Abstract
Purpose.
People with migraine are relatively poor at judging the direction of motion of coherently moving signal dots when interspersed with noise dots drifting in random directions, a task known as motion coherence. Although this has been taken as evidence of impoverished global pooling of motion signals, it could also arise from unreliable coding of local direction (of each dot), or an inability to segment signal from noise (noise-exclusion). The aim of this study was to determine how these putative limits contribute to impoverished motion processing in migraine.
Methods.
Twenty-two participants with migraine (mean age, 34.7 ± 8.3 years; 16 female) and 22 age- and sex-matched controls (mean age, 34.4 ± 6.2 years) performed a motion-coherence task and a motion-equivalent noise task, the latter quantifying local and global limits on motion processing. In addition, participants were tested on analogous equivalent noise paradigms involving judgments of orientation and size, so that the specificity of any findings (to visual dimension) could be ascertained.
Results.
Participants with migraine exhibited higher motion-coherence thresholds than controls (P = 0.01, independent t-test). However, this difference could not be attributed to deficits in either local or global processing since they performed normally on all equivalent noise tasks (P > 0.05, multivariate ANOVA).
Conclusions.
These findings indicate that motion perception in the participants with migraine was limited by an inability to exclude visual noise. We suggest that this is a defining characteristic of visual dysfunction in migraine, a theory that has the potential to integrate a wide range of findings in the literature.
Keywords: migraine, vision, noise, coherence, motion
Studies have shown that individuals with migraine are characterized by elevated motion-coherence thresholds. Here, using equivalent noise analysis and coherence paradigms, we show that this is due to a relative inability to exclude visual noise rather than impaired local or global processing.
Introduction
Migraine is an episodic disorder characterized by throbbing (commonly unilateral) head pain, which may be accompanied by nausea, vomiting, and an aversion to sound or light.1 In approximately 30% of cases, a transient sensory and/or motor disturbance known as an aura is also experienced.2 Certain visual stimuli can also trigger a migraine attack,3 and numerous studies have shown that individuals with migraine exhibit subtle differences in visual psychophysical performance, both ictally and interictally (see Refs. 4 and 5 for reviews). This is particularly the case for tasks involving judgments of visual motion.6
Processing of visual motion relies on at least two hierarchic processing stages. In the primary visual cortex (area V1), motion is processed locally (i.e., cells are sensitive to the direction of motion within a small region of space).7 This information is then relayed to the medial temporal (MT) and medial superior temporal (MST) areas, where it is integrated to form a global motion percept.8 People with migraine seemingly process local motion normally, since they perform as well as a control group when asked to discriminate or classify the direction of a stimulus containing a single direction of motion.6,9–11 However, people with migraine perform relatively poorly on motion coherence tasks where the participant must classify the direction of motion of a set of signal dots moving coherently (in one direction) but interspersed with noise dots drifting in random directions (Fig. 1A).6,9,10,12–14
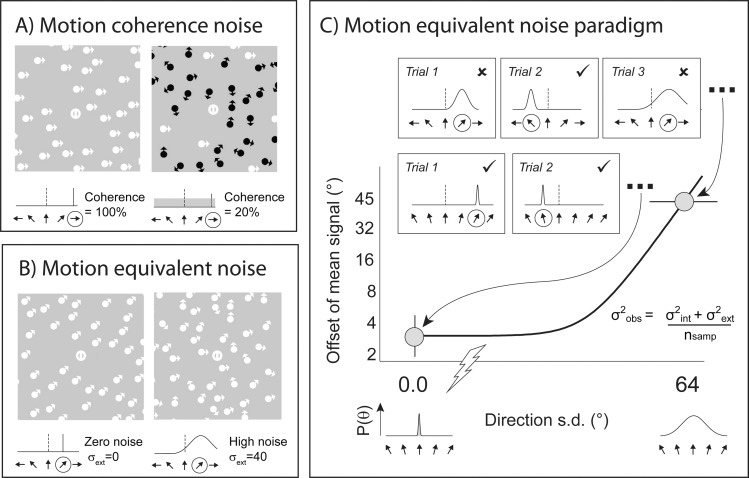
Figure 1.
Psychophysical procedures. (A) Examples of high (100%) and low (20%) coherence motion stimuli. Signal dots are shown in white and noise dots in black. Directions of motion are indicated by the orientation of the arrowheads. (Note: in the actual experiment, all dots were white.) Below each example stimulus is shown the corresponding distribution of signal values (solid black line) and noise values (dark gray shaded region). In the coherence task, noise was increased by changing the proportion of signal-to-noise dots. (B) Zero- and high-noise motion stimuli, with corresponding distributions of motion directions. In the equivalent noise task, noise was added by increasing the standard deviation of motion directions in the stimuli. In the plots of signal and noise distributions, the reference direction is denoted by a vertical black dotted line; the (average) direction of signal motion is circled. (C) The equivalent noise function (solid black line) is constrained by two data points: the “zero noise” threshold, which represents the minimum directional offset that can be reliably discriminated, and the “high noise” threshold, which represents the maximum level of noise that can be tolerated for a large directional offset. The function has two parameters (inset in [C]), providing estimates of internal noise and global sampling (see Supplementary Material).
