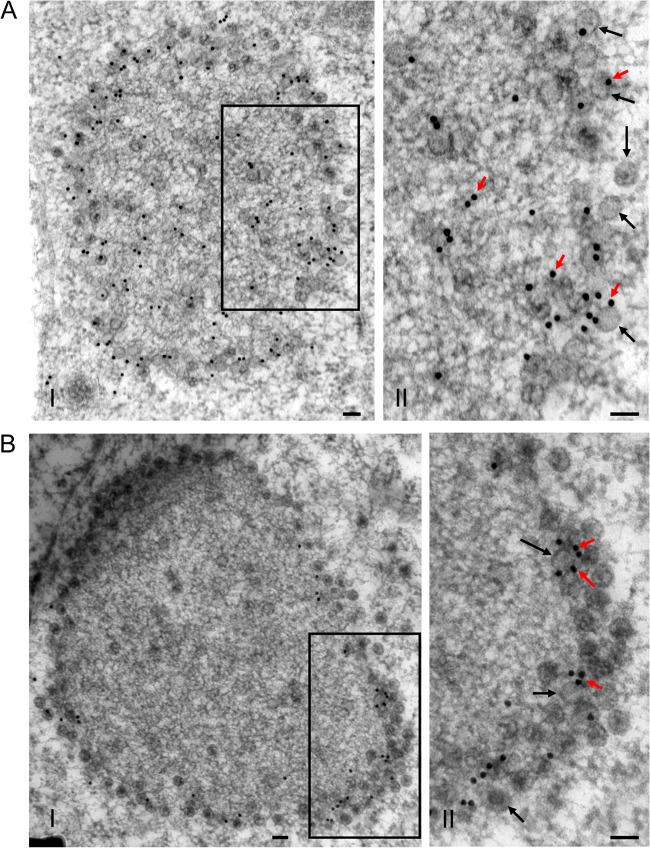
To further confirm whether the viroplasm matrix was the site for assembly of progeny virions during virus infection, we used immunoelectron microscopy to observe the distribution of the core capsid protein P3 and outer capsid protein P8 within the viroplasm. P3 antibodies reacted strongly with the viroplasm matrix and virions at the periphery of the viroplasm (Fig. 3A). Furthermore, P8 antibodies specifically reacted with virions at the periphery of the viroplasm, but not with the viroplasm matrix (Fig. 3B).
FIG 3.
Distribution of core capsid protein P3 and outer capsid protein P8 of RRSV within the viroplasm matrix in virus-infected VCMs at 84 hpi. VCMs were labeled for P3 (A) and P8 (B) with P3- and P8-specific antibodies, respectively, as primary antibodies, followed by anti-rabbit IgG conjugated to 15-nm gold particles as secondary antibodies. The II panels show enlarged images of the boxed areas in the I panels. Black arrows, virions; red arrows, gold particles. Bars, 100 nm.
To confirm our observations, we examined the distribution of structural proteins relative to viral inclusions of Pns6 using immunofluorescence microscopy. Infected cells were immunostained with core capsid protein P3-specific IgG conjugated to FITC (P3-FITC) and Pns6-rhodamine, with outer capsid protein P8-specific IgG conjugated to FITC (P8-FITC) and Pns6-rhodamine, or with P8-FITC and P3-specific IgG conjugated to rhodamine (P3-rhodamine). As early as 24 hpi, numerous small viral inclusions of Pns6 were clearly observed, but the structural proteins P3 and P8 still did not form clear fluorescent inclusions (Fig. 4A and B, 24 hpi). By 30 hpi, the core capsid protein P3 was colocalized with viral inclusions of Pns6, while the outer capsid protein P8 was localized to the ring-like structures at the margin of viral inclusions of Pns6 (Fig. 4A and B, 30 hpi). By 84 hpi, larger viral inclusions of Pns6 were observed, with the core capsid protein P3 concentrated in the center and the outer capsid protein P8 concentrated at the periphery (Fig. 4A, 84 hpi). All of these results indicated that progeny core particles assembled inside the viroplasm matrix, while progeny virions assembled at the periphery of the matrix.
FIG 4.
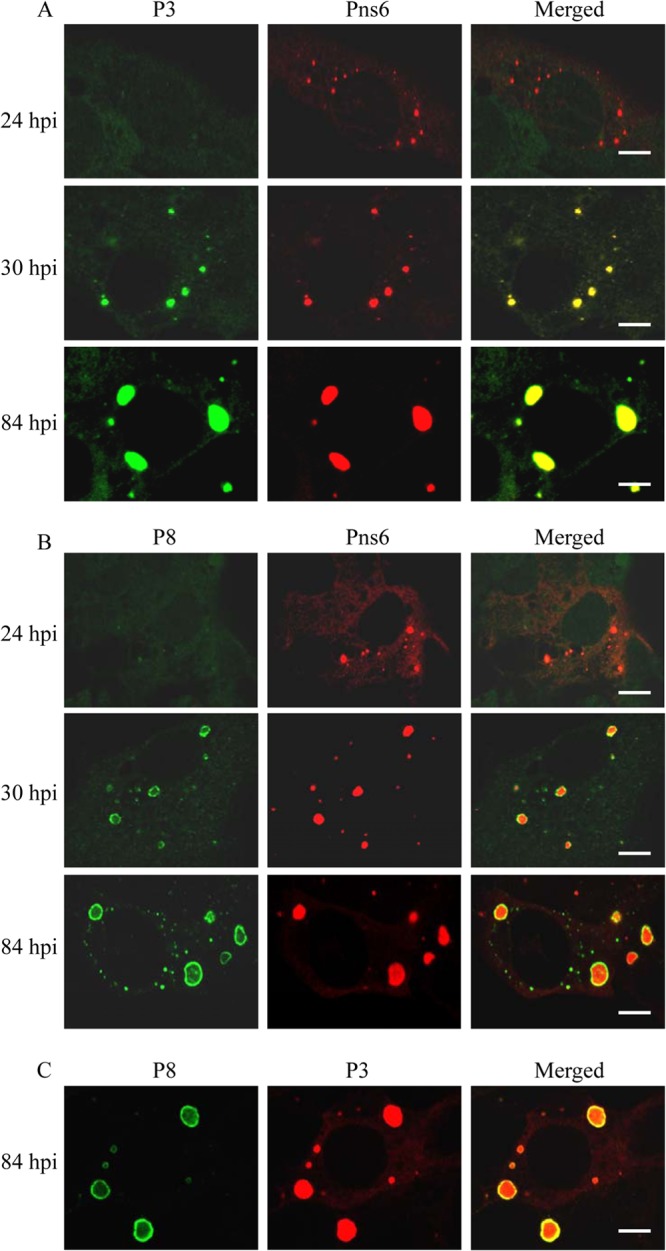
Confocal immunofluorescence micrographs of the colocalization of RRSV core protein P3 and outer capsid protein P8 with nonstructural protein Pns6 within the viroplasm matrix induced by RRSV infection in VCMs. RRSV-infected VCMs were immunostained with P3-FITC and Pns6-rhodamine (A), with P8-FITC and Pns6-rhodamine (B), or with P8-FITC and P3-rhodamine (C). The hours postinfection (hpi) are indicated. Bars, 5 μm.
