ABSTRACT
One of the major hurdles in treating tuberculosis (TB) is the time-consuming and difficult methodology for diagnosis. Stable-isotope breath tests hold great potential for rapidly diagnosing an infectious disease, monitoring therapy, and determining a bacterial phenotype in a rapid, point-of-care manner that does not require invasive sampling. Here we describe the preclinical development of a potentially highly selective TB diagnostic breath test based upon the organism’s CO dehydrogenase activity. After development of the test in vitro, we were able to use the breath test to discriminate between infected and control rabbits, demonstrating that a diagnosis can potentially be made and also that a complex bacterial phenotype can be noninvasively and rapidly studied in the host.
IMPORTANCE
Tuberculosis (TB) remains a major infectious cause of disease and death worldwide, and effective diagnosis and then treatment are the tools with which we fight TB. The more quickly and more specific the diagnosis can be made, the better, and this is also true of diagnosis being as close to the patient (point of care) as possible. Here we report our preclinical development of breath tests based upon specific mycobacterial metabolism that could, with development, allow rapid point-of-care diagnosis through measuring the mycobacterial conversion of labeled CO to labeled CO2.
INTRODUCTION
The fight against tuberculosis (TB) has been reinvigorated by a broad and sustained research effort that has produced a range of new drugs either receiving regulatory approval (bedaquiline, delamanid) or currently undergoing later-stage clinical trials (e.g., PA824 and SQ109), with many more in the preclinical and phase 1 stages. However, there is the potential for this burst of creativity to be severely hindered in the clinical trial process. There is great complexity in performing phase 2, phase 3, and postapproval trials of all these agents in the potentially wide range of drug combinations possible with organisms that have a range of drug resistance phenotypes (susceptible, monoresistant, multidrug resistant, extensively drug resistant). Even with innovative trial designs (1), this complexity can make recruitment and endpoint analysis difficult. Compounding this problem are the significant deficits in current endpoints of efficacy in clinical trials—sputum smear positivity, early bactericidal activity, or conversion to a negative sputum culture after a 2-month treatment—that have prompted a search for new and better biomarkers (2–4). Of course, once established and validated, these biomarkers might then prove useful in diagnosis or treatment outside clinical trials.
Furthermore, many of the most commonly used techniques for the diagnosis of TB are far from ideal, resulting in diagnostic delays that can both allow transmission and lead to inadequate antibiotic treatments that allow resistance to develop (5). Improved rapid and specific techniques for breath test-based diagnosis could also have a significant impact in increasing the speed of diagnosis.
Our groups recently reported preclinical studies upon a stable-isotope breath test approach delivering [13C]urea directly to the lung, where it underwent conversion by the mycobacterial virulence factor urease to 13CO2. By determining the extent of 13CO2 enrichment in breath, it was possible to track the progression of infection, effective treatment, and simulated treatment failure in a rabbit model (6). Because the test can be performed rapidly using approved point-of-care (POC) detectors, clinical translation of this approach is ongoing (using an oral delivery route for urea due to regulatory issues) to determine sensitivity and specificity in humans (clinical trial registration number NCT01301144).
However, urease is expressed by a range of pathogens of both the lung, such as Pseudomonas aeruginosa, Acinetobacter baumannii (7, 8), and Klebsiella pneumoniae (9), and other sites, such as the gastrointestinal (GI) pathogen Helicobacter pylori (10). Although direct lung delivery of [13C]urea (as a nebulized solution or inhaled dry powder) is expected to drive lung specificity and circumvent false positivity from GI urease expressers, more-specific tracer chemistry would circumvent false positives from lung infections with urease expressers, such as P. aeruginosa.
An extensive genomic and metabolomic search supported the hypothesis that the enzyme CO dehydrogenase (CODH) might provide a suitable and highly specific metabolomic route to enable TB detection. This enzyme oxidizes CO to CO2, as in reaction scheme 1 (CO + H2O → CO2 + 2H+ + 2e−), and activity has been reported by several groups (11–13) in several avirulent and environmental mycobacterial species.
Predicted active (nonpseudogene) genes for the large, small, and medium subunits of CODH are found in the genomes of several virulent tuberculous mycobacteria, such as Rv0373c, Rv0374c, and Rv0375c, respectively, in Mycobacterium tuberculosis, with analogs in virulent Mycobacterium bovis strains and the vaccine strain M. bovis BCG. We therefore hypothesized that, using appropriate stable isotopically labeled CO, we could use the mycobacterial conversion of labeled CO to labeled CO2 as a much more specific marker for mycobacterial lung infections and provide an improved breath test for TB diagnosis (Table 1).
TABLE 1 .
Common lung pathogens expressing urease do not express CODHa
| Pathogen | Expression of: |
|
|---|---|---|
| Urease (source) | CODH | |
| Pseudomonas aeruginosa | Yes (38) | No |
| Acinetobacter baumannii | Yes (8) | No |
| Klebsiella pneumoniae | Yes (9) | No |
| Haemophilus influenzae | Yes (39) | No |
| Staphylococcus aureus | Yes (40) | No |
Data are collected from prior work discussing genes or enzymatic activity. In this work, only genes were tested, and none of the tested strains expressed CODH.
Although it is known to be a toxic gas in sufficient quantity, CO is endogenously made by heme oxygenase and at limited exposures is also widely used clinically to measure lung diffusion capacity (14), specifically in patients with TB (15). Therefore, with this precedent, clinical translation may be possible upon a thorough evaluation of risk/benefit ratios. As is discussed later, CO-hemoglobin saturation in CO-treated rabbits used in detection ranged from 9 to 18%; in comparison, heavy smokers achieve 15% saturation (16).
