LETTER
In their Letter to the Editor “Elimination of Friend Virus in the Absence of CD8+ T Cells,” Tsuji-Kawahara and Miyazawa conclude from their recent study on Friend virus (FV) that “…CD8+ T cells contribute to control of FV infection, but are not essential for the elimination of FV in B6 [C57BL/6] mice” (1). On face value, this conclusion appears to further diminish the importance of the CD8+ T cell response in recovery from retroviral infection, consistent with previous assertions by these authors that CD8+ T cells are prematurely exhausted during acute FV infection (2). The question is whether this conclusion is warranted by the data. We contend that crucial caveats regarding the interpretation of the current results and the vast bulk of the published literature argue strongly against this conclusion (3–5). The authors compared virus clearance in wild-type (WT) and β2-microglobulin-deficient mice and found that while clearance was slower, the deficient mice were able to clear the virus after several weeks of infection. Since β2-microglobulin-deficient mice are also deficient in CD8+ T cells, they concluded that these cells were dispensable for controlling FV infection. Superficially, this conclusion appears sound, but it is well-known that deficiency in one T cell compartment can be compensated by enhanced responses in other cell subsets. For example, we have shown that CD4+ T cells do not normally develop cytotoxic function in response to FV infection, but in the absence of CD8+ T cells, they compensate by producing granzymes and in vivo cytotoxicity against virus-infected cells (6). Similar results have been described for lymphocytic choriomeningitis virus (LCMV) infections (7). Thus, it may be correct that β2-microglobolin-deficient mice, which have developed immune systems largely without CD8+ T cells, may not need such cells to control virus. However, it is overreaching to conclude that CD8+ T cells are also not required in normal C57BL/6 (B6) mice. Short-term antibody-mediated depletion experiments are more informative, since the time period for possible compensatory mechanisms is largely reduced. We previously performed such CD8 depletion experiments in FV-infected susceptible mice and found that they could no longer control either Friend murine leukemia helper virus (F-MuLV) titers or spleen focus-forming virus (SFFV)-induced splenomegaly (5). This result indicated that both viruses of the FV complex were restricted by CD8+ T cells. In fact, the Miyazawa lab has made similar findings (2). Finally, it has long been known that β2-microglobolin-deficient mice are not fully devoid of CD8+ T cells but contain functional CD8+ T cells that can mediate cytotoxicity (8). Such residual CD8+ T cells might have contributed to virus control in the Tsuji-Kawahara and Miyazawa experiment. Along this line, we found that FV titers in the bone marrow of Lyt-2-deficient B6 mice, which are completely devoid of CD8+ T cells (9), were on average more than 50 times higher than those in WT B6 mice at 12 days postinfection (Fig. 1). Overall, our results indicate a much more important role for CD8+ T cells in retrovirus control than attributed by Tsuji-Kawahara and Miyazawa.
FIG 1.
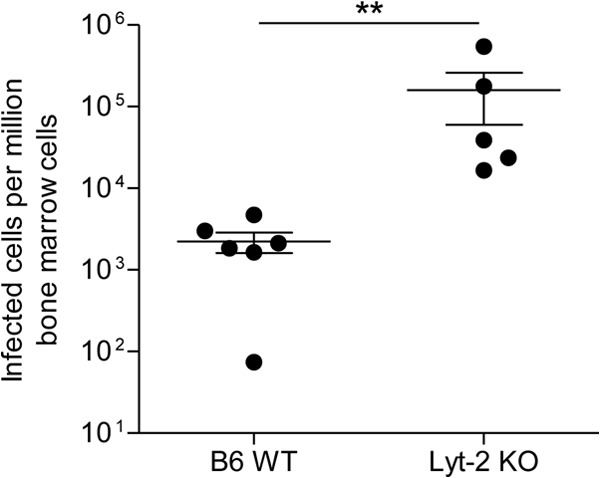
Viral loads in the bone marrow of FV-infected CD8-deficient mice. B6 wild-type (WT) and CD8-deficient Lyt-2 knockout (KO) mice were infected with 20,000 spleen focus-forming units (SFFV) lactate dehydrogenase-elevating virus (LDV)-free FV and assayed for bone marrow viral loads at 12 days postinfection using an infectious center assay. Each circle represents the value for a single mouse, and the bars indicate the means ± standard error of the means (SEM) (error bars) for the group of mice. Differences between the groups were analyzed by a t test (**, P < 0.005). These animal experiments were performed in strict accordance with the German regulations of the Society for Laboratory Animal Science (GV-SOLAS) and the European Health Law of the Federation of Laboratory Animal Science Associations (FELASA). Protocols were approved by the North Rhine-Westphalia State Agency for Nature, Environment and Consumer Protection (LANUV).
Footnotes
For the author reply, see doi:10.1128/JVI.00343-14.
REFERENCES
- 1.Tsuji-Kawahara S, Miyazawa M. 2014. Elimination of Friend retrovirus in the absence of CD8+ T cells. J. Virol. 88:1854–1855. 10.1128/JVI.03271-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takamura S, Tsuji-Kawahara S, Yagita H, Akiba H, Sakamoto M, Chikaishi T, Kato M, Miyazawa M. 2010. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. J. Immunol. 184:4696–4707. 10.4049/jimmunol.0903478 [DOI] [PubMed] [Google Scholar]
- 3.Zelinskyy G, Dietze K, Sparwasser T, Dittmer U. 2009. Regulatory T cells suppress antiviral immune responses and increase viral loads during acute infection with a lymphotropic retrovirus. PLoS Pathog. 5:e1000406. 10.1371/journal.ppat.1000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zelinskyy G, Dietze KK, Husecken YP, Schimmer S, Nair S, Werner T, Gibbert K, Kershaw O, Gruber AD, Sparwasser T, Dittmer U. 2009. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood 114:3199–3207. 10.1182/blood-2009-03-208736 [DOI] [PubMed] [Google Scholar]
- 5.Zelinskyy G, Myers L, Dietze KK, Gibbert K, Roggendorf M, Liu J, Lu M, Kraft AR, Teichgraber V, Hasenkrug KJ, Dittmer U. 2011. Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute Friend retrovirus infection but are highly cytotoxic and control virus replication. J. Immunol. 187:3730–3737. 10.4049/jimmunol.1101612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzke N, Akhmetzyanova I, Hasenkrug KJ, Trilling M, Zelinskyy G, Dittmer U. 2013. CD4+ T cells develop antiretroviral cytotoxic activity in the absence of regulatory T cells and CD8+ T cells. J. Virol. 87:6306–6313. 10.1128/JVI.00432-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller D, Koller BH, Whitton JL, LaPan KE, Brigman KK, Frelinger JA. 1992. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science 255:1576–1578. 10.1126/science.1347959 [DOI] [PubMed] [Google Scholar]
- 8.Glas R, Franksson L, Ohlen C, Hoglund P, Koller B, Ljunggren HG, Karre K. 1992. Major histocompatibility complex class I-specific and -restricted killing of beta 2-microglobulin-deficient cells by CD8+ cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 89:11381–11385. 10.1073/pnas.89.23.11381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung-Leung WP, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. 1991. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell 65:443–449. 10.1016/0092-8674(91)90462-8 [DOI] [PubMed] [Google Scholar]


