Abstract
Latent membrane protein 2A (LMP2A) of Epstein-Barr virus (EBV) is widely expressed in EBV-associated malignancies. We demonstrate that LMP2A has a transformation ability. This study shows that LMP2A-induced transformation in several human nonhematopoietic cell lines was blocked in those cells expressing an immunoreceptor tyrosine-based activation motif (ITAM) LMP2A mutant. The Syk inhibitor or Syk-specific small interfering RNA (siRNA) inhibited LMP2A-induced transformation. These results indicate that the interaction of the LMP2A ITAM with Syk is a key step for LMP2A-mediated transformation.
TEXT
Epstein-Barr virus (EBV), a ubiquitous human herpesvirus, is an important human tumor virus that is causally associated with various lymphoid and epithelioid malignancies, including Hodgkin's lymphoma, Burkitt's lymphoma, nasopharyngeal carcinoma, and gastric carcinoma (1). Characteristic of the herpesvirus family, EBV can persist in the human host as a lifelong latent infection; however, the molecular mechanisms underlying EBV's persistence and contribution to cancer remain poorly understood. Latent membrane protein 2A (LMP2A) is routinely detected in most EBV-related malignancies (1–4) and therefore may be an important risk factor in EBV-associated tumorigenesis.
LMP2A, consisting of a long N-terminal tail, 12 membrane-spanning domains, and a short C-terminal tail, forms aggregate patches on the surfaces of latent-infected cells and can also localize in perinuclear regions (5). The N-terminal tail of LMP2A contains eight constitutively phosphorylated tyrosine residues and several proline-rich regions that are essential for interactions between LMP2A and other cellular proteins (2, 6). The intracellular N terminus of LMP2A contains multiple functional domains, including an immunoreceptor tyrosine-based activation motif (ITAM) homologous to that found in B-cell receptor (BCR) Igα and Igβ signaling subunits (7–9). LMP2A associates with, in addition to the ITAM, Src family protein tyrosine kinases (PTKs) and Syk PTK, which normally form part of the BCR signaling complex (7, 10, 11). Further, LMP2A contains two polyproline motifs that have been shown to recruit Nedd4 ubiquitin ligases (8, 12, 13).
Previous studies have shown that LMP2A is capable of transforming cells, altering epithelial cell motility, and inhibiting epithelial cell differentiation (14–19). We showed previously that LMP2A induces anchorage-independent cell growth, a parameter indicative of transformation (20), in a human gastric carcinoma cell line, HSC-39 (16, 21). In addition, previous work on viral proteins with ITAMs (Kaposi's sarcoma-associated herpesvirus [KSHV] K1, EBV LMP2A, and mouse mammary tumor virus [MMTV] Env) indicated that expression of these proteins in nonhematopoietic cells results in transformation (16, 19, 22, 23). Further, the transforming ability of LMP2A in a human keratinocyte cell line, HaCaT, is variable (16, 19). LMP2A expression in a normal human foreskin keratinocyte (HFK) cell line also did not confer anchorage-independent cell growth or tumor formation in nude mice (8). Based on our studies and those of others exploring the functions of LMP2A in various cell types (4, 15, 16, 19), we propose that LMP2A's effect on virus-induced tumorigenesis arises from a combination of relative effects of (i) host cell type and origin, (ii) host cellular transformation activity, and (iii) LMP2A-induced signaling pathways (16).
In the relationship between these viral transmembrane ITAM-containing proteins and transformation activity, little is known about the role of the LMP2A ITAM in nonhematopoietic cell transformation. With everything taken into consideration, according to our proposal, the purposes of this study are to clarify the generality of the transforming ability of LMP2A in several human cell lines and to investigate the role of the LMP2A ITAM in LMP2A-induced cell transformation. Thus, to clarify the two primary purposes of this study, we used several human cell lines, a human gastric carcinoma cell line, HSC-39 (16, 21), a human breast cancer cell line, MCF-7 (24), and a human epithelial cell line transformed by DNA from adenovirus, 293 (25), as in vitro models for evaluating the ability of LMP2A to mediate transformation.
The LMP2A ITAM is required for LMP2A-mediated Akt phosphorylation and anchorage-independent cell growth in several human cell lines.
