ABSTRACT
Herpesviruses have evolved a unique mechanism for nuclear egress of nascent progeny nucleocapsids: the nucleocapsids bud through the inner nuclear membrane into the perinuclear space between the inner and outer nuclear membranes (primary envelopment), and enveloped nucleocapsids then fuse with the outer nuclear membrane to release nucleocapsids into the cytoplasm (de-envelopment). We have shown that the herpes simplex virus 1 (HSV-1) major virion structural protein UL47 (or VP13/VP14) is a novel regulator for HSV-1 nuclear egress. In particular, we demonstrated the following: (i) UL47 formed a complex(es) with HSV-1 proteins UL34, UL31, and/or Us3, which have all been reported to be critical for viral nuclear egress, and these viral proteins colocalized at the nuclear membrane in HSV-1-infected cells; (ii) the UL47-null mutation considerably reduced primary enveloped virions in the perinuclear space although capsids accumulated in the nucleus; and (iii) UL47 was detected in primary enveloped virions in the perinuclear space by immunoelectron microscopy. These results suggested that UL47 promoted HSV-1 primary envelopment, probably by interacting with the critical HSV-1 regulators for viral nuclear egress and by modulating their functions.
IMPORTANCE Like other herpesviruses, herpes simplex virus 1 (HSV-1) has evolved a vesicle-mediated nucleocytoplasmic transport mechanism for nuclear egress of nascent progeny nucleocapsids. Although previous reports identified and characterized several HSV-1 and cellular proteins involved in viral nuclear egress, complete details of HSV-1 nuclear egress remain to be elucidated. In this study, we have presented data suggesting (i) that the major HSV-1 virion structural protein UL47 (or VP13/VP14) formed a complex with known viral regulatory proteins critical for viral nuclear egress and (ii) that UL47 played a regulatory role in HSV-1 primary envelopment. Thus, we identified UL47 as a novel regulator for HSV-1 nuclear egress.
INTRODUCTION
Morphogenesis of herpes simplex virus 1 (HSV-1), like that of other herpesviruses, takes place in two different cellular compartments (1, 2). Viral DNA replication and transcription, capsid assembly, and packaging of nascent progeny virus genomes into preformed capsids take place in the nucleus, and final envelopment takes place in the cytoplasm (1, 2). Since herpesvirus nucleocapsids are too large to traverse the nuclear lamina or cross the inner and outer nuclear membranes (INM and ONM, respectively) through nuclear pores, these viruses appear to have evolved a unique nuclear egress mechanism in which progeny nucleocapsids acquire primary envelopes by budding through the INM into the space between the INM and ONM, the perinuclear space, and then the enveloped nucleocapsids fuse with the ONM to release de-enveloped nucleocapsids into the cytoplasm (1, 2).
In the present study, we focus on the first step of HSV-1 nuclear egress, the process by which progeny nucleocapsids acquire primary envelopes by budding through the INM into the perinuclear space (primary envelopment). It has been well established that a heterodimeric complex of HSV-1 proteins UL31 and UL34, which are conserved in all known herpesviruses, plays a crucial role in HSV-1 primary envelopment (1–5). In the absence of the HSV-1 UL31/UL34 complex, nucleocapsids accumulate in the nucleoplasm, and progeny virus intermediates and virions are barely detectable in the perinuclear space or cytoplasm or at the cell surface (5, 6). The HSV-1 UL31/UL34 complex and its homologs in other herpesviruses have been suggested to coordinate multiple events in the primary envelopment of nucleocapsids, including the following: (i) disruption of the nuclear lamina, which has been suggested to facilitate herpesvirus nucleocapsid access to the INM, by recruiting cellular protein kinases, such as protein kinase C isoforms, and by direct binding to components of the nuclear lamina (i.e., lamins A and C) and modifying their conformation (1, 2, 7–11); (ii) recruitment of nucleocapsids into primary envelopes by interaction of the UL31/UL34 complex and the capsid vertex-specific component (CVSC), which consists of the conserved capsid proteins UL17 and UL25 (12, 13); and (iii) budding of nucleocapsids into the INM (14, 15). In addition to UL31 and UL34, an HSV-1 serine/threonine protein kinase Us3 has been suggested to be involved in HSV-1 primary envelopment since Us3 phosphorylates and regulates proper localization of UL34 and UL31 at the nuclear membrane (4, 16, 17) and phosphorylates and modifies lamins A and C (7, 8, 10).
UL47 (or VP13/VP14) is a major structural protein in HSV-1 virion tegument (18). UL47 is an RNA binding protein (19) and shuttles between the cytoplasm and nucleus in HSV-1-infected cells (20). It has been suggested that UL47 may be a positive regulator of viral replication and pathogenicity, based on studies showing that recombinant UL47 mutant viruses have reduced growth in cell cultures and reduced pathogenicity in a mouse model (21, 22). Although the precise mechanisms by which UL47 functions in viral replication and pathogenicity remain largely unknown at present, the mechanisms by which UL47 acts in HSV-1-infected cells have been gradually elucidated as described below. Thus, (i) it has been reported that UL47 can regulate subcellular localization of some viral and cellular proteins that interact with it. For example, UL47 together with the HSV-1 regulatory protein ICP27 associates with and promotes nuclear translocation of the major form of the polyadenylate-binding protein PABC1 (23), and UL47 forms a complex with and promotes nuclear localization of Us3 in HSV-1-infected cells (21). (ii) UL47 was also shown to interact with capsid protein UL17 (24). As described above, UL17 forms a CVSC complex with UL25, which was suggested to recruit nucleocapsids for primary envelopes by interacting with the UL31/UL34 complex (12, 13). (iii) Recently, it has been reported that UL47 interacted with the viral endoribonuclease responsible for virus host protein synthesis shutoff (vhs) and attenuated vhs activity (25).
We previously reported that Us3 phosphorylated UL47 and promoted its nuclear localization in HSV-1-infected cells (21). In cells infected with an HSV-1 mutant encoding a Us3 kinase-dead mutant or carrying a mutation in the Us3 phosphorylation site in UL47, UL47 accumulated aberrantly in punctate structures at the nuclear membrane (21). During the course of the study, we noticed that the punctate structures containing UL47 induced in the absence of Us3 kinase activity in HSV-1-infected cells were reminiscent of the discrete foci containing the UL31/UL34 complex observed at the nuclear membrane in cells infected with HSV-1 mutants carrying a mutation abrogating either the expression or catalytic activity of Us3 (4, 26). These observations raised the possibility that UL47 interacted with the UL31/UL34 complex at the nuclear membrane and modulated the function(s) of the complex in HSV-1-infected cells. In the present study, we examined this possibility and showed that UL47 colocalized with UL31, UL34, and Us3 at the nuclear membrane and formed a complex with these viral proteins in HSV-1-infected cells. We also presented the data demonstrating that the UL47-null mutation considerably reduced primary enveloped virions in the perinuclear space although capsids accumulated in the nucleus. These results suggested that UL47 promoted HSV-1 primary envelopment, probably by interacting with the critical HSV-1 regulators for nuclear egress, the UL31/UL34 complex and Us3, and by modulating their functions.
MATERIALS AND METHODS
Cells and viruses.
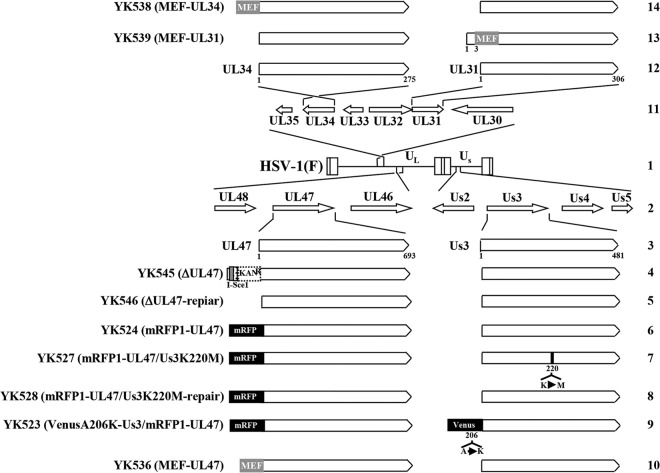
Vero, HEp-2, and rabbit skin cells were described previously (27, 28). The following viruses have been described previously (21, 29): HSV-1 wild-type strain HSV-1(F); recombinant virus YK524, encoding UL47 fused to the monomeric red fluorescent protein mRFP1 (mRFP1-UL47); recombinant virus YK527, encoding mRFP1-UL47 and carrying the Us3K220M mutation (mRFP1-UL47/Us3K220M); recombinant virus YK528, in which Us3K220M in YK527 was repaired (mRFP1-UL47/Us3K220M-repair); recombinant virus YK523, encoding mRFP1-UL47 and Us3 fused to the fluorescent protein VenusA206K (VenusA206K-Us3/mRFP1-UL47); recombinant virus YK545, a UL47-null mutant virus in which the UL47 gene was disrupted by insertion of a foreign gene cassette just downstream of the UL47 start codon (ΔUL47); and recombinant virus YK546, in which the foreign gene cassette inserted into the UL47 locus of YK545 (ΔUL47) was excised (ΔUL47-repair) (Fig. 1).