RESULTS AND DISCUSSION
CODH metabolism of 13CO to 13CO2 in vitro.
Initially, the conversion of 13CO to 13CO2 was measured in vitro in M. bovis BCG and exhibited the anticipated increases in headspace δ-13CO2 that were dependent upon 13CO dose (Fig. 1A), bacterial density (Fig. 1B), and incubation time (Fig. 1C). However, the magnitude of the increases in δ-13CO2 were much lower than we observed previously in vitro using [13C]urea (6). This suggested that if used as a breath test in vivo (where any CODH-derived 13CO2 would be mixed with large amounts of exhaled CO2), the changes in δ-13CO2 would not be detectable.
FIG 1 .
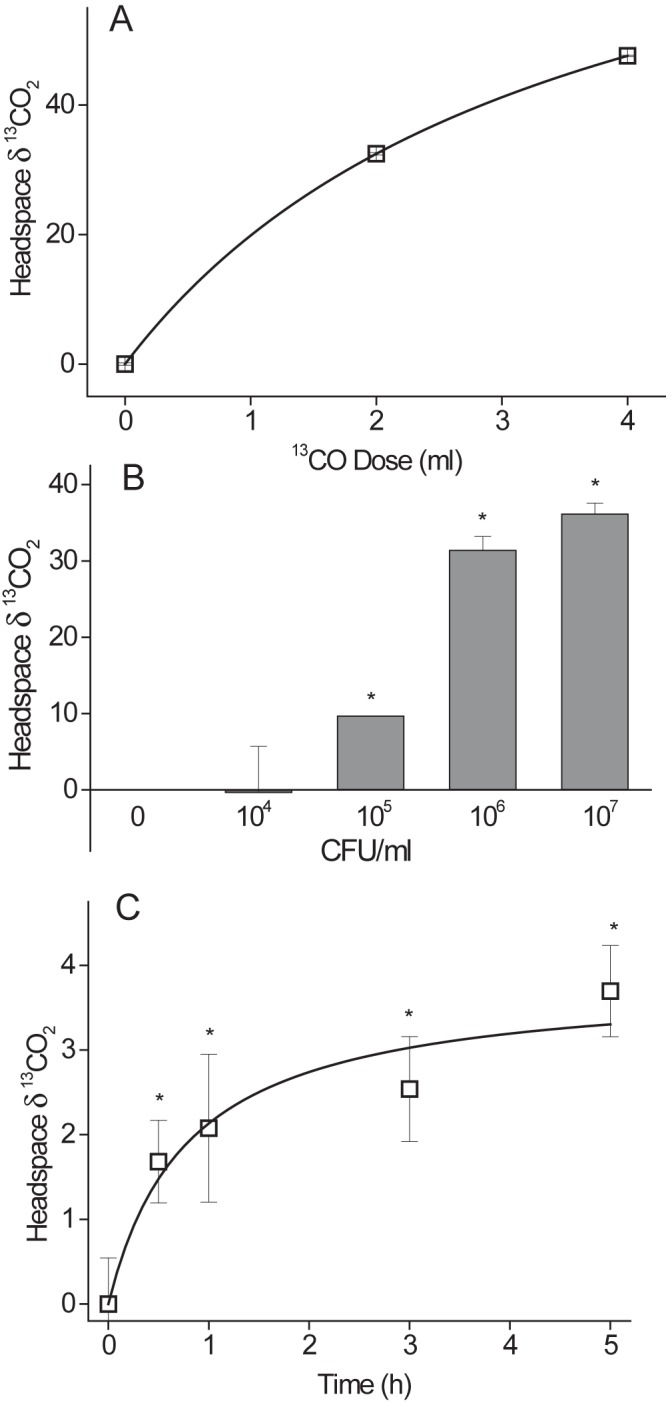
Dose, CFU, and time dependence of CODH activity using 13CO. (A) M. bovis BCG (3 ml, 1 × 107 CFU/ml) was incubated with increasing doses of 13CO at 37°C for 4 h. Headspace δ-13CO2 was determined by IRMS. Numbers are means ± SD (n = 4) of results from separate biological replicates. By one-way ANOVA with the Tukey test as a post hoc test, P was <0.001 (0 ml versus 2 or 4 ml). (B) Different dilutions of M. bovis BCG (3 ml) were incubated with 2 ml 13CO for 4 h. Headspace δ-13CO2 was determined by IRMS. Numbers are means ± SD of results from separate biological replicates with 1 × 105, 1 × 107, or 1 × 108 CFU (n = 3) or 0 or e6 CFU (n = 2). By one-way ANOVA with the Tukey test as a post hoc test, P was <0.05 (1 × 105 versus 1 × 107 and 1 × 108; the significance for 1 × 106 cannot be determined). (C) M. bovis BCG (3 ml, 1 × 107 CFU/ml) was incubated with 2 ml 13CO for differing time points. Headspace δ-13CO2 was determined by IRMS. Numbers are means ± SD (n = 4) of results from separate biological replicates. By one-way ANOVA with the Tukey test as a post hoc test, P was <0.05 (0 h versus 0.5, 1, 3, or 5 h).