LMP2A activates the phosphoinositol 3-kinase (PI3-K)/Akt pathway in B cells and epithelial cells (19, 26, 27) and induces anchorage-independent cell growth in a human gastric carcinoma cell line, HSC-39, through constitutive activation of the Ras/PI3-K/Akt pathway (16). To determine if the ITAM of LMP2A is critical for LMP2A-mediated Akt phosphorylation and anchorage-independent cell growth, HSC-39, MCF-7, and 293 cells were transduced with a control retroviral vector (164, a pLXSN-based retroviral vector) or one that expresses LMP2A or LMP2A with a mutant ITAM (in which tyrosines at positions 74 and 85 are mutated to phenylalanine [LMP2A-Y74/85F]) (7). Following transduction, stable G418-resistant clones were selected and subjected to immunoblotting for analysis of LMP2A expression and Akt phosphorylation (Fig. 1A). As shown previously, LMP2A induced Akt phosphorylation and anchorage-independent cell growth in HSC-39, MCF-7, and 293 cell clones (clone 1 [Cl-1], Cl-2, Cl-3); however, these effects, significantly, were not observed in LMP2A-Y74/85F-expressing HSC-39, MCF-7, and 293 cell clones (Cl-1, Cl-2, Cl-3) or in vector control clones (Cl-1, Cl-2) (Fig. 1) under optimal conditions. In addition, HSC-39 cell clones expressing wild-type LMP2A formed colonies that were significantly larger (diameter, >200 μm) than those of clones expressing LMP2A-Y74/85F (Fig. 1B). Furthermore, LMP2A-expressing HSC-39, MCF-7, and 293 cells formed colonies and exhibited a spherical shape in soft agarose (Fig. 1B).
FIG 1.
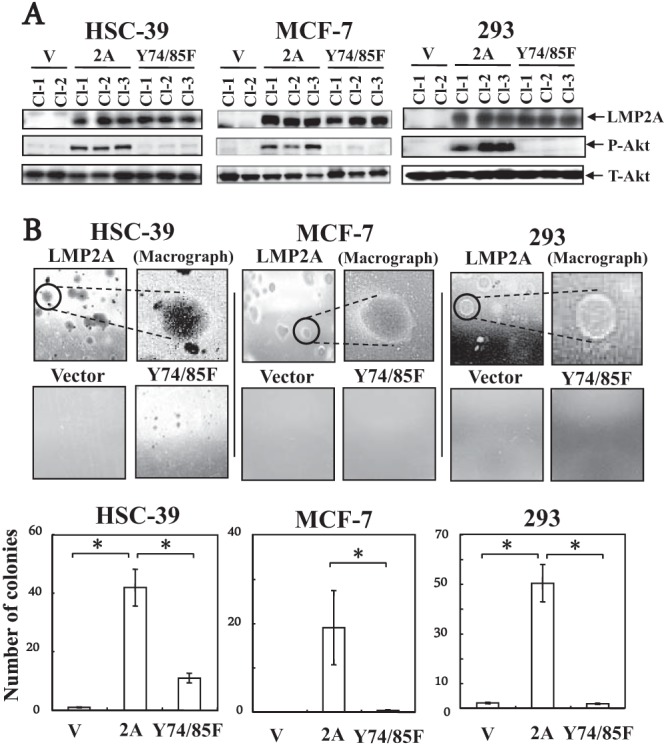
(A) Effect of LMP2A or ITAM mutant LMP2A-Y74/85F expression on Akt phosphorylation and anchorage-independent cell growth in HSC-39, MCF-7, and 293 cells. Cells were stably transduced with an LMP2A (2A), an ITAM mutant LMP2A-Y74/85F (Y74/85F), or a retroviral vector control (V) expression construct. After 2 h of serum starvation, whole-cell extracts were separated by SDS-PAGE and the expression levels of LMP2A (54 kDa) and phospho-specific Akt on serine 473 (P-Akt) (60 kDa) were determined by immunoblotting with anti-phospho-Akt (serine 473) or Akt (New England BioLabs) antibody in the vector control, LMP2A-expressing, and ITAM mutant LMP2A-Y74/85F-expressing HSC-39, MCF-7, and 293 cell clones. The lower panels show equal loadings of proteins and the expression of total Akt (T-Akt) (60 kDa). (B) Soft-agar colony formation assay of HSC-39, MCF-7, and 293 cells stably expressing the vector (Vector), LMP2A, or ITAM mutant LMP2A-Y74/85F (Y74/85F). Vector control, LMP2A-expressing, or ITAM mutant LMP2A-expressing HSC-39, MCF-7, and 293 cells (1 × 104 cells) were cultured in soft agar for 3 weeks. Colonies were defined and scored as cell clusters when they exceeded 200 μm in diameter. Representative images from one of three independent experiments are shown. The photomacrographs are of LMP2A-expressing HSC-39, MCF-7, and 293 cells. The average numbers of foci formed in wells from three independent experiments with HSC-39, MCF-7, and 293 cells transduced with the vector control, LMP2A, or ITAM mutant LMP2A are shown in the graph; error bars indicate standard deviations. Data were analyzed by Student's t test. Values that were statistically significantly different (P < 0.05) from each other are indicated (*).
Interaction of LMP2A with Syk.