FIG 1.
Schematic diagrams of the genome structures of wild-type HSV-1(F) and the relevant domains of the recombinant viruses used in this study. Line 1, wild-type HSV-1(F) genome; line 2, domains of the UL46 to UL48 and Us2 to Us5 genes; line 3, domains of the UL47 and Us3 genes; lines 4 to 10, recombinant viruses with mutations in the UL47 and/or Us3 genes; line 11, domains of the UL30 to UL35 genes; line 12, domains of the UL34 and UL31 genes; line 13, recombinant virus encoding MEF-tagged UL31; line 14, recombinant virus encoding MEF-tagged UL34.
Plasmids.
To generate a fusion protein of maltose binding protein (MBP) and either part of HSV-1 UL31 or part of HSV-2 UL31, plasmid pMAL-UL31-C or pMAL-UL31(2)-Pii, respectively, was constructed by cloning the HSV-1 UL31 domain consisting of codons 50 to 307 amplified by PCR from pBC1007 (30) or the HSV-2 UL31 domain consisting of codons 183 to 306 amplified by PCR from pYEbac356, a full-length infectious HSV-2 186 clone (31), respectively, into pMAL-c (New England BioLabs) in frame with the MBP. To generate fusion proteins of MBP and parts of UL47, plasmids pMAL-UL47-Pi, pMAL-UL47-Pii, and pMAL-UL47-Piii were constructed by cloning the UL47 domain consisting of codons 1 to 120, 121 to 390, and 380 to 693, respectively, amplified by PCR from pBC1007 into pMAL-c.
Mutagenesis of viral genomes in Escherichia coli and generation of recombinant HSV-1.
To generate recombinant viruses YK536 with the UL47 protein carrying an MEF (for myc tag, the tobacco etch virus protease cleavage site, and FLAG tag) tag (MEF-UL47), YK538 (MEF-UL34), and YK539 (MEF-UL31) (Fig. 1), a two-step Red-mediated mutagenesis procedure was carried out using E. coli GS1783 containing pYEbac102 (28), a full-length infectious HSV-1(F) clone, as described previously (29) except with the primers listed in Table 1.
TABLE 1.
Oligonucleotide primers used for construction of the recombinant viruses in this study
| Mutation | Primer | Sequence (5′–3′) |
|---|---|---|
| MEF-UL34 | Forward | GAACCCTTTGGTGGGTTTACGCGGGCACGCACGCTCCCATCGCGGGCGCCATGGAGCAAAAGCTCATTTC |
| Reverse | CCCTCGAAGGCGTCACCTGGGTGGCCGGTGTAGGGCTTGCCCAGTCCCGCATCTTTGTCATCGTCGTCCT | |
| MEF-UL31 | Forward | CTCGATCTCGCTCCTGTCCCTGGAGCACACCCTGTGTACCTATGTATGACGAGCAAAAGCTCATTTCTGA |
| Reverse | TCCTTGCCGTGATAGGGCCCGGGCCGGGAGCCGCGGCGATGGGGGTCGGTATCTTTGTCATCGTCGTCCT | |
| MEF-UL47 | Forward | TTCTTTTTTGGGGGGTAGCGGACATCCGATAACCCGCGTCTATCGCCACCATGGAGCAAAAGCTCATTTC |
| Reverse | CGGGGGCGGGTGGATGCGCGCCTCCTACGCCCCGCGGGTTCGCGAGCCGAATCTTTGTCATCGTCGTCCT |
Production and purification of MBP fusion proteins in E. coli.
MBP fusion proteins MBP-UL31-C, MBP-UL31(2)-Pii, MBP-UL47-Pi, MBP-UL47-Pii, and MBP-UL47-Piii were expressed in E. coli that had been transformed with pMAL-UL31-C, pMAL-UL31(2)-Pii, pMAL-UL47-Pi, pMAL-UL47-Pii, and pMAL-UL47-Piii, respectively, and purified as described previously (16, 30).
Antibodies.
To generate rabbit polyclonal antibody to UL31 or UL47, rabbits were immunized, respectively, with purified MBP-UL31(2)-Pii or with a mixture of MBP-UL47-Pi, MBP-UL47-Pii, and MBP-UL47-Piii as described previously (27). Serum from the immunized rabbits was used as anti-UL31 or anti-UL47 rabbit polyclonal antibody. To generate mouse polyclonal antibody to UL31, BALB/c mice were immunized once with purified MBP-UL31-C with TiterMax Gold adjuvant (TiterMax USA, Inc.). Serum from the immunized mice was used as anti-UL31 mouse polyclonal antibody. Commercial rabbit polyclonal antibody against VP23 (CAC-CT-HSV-UL18; CosmoBio) and commercial mouse monoclonal antibodies against Flag (M2; Sigma), Myc (PL14; MBL), and α-tubulin (DM1A; Sigma) were used in this study. Rabbit polyclonal antibody to UL34 and chicken polyclonal antibody to UL34 were described previously (29, 31). Rabbit polyclonal antibodies to Us3, UL46, and UL48 were described previously (29, 31, 32).
Ethics statement.
All animal experiments were carried out in accordance with the Guidelines for Proper Conduct of Animal Experiments, Science Council of Japan. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Institute of Medical Science, The University of Tokyo (IACUC protocol approval number 19-26).
Antibody analyses.
Immunoprecipitation, immunoblotting, and immunofluorescence were performed as described previously (27, 30).
Electron microscopic analysis.
Vero cells infected with wild-type HSV-1(F), YK545 (ΔUL47), or YK546 (ΔUL47-repair) at a multiplicity of infection (MOI) of 5 for 18 h were examined by ultrathin-section electron microscopy as described previously (31). Immunoelectron microscopy was performed as described previously (33, 34). Briefly, Vero cells infected with wild-type HSV-1(F) or YK536 (MEF-UL47) at an MOI of 5 for 18 h were fixed with 2% paraformaldehyde and 1% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) on ice for 1 h. After a wash with the same buffer, the cells were postfixed with 2% osmium tetroxide on ice for 1 h, washed with distilled water, dehydrated with an ethanol gradient series, incubated in propylene oxide, and embedded in an Epon 812 resin mixture. Ultrathin sections were prepared on nickel grids as described previously (34) and incubated with a saturated sodium periodate solution (33), followed by 0.2 M glycine in phosphate-buffered saline (PBS) buffer. After a PBS wash, the sections were incubated with 1% bovine serum albumin in PBS and then with anti-Myc mouse monoclonal antibody. The sections were then washed with PBS and incubated with goat anti-mouse IgG conjugated to 10-nm gold particles. After immunostaining, the sections were stained with 2% uranyl acetate and Reynold's lead citrate and examined by transmission electron microscopy.
RESULTS
Localization of UL47, UL31, and UL34 in the presence or absence of the Us3 catalytic activity in HSV-1-infected cells.