Accumulation of newly synthesized viral RNAs within the viroplasm matrix.
Our electron and confocal microscopic observations indicated that core particles of RRSV are embedded in the viroplasm matrix in infected cells. Because core particles synthesize the plus-strand RNAs (1, 26), we hypothesized that the localization of core particles of RRSV to the viroplasm may result in the production of viral RNAs within the viroplasm during infection. To test this hypothesis, we investigated the distribution of newly synthesized viral RNAs within the viroplasm matrix by colocalization of BrUTP and Pns6. Our results showed that RRSV RNAs were accumulated within the viroplasm matrix at 30 and 58 hpi (Fig. 5), supporting our hypothesis that the newly synthesized viral RNAs accumulated inside the viroplasm matrix during viral infection.
FIG 5.
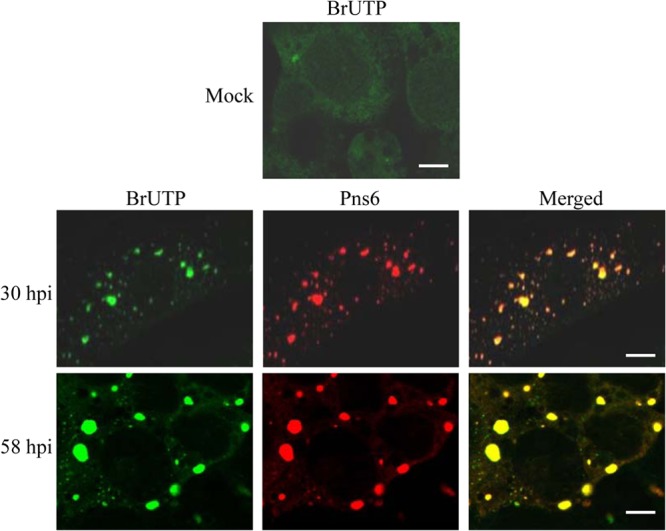
Intracellular sites of viral RNAs synthesis in mock- or RRSV-infected VCMs. BrUTP-labeled viral RNA was immunostained with anti-BrdU from mouse, followed by anti-mouse IgG conjugated to FITC. Viroplasm matrix is immunostained with Pns6-rhodamine. The hours postinfection (hpi) are indicated. Bar, 5 μm.
Core protein P3 was recruited to viroplasm through interaction with Pns6.
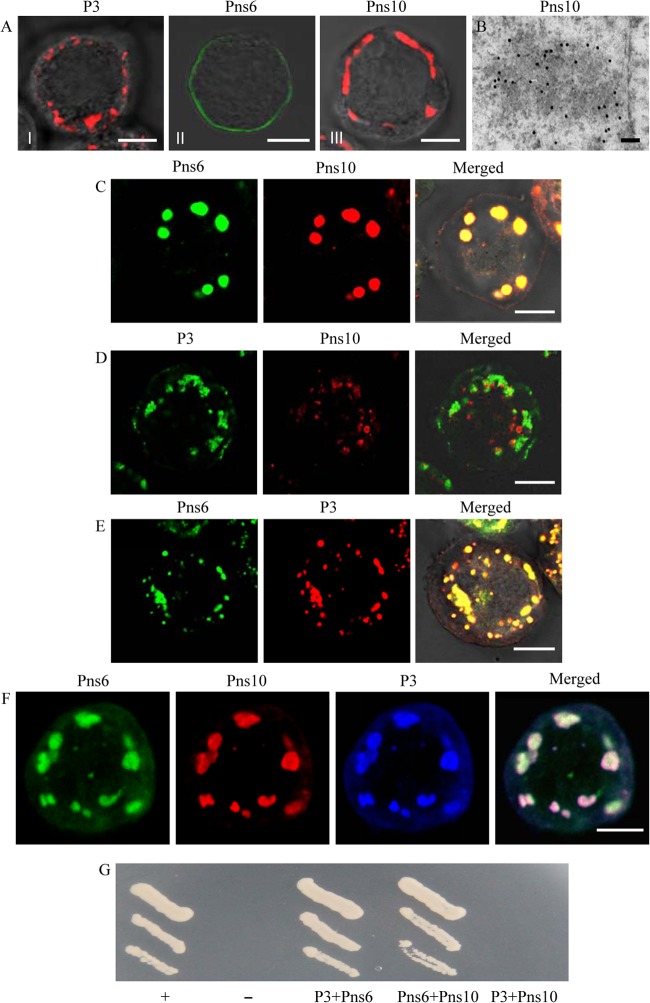
Given our observations that viral nonstructural proteins Pns6 and Pns10, as well as the core protein P3, are concentrated within the viroplasm matrix in infected cells, we hypothesized that Pns6 or Pns10 may recruit and concentrate P3 to the viroplasm. To address this hypothesis, we coinfected Sf9 cells with recombinant baculoviruses containing P3, Pns6, or Pns10 and examined the localization by immunofluorescence microscopy. When expressed alone, P3 or Pns10 aggregated to form punctate inclusions in the cytoplasm, and Pns6 was associated with the plasma membrane (Fig. 6A). Furthermore, immunoelectron microscopy showed that Pns10 antibodies reacted with the electron-dense inclusions, whose morphology was similar to viroplasm matrix (Fig. 6B). The coinfection led to the redistribution of Pns6 into the inclusions of Pns10 (Fig. 6C), as described previously (20). In contrast, the coinfection showed that the inclusions of P3 did not overlap the inclusions of Pns10 (Fig. 6D). However, Pns6 was clearly associated with P3 on the inclusions (Fig. 6E). As expected, these three proteins could colocalize on the inclusions when we triply infected Sf9 cells with recombinant baculoviruses containing P3, Pns6, or Pns10 (Fig. 6F). The yeast two-hybrid assay further confirmed that Pns6 interacted directly with P3 or Pns10, whereas Pns10 did not interact with P3 (Fig. 6G). These findings, combined with our observation that nonstructural proteins Pns6 and Pns10, preceded the core capsid protein P3 in localization to viroplasms (Fig. 4), provided compelling evidence that the core capsid protein P3 was recruited to the viroplasm matrix via its direct interaction with Pns6 in virus-infected cells.
FIG 6.