In a previous study, Katz et al. (22) demonstrated that expression of the MMTV ITAM-containing protein Env in nonhematopoietic cells (NMuMG murine mammary and MCF-10F human mammary epithelial cells) resulted in transformation via an ITAM/Syk-dependent pathway (22). Compared with the results of the anchorage-independent cell growth of MCF-7 and 293 cells, the size and number of colonies in LMP2A-Y74/85F-expressing HSC-39 cells were significantly smaller than those of LMP2A-expressing HSC-39 cells; however, there appeared to be a significant number of colonies in the LMP2A-Y74/85F-expressing HSC-39 cells (Fig. 1B). Therefore, to explore the role of the LMP2A ITAM in human cell transformation, we performed further molecular studies with HSC-39 cells. First, we tested immunofluorescent confocal microscopic imaging to determine whether LMP2A expressed in HSC-39 cells colocalizes and interacts with endogenous Syk. Most of the LMP2A colocalized with Syk in the cytoplasm of LMP2A-expressing HSC-39 cells (data not shown).
Many protein kinases are implicated in transformations that lead to malignancies. Protein tyrosine kinases are thought to be important regulators of intracellular signal transduction pathways and malignant transformation (28). Phosphorylation of EBV LMP2A is constitutive in both lymphoblastoid cell lines and LMP2A-expressing nonhematopoietic cells (10, 29–31). Second, by performing in vitro kinase assays with immune complexes immunoprecipitated with anti-LMP2A antibody (32) or anti-Syk antibody, respectively, we confirmed that LMP2A and Syk proteins in HSC-39 cells were phosphorylated (Fig. 2A and B). Elimination of LMP2A and Syk protein phospholabeling following phosphatase treatment confirmed that LMP2A and Syk were phosphorylated with [γ-32P]ATP (Fig. 2A and B). The expression and migration positions of radiolabeled LMP2A and Syk proteins following SDS-PAGE were verified by Western blot analysis (Fig. 2A and B, lower panels). Next, coimmunoprecipitations with anti-Syk and anti-LMP2A antibodies were performed to further investigate LMP2A-Syk interactions. LMP2A was immunoprecipitated from HSC-39 cells not expressing LMP2A (vector control) or expressing LMP2A or LMP2A-Y74/85F and immunoblotted with anti-LMP2A antibody to verify expression levels in each line (Fig. 2C). Similarly, Syk was immunoprecipitated from HSC-39 cells not expressing LMP2A (vector control) or expressing LMP2A or LMP2A-Y74/85F and immunoblotted with anti-Syk antibody to verify expression levels (Fig. 2D). There was a significant decrease in the expression level of Syk in LMP2A-expressing HSC-39 cells relative to those in vector control- and LMP2A-Y74/85F-expressing HSC-39 cells (Fig. 2D). A recent study reveals that utilization of the LMP2A ITAM by the signaling scaffold protein Shb regulates the stability of Syk in LMP2A-expressing HEK-293 cells (33). These results suggest that LMP2A might utilize ubiquitin-dependent processes to modulate cellular signaling pathways in HSC-39 cells. In LMP2A-expressing HSC-39 cells, where LMP2A coimmunoprecipitated with Syk, both LMP2A (54 kDa) and Syk (72 kDa) were seen in antiphosphotyrosine antibody immunoblots (Fig. 2C and D). In contrast, LMP2A-Y74/85F-expressing HSC-39 cells demonstrated tyrosine phosphorylation of LMP2A-Y74/85F but not Syk (Fig. 2C and D). These results demonstrate that the LMP2A-Y74/85F mutant expressed in HSC-39 cells is unable to bind Syk and indicate that the LMP2A ITAM is required for LMP2A-Syk interaction. These results also indicate that tyrosine residues other than Y74/85 are also phosphorylated in LMP2A-expressing HSC-39 cells.
FIG 2.