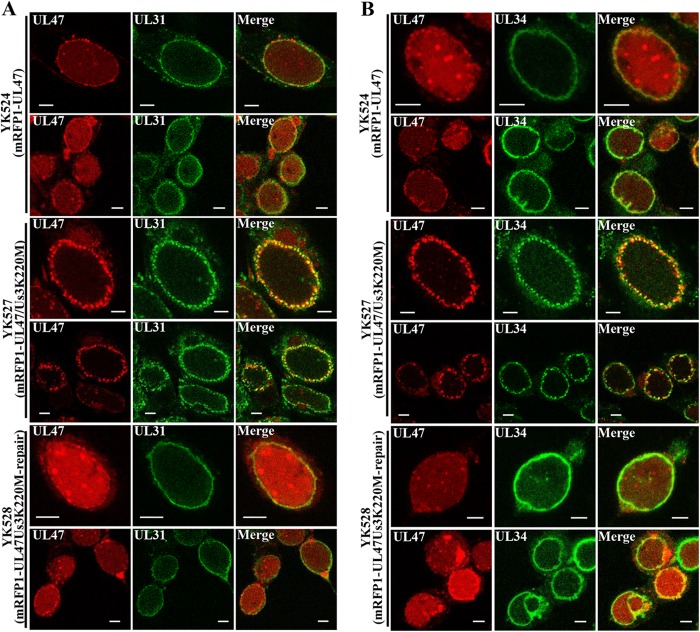
As described above, Us3 has been reported to phosphorylate UL31, UL34, and UL47 (16, 17, 21) and to regulate their proper localization at the nuclear membrane in HSV-1-infected cells (4, 21). In the absence of the Us3 catalytic activity, the UL31/UL34 complex and UL47 were shown to localize aberrantly in similar punctate structures at the nuclear membrane in HSV-1-infected cells (4, 21, 26, 35). To examine whether UL47 colocalized with the UL31/UL34 complex in the presence or absence of the Us3 catalytic activity in HSV-1-infected cells, Vero cells were infected with YK524 (mRFP1-UL47) encoding mRFP1-UL47, YK527 (mRFP1-UL47/Us3K220M) encoding mRFP1-UL47, and Us3 with the kinase-dead K220M mutation or YK528 (mRFP1-UL47/Us3K220M-repair) in which the Us3 K220M mutation in YK527 was repaired (Fig. 1). At 18 h postinfection, infected cells were fixed and stained with anti-UL34 or anti-UL31 antibody, and localization of mRFP1-UL47 in combination with UL34 or UL31 was examined by confocal microscopy. It has been noted that the anti-UL47 antibody reported to date was not useful for immunofluorescence assays because it showed nonspecific staining (24). In agreement with the report, the anti-UL47 antibody generated in this study was not useful for immunofluorescence (data not shown). Therefore, we used YK524 (mRFP1-UL47), YK527 (mRFP1-UL47/Us3K220M), and YK528 (mRFP1-UL47/Us3K220M-repair) expressing UL47 tagged with the fluorescent protein mRFP1 (21) to detect UL47 localization. The mRFP1 tag on UL47 appeared to have little effect on the function(s) of UL47 in HSV-1-infected Vero cells since we previously reported that YK524 (mRFP1-UL47) showed growth kinetics similar to that of the wild-type HSV-1(F) in Vero cells (21) and since, as shown in Fig. 2A, Vero cells infected with YK524 (mRFP1-UL47) produced UL47 protein at a level similar to that in cells infected with wild-type HSV-1(F). As shown in Fig. 3, mRFP1-UL47 was localized throughout the nuclei of Vero cells infected with YK524 (mRFP1-UL47) or YK528 (mRFP1-UL47/Us3K220M-repair) and colocalized at the nuclear rim with UL31 and UL34, which were detected smoothly along the nuclear rim. In Vero cells infected with YK527 (mRFP1-UL47/Us3K220M), mRFP1-UL47 accumulated in the punctate structures at the nuclear rim and colocalized with UL34 and UL31 in these punctate structures (Fig. 3).
FIG 2.
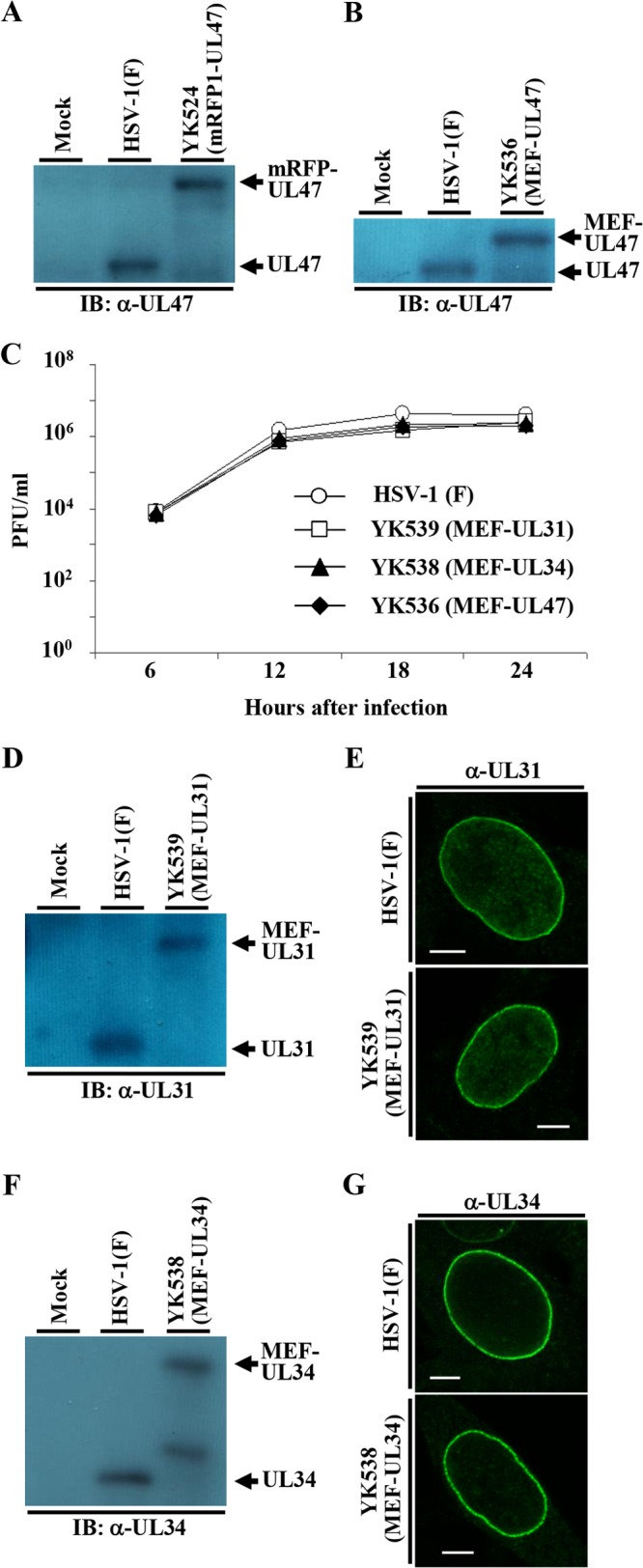
Characterization of the recombinant viruses used in this study. (A) Vero cells mock infected or infected with wild-type HSV-1(F) or YK524 (mRFP1-UL47) at an MOI of 5 for 18 h were analyzed by immunoblotting (IB) with antibody to UL47. (B) Vero cells mock infected or infected with wild-type HSV-1(F) or YK536 (MEF-UL47) at an MOI of 5 for 18 h were analyzed by immunoblotting with antibody to UL47. (C) Vero cells were infected with wild-type HSV-1(F), YK539 (MEF-UL31), YK538 (MEF-UL34), or YK536 (MEF-UL47) at an MOI of 5. Total viruses from cell culture supernatants and infected cells was harvested at the indicated times and assayed on Vero cells. (D) Vero cells mock infected or infected with wild-type HSV-1(F) or YK539 (MEF-UL31) at an MOI of 5 for 18 h were analyzed by immunoblotting with antibody to UL31. (E) Vero cells infected with wild-type HSV-1(F) or YK539 (MEF-UL31) at an MOI of 3 for 18 h were analyzed by immunofluorescence with antibody to UL31. Scale bar, 5 μm. (F) Vero cells mock infected or infected with wild-type HSV-1(F) or YK538 (MEF-UL34) at an MOI of 5 for 18 h were analyzed by immunoblotting with antibody to UL34. (G) Vero cells infected with wild-type HSV-1(F) or YK538 (MEF-UL34) at an MOI of 3 for 18 h were analyzed by immunofluorescence with antibody to UL34. Scale bar, 5 μm. α, anti.