Core protein P3 was recruited to the viroplasm matrix through interaction with Pns6. (A to D) The localization of Pns6, Pns10, and P3 in the absence of viral infection. Sf9 cells infected with recombinant baculoviruses containing Pns6, Pns10, or P3 were fixed 3 days after infection and processed for immunofluorescence and immunoelectron microscopy. (A) Pns6, Pns10, and P3 were expressed alone. Sf9 cells were immunostained with P3-rhodamine, Pns6-specific IgG conjugated to FITC (Pns6-FITC) or Pns10-specific IgG conjugated to rhodamine (Pns10-rhodamine). Bars, 5 μm. (B) Immunogold labeling of Pns10 associated with electron-dense inclusion in Sf9 cells infected with recombinant baculovirus containing Pns10. Cells were immunostained with Pns10 antibodies and goat antibodies against rabbit IgG that had been conjugated with 15-nm-diameter gold particles as secondary antibodies. Bar, 100 nm. (C) Pns6 and Pns10 were coexpressed. Sf9 cells were immunostained with Pns6-FITC and Pns10-rhodamine. Bar, 5 μm. (D) P3 and Pns10 were coexpressed. Cells were immunostained with P3-FITC and Pns10-rhodamine. Bar, 5 μm. (E) P3 and Pns6 were coexpressed. Sf9 cells were immunostained with Pns6-FITC and P3-rhodamine. Bar, 5 μm. (F) Pns6, Pns10, and P3 were coexpressed. Sf9 cells were immunostained with Pns6-FITC, Pns10-rhodamine, and P3-specific IgG conjugated to Alexa Fluor 647-carboxylic acid. Bar, 5 μm. (G) Yeast two-hybrid assay of protein-protein interactions among the RRSV P3, Pns6, and Pns10 proteins. Transformants were plated on SD/−Leu/−Trp/−His/−Ade medium for 4 days. +, Positive control, i.e., pBT3-STE+pOst1-NubI; –, negative control, i.e., pBT3-STE+pPR3-N; P3+Pns10, pBT3-STE-P3+pPR3-N-Pns10; Pns6+Pns10, pBT3-STE-Pns6+pPR3-N-Pns10; P3+Pns6, pBT3-STE-P3+pPR3-N-Pns6.
RNAi induced by dsRNAs from Pns6 and Pns10 genes inhibited the assembly of viroplasms and viral infection in insect vector cell cultures.
Our previous study showed that RNA interference (RNAi) induced by ingestion of dsRNA from Pns10 gene of RRSV via membrane feeding knocked down Pns10 expression and strongly inhibited viroplasm formation, preventing efficient viral infection in its insect vectors (20). To determine whether Pns6 and Pns10 play a crucial role in viral replication or assembly in cultured insect vector cells, we use RNAi strategy to knock down the expression of Pns6 or Pns10 and then analyzed the effects on the formation of viroplasm and viral infection in virus-infected VCMs. At 30 or 84 hpi, immunofluorescence microcopy showed that the treatment of dsPns6 or dsPns10 significantly inhibited the formation of viroplasm containing Pns6 and Pns10, and the accumulation of core or virions containing P3 or P8 (Fig. 7 and 8 and data not shown). As expected, the expression viral proteins (Pns6, Pns10, P3, and P8) and the synthesis of viral dsRNAs were significantly decreased in VCMs transfected with dsPns6 or dsPns10 (Fig. 9). Taken together, all of these results suggested that Pns6 and Pns10 of RRSV played critical roles in viral replication and assembly during viral infection in insect vector cells.
FIG 7.