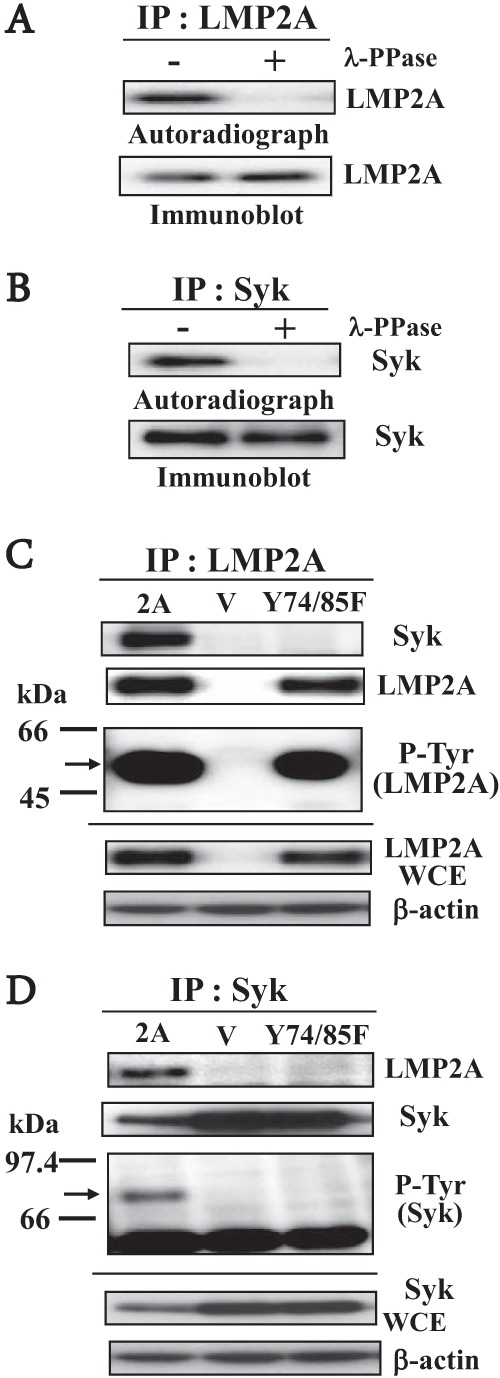
Interaction of endogenous Syk with wild-type or ITAM mutant LMP2A. (A and B) LMP2A or Syk immunoprecipitated (IP) from stably expressed HSC-39 cell lysates were used for immune complex kinase assays. Whole-cell extracts from LMP2A-expressing HSC-39 cells were immunoprecipitated with anti-LMP2A antibody (14B7-1) (A) or anti-Syk antibody (4D-10; Santa Cruz) (B). Immunoprecipitates incubated in kinase buffer containing [γ-32P]ATP were then either mock treated (−) or treated with gamma protein phosphatase (λ-PPase) (+), separated on a denaturing gel, transferred to a nitrocellulose membrane, and analyzed by autoradiography (upper panels). (Lower panels) Immunoblots of the nitrocellulose membrane using anti-LMP2A antibody. (C and D) Tyrosine phosphorylation of LMP2A or Syk in vector control (V), LMP2A-expressing (2A), or ITAM mutant LMP2A-Y74/85F (Y74/85F)-expressing HSC-39 cells. Cell lysates were immunoprecipitated with anti-LMP2A antibody (C) or anti-Syk antibody (D), and then immunoblot analysis was performed with anti-Syk antibody, anti-LMP2A antibody, or anti-phosphotyrosine antibody (P-Tyr) (4G10; Upstate). Whole-cell extracts (WCE) were separated by SDS-PAGE, and the expression levels of LMP2A (54 kDa; arrow) (C) or Syk (72 kDa; arrow) (D) were determined by immunoblotting in vector control, LMP2A-expressing, and ITAM mutant LMP2A-Y74/85F-expressing HSC-39 cells (lower panels). The amount of protein loaded in each lane was assessed using a specific antibody for β-actin (Sigma-Aldrich).
The role of LMP2A and Syk phosphorylation in LMP2A-mediated transformation activity.
We have shown that LMP2A mediates the transformation of HSC-39 cells by constitutive activation of the Ras/PI3-K/Akt pathway (16). To explore the role of LMP2A and Syk phosphorylation in LMP2A-mediated Akt phosphorylation and anchorage-independent cell growth, we treated LMP2A-expressing cells with genistein (a tyrosine kinase-specific inhibitor), piceatannol (a Syk-specific inhibitor), or LY294002 (a PI3-K inhibitor [control inhibitor]) (16, 27). Specific inhibition of Akt serine 473 phosphorylation after treatment with genistein at 30 μg/ml for 30 min or piceatannol at 25 μM for 1 h was confirmed in LMP2A-expressing HSC-39, MCF-7, and 293 cell clones (Fig. 3A). Genistein or piceatannol resulted in a significant reduction in the number and size of LMP2A-expressing HSC-39, MCF-7, and 293 cell colonies relative to those of untreated cells (Fig. 3B). These results suggest that Syk tyrosine phosphorylation and its interaction with LMP2A are necessary to induce LMP2A-mediated Akt phosphorylation and anchorage-independent cell growth in HSC-39, MCF-7, and 293 cells.
FIG 3.