FIG 3.
Effect of Us3 kinase activity on localization of mRFP1-UL47, UL31, and UL34 in HSV-1-infected cells. Vero cells were infected with YK524 (mRFP1-UL47), YK527 (mRFP1-UL47/Us3K220M), or YK528 (mRFP1-UL47/Us3K220M-repair) at an MOI of 3, fixed at 18 h postinfection, permeabilized, stained with anti-UL31 (A) or anti-UL34 (B) antibody, and examined by confocal microscopy. Scale bar, 5 μm.
Interactions among UL47, UL31, UL34, and Us3 in HSV-1-infected cells.
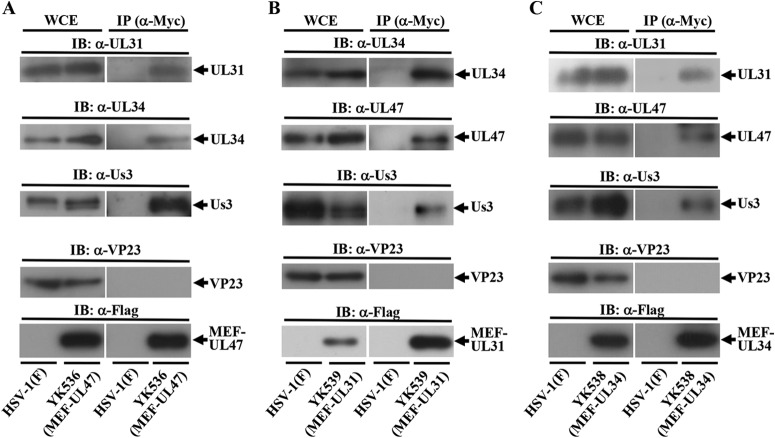
The data above showing that UL47 colocalized well with UL31 and UL34 at the nuclear membrane in the presence or absence of the Us3 catalytic activity in HSV-1-infected cells and our previous observation that UL47 formed a complex with Us3 in HSV-1-infected cells (21) raised the possibility that UL47 interacted with UL31, UL34, and Us3 at the nuclear membrane in HSV-1-infected cells. To test this possibility, we performed two series of experiments. In the first series of experiments, Vero cells were infected with wild-type HSV-1(F), YK536 (MEF-UL47) encoding MEF-tagged UL47, YK538 (MEF-UL34) encoding MEF-tagged UL34, or YK539 (MEF-UL31) encoding MEF-tagged UL31 (Fig. 1) and, at 18 h postinfection, lysed and immunoprecipitated with anti-Myc antibody; the immunoprecipitates were analyzed by immunoblotting with antibodies to the viral proteins shown in Fig. 4. As shown in Fig. 4A, anti-Myc antibody coprecipitated UL31, UL34, and Us3 with MEF-tagged UL47 from lysates of YK536 (MEF-UL47)-infected Vero cells but did not coprecipitate capsid protein VP23. In contrast, the anti-Myc antibody did not immunoprecipitate any of these viral proteins from lysates of wild-type HSV-1(F)-infected cells (Fig. 4A). These results indicated that UL47 formed a complex(es) with UL31, UL34, and/or Us3 in HSV-1-infected cells. Similarly, anti-Myc antibody coprecipitated UL34, Us3, and UL47 with MEF-tagged UL31 from lysates of YK539 (MEF-UL31)-infected cells (Fig. 4B) and coprecipitated UL31, UL47, and Us3 with MEF-tagged UL34 from lysates of YK538 (MEF-UL34)-infected cells (Fig. 4C). These results indicated that UL31 formed a complex(es) with UL34, Us3, and/or UL47 and that UL34 formed a complex(es) with UL31, Us3, and/or UL47 in HSV-1-infected cells. We noted that the MEF tag on UL47, UL31, and UL34 appeared to have little effect on the functions of these proteins in HSV-1-infected Vero cells, as follows: (i) YK536 (MEF-UL47), YK539 (MEF-UL31), and YK538 (MEF-UL34) showed growth kinetics similar to that of the wild-type HSV-1(F) in Vero cells (Fig. 2C); (ii) Vero cells infected with YK536 (MEF-UL47), YK539 (MEF-UL31), and YK538 (MEF-UL34) produced UL47, UL31, and UL34 proteins, respectively, at levels similar to those in cells infected with wild-type HSV-1(F) (Fig. 2B, D, and F); and (iii) localization of UL31 and UL34 proteins in Vero cells infected with YK539 (MEF-UL31) and YK538 (MEF-UL34), respectively, was identical to that in cells infected with wild-type HSV-1(F) (Fig. 2E and G).
FIG 4.
Interactions among UL47, UL31, UL34, and Us3 in HSV-1-infected cells. Vero cells infected with wild-type HSV-1(F) (A to C) and YK536 (MEF-UL47) (A), YK539 (MEF-UL31) (B), or YK538 (MEF-UL34) (C) at an MOI of 5 for 18 h were harvested, immunoprecipitated (IP) with anti-Myc antibody (α-Myc), and analyzed by immunoblotting (IB) with the indicated antibodies. WCE, whole-cell extract.
In the second series of experiments, we examined whether UL47 colocalized with not only UL31 and UL34 but also Us3 at the nuclear membrane in HSV-1-infected cells. As with the anti-UL47 antibody described above, it has also been noted by us and by other laboratories that the anti-Us3 antibodies reported to date were not useful for immunofluorescence assays because they showed nonspecific staining (29, 36). Therefore, we used YK523 (VenusA206K-Us3/mRFP1-UL47) expressing Us3 and UL47 tagged with fluorescent proteins VenusA206K and mRFP1 (21), respectively, to detect Us3 and UL47 localizations (Fig. 1). Vero cells infected with YK523 (VenusA206K-Us3/mRFP1-UL47) were fixed at 18 h postinfection and stained with anti-UL34 or -UL31 antibody to enable simultaneous localization of combinations of these proteins to be observed by confocal microscopy. As shown in Fig. 5, VenusA206K-Us3 and mRFP1-UL47 colocalized with UL34 and UL31 at the nuclear rim in Vero cells infected with YK523 (VenusA206K-Us3/mRFP1-UL47). Similar results were also obtained with YK523 (VenusA206K-Us3/mRFP1-UL47)-infected Vero cells at 12 and 24 h postinfection (data not shown). These results indicated that these viral proteins colocalized at the nuclear membrane in HSV-1-infected cells.
FIG 5.

Localization of UL47, UL31, UL34, and Us3 in HSV-1-infected cells. Vero cells were infected with YK523 (VenusA206K-Us3/mRFP1-UL47) at an MOI of 3, fixed at 18 h postinfection, permeabilized, stained with anti-UL34 (upper panels) or anti-UL31 (lower panels) antibody, and examined by confocal microscopy. Scale bar, 5 μm.
Effect of the UL47-null mutation on viral nuclear egress.
The data above showing the interactions of UL47 with the critical HSV-1 regulators for viral nuclear egress including UL31, UL34, and Us3 led us to hypothesize that UL47 played a role(s) in viral nuclear egress. To test this hypothesis, we investigated the effect of the UL47-null mutation on viral morphogenesis by quantitating the number of virus particles at different morphogenetic stages by electron microscopy of Vero cells infected with wild-type HSV-1(F), YK545 (ΔUL47), or YK546 (ΔUL47-repair). In Vero cells infected with wild-type HSV-1(F) and YK546 (ΔUL47-repair), 19.9 and 15.9%, respectively, of the total number of virus particles were primary enveloped virions in the perinuclear space (Table 2). However, cells infected with YK545 (ΔUL47) had almost no (0.4%) primary enveloped virions in the perinuclear space, which was 49.8- and 39.8-fold less than that in cells infected with wild-type HSV-1(F) and YK546 (ΔUL47-repair), respectively (Table 2 and Fig. 6). In contrast, capsids appeared to accumulate in the nucleus in cells infected with YK545 (ΔUL47) (Table 2). While 34.1 and 38.3% of total virus particles were capsids in the nucleus in cells infected with wild-type HSV-1(F) and YK546 (ΔUL47-repair), respectively, the fraction of total virus particles that were capsids in the nucleus in cells infected with YK545 (ΔUL47) increased to 63.8% (Table 2). Similar results were also obtained with HEp-2 cells infected with wild-type HSV-1(F), YK545 (ΔUL47), or YK546 (ΔUL47-repair) (Table 3). In addition, the UL47-null mutation in YK545 (ΔUL47) had no effect on expression of the neighboring UL46 and UL48 genes (Fig. 7). These results indicated that the UL47-null mutation resulted in a decrease in the fraction of virus particles that were primary enveloped virions in the perinuclear space and an increase in the fraction of virus particles that were capsids in the nucleus.