To increase the sensitivity of CODH detection in a breath test format, it was apparent that measurement of a rare CO2 isotopologue would be needed, so that the CODH-derived signal could be observed. The conversion of 13C18O to 13C18O16O by CODH was predicted to allow a much greater increase in sensitivity, due to the extremely low abundance of the clumped isotope 13C18O16O in nature. Only about 0.002% of exhaled metabolic CO2 from non-CODH sources will be 13C18O16O.
CODH metabolism of 13C18O to 13C18O16O in vitro.
In order to validate the potential of the assay for breath test measurements, we tested key features of the assay in vitro, namely, the dependence of Δ47 (the index of CODH metabolism of 13C18O to 13C18O16O) on the following: (i) the dose of 13C18O used, to understand sensitivity limits; (ii) the bacterial density (measured as numbers of CFU/ml), as it is highly desirable that the magnitude of δ47 changes with bacterial burden, so that response to treatment can be observed {δ47 = 1,000 ⋅ [rR47/44(sample) − rR47/44(standard)] divided by the rR47/44(standard), where rR47/44 is the relative ratio of masses 47 (13C18O16O) and 44 (CO2)}; and (iii) incubation time, so that appropriate time points after administration for in vivo measurements are used.
Figure 2 demonstrates the relationship between 13C18O dose and headspace Δ47 above those of cultures of M. bovis BCG, with a linear relationship over the dose range chosen. This indicates that within the limits of the experiment, saturation of the assay’s ability to report CODH does not occur, so that increased sensitivity in vivo should be achieved using more 13C18O (although the total dose will be limited by CO toxicity). The relationship between bacterial density and headspace Δ47 above that of cultures of M. bovis BCG is shown in Fig. 3, demonstrating that the assay would be able to detect decreasing levels of bacteria during drug treatment and that 6 × 105 total CFU were readily detected. About 107 to 109 bacilli can be routinely cultured from a single human pulmonary cavity (17), so the assay sensitivity may be suitable. The Δ47 signal saturates at between 106 and 107 CFU/ml in this in vitro assay, although it is as yet uncertain why this occurs.
FIG 2 .
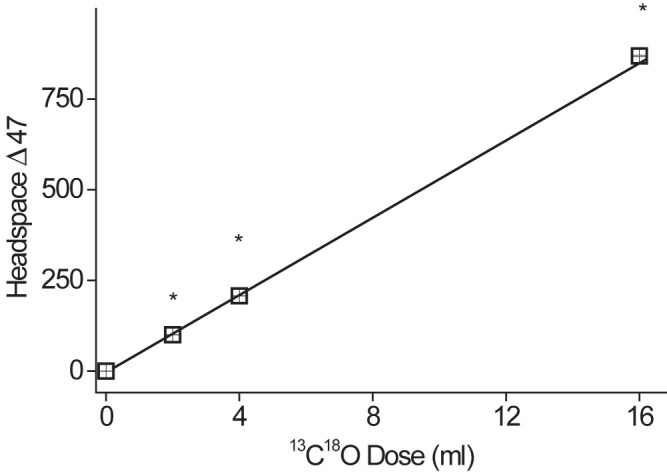
13C18O dose curve. M. bovis BCG (3 ml, 1 × 107 CFU/ml) was incubated with 13C18O gas for 22 h. Headspace gas was analyzed by isotope ratio mass spectrometry (IRMS) to determine Δ47. Data represent means ± SD (n = 4) of results from separate biological replicates. By one-way ANOVA with the Tukey test as a post hoc test (ANOVA between groups), P was <0.001 (*) versus results with a 0-ml CO dose.
FIG 3 .
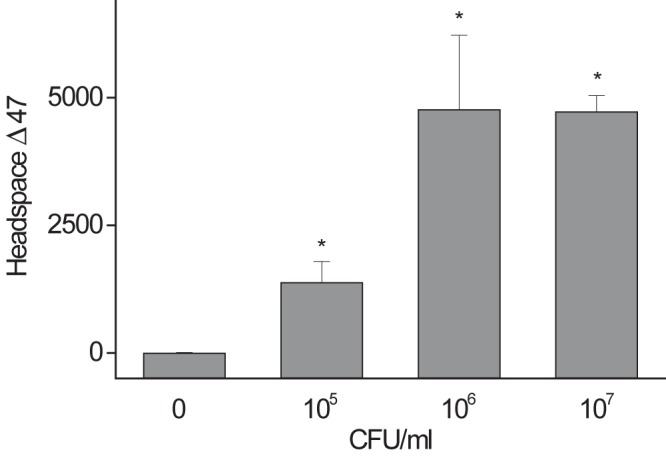
Cell density-dependent conversion of 13C18O to 13C18O16O. M. bovis BCG culture (6 ml) was incubated with 1 ml of 13C18O gas for 0.5 h. Headspace gas was measured to determine Δ47. Data represent means ± SD (n = 4) of results from separate biological replicates. By one-way ANOVA with the Tukey test as a post hoc test, P was <0.001 (*) versus 0 CFU/ml.
The time dependence of the metabolism of 13C18O (corrected for the number of CFU present and the CO dose) for a range of mycobacteria is shown in Fig. 4. It can be seen that Δ47 rapidly increased for all species studied, followed by a decline. The reasons for lower values for M. tuberculosis than for M. bovis strains are not known and may be complex, including relative loss of activity of the heavily laboratory adapted H37Rv strain. In this light, relative assessments of activity between species and strains should be made in vivo, where host-imposed CODH pressures may bring about upregulation, although this is beyond the scope of this work. We were unable to measure any conversion of 13C18O into 13C18O16O in similarly treated cultures of Pseudomonas aeruginosa or Escherichia coli. This is in accord with neither of these organisms’ genomes possessing any known CODH genes.