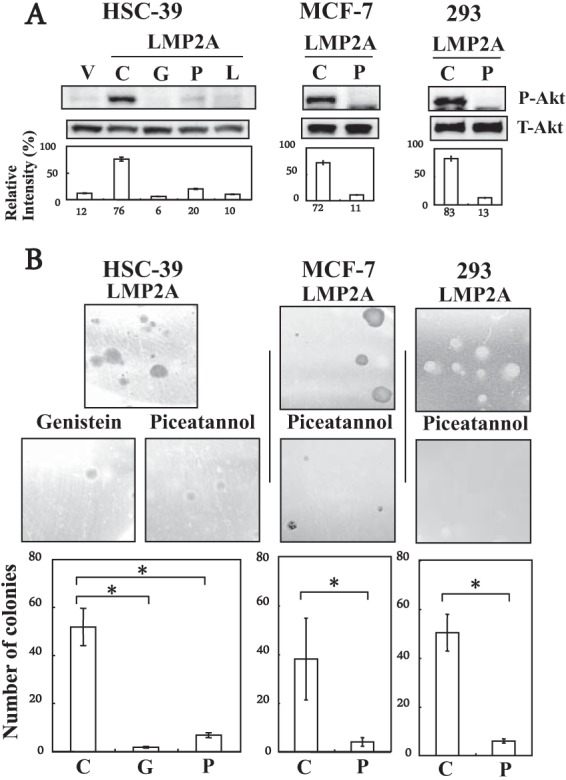
Effect of genistein or piceatannol on Akt phosphorylation and anchorage-independent cell growth in LMP2A-expressing HSC-39, MCF-7, and 293 cells. (A) Immunoblot analysis. Vector control (V), and LMP2A-expressing cells (LMP2A) were cultured for 1 h without serum, and then the cells were incubated for 1 h without inhibitors (control [C]) or with genistein (G) (30 μg/ml), piceatannol (P) (20 μM), or LY294002 (L) (control inhibitor) (25 μM). Equal amounts of protein from the respective cells were separated by SDS-PAGE. The levels of phospho-specific Akt (P-Akt) (on serine 473) (60 kDa) were determined by immunoblotting. The bottom blots show equal loadings of protein and expression of total Akt (T-Akt) (60 kDa). The extent of expression of P-Akt and T-Akt proteins was quantified as described in the legend to Fig. 1A. (B) Colony formation assay. LMP2A-expressing cells (1 × 104 cells) were cultured in soft agar without inhibitors (Control) or with genistein (30 μg/ml) or piceatannol (20 μM) for 3 weeks. Colonies were defined and scored as cell clusters greater than 400 μm in diameter. Representative images from one of three independent experiments are shown. The average number of foci formed from LMP2A-expressing cells cultured without inhibitors (control [C]) or with genistein (G) or piceatannol (P) in a well from three independent experiments is shown; error bars indicate standard deviations. Data were analyzed by Student's t test. Values that were statistically significantly different (P < 0.05) from each other are indicated (*).
Syk tyrosine kinase contributes to LMP2A-mediated transformation activity.
The critical role of LMP2A-Syk interactions in LMP2A-mediated transformation was analyzed using small interfering RNA (siRNA) inhibition of Syk and measuring its effect on LMP2A-induced colony formation. At 48 and 72 h after transfection of LMP2A-expressing HSC-39 cells with nontargeting siRNA or Syk siRNAs, Syk expression was markedly downregulated specifically with Syk siRNAs and not with nontargeting control siRNA (Fig. 4A). LMP2A-induced colony formation in soft agarose was also significantly inhibited by Syk siRNAs relative to the colony formation of a nontargeting control siRNA (Fig. 4B).
FIG 4.
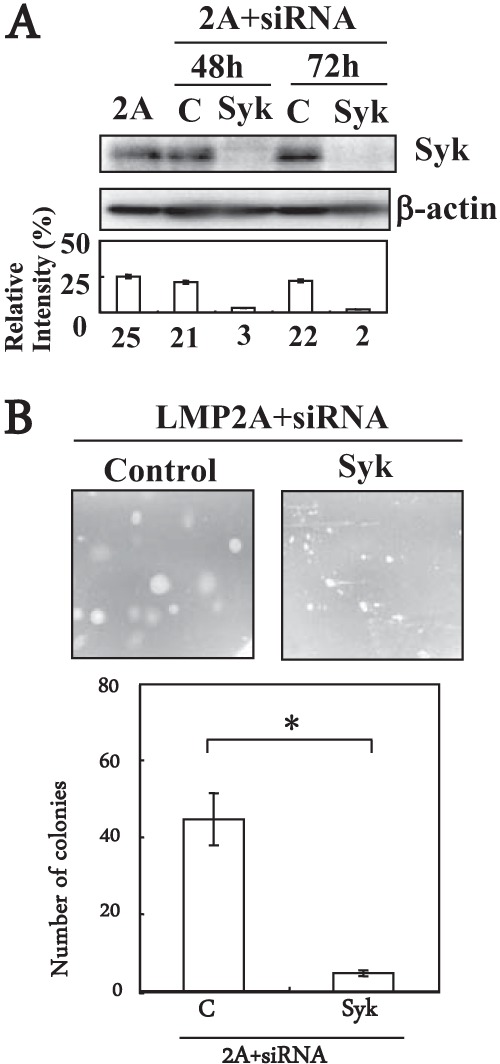
The role of Syk in LMP2A-mediated anchorage-independent cell growth in HSC-39 cells. (A) Immunoblot of LMP2A-expressing HSC-39 cells transfected with Syk siRNA or control siRNA. LMP2A-expressing HSC-39 cells cells were transfected with siRNA (0.1 μM) targeted to Syk (SMARTpool siGENOME Syk siRNA; Dharmacon) or with nontargeting (control) siRNA (SiGENOME SMARTpool siRNAs, catalog no. M-003176-03; Dharmacon) for 48 h or 72 h. Whole-cell extracts were separated by SDS-PAGE, and the expression level of Syk (72 kDa) was determined by immunoblotting. The amount of protein loaded in each lane was assessed using a antibody specific for β-actin. Expression of Syk and β-actin proteins was quantified using ImageJ software. Relative levels were calculated as the ratio of Syk to β-actin protein levels. Results are expressed as the means ± standard deviations (error bars) of results from three experiments. (B) Soft-agar colony formation assay of cells transfected with Syk siRNA or nontargeting siRNA. LMP2A-expressing HSC-39 cells were transfected with siRNA (0.1 μM) targeted to Syk or with nontargeting (control) siRNA for 48 h or 72 h, and cells (1 × 104 cells) were then cultured in soft agar for 3 weeks. Colonies were defined and scored as cell clusters greater than 200 μm in diameter. Representative images from one of three independent experiments are shown. The average number of foci formed from LMP2A-expressing HSC-39 cells transfected with Syk siRNA (Syk) or nontargeting siRNA (control) in a well from three independent experiments is shown; error bars indicate standard deviations. Data were analyzed by Student's t test. Values that were statistically significantly different (P < 0.05) from each other are indicated (*).