TABLE 2.
Effect of the UL47-null mutation on distribution of virus particles in infected Vero cells
| Virus | Avg ± SE (%) virus particles in the indicated morphogenetic stagea |
Total no. of particles counted | ||||
|---|---|---|---|---|---|---|
| Nucleocapsids in the nucleus | Enveloped virions in the perinuclear space | Nucleocapsids in the cytoplasm | Enveloped virions in the cytoplasm | Extracellular enveloped virions | ||
| HSV-1(F) | 34.1 ± 2.5d (1,040) | 19.9 ± 3.2d (606) | 11.0 ± 1.4b (336) | 13.7 ± 1.6d (419) | 21.3 ± 2.4e (650) | 3,051 |
| YK545 (ΔUL47) | 63.8 ± 2.4 (662) | 0.4 ± 0.2 (4) | 5.3 ± 1.2 (56) | 3.8 ± 0.9 (39) | 26.7 ± 2.7 (277) | 1,038 |
| YK546 (ΔUL47-repair) | 38.3 ± 2.3d (1,102) | 15.9 ± 3.0c (458) | 9.8 ± 2.2e (281) | 12.3 ± 1.8c (356) | 23.7 ± 2.0e (684) | 2,881 |
Numbers in parenthesis are the numbers of virus particles. A total of 20 cells were counted in each case.
Statistically significant difference from YK545 (ΔUL47) at P < 0.05.
Statistically significant difference from YK545 (ΔUL47) at P < 0.0005.
Statistically significant difference from YK545 (ΔUL47) at P < 0.00005.
Statistically nonsignificant difference from YK545 (ΔUL47).
FIG 6.
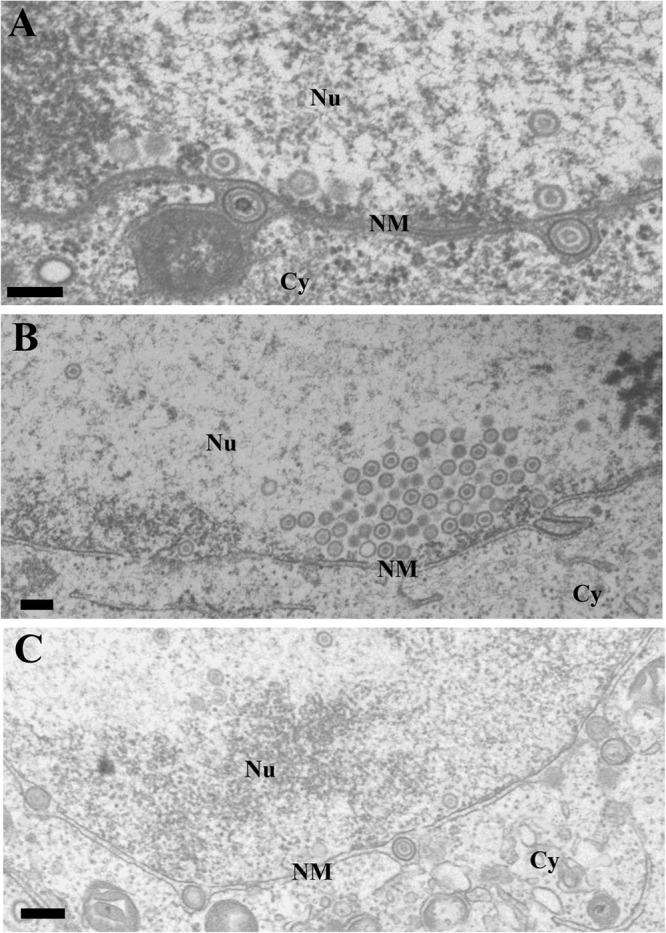
Ultrastructural analysis of the effect of UL47 on HSV-1 nuclear egress. Vero cells infected with wild-type HSV-1(F) (A), YK545 (ΔUL47) (B), or YK546 (ΔUL47-repair) (C) at an MOI of 5 were fixed at 18 h postinfection, embedded, sectioned, stained, and examined by transmission electron microscopy. Nu, nucleus; Cy, cytoplasm; NM, nuclear membrane. Scale bar, 200 nm.
TABLE 3.
Effect of the UL47-null mutation on distribution of virus particles in infected HEp-2 cells
| Virus | Avg ± SE (%) virus particles in the indicated morphogenetic stagea |
Total no. of particles counted | ||||
|---|---|---|---|---|---|---|
| Nucleocapsids in the nucleus | Enveloped virions in the perinuclear space | Nucleocapsids in the cytoplasm | Enveloped virions in the cytoplasm | Extracellular enveloped virions | ||
| HSV-1(F) | 26.6 ± 2.8c (579) | 13.8 ± 1.3c (300) | 9.7 ± 1.0b (211) | 19.9 ± 1.8c (434) | 30.0 ± 2.4d (652) | 2,176 |
| YK545 (ΔUL47) | 61.7 ± 3.0 (587) | 0.3 ± 0.3 (3) | 5.8 ± 1.3 (55) | 4.8 ± 1.1 (46) | 27.4 ± 2.7 (266) | 952 |
| YK546 (ΔUL47-repair) | 30.2 ± 2.2c (687) | 13.1 ± 1.2c (298) | 9.2 ± 0.9b (208) | 19.4 ± 1.6c (442) | 28.1 ± 2.3d (638) | 2,273 |
Numbers in parentheses are the numbers of virus particles. A total of 20 cells were counted in each case.
Statistically significant difference from YK545 (ΔUL47) at P < 0.05.
Statistically significant difference from YK545 (ΔUL47) at P < 0.0000005.
Statistically nonsignificant difference from YK545 (ΔUL47).
FIG 7.
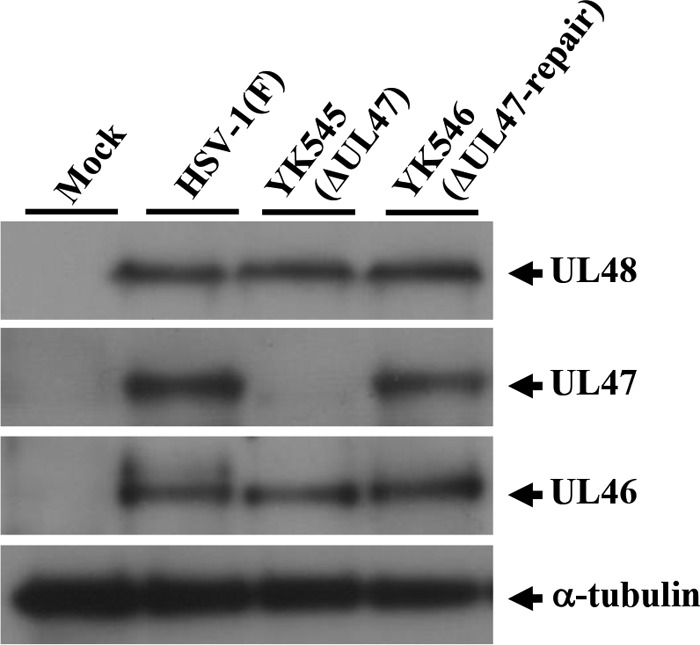
Effect of the null mutation in UL47 on expression of neighboring genes UL48 and UL46. Vero cells were mock infected or infected with wild-type HSV-1(F), YK545 (ΔUL47), or YK546 (ΔUL47-repair) at an MOI of 5, harvested at 18 h postinfection, lysed, and analyzed by immunoblotting with antibodies to the indicated proteins.