FIG 4 .
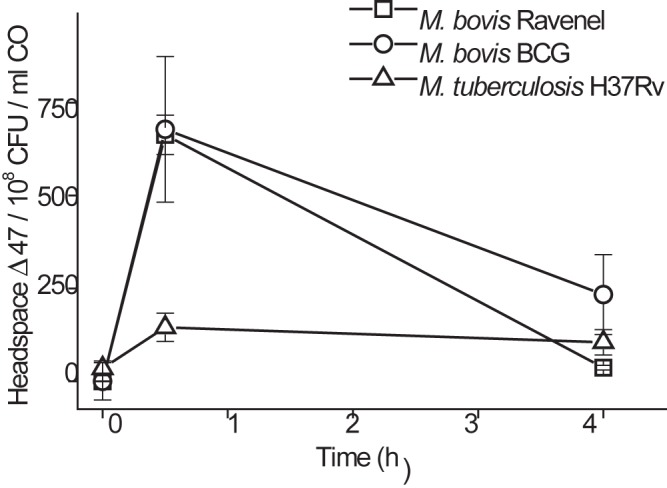
Time-dependent conversion of 13C18O to mass 47 (13C18OO). M. bovis BCG or M. tuberculosis H37Rv (6 ml, 3 × 107 CFU/ml) was incubated with 1 ml of 13C18O gas; M. bovis Ravenel (3 ml of 1 × 108 CFU/ml) was incubated with 3 ml 13C18O. Headspace gas Δ47 was measured, and Δ47 per 1 × 108 CFU per ml added CO was calculated and plotted. Data represent means ± SD (n = 4) of results from separate biological replicates. By one-way ANOVA with the Tukey test as a post hoc test, for 0 h versus 0.5 h, P was <0.001 for all groups; for 0 h versus 4 h, P was <0.01 for M. tuberculosis H37Rv only; and differences were not significant for M. bovis BCG and M. bovis Ravenel.
The rapid kinetics of conversion supported the potential of this assay to provide a rapid breath test, with lengthy periods after tracer administration likely to be unnecessary. The decline at 4 h was, however, unexpected and was not seen for measures of 13CO to 13CO2 (Fig. 1A). We hypothesized that the loss of the δ47 signal was due to loss of the 18O of 13C18O16O, catalyzed by sequential hydration and dehydration of the CO2 by the enzyme carbonic anhydrase, as shown in reaction scheme 2 (18): 13C18O16O + H216O → H213C18O16O2 and H213C18O16O2 → 13C16O2 + H218O. Thus, loss of 18O from 13C18O16O would decrease the Δ47 with time, but not the δ-13C value, which is what was observed. Carbonic anhydrases are ubiquitous enzymes, are known to be expressed and active in mycobacteria such as M. tuberculosis (19), and represent potential drug targets against TB. However, although the carbonic anhydrase inhibitor drugs, such as acetazolamide (Diamox), inhibit isolated bacterial enzymes in vitro (20), they had no effect in whole M. tuberculosis cells due to their inability to penetrate the waxy mycolic acid cell wall (19). Accordingly, concentrations of acetazolamide up to 9 mM had no observed effect upon the loss of Δ47 associated at longer incubation times (not shown). However, since the lungs are known to have high levels of carbonic anhydrase activity (21) that would have similar effects, later in vivo studies used acetazolamide pretreatment at a previously validated dosage (22) to inhibit any lung carbonic anhydrase loss of δ47 that would decrease detection.
Detection of infection by CODH metabolism of 13C18O to 13C18O16O in vivo.
Groups of rabbits were assigned to a control group (no infection) or infected with virulent M. bovis Ravenel, which has been shown by our group to be a highly representative rabbit model of the human disease condition (23, 24). Approximately 107 to 109 bacilli can typically be cultured from a single human pulmonary cavity (17), while in this model, M. bovis infections generated cavitary CFU counts of 106 to 109 bacilli. Figure 5 shows the δ47 in the exhaled breath samples of the two groups after administration of 13C18O, which increased over the course of 15 min in the infected group, significantly greater than in the uninfected control group (by analysis of variance [ANOVA] with a split plot, P = 0.018). The Holm-Šidák significance between results for infected and control groups increased with time; differences were not significant at 0 and 5 min (P = 0.14 and 0.13), they approached significance at 10 min (P = 0.055), and they were significantly different (P < 0.001) at 15 min, reflecting greater conversions of CO to CODH with time. Thus, the breath test exhibited potentially suitable characteristics for detecting tuberculosis, but it appears that, in this model at least, a period of 15 min to allow for more significant production of 13C18O16O would be optimal. The magnitude of δ47 values in vivo (0 to 15) were significantly lower than those observed in vitro (up to several thousand) (Fig. 2 to 4), likely reflecting both dilution by endogenous (non-CODH-mediated) 13C18O16O and absorption of the 13C18O by hemoglobin.
FIG 5 .