Conclusions.
We have shown that LMP2A promotes cell transformation and cell survival through the activity of host cellular signaling pathways (16, 27, 34). In the analysis described in the current paper, we chose to focus on the generality of the ability of LMP2A to induce transformation and the importance of the ITAM in LMP2A-induced signaling pathways and transformation activity in several human cell lines.
Previous studies of the contribution of ITAM and Syk for LMP2A-mediated migration in 293 cells have been reported using a three-dimensional collagen gel assay (17). The migration of cells through collagen matrices requires the activities of matrix metalloproteinases (MMPs) (35). In contrast to the results of previous collagen gel assays (invasion ability) in 293 cells (spindle-like shape) (14, 17), LMP2A-expressing HSC-39, MCF-7, and 293 cells exhibited a spherical shape and anchorage-independent cell growth in soft agarose (Fig. 1B). Several reports have shown that LMP2A-induced migration activity correlates with LMP2A and its ITAM and Syk signaling (15, 27). Collectively, this study is the first report to clarify the direct relationship between the ITAM of LMP2A and LMP2A-mediated transformation activity.
Although signal transduction pathways generally initiate with ligand binding to a receptor, several reports indicate that ITAMs also signal in a ligand-independent manner referred to as tonic signaling (36, 37). The work presented here suggests a model in which the tyrosine kinase-interacting membrane protein LMP2A also interacts with non-receptor tyrosine kinase Syk through the ITAM. Tyrosine phosphorylation of both LMP2A and Syk subsequently induces downstream signals and transformation activity. Evidence supporting this model includes coprecipitation of expressed LMP2A with endogenous Syk and tyrosine phosphorylation of both LMP2A and Syk in vitro, as well as in LMP2A-expressing HSC-39 cells (Fig. 2) (16, 20).
While LMP2A-induced Akt phosphorylation and anchorage-independent cell growth in HSC-39, MCF-7, and 293 cells was diminished with the Y74/85F mutant LMP2A (Fig. 1), the mutant protein was still phosphorylated in HSC-39 cells (Fig. 2). Further, the addition of genistein or piceatannol to the LMP2A-expressing HSC-39, MCF-7, and 293 cells inhibited the phosphorylation of Akt; however, several colonies were observed in the presence of those inhibitors (Fig. 3). Moreover, effects of the inhibitor's treatment on cell growth and cell toxicity were assessed with a cell-counting kit and trypan blue dye exclusion assay. In agreement with previous reports (34, 38, 39), genistein or piceatannol inhibited the growth of parental and LMP2A-expressing HSC-39, MCF-7, and 293 cells; also, genistein had more cytotoxic effects in those cells (data not shown). These results suggest that LMP2A-induced anchorage-independent cell growth is associated with general cell growth. In addition, these results indicate that other LMP2A protein-protein interaction motifs may interact with other protein kinases in the cells or that multiple interactions of LMP2A with protein kinases may be necessary for activation of LMP2A-mediated downstream signaling cascades (7, 10, 11) and LMP2A-induced transformation activity. Further, these results support our hypothesis (16), and it is interesting to clarify the generality and further molecular mechanisms of LMP2A-induced transformation activity and cell growth in various kinds of cells.