Localization of UL47 in HSV-1-infected cells by immunoelectron microscopy.
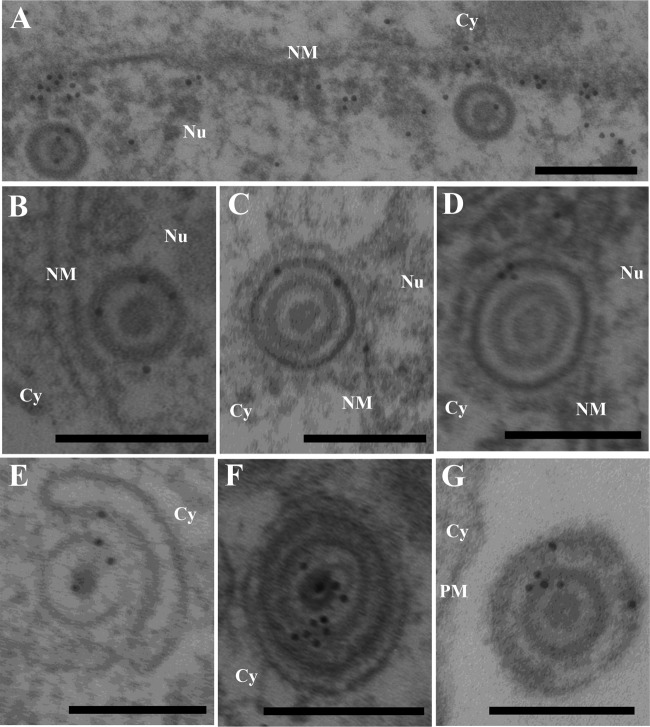
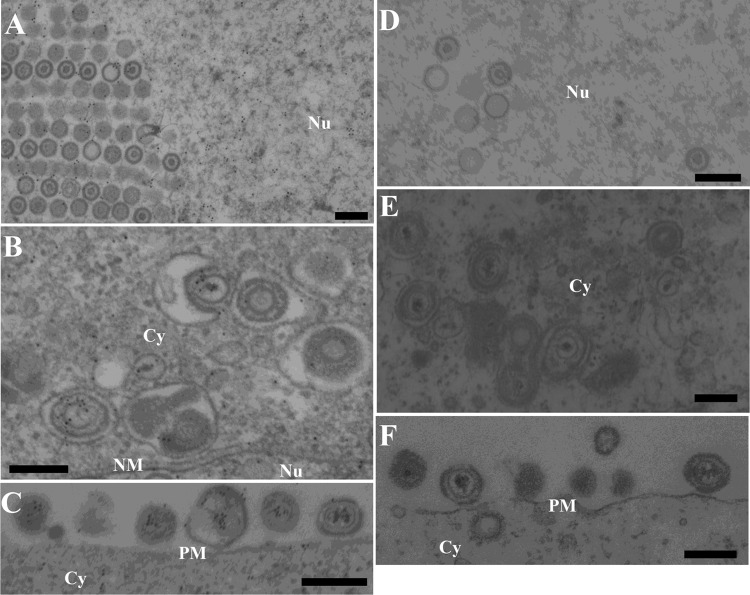
UL31, UL34, and Us3 have been reported to localize at the nuclear membrane and to be components of primary enveloped virions in the perinuclear space, and Us3 has been reported to localize at cytoplasmic membranes and extranuclear virions (36). Finally, we attempted to localize UL47 in HSV-1-infected cells at the ultrastructural level. Preliminary experiments indicated that the anti-UL47 antibody generated in this study was not useful for immunoelectron microscopy (data not shown). Therefore, we attempted to detect tagged UL47 in Vero cells infected with YK524 (mRFP1-UL47) or YK536 (MEF-UL47) using various rabbit polyclonal and mouse monoclonal antibodies to the mRFP1, Flag, and Myc tags. Among the antibodies tested, only anti-Myc mouse monoclonal antibody bound significantly to Vero cells infected with YK536 (MEF-UL47) (Fig. 8 and 9A to C) but not to wild-type HSV-1(F)-infected Vero cells (Fig. 9D to F). MEF-tagged UL47 was detected throughout the nucleus (Fig. 9A) and at the nuclear membrane (Fig. 8A) by immunoelectron microscopy of YK536 (MEF-UL47)-infected Vero cells, in agreement with the localization of mRFP1-UL47 by fluorescence microscopy (Fig. 3 and 5). We noted that MEF-tagged UL47 was detected on nuclear capsids, but the density of immunostained MEF-tagged UL47 in nuclear domains with capsid aggregates was approximately the same as in domains without capsids (Fig. 9A). These observations raised the possibility that MEF-UL47 was not specifically associated with nuclear capsids. MEF-tagged UL47 was also detected on most primary enveloped virions in the perinuclear space, secondary enveloped virions in the cytoplasm, and extracellular virions (Fig. 8C to G and 9B and C), suggesting that UL47 was a component of both primary and secondary enveloped virions.
FIG 8.
Localization of UL47 in HSV-1-infected cells by immunoelectron microscopy. Vero cells were infected with YK536 (MEF-UL47) at an MOI of 5, fixed at 18 h postinfection, embedded, sectioned, stained with mouse anti-Myc monoclonal antibody followed by goat anti-mouse IgG conjugated onto 10-nm gold particles, and examined by transmission electron microscopy. UL47 was detected in the nucleoplasm (A), along the nuclear membrane (A), on capsids in the nucleus (A and B) and cytoplasm (E), on primary enveloped virions in the perinuclear space (C and D), and on secondary enveloped virions in the cytoplasm (F) and extracellular space (G). Nu, nucleus; Cy, cytoplasm; NM, nuclear membrane; PM, plasma membrane. Scale bar, 200 nm.
FIG 9.
Immunoelectron microscopy of Vero cells infected with YK536 (MEF-UL47) and wild-type HSV-1(F). Vero cells were infected with YK536 (MEF-UL47) (A to C) or wild-type HSV-1(F) (D to F) at an MOI of 5, fixed at 18 h postinfection, embedded, sectioned, stained with mouse anti-Myc monoclonal antibody followed by goat anti-mouse IgG conjugated onto 10-nm gold particles, and examined by transmission electron microscopy. UL47 was detected in the nucleoplasm (A), on capsids in the nucleus (A) and cytoplasm (B), and on secondary enveloped virions in the cytoplasm (B) and extracellular space (C). Nu, nucleus; Cy, cytoplasm; NM, nuclear membrane; PM, plasma membrane. Scale bar, 200 nm.
DISCUSSION
In this study, we showed the following: that MEF-tagged UL47 coimmunoprecipitated with UL34, UL31, and Us3; that MEF-tagged UL31 coimmunoprecipitated with UL34, Us3, and UL47; and that MEF-tagged UL34 coimmunoprecipitated with UL31, UL47, and Us3. Taken together, these results indicated that UL47 formed a complex with UL34, UL31, and/or Us3 in HSV-1-infected cells. This conclusion was in agreement with previous reports (12, 21, 24) that, in HSV-1-infected cells, UL47 interacted with viral capsid protein UL17, which further formed a complex with UL31 and viral capsid protein UL25, and that UL47 interacted with Us3. At present it remains to be determined whether UL47, UL31, UL34, and Us3 form a high-order complex in HSV-1-infected cells. However, the reciprocal coimmunoprecipitation experiments in this and previous studies (4, 21) showing coimmunoprecipitation of Us3 and UL47 and of UL31 and UL34 strongly suggested that these interactions form a high-order complex of UL47, UL31, UL34, and Us3 in HSV-1-infected cells. In particular, UL31 and UL34 have been shown to be predominantly detected at the nuclear membrane in wild-type HSV-1-infected cells by immunofluorescence microscopy (4). Therefore, it is likely that the interactions of UL31 and UL34 with UL47 and Us3 observed in this study occurred mainly at the nuclear membrane in HSV-1-infected cells. Furthermore, we showed here that, in the absence of Us3 kinase activity, UL47, UL31, and UL34 were all aberrantly localized and colocalized in punctate structures at the nuclear membrane. This result suggested that localization of UL47, UL31, and UL34 at the nuclear membrane were all regulated by Us3 kinase activity and supported the hypothesis that these viral proteins and probably Us3 formed a complex at the nuclear membrane in HSV-1-infected cells. Based on the immunoelectron microscopy results in this and previous studies showing that UL47, UL31, UL34, and Us3 are components of primary enveloped virions, it seemed possible that coimmunoprecipitation of UL31, UL34, UL47, and Us3 indicated that these viral proteins may associate in intact capsids. However, since we found that none of these MEF-tagged viral proteins coimmunoprecipitated with HSV-1 capsid protein VP23, this possibility seemed less likely.