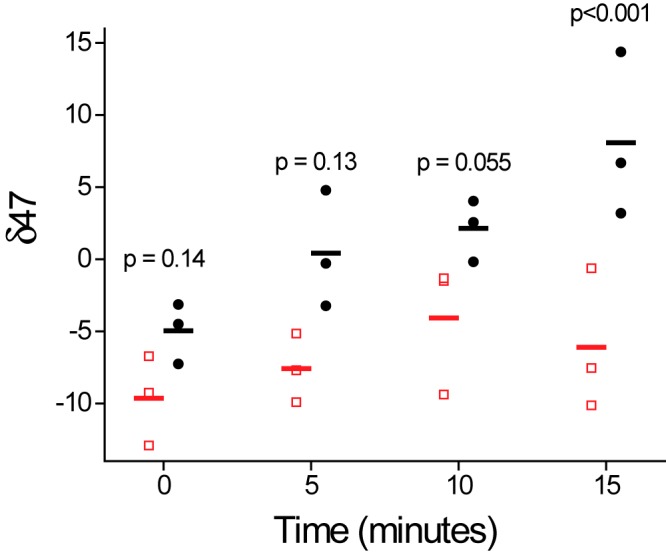
Conversion of 13C18O to mass 47 (13C18OO gas) by M. bovis Ravenel-infected rabbits. Detailed methods are described in Materials and Methods. Exhaled breath was measured to determine δ47. ●, infected rabbits; □, uninfected rabbits. Data represent individual data for separate animals, with the bar representing the mean. An ANOVA split plot was used as a measure of between-subjects effects (uninfected versus infected), and the slopes were significantly different (P = 0.018). Individual time points were compared between infected and control animals using one-way ANOVA with the Holm-Šidák test as a post hoc test.
Conclusions.
We here demonstrated that stable-isotope-based assays of CODH offer the potential to rapidly detect tuberculous mycobacteria in vitro and in vivo. While the additional sensitivity afforded by using doubly labeled 13C18O enabled successful in vivo detection, the potential for carbonic anhydrase-mediated loss of 18O from resultant 13C18O16O entailed the use of acetazolamide or a similar drug. The key advantage of the CODH breath test over the urease breath test (6) is the likely very high specificity compared to that of the urease test. Many important lung pathogens express urease (Table 1), and so urease may lead to a false positive if used to diagnose tuberculosis. None of these (Table 1) or any other recognized lung pathogens possess genes for CODH or have been shown to oxidize CO to CO2. Thus, while urease activity might provide a rapid POC screen for subsequent TB diagnosis or a suitable biomarker for monitoring treatment in already-diagnosed TB patients, the CODH breath test has the potential for rapid POC diagnosis of TB. However, it is clear that the enhanced specificity of CODH detection comes at the cost of decreased sensitivity compared to that of urease detection.
A key issue in translating the procedure for clinical studies will be the need to balance the requirement for sufficient 13C18O gas to be inhaled so as to improve sensitivity with the need to maintain sufficiently low carboxyhemoglobin levels to maintain safety. Increasing the sensitivity of 13C18O16O detection would greatly assist in balancing these opposing requirements. A potential alternative would be to use a microdose of 14CO with conventional counting, as has been performed for urease testing (25), or to use more sensitive, potentially transportable alternatives to accelerator mass spectrometry (MS) (26). Such approaches would also eliminate the need for acetazolamide pretreatment due to their independence from oxygen atom exchange. For use with a biomarker of treatment response in drug trials, centralized accelerator MS could be feasible, with reporting times dependent upon sample transport.
It has become increasingly clear that host-derived CO from heme oxygenase, in addition to potentially being used as a diagnostic tool, is an important modulator of mycobacterial signaling (27, 28) and is involved in mycobacterial control (29, 30) in a manner that appears to be clinically important (31). Yet it is far from clear how to resolve the differing effects of CO, as it may function both as a host-imposed controller of mycobacterial signaling and as a host-derived source of mycobacterial energy. Although advances in whole-cell metabolomics through nuclear magnetic resonance (NMR) allow powerful analysis of drug effects in cultures (32), currently these techniques are suitable only with culture. The ability to study, in situ in the host, the CODH phenotype developed here will assist in the resolution of this conundrum. Furthermore, this work demonstrates that even complex bacterial metabolic phenotypes can be detected and studied in situ in the host through appropriate labeling and detection chemistries.
MATERIALS AND METHODS
Materials.
Unlabeled CO gas was purchased from Sigma-Aldrich, and 13CO and 13C18O gasses were bought from Cambridge Isotope Laboratory (Andover, MA) and were compressed. 7H9 medium was purchased from BD (Franklin Lakes, NJ). A CO detector (Fluke carbon monoxide detector; Cole-Parmer, Vernon Hills, IL) was purchased from Fisher Scientific (Pittsburgh, PA). Unless otherwise mentioned, other reagents were purchased from Sigma-Aldrich. Isotope ratio MS (IRMS) was performed using a DELTAplusXL spectrometer (Thermo Scientific Inc., Waltham, MA).
Bacterial cultures.
Mycobacterium cultures were prepared by thawing frozen stock aliquots of Mycobacterium tuberculosis H37Rv, M. bovis Ravenel, or M. bovis BCG as described previously for urease experiments (6). They were grown aerobically at 37°C in 7H9 Middlebrook liquid medium supplemented with oleic acid, albumin, dextrose, and catalase (Becton, Dickinson, Inc., Sparks, MD), 0.5% glycerol, and 0.05% Tween 80. M. bovis BCG was grown in the same culture medium but without oleic acid.
In vitro CODH assay.