In conclusion, the ITAM of LMP2A is essential for LMP2A-mediated transformation of HSC-39, MCF-7, and 293 cells. As in some other virus-host interactions, LMP2A interactions with Syk are mediated by the ITAM, suggesting that interactions with host cellular protein kinases can activate the function of some viral membrane proteins. We propose that LMP2A-mediated functions (pathogenesis) that arise from phosphorylation with host cellular protein kinases result in the stimulation and dysregulation of cellular signal transduction pathways.
ACKNOWLEDGMENTS
We kindly thank K. Yanagihara for HSC-39 cells and A. Aiyar for the 164 retroviral vector.
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, Culture and Technology of Japan (to Y.K. and grant 20590466 to M.F.).
Footnotes
Published ahead of print 19 February 2014
REFERENCES
- 1.Rickinson AB, Kieff E. 2007. Epstein-Barr virus, p 2655–2700 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott, Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 2.Longnecker R. 2000. Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence? Adv. Cancer Res. 79:175–200. 10.1016/S0065-230X(00)79006-3 [DOI] [PubMed] [Google Scholar]
- 3.Thorley-Lawson DA, Gross A. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350:1328–1337. 10.1056/NEJMra032015 [DOI] [PubMed] [Google Scholar]
- 4.Young LS, Rickinson AB. 2004. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 4:757–768. 10.1038/nrc1452 [DOI] [PubMed] [Google Scholar]
- 5.Lynch DT, Zimmerman JS, Rowe DT. 2002. Epstein-Barr virus latent membrane protein 2B (LMP2B) co-localizes with LMP2A in perinuclear regions in transiently transfected cells. J. Gen. Virol. 83:1025–1035 http://vir.sgmjournals.org/content/83/5/1025.long [DOI] [PubMed] [Google Scholar]
- 6.Ikeda M, Fukuda M, Longnecker R. 2005. Function of latent membrane protein 2A, p 531–550 In Robertson E. (ed), Epstein-Barr virus. Caister Academic Press, Wymondham, England [Google Scholar]
- 7.Fruehling S, Longnecker R. 1997. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology 235:241–251. 10.1006/viro.1997.8690 [DOI] [PubMed] [Google Scholar]
- 8.Morrison JA, Raab-Traub N. 2005. Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of β-catenin signaling. J. Virol. 79:2375–2382. 10.1128/JVI.79.4.2375-2382.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reth M. 1989. Antigen receptor tail clue. Nature 338:383–384. 10.1038/338383b0 [DOI] [PubMed] [Google Scholar]
- 10.Burkhardt AL, Bolen JB, Kieff E, Longnecker R. 1992. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinases. J. Virol. 66:5161–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fruehling S, Swart R, Dolwick KM, Kremmer E, Longnecker R. 1998. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J. Virol. 72:7796–7806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda M, Ikeda A, Longan LC, Longnecker R. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178–191. 10.1006/viro.1999.0166 [DOI] [PubMed] [Google Scholar]
- 13.Winberg G, Matskova L, Chen F, Plant P, Rotin D, Gish G, Ingham R, Ernberg I, Pawson T. 2000. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 20:8526–8535. 10.1128/MCB.20.22.8526-8535.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SY, Lu J, Shih YC, Tsai CH. 2002. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J. Virol. 76:9556–9561. 10.1128/JVI.76.18.9556-9561.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fotheringham JA, Coalson NE, Raab-Traub N. 2012. Epstein-Barr virus latent membrane protein-2A induces ITAM/Syk- and Akt-dependent epithelial migration through αV-integrin membrane translocation. J. Virol. 86:10308–10320. 10.1128/JVI.00853-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda M, Longnecker R. 2007. Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/ PI3-K/Akt pathway. J. Virol. 81:9299–9306. 10.1128/JVI.00537-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu J, Lin WH, Chen SY, Longnecker R, Tsai SC, Chen CL, Tsai CH. 2006. Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J. Biol. Chem. 281:8806–8814. 10.1074/jbc.M507305200 [DOI] [PubMed] [Google Scholar]
- 18.Pegtel DM, Subramanian A, Sheen TS, Tsai CH, Golub TR, Thorley-Lawson DA. 2005. Epstein-Barr-virus-encoded LMP2A induces primary epithelial cell migration and invasion: possible role in nasopharyngeal carcinoma metastasis. J. Virol. 79:15430–15442. 10.1128/JVI.79.24.15430-15442.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholle F, Bendt KM, Raab-Traub N. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681–10689. 10.1128/JVI.74.22.10681-10689.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman VH, Shin SI. 1974. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell 3:355–359. 10.1016/0092-8674(74)90050-6 [DOI] [PubMed] [Google Scholar]