Quantitative electron microscopic analysis of HSV-1-infected Vero and HEp-2 cells showed that, in the absence of UL47, primary enveloped virions in the perinuclear space were barely detectable and that the prevalence of this type of virion was substantially reduced. In contrast, the frequency of nuclear capsids increased. The accumulation of nuclear capsids and the lack of primary enveloped virions in the perinuclear space in the absence of UL47 likely reflected an imbalance between the rate of virion delivery into the perinuclear space and the rate of egress from this region. Thus, it appeared that the rate of viral egress from the nucleoplasm decreased in the absence of UL47 compared to that in the presence of UL47, but the rate of egress from the perinuclear space in the absence of UL47 was similar to that in the presence of UL47. Based on these results, UL47 appeared to be required for efficient primary envelopment of nucleocapsids in HSV-1 nuclear egress. In support of this hypothesis, we showed that UL47 was detected at the inner nuclear membrane by immunoelectron microscopy. It has been reported that, in cells infected with a UL34- or UL31-null mutant HSV-1, no enveloped virions were detected in the perinuclear space or cytoplasm or at the cell surface (5, 6), indicating that UL31 and UL34 functions were required for primary envelopment of nucleocapsids. In this study, primary enveloped virions in the perinuclear space were barely detected in cells infected with the UL47-null mutant HSV-1, as was observed in cells infected with the UL31- or UL34-null mutant HSV-1, but the prevalence of enveloped virions in the cytoplasm of cells infected with the UL47-null mutant HSV-1, although detectable, was decreased. These observations suggested that UL47 was not as essential for primary envelopment of nucleocapsids at the nuclear membrane as UL34 and UL31 but played a regulatory role in this process. UL47 may regulate the optimal primary envelopment activity of the UL34/UL31 complex by interaction with the complex. This hypothesis was supported by the observation in this study that UL47 interacted with UL31 and UL34 in HSV-1-infected cells and that UL47 was a component of primary enveloped virions, which enabled UL47 to interact with the UL34/UL31 complex during primary envelopment. As described above, UL47 was reported to form a complex with UL17, a component of the HSV-1 CVSC, and, therefore, may interact with UL31 to recruit HSV nucleocapsids for primary envelopment (12, 13). Therefore, UL47 may upregulate the primary envelopment of nucleocapsids by promoting recruitment of nucleocapsids through interaction with the UL17/UL25/UL31 complex, which may stabilize the association of capsids with the UL34/UL31 complex at the nuclear membrane. It was interesting that, although the frequencies of virions in the perinuclear space and the cytoplasm in cells infected with the UL47-null mutant HSV-1 were reduced compared to those in cells infected with wild-type HSV-1, the prevalence rates of extracellular virions were similar in cells infected with the mutant and wild-type HSV-1. This observation raised the possibility that UL47 might promote an HSV-1 virion maturation step(s) after primary envelopment, probably de-envelopment, secondary envelopment, and/or transport of secondary enveloped virions from the cytoplasmic vesicles to the extracellular space.
Our immunoelectron microscopy data showing that HSV-1 UL47 was a component of both primary enveloped virions and extracellular virions was not in agreement with previous reports by Naldinho-Souto et al. (37) that HSV-1 UL47 tagged with yellow fluorescent protein (YFP) was not detected in primary enveloped virions by immunoelectron microscopy but was detected in extracellular virions. A similar observation was also obtained with VP22 tagged with green fluorescent protein (GFP) (37). However, biochemical isolation and characterization of wild-type HSV-1 primary enveloped virions by Padula et al. (38) showed that untagged VP22 was detected as a component of primary enveloped virions. Therefore, it may be more difficult to detect a fluorescence-tagged protein component of primary enveloped virions by immunoelectron microscopy than to detect it in extracellular virions. Since El Bilali et al. (39) recently reported that tagging tegument proteins with a fluorescent protein had a significant effect on incorporation of the tagged proteins into virions, UL47 tagged with a fluorescent protein may be incorporated into primary enveloped virions much less efficiently than untagged UL47 or UL47 tagged with MEF, which is much smaller than the fluorescent proteins. Our data also were not in agreement with a previous report that pseudorabies virus (PRV) UL47 was not present in primary enveloped virions (40). It appears that, despite their genetic similarities, HSV-1 and PRV differ in the compositions of their virions since HSV-1 primary enveloped virions contain gB, gD, gH/gL, gM, VP22, VHS, VP16, and UL11, but PRV does not (1, 37, 38, 41–45). Alternatively, UL47 may have been present in PRV primary enveloped virions but could not be detected with the antibody used in that study.
In conclusion, the data presented here begin to elucidate the novel function of HSV-1 UL47 in regulating HSV-1 primary envelopment during viral nuclear egress. The vesicle-mediated viral nuclear egress process may involve viral and cellular proteins other than those reported to date. Further studies to identify other novel viral and cellular proteins that regulate the vesicle-mediated viral egress process and to elucidate the mechanisms of these regulatory proteins, including UL47, in this process will be needed and are under way in this laboratory.
ACKNOWLEDGMENTS
We thank Tomoko Ando and Shihoko Koyama for excellent technical assistance.
This study was supported by the Funding Program for Next Generation World-Leading Researchers and Grants for Scientific Research from the Japan Society for the Promotion of Science, a contract research fund for the Program of Japan Initiative for Global Research Network on Infectious Diseases, a grant for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Science, Sports and Technology (MEXT) of Japan, and grants from the Takeda Science Foundation, the Naito Foundation, the Sumitomo Foundation, and the Tokyo Biochemical Research Foundation. Z.L. is supported by a MEXT scholarship for foreign researchers.