Three milliliters of mycobacterial culture within 2 to 3 days of approaching stationary phase (from previous growth curves obtained using an optical density at 600 nm) was grown aerobically and was incubated with CO in air in 12-ml Exetainer vials with rubber septa (Labco Ltd., Ceredigion, United Kingdom) for 1 h at 37°C with rotary shaking at 250 rpm unless otherwise indicated, using culture medium as indicated above. The CO was injected through the septa with a gas syringe after withdrawal of the same volume of air to maintain atmospheric pressure. Collected headspace gas (1 ml) was similarly obtained after injection of 1 ml air, double-filtered through 0.25-μm syringe filters, and transferred into helium-flushed Exetainer vials for IRMS. Unless otherwise mentioned, incubation was for 30 min using the addition of 1 ml of CO gas.
Measurement of stable-isotope ratios in CO2.
Determinations were by gas isotope ratio mass spectrometry (DELTAplusXL; Thermo Scientific Inc., Waltham, MA). Unenriched CO2 gas (Matheson Tri-Gas, Albuquerque, NM) was used as reference gas. For detection of 13CO conversion to 13CO2, we used IRMS as previously described (6) and report enrichment in headspace 13CO2 using conventional δ-13C notation (33). For detection of conversion of 13C18O to 13C18O16O, we measured the relative ratios of masses 47 (13C18O16O) and 44 (CO2) (rR47/44) and defined δ47 as 1,000 ⋅ [rR47/44(sample) − rR47/44(standard)] divided by rR47/44(standard).
As is customary in stable-isotope breath testing, the increase in isotopic abundance after administration of the tracer is reported so as to correct for minor deviations in baseline that occur naturally, and the result was termed Δ47, where Δ47 = δ47(after CO) − δ47(baseline before CO). Because of the potential for greater variance in vivo in the baseline data to affect values through the series (due to its constant subtraction from data), δ47 is presented for data from rabbits.
Rabbits and infections.
All protocols were approved by the Institutional Animal Care and Use Committees at the Johns Hopkins University (protocol number RB11M466). Pathogen-free outbred New Zealand White rabbits (3.5 to 4.1 kg) obtained from Covance Research Products, Inc. (Denver, PA), were used. Animals were anesthetized for infection and breath tests with ketamine (15 to 25 mg/kg of body weight) and xylazine (5 to 10 mg/kg). Reversal of sedation was achieved with yohimbine (0.1 to 0.2 mg/kg). The rabbits were infected by intratracheal insertion of a bacterial suspension containing 106 cells of Mycobacterium bovis Ravenel using a feeding tube through an inserted pediatric endotracheal tube. The rabbit was kept after infection on its right side and the head up until reverse of sedation. Rabbits were tested 4 weeks after infection or mock infection. Clinical signs and symptoms supported infection, with uninfected rabbits gaining 0.4, 0.4, and 0.6 kg and infected rabbits losing 0.1, 0.6, and 0.3 kg over the same time (P < 0.01 by one-way ANOVA). The rabbit model was chosen, as it is the smallest validated TB animal model that allows bronchoscopic infection for high levels of inoculum certainty (as opposed to the Glas-Col system or similar infection models in which the infection inoculum is much more varied) and also allows for bronchoscopic collection of exhaled breath samples for analysis.
In vivo breath tests.
Rabbits were pretreated with acetazolamide (AZZ) (80 mg/kg) to inhibit carbonic anhydrase-mediated isotopic scrambling of 13C18O16O to 13C16O16O. Anesthetized rabbits were then treated with a respiratory bag (with a total volume of 750 ml) connected to an inserted endotracheal tube for delivery of the carbon monoxide (CO). The CO was injected with a syringe into the respiratory bag, which was kept connected for 1 min before breath samples were taken at several time points (5, 10, and 15 min after removal of the respiratory bag). A 14 French feeding tube connected to a 30-ml syringe was then introduced through the endotracheal tube to the level of the carina to aspirate the exhaled air. AZZ (80 mg/kg) was injected intravenously 5 min before CO treatment, and 5 baseline gas samples were taken. Exhaled breath gas was filtered with 0.35-μm membrane filters into air pouches (Otsuka, Japan) and then sealed vacuum tubes (Becton, Dickinson, Franklin Lakes, NJ). For IRMS, filtered samples were transferred to helium-flushed Exetainer vials.
In vivo CO dosing.
Below a carboxyhemoglobin level of saturation of about 20%, effects on human coordination are mild (34), and below 11 to 13%, no effects are detected (35) and so we targeted a CO dose that would lead to less than 20% but more than 11% carboxyhemoglobin in the rabbits. After calculation, we administered 30 ml of 13C18O gas in the respiratory bag inflated with 500 ml of air for 1 min (5.7% CO; total exposure, 0.475% per hour equivalent) and then removed this and allowed rabbits to breath room air. Exhaled breath was collected from 0 to 15 min after CO delivery. CO saturation was estimated from the concentration in exhaled air (36) and ranged from 9.3 to 18.2%. Rabbits did not show any abnormal behavior; clinical respiratory signs were normal. We therefore used this dose in subsequent breath testing. Extended studies of breathing 0.19% CO (5 h, 0.95% per hour equivalent) showed no morphological changes in rabbit lungs (37).
Statistics.
All numbers are mean values ± standard deviations (SD) from independent biological replicates, with n and the statistical test used described in the appropriate figure legends. For in vitro studies, the biological replicates were of the same cultures but split into independent Exetainer tubes, with gases from each tube analyzed individually. For in vivo studies, the biological replicates were of individual animals that were either infected (n = 3) or uninfected (n = 3) controls. Individual time points were compared using one-way ANOVA with the post hoc Holm-Šidák comparison. Split-plot ANOVA was used as the measure of between-subject effects (uninfected versus infected).