- 21.Yanagihara K, Seyama T, Tsumuraya M, Kamada N, Yokoro K. 1991. Establishment and characterization of human signet ring cell gastric carcinoma cell lines with amplification of the c-myc oncogene. Cancer Res. 51:381–386 [PubMed] [Google Scholar]
- 22.Katz E, Lareef MH, Rassa JC, Grande SM, King LB, Russo J, Ross SR, Monroe JG. 2005. MMTV Env encodes an ITAM responsible for transformation of mammary epithelial cells in three-dimensional culture. J. Exp. Med. 201:431–439. 10.1084/jem.20041471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H, Veazey R, Williams K, Li M, Guo J, Neipel F, Fleckenstein B, Lackner A, Desrosiers RC, Jung JU. 1998. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat. Med. 4:435–440. 10.1038/nm0498-435 [DOI] [PubMed] [Google Scholar]
- 24.Soule HD, Vazguez J, Long A, Albert S, Brennan M. 1973. A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl. Cancer Inst. 51:1409–1416 [DOI] [PubMed] [Google Scholar]
- 25.Graham FL, Smiley J, Russell WC, Nairn R. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59–74. 10.1099/0022-1317-36-1-59 [DOI] [PubMed] [Google Scholar]
- 26.Swart R, Ruf IK, Sample J, Longnecker R. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838–10845. 10.1128/JVI.74.22.10838-10845.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuda M, Longnecker R. 2004. Latent membrane protein 2A inhibits transforming growth factor-beta 1-induced apoptosis through the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 78:1697–1705. 10.1128/JVI.78.4.1697-1705.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blume-Jensen P, Hunter T. 2001. Oncogenic kinase signalling. Nature 411:355–365. 10.1038/35077225 [DOI] [PubMed] [Google Scholar]
- 29.Longnecker R, Druker B, Roberts TM, Kieff E. 1991. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J. Virol. 65:3681–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholle F, Longnecker R, Raab-Traub N. 1999. Epithelial cell adhesion to extracellular matrix proteins induces tyrosine phosphorylation of the Epstein-Barr virus latent membrane protein 2: a role for C-terminal Src kinase. J. Virol. 73:4767–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholle F, Longnecker R, Raab-Traub N. 2001. Analysis of the phosphorylation status of Epstein-Barr virus LMP2A in epithelial cells. Virology 291:208–214. 10.1006/viro.2001.1197 [DOI] [PubMed] [Google Scholar]
- 32.Fruehling S, Lee SK, Herrold R, Frech B, Laux G, Kremmer E, Grasser FA, Longnecker R. 1996. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J. Virol. 70:6216–6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matskova LV, Helmstetter C, Ingham RJ, Gish G, Lindholm CK, Ernberg I, Pawson T, Winberg G. 2007. The Shb signalling scaffold binds to and regulates constitutive signals from the Epstein-Barr virus LMP2A membrane protein. Oncogene 26:4908–4917. 10.1038/sj.onc.1210298 [DOI] [PubMed] [Google Scholar]
- 34.Fukuda M, Longnecker R. 2005. Epstein-Barr virus (EBV) latent membrane protein 2A regulates B-cell receptor-induced apoptosis and EBV reactivation through tyrosine phosphorylation. J. Virol. 79:8655–8660. 10.1128/JVI.79.13.8655-8660.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egeblad M, Werb Z. 2002. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2:161–174. 10.1038/nrc745 [DOI] [PubMed] [Google Scholar]
- 36.Bannish G, Fuentes-Panana EM, Cambier JC, Pear WS, Monroe JG. 2001. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J. Exp. Med. 194:1583–1596. 10.1084/jem.194.11.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monroe JG. 2006. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat. Rev. Immunol. 6:283–294. 10.1038/nri1808 [DOI] [PubMed] [Google Scholar]
- 38.El-Rayes BF, Ali S, Ali IF, Philip PA, Abbruzzese J, Sarkar FH. 2006. Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor-kappaB. Cancer Res. 66:10553–10559. 10.1158/0008-5472.CAN-06-2333 [DOI] [PubMed] [Google Scholar]
- 39.Cheng S, Coffey G, Zhang XH, Shaknovich R, Song Z, Lu P, Pandey A, Melnick AM, Sinha U, Wang YL. 2011. SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood 118:6342–6352. 10.1182/blood-2011-02-333773 [DOI] [PubMed] [Google Scholar]