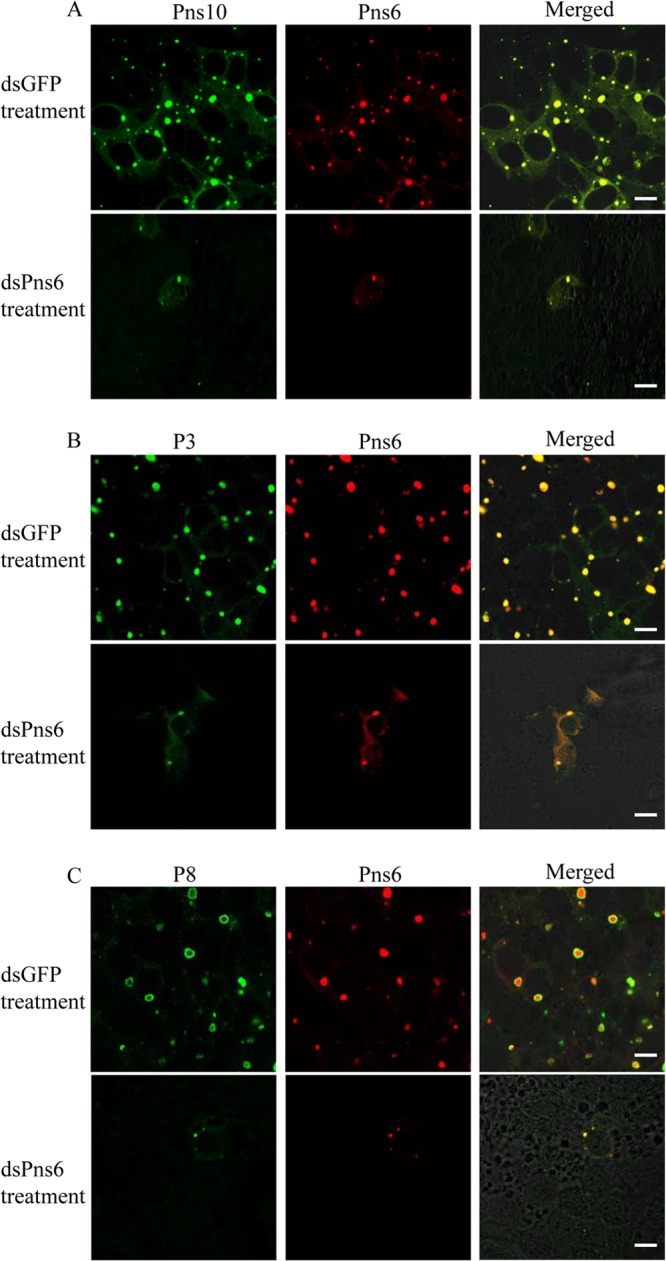
RNAi induced by dsPns6 inhibited the assembly of viroplasm and the infection of RRSV in VCMs. At 24 h after transfection with dsGFP or dsPns6, VCMs were inoculated with RRSV. At 84 hpi, VCMs were immunostained with Pns10-FITC and Pns6-rhodamine (A), with P3-FITC and Pns6-rhodamine (B), or with P8-FITC and Pns6-rhodamine (C) and then examined with confocal microscopy. Images are representative results of multiple experiments with multiple preparations. Bars, 10 μm.
FIG 8.
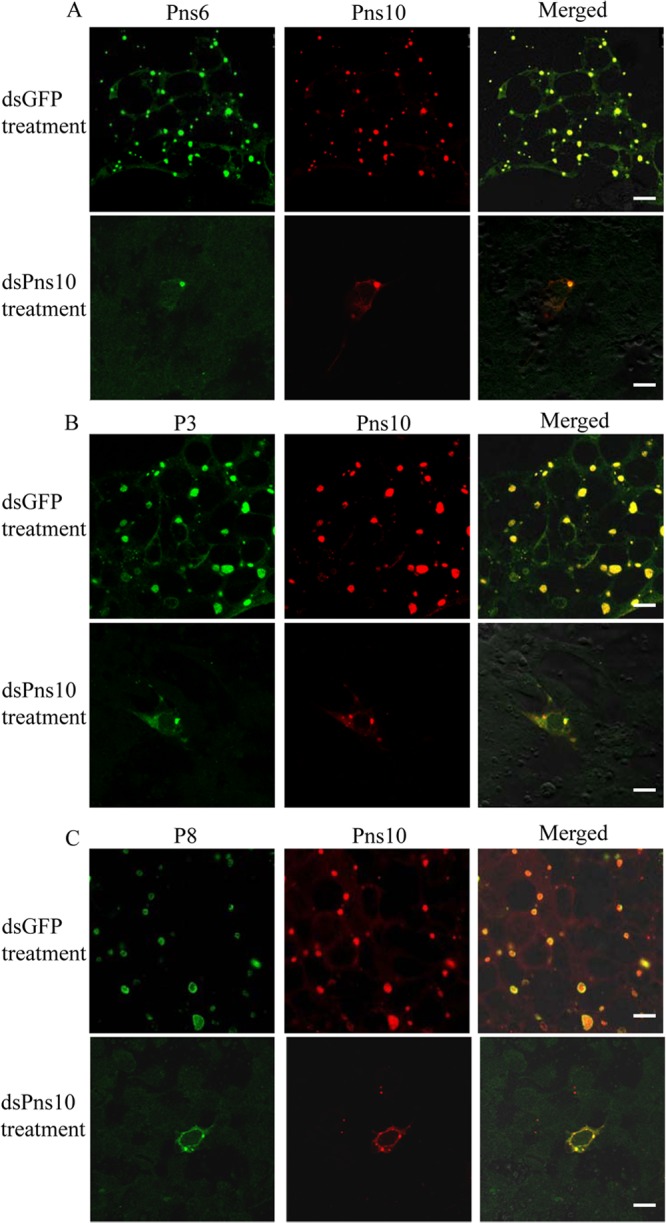
RNAi induced by dsPns10 inhibited the assembly of viroplasm and the infection of RRSV in VCMs. At 24 h after transfection with dsGFP or dsPns10, VCMs were inoculated with RRSV. At 84 hpi, VCMs were immunostained with Pns6-FITC and Pns10-rhodamine (A), with P3-FITC and Pns10-rhodamine (B), or with P8-FITC and Pns10-rhodamine (C) and then examined with confocal microscopy. Images are representative results of multiple experiments with multiple preparations. Bars, 10 μm.