Footnotes
Citation Maiga M, Choi SW, Atudorei V, Maiga MC, Sharp ZD, Bishai WR, Timmins GS. 2014. In vitro and in vivo studies of a rapid and selective breath test for tuberculosis based upon mycobacterial CO dehydrogenase. mBio 5(2):e00990-14. doi:10.1128/mBio.00990-14.
REFERENCES
- 1. Phillips PP, Gillespie SH, Boeree M, Heinrich N, Aarnoutse R, McHugh T, Pletschette M, Lienhardt C, Hafner R, Mgone C, Zumla A, Nunn AJ, Hoelscher M. 2012. Innovative trial designs are practical solutions for improving the treatment of tuberculosis. J. Infect. Dis. 205:S250–S257. 10.1093/infdis/jis041 [DOI] [PubMed] [Google Scholar]
- 2. Perrin FM, Lipman MC, McHugh TD, Gillespie SH. 2007. Biomarkers of treatment response in clinical trials of novel antituberculosis agents. Lancet Infect. Dis. 7:481–490. 10.1016/S1473-3099(07)70112-3 [DOI] [PubMed] [Google Scholar]
- 3. Wallis RS, Doherty TM, Onyebujoh P, Vahedi M, Laang H, Olesen O, Parida S, Zumla A. 2009. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect. Dis. 9:162–172. 10.1016/S1473-3099(09)70042-8 [DOI] [PubMed] [Google Scholar]
- 4. Walzl G, Ronacher K, Djoba Siawaya JF, Dockrell HM. 2008. Biomarkers for TB treatment response: challenges and future strategies. J. Infect. 57:103–109. 10.1016/j.jinf.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 5. Storla DG, Yimer S, Bjune GA. 2008. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 8:15. 10.1186/1471-2458-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jassal M, Nedeltchev GG, Lee JH, Choi SW, Atudorei V, Sharp ZD, Deretic V, Timmins GS, Bishai WR. 2010. 13 [C]-urea breath test as a novel point-of-care biomarker for tuberculosis treatment and diagnosis. PLoS One 5: e12451. 10.1371/journal.pone.0012451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yavankar SP, Pardesi KR, Chopade BA. 2007. Species distribution and physiological characterization of Acinetobacter genospecies from healthy human skin of tribal population in India. Indian J. Med. Microbiol. 25:336-345. 10.4103/0255-0857.37335 [DOI] [PubMed] [Google Scholar]
- 8. Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, Snyder M. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 21:601–614. 10.1101/gad.1510307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osaki T, Mabe K, Hanawa T, Kamiya S. 2008. Urease-positive bacteria in the stomach induce a false-positive reaction in a urea breath test for diagnosis of Helicobacter pylori infection. J. Med. Microbiol. 57:814–819. 10.1099/jmm.0.47768-0 [DOI] [PubMed] [Google Scholar]
- 10. Graham DY, Klein PD, Evans DJ, Evans DG, Alpert LC, Opekun AR, Boutton TW. 1987. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet i:1174–1177 [DOI] [PubMed] [Google Scholar]
- 11. King GM. 2003. Uptake of carbon monoxide and hydrogen at environmentally relevant concentrations by mycobacteria. Appl. Environ. Microbiol. 69:7266–7272. 10.1128/AEM.69.12.7266-7272.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park SW, Hwang EH, Park H, Kim JA, Heo J, Lee KH, Song T, Kim E, Ro YT, Kim SW, Kim YM. 2003. Growth of mycobacteria on carbon monoxide and methanol. J. Bacteriol. 185:142–147. 10.1128/JB.185.1.142-147.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartholomew GW, Alexander M. 1979. Microbial metabolism of carbon monoxide in culture and in soil. Appl. Environ. Microbiol. 37:932–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hegewald MJ. 2009. Diffusing capacity. Clin. Rev. Allergy Immunol. 37:159–166. 10.1007/s12016-009-8125-2 [DOI] [PubMed] [Google Scholar]
- 15. Pipavath SN, Sharma SK, Sinha S, Mukhopadhyay S, Gulati MS. 2007. High resolution CT (HRCT) in miliary tuberculosis (MTB) of the lung: correlation with pulmonary function tests & gas exchange parameters in north Indian patients. Indian J. Med. Res. 126:193–198 [PubMed] [Google Scholar]
- 16. Raub JA, Mathieu-Nolf M, Hampson NB, Thom SR. 2000. Carbon monoxide poisoning—a public health perspective. Toxicology 145:1–14. 10.1016/S0300-483X(99)00217-6 [DOI] [PubMed] [Google Scholar]
- 17. Canetti G. 1955. The tubercle bacillus in the pulmonary lesion of man; histobacteriology and its bearing on the therapy of pulmonary tuberculosis, American rev ed, p 226. Springer Publishing Co, New York, NY. [Google Scholar]
- 18. Itada N, Forster RE. 1977. Carbonic anhydrase activity in intact red blood cells measured with 18O exchange. J. Biol. Chem. 252:3881–3890 [PubMed] [Google Scholar]
- 19. Nishimoria I, Minakuchi T, Maresca A, Carta F, Scozzafava A, Supuran CT. 2010. The-carbonic anhydrases from mycobacterium tuberculosis as drug targets. Curr. Pharm. Des. 16:3300–3309. 10.2174/138161210793429814 [DOI] [PubMed] [Google Scholar]