Since the signal-direction in a coherence task cannot be determined from a single dot's trajectory, the participant must make a judgment of global motion direction. As a result, high motion coherence thresholds are often taken as evidence of a selective deficit in global motion pooling. However, motion coherence judgments can be limited not only by global integration, but also by unreliable local processing.15 This could be the case, for example, if higher cortical areas inherit input from V1 cells prone to high levels of random firing (i.e., elevated internal noise). A further limit on motion coherence performance is defined by an observer's ability to segregate signal from noise dot directions. Thus, computational models show that human observers perform much better on coherence tasks than would be expected if they used a pure pooling strategy,15,16 suggesting that they are capable of selectively monitoring directions of interest.
To try and disentangle these putative limits to motion processing, we used a technique known as equivalent noise (EN) analysis. This psychophysical paradigm allows performance to be parcellated into independent estimates of local and global processing.17 Similar to the motion coherence paradigm, EN analysis requires participants to classify the direction of motion of signal dots that are corrupted by noise.15 However, in EN analysis, noise is added by manipulating the standard deviation of the distribution of directions presented, rather than adding noise dots that drift in random directions (Fig. 1B). As a result, every dot contributes to the signal, and the optimum strategy is to integrate all directions of motion in the stimulus. Consequently, an estimate of global processing is obtained that does not rely on the participant's ability to exclude noise. Further, by measuring performance in the absence (as well as in the presence) of noise, an independent estimate of a participant's ability to process information locally is also available.
We sought to determine if motion processing in migraine is (1) limited by local processing, global processing, and/or noise exclusion; and (2) part of a more general integration deficit. To this end, participants with and without migraine were tested on a series of matched psychophysical tasks. A motion coherence paradigm was used to assess each participant's ability to classify the direction of signal motion whilst excluding random noise. Independent estimates of local and global motion processing performance were obtained using a motion EN paradigm. Finally, to assess the specificity of any findings to motion processing, participants undertook analogous EN tasks that probed local and global processing for judgments of orientation and size.
Materials and Methods
Ethics approval was granted by the University of East London Psychology Research Ethics Committee and the Department of Psychological Sciences Ethics Committee at Birkbeck College. Informed written consent was obtained from each participant, and all subjects were treated in accordance with the Declaration of Helsinki.
Participants
Data were gathered from 22 participants with migraine (MG) and 22 migraine-free control participants (CON) (Table 1). The two groups were matched for sex (16 female) and did not differ significantly with respect to age (mean age, 34.7 ± 8.3 [MG] and 34.4 ± 6.2 years [CON]; t(42) = 0.04, P = 0.97). All participants with migraine fulfilled the International Headache Society (2004) diagnostic criteria for migraine without aura (MO) or migraine with visual aura (VA) and had been diagnosed previously by a general practitioner or neurologist. All participants had a minimum visual acuity of 20/20 binocularly (with or without optometric correction). No participant had a history of mental illness, and none were taking daily medication at the time of testing.
Table 1.
Migraine Group Demographics and Details of Migraine History
|
Type |
Sex |
Age, y |
Onset* |
Freq 1† |
Freq 2‡ |
Last§ |
Duration‖ |
Severity¶ |
| MO | F | 21 | 13.5 | 1 | 3.5 | 8 | 60 | 144 |
| MO | F | 25 | 23 | 4 | 16 | 2 | 6.5 | 192 |
| MO | F | 38 | 16 | 4 | 20 | 1 | 24 | 384 |
| MO | F | 39 | 30 | 3.5 | 12 | 1 | 24 | 144 |
| MO | F | 40 | 5 | 6 | 24 | 2 | 48 | 517.5 |
| MO | F | 43 | 32 | 3 | 10 | 4 | 60 | 108 |
| MO | M | 23 | 16 | 2 | 6.5 | 4 | 24 | 32 |
| MO | M | 34 | 11.5 | 2 | 3 | 5 | 96 | 26.25 |
| MO | M | 38 | 28 | 3 | 10 | 3 | 4 | 22.5 |
| MO | M | 40 | 5.5 | 3 | 15 | 2.5 | 60 | 80 |
| VA | F | 21 | 10 | 3 | 12 | 2 | 6.5 | 100 |
| VA | F | 24 | 19 | 12 | 182 | 0.29 | 4.5 | 1536 |
| VA | F | 29 | 22 | 1 | 7.5 | 1.5 | 36 | 132 |
| VA | F | 30 | 14 | 3 | 12 | 3 | 6.5 | 440 |
| VA | F | 32 | 28 | 5 | 20 | 2 | 24 | 52.5 |
| VA | F | 33 | 10.5 | 0 | 1 | 30 | 24 | 840 |
| VA | F | 36 | 18 | 1.5 | 8 | 2 | 60 | 1575 |
| VA | F | 40 | 32 | 8 | 18 | 1 | 72 | 67.5 |
| VA | F | 44 | 28 | 5 | 24 | 1 | 10 | 45.5 |
| VA | F | 51 | 25 | 2 | 6.5 | 3 | 48 | 169 |
| VA | M | 38 | 6 | 12 | 48 | 0.29 | 12 | 110 |
| VA | M | 44 | 12.5 | 0 | 50 | 16 | 24 | 910 |
| Mean | 34.68 | 18.43 | 3.82 | 23.14 | 4.30 | 33.36 | 346.72 | |
| SD | 8.25 | 8.81 | 3.26 | 37.65 | 6.66 | 25.89 | 463.62 |
F, female; M, male.