FIG 9.
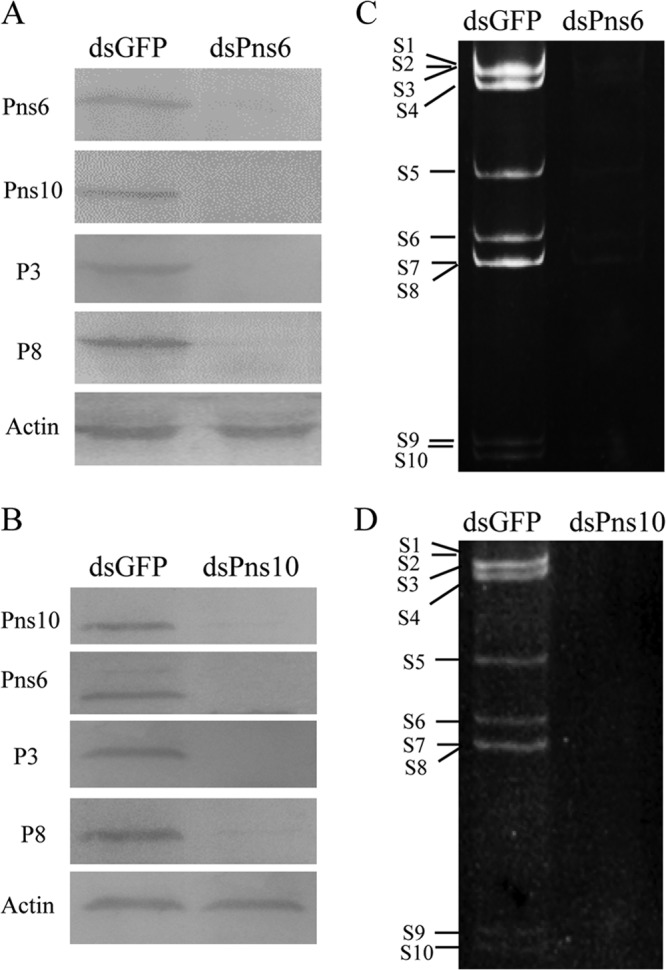
Inhibition of viral protein expression and dsRNA synthesis in VCMs by RNAi induced by dsPns6 or dsPns10. At 24 h after transfection with dsGFP, dsPns6, or dsPns10, VCMs were inoculated with RRSV and kept in cultured medium for 84 h. (A and B) Lysates prepared from infected cells were analyzed by immunoblotting with polyclonal antisera against P3, P8, Pns6, and Pns10 of RRSV. An actin-specific antibody was used as control. (C and D) Viral dsRNAs from infected VCMs was resolved by electrophoresis in 10% polyacrylamide gels and analyzed by ethidium bromide staining. The size classes of viral dsRNA segments are indicated.
DISCUSSION
The brown planthopper (BPH; Nilaparvata lugens), a typical large-scale migratory rice pest in Asia, is a major vector of RRSV. In the present study, for the first time, we successfully established the continuous cell cultures derived from BPH. The unique uniformity of BPH cells in the monolayer (VCM) supports a consistent, synchronous infection of its insect vector cells by RRSV, allowing us to define the early process of viral replication and assembly during viral infection. After the entry of the virus into the cytoplasm of the VCMs, the RRSV core particles transcribe viral plus-strand RNAs, as in the case of other viruses in the family Reoviridae (1, 26). The nonstructural proteins Pns6 and Pns10 of RRSV were expressed first by the host's cellular translational machinery, then associated together to form the initial viroplasm matrix, the site for viral replication and assembly (Fig. 2). Pns10 formed viroplasm-like inclusions in the absence of viral infection, suggesting that the viroplasm matrix induced by RRSV infection was formed primarily by Pns10 (20). Pns6 was recruited in the viroplasm matrix by direct interaction with Pns10 (Fig. 6). Thus, both Pns6 and Pns10 seem to play important roles in the early stages of the viral life cycle by recruiting or retaining components necessary for viral replication and assembly.
To determine whether Pns6 and Pns10 of RRSV play a crucial role in viral replication and assembly, we used the RNAi strategy to knock down the expression of Pns6 and Pns10 in virus-infected VCMs. Our results indicated that the knockdown of Pns6 and Pns10 expression due to RNAi induced by dsPns6 and dsPns10 led to the significant inhibition of viroplasm formation, virion assembly, viral protein expression, and viral dsRNA synthesis in VCMs (Fig. 7, 8, and 9). This finding, together with the observation that Pns6 and Pns10 were the components of the viroplasm matrix, suggested that Pns6 and Pns10 were essential for viral replication and assembly in its insect vector cells.