- 20. Güzel O, Maresca A, Scozzafava A, Salman A, Balaban AT, Supuran CT. 2009. Discovery of low nanomolar and subnanomolar inhibitors of the mycobacterial beta-carbonic anhydrases Rv1284 and Rv3273. J. Med. Chem. 52:4063–4067. 10.1021/jm9004016 [DOI] [PubMed] [Google Scholar]
- 21. Effros RM, Shapiro L, Silverman P. 1980. Carbonic anhydrase activity of rabbit lungs. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 49:589–600 [DOI] [PubMed] [Google Scholar]
- 22. Holm L, Flemström G, Nylander O. 1990. Duodenal alkaline secretion in rabbits: influence of artificial ventilation. Acta Physiol. Scand. 138:471–478. 10.1111/j.1748-1716.1990.tb08874.x [DOI] [PubMed] [Google Scholar]
- 23. Converse PJ, Dannenberg AM, Estep JE, Sugisaki K, Abe Y, Schofield BH, Pitt ML. 1996. Cavitary tuberculosis produced in rabbits by aerosolized virulent tubercle bacilli. Infect. Immun. 64:4776–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nedeltchev GG, Raghunand TR, Jassal MS, Lun S, Cheng QJ, Bishai WR. 2009. Extrapulmonary dissemination of Mycobacterium bovis but not Mycobacterium tuberculosis in a bronchoscopic rabbit model of cavitary tuberculosis. Infect. Immun. 77:598–603. 10.1128/IAI.01132-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peura DA, Pambianco DJ, Dye KR, Lind C, Frierson HF, Hoffman SR, Combs MJ, Guilfoyle E, Marshall BJ. 1996. Microdose 14C-urea breath test offers diagnosis of Helicobacter pylori in 10 minutes. Am. J. Gastroenterol. 91:233–238 [PubMed] [Google Scholar]
- 26. Murnick DE, Dogru O, Ilkmen E. 2008. Intracavity optogalvanic spectroscopy. An analytical technique for 14C analysis with subattomole sensitivity. Anal. Chem. 80:4820–4824. 10.1021/ac800751y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar A, Deshane JS, Crossman DK, Bolisetty S, Yan BS, Kramnik I, Agarwal A, Steyn AJ. 2008. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 283:18032–18039. 10.1074/jbc.M802274200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shiloh MU, Manzanillo P, Cox JS. 2008. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3:323–330. 10.1016/j.chom.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Regev D, Surolia R, Karki S, Zolak J, Montes-Worboys A, Oliva O, Guroji P, Saini V, Steyn AJ, Agarwal A, Antony VB. 2012. Heme oxygenase-1 promotes granuloma development and protects against dissemination of mycobacteria. Lab. Invest. 92:1541–1552. 10.1038/labinvest.2012.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silva-Gomes S, Appelberg R, Larsen R, Soares MP, Gomes MS. 2013. Heme catabolism by heme oxygenase-1 confers host resistance to mycobacterium infection. Infect. Immun. 81:2536–2545. 10.1128/IAI.00251-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrade BB, Pavan Kumar N, Mayer-Barber KD, Barber DL, Sridhar R, Rekha VV, Jawahar MS, Nutman TB, Sher A, Babu S. 2013. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One 8:e62618. 10.1371/journal.pone.0062618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Halouska S, Fenton RJ, Barletta RG, Powers R. 2012. Predicting the in vivo mechanism of action for drug leads using NMR metabolomics. ACS Chem. Biol. 7:166–171. 10.1021/cb200348m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharp Z. 2007. Principles of stable isotope geochemistry. Pearson Education, Upper Saddle River, NJ [Google Scholar]
- 34. NIOSH 1973. Criteria for a recommended standard: occupational exposure to carbon monoxide, vol HSM:73-11000 U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, DHEW (NIOSH), Cincinnati, OH. [Google Scholar]
- 35. Stewart RD. 1975. The effect of carbon monoxide on humans. Annu. Rev. Pharmacol. 15:409–423. 10.1146/annurev.pa.15.040175.002205 [DOI] [PubMed] [Google Scholar]
- 36. Jarvis MJ, Belcher M, Vesey C, Hutchison DC. 1986. Low cost carbon monoxide monitors in smoking assessment. Thorax 41:886–887. 10.1136/thx.41.11.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hugod C. 1980. The effect of carbon monoxide exposure on morphology of lungs and pulmonary arteries in rabbits. A light- and electron-microscopic study. Arch. Toxicol. 43:273–281. 10.1007/BF00366183 [DOI] [PubMed] [Google Scholar]
- 38. Iskhakova K. 1985. Characteristics of hospital strains of Pseudomonas aeruginosa. Zh. Mikrobiol. Epidemiol. Immunobiol. 2:11–14 (In Russian.) [PubMed] [Google Scholar]
- 39. Juni BA, Rysavy JM, Blazevic DJ. 1982. Rapid biotyping of Haemophilus influenzae and Haemophilus parainfluenzae with PathoTec strips and spot biochemical tests. J. Clin. Microbiol. 15:976–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nishida H. 2009. Ureaplasma urease genes have undergone a unique evolutionary process. Open Syst. Biol. J. 2:1. 10.2174/1876392800902010001 [DOI] [Google Scholar]