Footnotes
Published ahead of print 12 February 2014
REFERENCES
- 1.Mettenleiter TC, Muller F, Granzow H, Klupp BG. 2013. The way out: what we know and do not know about herpesvirus nuclear egress. Cell Microbiol. 15:170–178. 10.1111/cmi.12044 [DOI] [PubMed] [Google Scholar]
- 2.Johnson DC, Baines JD. 2011. Herpesviruses remodel host membranes for virus egress. Nat. Rev. Microbiol. 9:382–394. 10.1038/nrmicro2559 [DOI] [PubMed] [Google Scholar]
- 3.Roizman B, Knipe DM, Whitley RJ. 2013. Herpes simplex viruses, p 1823–1825 In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Rancaniello VR, Roizman B. (ed), Fields virology, 6th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 4.Reynolds AE, Ryckman BJ, Baines JD, Zhou Y, Liang L, Roller RJ. 2001. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803–8817. 10.1128/JVI.75.18.8803-8817.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roller RJ, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D. 2000. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J. Virol. 74:117–129. 10.1128/JVI.74.1.117-129.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YE, Van Sant C, Krug PW, Sears AE, Roizman B. 1997. The null mutant of the UL31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J. Virol. 71:8307–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mou F, Wills EG, Park R, Baines JD. 2008. Effects of lamin A/C, lamin B1, and viral US3 kinase activity on viral infectivity, virion egress, and the targeting of herpes simplex virus UL34-encoded protein to the inner nuclear membrane. J. Virol. 82:8094–8104. 10.1128/JVI.00874-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mou F, Forest T, Baines JD. 2007. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 81:6459–6470. 10.1128/JVI.00380-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park R, Baines JD. 2006. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J. Virol. 80:494–504. 10.1128/JVI.80.1.494-504.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjerke SL, Roller RJ. 2006. Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology 347:261–276. 10.1016/j.virol.2005.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds AE, Liang L, Baines JD. 2004. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes UL31 and UL34. J. Virol. 78:5564–5575. 10.1128/JVI.78.11.5564-5575.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang K, Baines JD. 2011. Selection of HSV capsids for envelopment involves interaction between capsid surface components pUL31, pUL17, and pUL25. Proc. Natl. Acad. Sci. U. S. A. 108:14276–14281. 10.1073/pnas.1108564108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toropova K, Huffman JB, Homa FL, Conway JF. 2011. The herpes simplex virus 1 UL17 protein is the second constituent of the capsid vertex-specific component required for DNA packaging and retention. J. Virol. 85:7513–7522. 10.1128/JVI.00837-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai PJ, Pryce EN, Henson BW, Luitweiler EM, Cothran J. 2012. Reconstitution of the Kaposi's sarcoma-associated herpesvirus nuclear egress complex and formation of nuclear membrane vesicles by coexpression of ORF67 and ORF69 gene products. J. Virol. 86:594–598. 10.1128/JVI.05988-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klupp BG, Granzow H, Fuchs W, Keil GM, Finke S, Mettenleiter TC. 2007. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc. Natl. Acad. Sci. U. S. A. 104:7241–7246. 10.1073/pnas.0701757104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato A, Yamamoto M, Ohno T, Kodaira H, Nishiyama Y, Kawaguchi Y. 2005. Identification of proteins phosphorylated directly by the Us3 protein kinase encoded by herpes simplex virus 1. J. Virol. 79:9325–9331. 10.1128/JVI.79.14.9325-9331.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purves FC, Spector D, Roizman B. 1991. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 65:5757–5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loret S, Guay G, Lippe R. 2008. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J. Virol. 82:8605–8618. 10.1128/JVI.00904-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnelly M, Verhagen J, Elliott G. 2007. RNA binding by the herpes simplex virus type 1 nucleocytoplasmic shuttling protein UL47 is mediated by an N-terminal arginine-rich domain that also functions as its nuclear localization signal. J. Virol. 81:2283–2296. 10.1128/JVI.01677-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnelly M, Elliott G. 2001. Nuclear localization and shuttling of herpes simplex virus tegument protein VP13/14. J. Virol. 75:2566–2574. 10.1128/JVI.75.6.2566-2574.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato A, Liu Z, Minowa A, Imai T, Tanaka M, Sugimoto K, Nishiyama Y, Arii J, Kawaguchi Y. 2011. Herpes simplex virus 1 protein kinase Us3 and major tegument protein UL47 reciprocally regulate their subcellular localization in infected cells. J. Virol. 85:9599–9613. 10.1128/JVI.00845-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Sirko DA, McKnight JL. 1991. Role of herpes simplex virus type 1 UL46 and UL47 in alpha TIF-mediated transcriptional induction: characterization of three viral deletion mutants. J. Virol. 65:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobrikova E, Shveygert M, Walters R, Gromeier M. 2010. Herpes simplex virus proteins ICP27 and UL47 associate with polyadenylate-binding protein and control its subcellular distribution. J. Virol. 84:270–279. 10.1128/JVI.01740-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholtes LD, Yang K, Li LX, Baines JD. 2010. The capsid protein encoded by UL17 of herpes simplex virus 1 interacts with tegument protein VP13/14. J. Virol. 84:7642–7650. 10.1128/JVI.00277-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu M, Taddeo B, Zhang W, Roizman B. 2013. Selective degradation of mRNAs by the HSV host shutoff RNase is regulated by the UL47 tegument protein. Proc. Natl. Acad. Sci. U. S. A. 110:E1669–E1675. 10.1073/pnas.1305475110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryckman BJ, Roller RJ. 2004. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 78:399–412. 10.1128/JVI.78.1.399-412.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugimoto K, Uema M, Sagara H, Tanaka M, Sata T, Hashimoto Y, Kawaguchi Y. 2008. Simultaneous tracking of capsid, tegument, and envelope protein localization in living cells infected with triply fluorescent herpes simplex virus 1. J. Virol. 82:5198–5211. 10.1128/JVI.02681-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka M, Kagawa H, Yamanashi Y, Sata T, Kawaguchi Y. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J. Virol. 77:1382–1391. 10.1128/JVI.77.2.1382-1391.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato A, Tanaka M, Yamamoto M, Asai R, Sata T, Nishiyama Y, Kawaguchi Y. 2008. Identification of a physiological phosphorylation site of the herpes simplex virus 1-encoded protein kinase Us3 which regulates its optimal catalytic activity in vitro and influences its function in infected cells. J. Virol. 82:6172–6189. 10.1128/JVI.00044-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawaguchi Y, Van Sant C, Roizman B. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328–7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto T, Arii J, Tanaka M, Sata T, Akashi H, Yamada M, Nishiyama Y, Uema M, Kawaguchi Y. 2009. Differences in the regulatory and functional effects of the Us3 protein kinase activities of herpes simplex virus 1 and 2. J. Virol. 83:11624–11634. 10.1128/JVI.00993-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato K, Daikoku T, Goshima F, Kume H, Yamaki K, Nishiyama Y. 2000. Synthesis, subcellular localization and VP16 interaction of the herpes simplex virus type 2 UL46 gene product. Arch. Virol. 145:2149–2162. 10.1007/s007050070045 [DOI] [PubMed] [Google Scholar]
- 33.Noda T, Sugita Y, Aoyama K, Hirase A, Kawakami E, Miyazawa A, Sagara H, Kawaoka Y. 2012. Three-dimensional analysis of ribonucleoprotein complexes in influenza A virus. Nat. Commun. 3:639. 10.1038/ncomms1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noda T, Sagara H, Yen A, Takada A, Kida H, Cheng RH, Kawaoka Y. 2006. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439:490–492. 10.1038/nature04378 [DOI] [PubMed] [Google Scholar]
- 35.Mou F, Wills E, Baines JD. 2009. Phosphorylation of the UL31 protein of herpes simplex virus 1 by the US3-encoded kinase regulates localization of the nuclear envelopment complex and egress of nucleocapsids. J. Virol. 83:5181–5191. 10.1128/JVI.00090-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939–8952. 10.1128/JVI.76.17.8939-8952.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naldinho-Souto R, Browne H, Minson T. 2006. Herpes simplex virus tegument protein VP16 is a component of primary enveloped virions. J. Virol. 80:2582–2584. 10.1128/JVI.80.5.2582-2584.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padula ME, Sydnor ML, Wilson DW. 2009. Isolation and preliminary characterization of herpes simplex virus 1 primary enveloped virions from the perinuclear space. J. Virol. 83:4757–4765. 10.1128/JVI.01927-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Bilali N, Radtke K, Lippe R. 2013. Analysis of the HSV-1 tegument by flow cytometry, abstr 4.07, p 100 Abstr. 38th Annu. Int. Herpesvirus Workshop, Grand Rapids, MI [Google Scholar]
- 40.Granzow H, Klupp BG, Mettenleiter TC. 2004. The pseudorabies virus US3 protein is a component of primary and of mature virions. J. Virol. 78:1314–1323. 10.1128/JVI.78.3.1314-1323.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Read GS, Patterson M. 2007. Packaging of the virion host shutoff (Vhs) protein of herpes simplex virus: two forms of the Vhs polypeptide are associated with intranuclear B and C capsids, but only one is associated with enveloped virions. J. Virol. 81:1148–1161. 10.1128/JVI.01812-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farnsworth A, Wisner TW, Webb M, Roller R, Cohen G, Eisenberg R, Johnson DC. 2007. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc. Natl. Acad. Sci. U. S. A. 104:10187–10192. 10.1073/pnas.0703790104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baines JD, Wills E, Jacob RJ, Pennington J, Roizman B. 2007. Glycoprotein M of herpes simplex virus 1 is incorporated into virions during budding at the inner nuclear membrane. J. Virol. 81:800–812. 10.1128/JVI.01756-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stannard LM, Himmelhoch S, Wynchank S. 1996. Intra-nuclear localization of two envelope proteins, gB and gD, of herpes simplex virus. Arch. Virol. 141:505–524. 10.1007/BF01718314 [DOI] [PubMed] [Google Scholar]
- 45.Baines JD, Jacob RJ, Simmerman L, Roizman B. 1995. The herpes simplex virus 1 UL11 proteins are associated with cytoplasmic and nuclear membranes and with nuclear bodies of infected cells. J. Virol. 69:825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]