How is Pns6 of RRSV involved in viral replication and assembly in virus-infected insect vector cells? Previous data showed that Pns6 is a viral movement protein and a viral RNA-silencing suppressor in plant hosts (16, 17). Furthermore, Pns6 of RRSV binds to both dsRNA and ssRNA in a sequence-independent manner, showing a preference for RRSV ssRNA over the rice ssRNA (27). Here, we showed that Pns6 rather than Pns10 specifically interacted with core capsid P3 (Fig. 6). Thus, Pns6 may be involved in the recruitment or retention of viral RNAs and core proteins to viroplasm matrix during viral replication or assembly. The direct interaction of Pns6 with core capsid protein P3 indicated that Pns6 has the ability to bind core particles within the interior regions of viroplasm. In RRSV-infected VCMs, core particles localized to the interior regions of viroplasms, while intact virions accumulated in peripheral regions of the viroplasms (Fig. 2, 3, and 4). It seems that the peripheral zone of the viroplasms, which contains both viral core and outer capsid proteins (Fig. 2, 3, and 4), is the site of assembly of progeny virions where outer capsid proteins are attached to core particles. For viruses in the family Reoviridae, the core particles, rather than intact virions, serve as the transcriptase particles for the production of viral plus-strand RNAs (1, 26). Based on these analyses, we deduced that the binding of Pns6 with core capsid protein P3 within the interior regions of viroplasm might delay the attachment of outer capsid proteins to core particles, allowing core particles to continue synthesizing more viral plus-strand RNAs. This hypothesis was supported by our finding that the newly synthesized viral RNAs accumulated abundantly in viroplasm matrix induced by RRSV infection (Fig. 5). In the case of viruses in the family Reoviridae, due to the lack of ribosomes within the viroplasm, viral RNAs produced by core particles could be released into the cytoplasm surrounding the viroplasm for translation of viral proteins (1). Thus, our results suggested that the binding of Pns6 with core capsid protein P3 might benefit the replication and assembly of RRSV in its insect vector cells by enhancing the synthesis of viral RNAs and the expression of viral proteins. The RNA-binding activity of Pns6 suggested that this protein might also function during the assembly of core particles, possibly in the packaging of the 10 different viral genome segments of RRSV in the early steps of progeny core particle assembly. All of these analyses support our hypothesis that Pns6 of RRSV plays important roles in the formation of viroplasm matrix and in the recruitment or retention viral RNAs and proteins to viroplasms for viral replication and assembly.
Viruses in the family Reoviridae encode specific nonstructural proteins that function in forming the organizational viroplasm matrix. For example, μNS of reoviruses, NS2 of orbiviruses, NSP2/NSP5 of rotaviruses, Pns12 of the phytoreovirus RDV, P9-1/P5 of the fijivirus SRBSDV, and Pns10 of RRSV, the oryzavirus under study here, have been shown to be the viral proteins whose expression is required for forming viroplasm-like structures in the absence of viral infection (2, 4, 7, 20, 28–34), suggesting that these proteins might play similar roles in formation of the viroplasm matrix during virus replication cycles. Furthermore, these viroplasm matrix proteins, such as reovirus μNS, orbivirus NS2, rotavirus NSP5, are involved in recruiting or retaining core capsid proteins to their respective viroplasm matrices (32–34). However, in the case of RRSV, an oryzavirus, the core capsid protein is recruited or retained by the nonstructural protein Pns6, rather than the viroplasm matrix protein Pns10, to the viroplasm. Thus, Pns6 and Pns10 of RRSV appeared to “split” the functional duties of reovirus μNS, orbivirus NS2, or rotavirus NSP5.
Based on this discussion, we propose a model for the genesis and maturation of viroplasm induced by RRSV in its insect vector cells. Early in the infection process, Pns6 and Pns10 of RRSV associated together to form the initial viroplasm matrix. During infection, Pns6 might act as a scaffold for the recruitment of core capsid protein P3, whereas other structural proteins are recruited to the viroplasms through interactions with as-yet-unknown factors. RRSV RNA was bound by Pns6 or other viral proteins to form replication and assembly complexes for the production of progeny core particles within the interior regions of viroplasm. Pns6 might bind to core particles to enhance the production of viral RNA by blocking or delaying outer capsid assembly on these particles. The intact virions were assembled at the periphery of the viroplasm. Thus, the development of the continuous BPH cell culture can further advance our understanding of the early infection process of oryzaviruses and related pathogens in their insect vector cells.
By development continuous insect vector cell cultures, we already revealed the mechanisms for the genesis and maturation of the viroplasms induced by plant reoviruses, including phytoreovirus RDV, oryzavirus RRSV, and fijivirus SRBSDV (4, 7; the present study). The viroplasm matrices of RDV and RRSV consist of a granular region for viral replication and the assembly of progeny virions (Fig. 2, 3, and 4) (7). However, in the case of SRBSDV, the viroplasm matrices consist of a granular region for the accumulation of viral RNAs and a filamentous region for the assembly of progeny virions (2, 4). It is interesting to find that the granular region of SRBSDV viroplasm was not suitable for the assembly of progeny virions (4). We determined that the filamentous region of SRBSDV viroplasm would provide a larger surface area for producing many more progeny virions than RDV or RRSV can in their respective insect vector cells (4). These analyses suggest that SRBSDV, a new identified plant reovirus, has evolved to be well adapted for persistent transmission by its WBPH vector, which is consistent with the fact that the minimum latent periods for RDV, RRSV and SRBSDV in their insect vectors are 6, 9, and 14 days, respectively (1, 9, 35, 36).
ACKNOWLEDGMENTS
This study was supported by grants from the programs for the National Basic Research Program of China (2014CB138400 and 2010CB126203), the Specialized Research Fund for the Ministry of Agriculture (201003031 and 201303021), the National Natural Science Foundation of China (31130044 and 31200118), and the Natural Science Foundation of Fujian Province (2012J01087).
Footnotes
Published ahead of print 29 January 2014
REFERENCES
- 1.Attoui H, Mertens PPC, Becnel J, Belaganahalli S, Bergoin M, Brussaard CP, Chappell JD, Ciarlet M, del Vas M, Dermody TS, Dormitzer PR, Duncan R, Fcang Q, Graham R, Guglielmi KM, Harding RM, Hillman B, Makkay A, Marzachì C, Matthijnssens J, Milne RG, Mohd JF, Mori H, Noordeloos AA, Omura T, Patton JT, Rao S, Maan M, Stoltz D, Suzuki N, Upadhyaya NM, Wei C, Zhou H. 2012. Family Reoviridae, p 541–637 In King AMQ, Adams MJ, Carstens EB, Lefkowits EJ. (ed), Virus taxonomy: ninth report of the International Committee for the Taxonomy of Viruses. Academic Press, Inc, New York, NY [Google Scholar]
- 2.Jia D, Chen H, Zheng A, Chen Q, Liu Q, Xie L, Wu Z, Wei T. 2012. Development of an insect vector cell culture and RNA interference system to investigate the functional role of fijivirus replication protein. J. Virol. 86:5800–5807. 10.1128/JVI.07121-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura I, Omura T. 1988. Leafhopper cell cultures as a means for phytoreovirus research. Adv. Dis. Vector Res. 5:111–135 [Google Scholar]
- 4.Mao Q, Zheng S, Han Q, Chen H, Ma Y, Jia D, Chen Q, Wei T. 2013. New model for the genesis and maturation of viroplasms induced by fijiviruses in insect vector cells. J. Virol. 87:6819–6828. 10.1128/JVI.00409-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei T, Chen H, Ichiki-Uehara T, Hibino H, Omura T. 2007. Entry of Rice dwarf virus into cultured cells of its insect vector involves clathrin-mediated endocytosis. J. Virol. 81:7811–7815. 10.1128/JVI.00050-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei T, Kikuchi A, Moriyasu Y, Suzuki N, Shimizu T, Hagiwara K, Chen H, Takahashi M, Ichiki-Uehara T, Omura T. 2006. The spread of Rice dwarf virus among cells of its insect vector exploits virus-induced tubular structures. J. Virol. 80:8593–8602. 10.1128/JVI.00537-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei T, Shimizu T, Hagiwara K, Kikuchi A, Moriyasu Y, Suzuki N, Chen H, Omura T. 2006. Pns12 protein of Rice dwarf virus is essential for formation of viroplasms and nucleation of viral-assembly complexes. J. Gen. Virol. 87:429–438. 10.1099/vir.0.81425-0 [DOI] [PubMed] [Google Scholar]
- 8.Hibino H, Roechan M, Suoarisman S, Tantera DM. 1977. A virus disease of rice (kerdil hampa) transmitted by brown planthopper, Nilaparvata lugens Stål, in Indonesia. Contr. Centr. Res. Inst. Agric. Bogor. 35:1–15 [Google Scholar]
- 9.Hibino H, Saleh N, Roechan M. 1979. Reovirus-like particles associated with rice ragged stunt diseased rice and insect vector cells. Ann. Phytopathol. Soc. Japan 45:228–239. 10.3186/jjphytopath.45.228 [DOI] [Google Scholar]
- 10.Wang H, Xu D, Pu L, Zhou GH. 2014. Southern rice black-streaked dwarf virus alters insect vectors' host orientation preferences to enhance spread and increase Rice ragged stunt virus coinfection. Phytopathology 104:196–201. 10.1094/PHYTO-08-13-0227-R [DOI] [PubMed] [Google Scholar]
- 11.Hagiwara K, Minobe Y, Nozu Y, Hibino H, Kimura I, Omura T. 1986. Component proteins and structures of Rice ragged stunt virus. J. Gen. Virol. 67:1711–1715. 10.1099/0022-1317-67-8-1711 [DOI] [Google Scholar]
- 12.Miyazaki N, Uehara-Ichiki T, Xing L, Bergman L, Higashiura A, Nakagawa A, Omura T, Cheng R. 2008. Structural evolution of Reoviridae revealed by oryzavirus in acquiring the second capsid shell. J. Virol. 82:11344–11353. 10.1128/JVI.02375-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upadhyaya NM, Zinkowsky E, Kositratana W, Waterhouse PM. 1996. The Mr 43K major capsid protein of rice ragged stunt oryzavirus is a posttranslationally processed product of an Mr 67,348 polypeptide encoded by genome segment 8. Arch. Virol. 141:1689–1701. 10.1007/BF01718292 [DOI] [PubMed] [Google Scholar]
- 14.Upadhyaya NM, Ramm K, Gellatly JA, Li Z, Kositratana W, Waterhouse PM. 1997. Rice ragged stunt oryzavirus genome segments S7 and S10 encode nonstructural proteins of Mr 68,025 (Pns7) and Mr 32,364 (Pns10). Arch. Virol. 142:1719–1726. 10.1007/s007050050193 [DOI] [PubMed] [Google Scholar]
- 15.Upadhyaya NM, Ramm K, Gellatly JA, Li Z, Kositratana W, Waterhouse PM. 1998. Rice ragged stunt Oryzavirus genome segment S4 could encode an RNA-dependent RNA polymerase and a second protein of unknown function. Arch. Virol. 143:1815–1822. 10.1007/s007050050419 [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Du Z, Wang C, Cai L, Hu M, Lin Q, Wu Z, Li Y, Xie L. 2010. Identification of Pns6, a putative movement protein of RRSV, as a silencing suppressor. Virol. J. 7:335. 10.1186/1743-422X-7-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, Wu J, Adkins S, Xie L, Li W. 2010. Rice ragged stunt virus segment S6-encoded nonstructural protein Pns6 complements cell-to-cell movement of Tobacco mosaic virus-based chimeric virus. Virus Res. 152:176–179. 10.1016/j.virusres.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 18.Supyani S, Hillman BI, Suzuki N. 2007. Baculovirus expression of the 11 mycoreovirus-1 genome segments and identification of the guanylyltransferase-encoding segment. J. Gen. Virol. 88:342–350. 10.1099/vir.0.82318-0 [DOI] [PubMed] [Google Scholar]
- 19.Zhou GY, Lu XB, Lu HJ, Lei JL, Chen SX, Gong ZX. 1999. Rice ragged stunt oryzavirus: role of the viral spike protein in transmission by the insect vector. Ann. Appl. Biol. 135:573–578. 10.1111/j.1744-7348.1999.tb00888.x [DOI] [Google Scholar]
- 20.Jia D, Guo N, Chen H, Akita F, Xie L, Omura T, Wei T. 2012. Assembly of the viroplasm by viral nonstructural protein Pns10 is essential for persistent infection of rice ragged stunt virus in its insect vector. J. Gen. Virol. 93:2299–2309. 10.1099/vir.0.042424-0 [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Wu W, Chen H, Liu Q, Jia D, Mao Q, Chen Q, Wu Z, Wei T. 2013. An insect cell line derived from the small brown planthopper supports replication of rice stripe virus, a tenuivirus. J. Gen. Virol. 94:1421–1425. 10.1099/vir.0.050104-0 [DOI] [PubMed] [Google Scholar]
- 22.Kimura I. 1986. A study of rice dwarf virus in vector cell monolayers by fluorescent antibody focus counting. J. Gen. Virol. 67:2119–2124. 10.1099/0022-1317-67-10-2119 [DOI] [Google Scholar]
- 23.Chen Q, Chen H, Mao Q, Liu Q, Shimizu T, Uehara-Ichiki T, Wu Z, Xie L, Omura T, Wei T. 2012. Tubular structure induced by a plant virus facilitates viral spread in its vector insect. PLoS Pathog. 8:e1003032. 10.1371/journal.ppat.1003032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu FL. 1980. Selective inhibition of rat liver nuclear RNA polymerase II by actinomycin D in vivo. Carcinogenesis 1:577–581. 10.1093/carcin/1.7.577 [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Chappell JD, Danthi P, Dermody TS. 2006. Gene-specific inhibition of reovirus replication by RNA interference. J. Virol. 80:9053–9063. 10.1128/JVI.00276-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahlquist P. 2006. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat. Rev. Microbiol. 4:371–382. 10.1038/nrmicro1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao CG, Lü HJ, Wu JH, Gong ZX. 2004. Nucleic acid binding activity of pns6 encoded by genome segment 6 of rice ragged stunt oryzavirus. Acta Biochim. Biophys. Sin. 36:457–466. 10.1093/abbs/36.7.457 [DOI] [PubMed] [Google Scholar]
- 28.Fabbretti E, Afrikanova I, Vascotto F, Burrone OR. 1999. Two nonstructural rotavirus proteins, NSP2 and NSP5, form viroplasm-like structures in vivo. J. Gen. Virol. 80:333–339 [DOI] [PubMed] [Google Scholar]
- 29.Miller CL, Arnold MM, Broering TJ, Hastings CE, Nibert ML. 2010. Localization of mammalian orthoreovirus proteins to cytoplasmic factory-like structures via nonoverlapping regions of microNS. J. Virol. 84:867–882. 10.1128/JVI.01571-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theron J, Huismans H, Nel LH. 1996. Site-specific mutations in the NS2 protein of epizootic haemorrhagic disease virus markedly affect the formation of cytoplasmic inclusion bodies. Arch. Virol. 141:1143–1151. 10.1007/BF01718617 [DOI] [PubMed] [Google Scholar]
- 31.Thomas CP, Booth TF, Roy P. 1990. Synthesis of bluetongue virus-encoded phosphoprotein and formation of inclusion bodies by recombinant baculovirus in insect cells: it binds the single-stranded RNA species. J. Gen. Virol. 71:2073–2083. 10.1099/0022-1317-71-9-2073 [DOI] [PubMed] [Google Scholar]
- 32.Broering TJ, Kim J, Miller CL, Piggott CD, Dinoso JB, Nibert ML, Parker JS. 2004. Reovirus nonstructural protein mu NS recruits viral core surface proteins and entering core particles to factory-like inclusions. J. Virol. 78:1882–1892. 10.1128/JVI.78.4.1882-1892.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contin R, Arnoldi F, Campagna M, Burrone OR. 2010. Rotavirus NSP5 orchestrates recruitment of viroplasmic proteins. J. Gen. Virol. 91:1782–1793. 10.1099/vir.0.019133-0 [DOI] [PubMed] [Google Scholar]
- 34.Kar AK, Bhattacharya B, Roy P. 2007. Bluetongue virus RNA binding protein NS2 is a modulator of viral replication and assembly. BMC Mol. Biol. 8:4. 10.1186/1471-2199-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pu LL, Xie GH, Ji CY, Ling B, Zhang MX, Xu DL, Zhou GH. 2012. Transmission characteristics of Southern rice black-streaked dwarf virus by rice planthoppers. Crop Prot. 41:71–76. 10.1016/j.cropro.2012.04.026 [DOI] [Google Scholar]
- 36.Honda K, Wei T, Hagiwara K, Higashi T, Kimura I, Akutsu K, Omura T. 2007. Retention of rice dwarf virus by descendants of pairs of viruliferous vector insects after rearing for 6 years. Phytopathology 97:712–716. 10.1094/PHYTO-97-6-0712 [DOI] [PubMed] [Google Scholar]