Onset: age (in years) of migraine onset.
Freq 1: number of migraine attacks experienced within the last 3 months.
Freq 2: number of migraine attacks experienced within the last year.
Last: time elapsed, in weeks, since last migraine attack.
‖ Duration: average duration, in hours, of a migraine attack when painkillers are administered.
Severity: index of migraine severity, derived from the multiplication of average migraine duration by the number of years migraine has been experienced.
General Procedure
The experiment lasted 60 to 75 minutes and consisted of (1) a brief test of visual acuity (assessed using a handheld logMar near visual acuity chart); (2) a customized questionnaire about basic demographics and migraine history; (3) a motion coherence paradigm; and (4) three EN paradigms, which probed local and global processing for judgments of visual orientation, motion, and size (separately). Individual EN and coherence tasks were blocked and presented in a random order to avoid sequence effects. All responses were given verbally and relayed to the computer by the experimenter.
Motion Coherence Procedure
Participants classified the direction of motion of a number of coherently moving dots (the signal) embedded in noise. All signal dots were restricted to motion in the horizontal plane (all left or all right on any given trial). Noise was added to the stimulus by assigning a subset of dots directions of motion that were randomly sampled from a flat distribution (Fig. 1A). Under the control of QUEST,18 an adaptive staircase procedure manipulated the level of coherence on each trial, where coherence was defined as the percentage of dots that constituted the signal. The staircase converged on the level of coherence necessary for each participant to correctly ascertain the direction of motion on 82% of trials: the motion coherence threshold (see Supplementary Fig. S1A for further details). Lower coherence thresholds, therefore, reflected superior performance, indicating that the participant needed fewer signal dots to correctly identify the direction of signal motion. The staircase terminated after 75 trials and was preceded by 15 practice trials.
Equivalent Noise Procedure
A fast, efficient version of the EN paradigm, adapted for use with clinical populations, was used to assess local and global processing limits. In the EN tasks, participants judged whether a number of signal elements presented for a brief duration were, on average, drifting clockwise or anticlockwise of vertical-upward motion (motion task; Fig. 1B), tilted to the left or right of vertical (orientation task; Supplementary Fig. S2A), or smaller or larger than a reference (size task; Supplementary Fig. S2B). The reference direction, orientation, and size were defined by the fixation guide itself, which consisted of a small white circle bisected by a vertical line (identical in all tasks).
Two independent staircases were randomly interleaved: a “zero noise” and a “high noise” condition (Fig. 1C). In the zero noise condition, external noise was set to zero, and the staircase tracked the minimum orientation offset from vertical (orientation task), directional offset from vertical (motion task), or size offset from reference (size task) that could be reliably classified (Supplementary Fig. S1B). In the high noise condition, the staircase tracked the maximum level of external noise that could be tolerated for a large (fixed) signal offset (Supplementary Fig. S1C). In this condition, the signal level was fixed at ±22.5° for the orientation, ±45° for the motion, and ±0.5 octaves for the size task. These values were selected on the basis of previous studies and pilot data.15,19,20 Both staircases terminated after 75 trials each. As per the coherence task, the staircases were under the control of QUEST and converged on 82% correct thresholds. For each participant and task a two-parameter EN function was fit to their data, providing estimates of internal noise (a measure of local processing) and sampling (global processing; see Fig. 1C and Supplementary Material). To accustom participants to the nature of the task, all test blocks were preceded by 15 practice trials. In addition, for a subset of observers (10 participants with migraine and 8 without), 15 catch trials were randomly interleaved into each EN paradigm. On each catch trial, the stimulus was presented at a large signal level in the absence of external noise (±22.5°, ±45°, and ±0.5 octaves for orientation, motion, and size tasks, respectively).
Stimulus Parameters
All stimuli were generated in Matlab (MathWorks, Cambridge, MA, USA) using the Psychophysics Toolbox extensions21,22 and were presented on a MacBook Pro laptop computer connected to a luminance-calibrated LCD monitor at a spatial and temporal resolution of 1920 × 1080 pixels and 60 Hz, respectively.
Test images were generated by randomly dropping 100 elements (disks) within a circular region with a diameter of 15°. For motion and size judgments, individual elements could overlap. In the motion task, overlapping elements led to occlusion. In the size task, the contrasts of overlapping elements were summed. For the orientation task, element overlap was avoided by ensuring that adjacent elements were separated by a minimum distance equal to twice their diameter. The resulting images were presented in the center of the screen for 400 ms against a background gray display. Stimuli were viewed in a dark room from a distance of 51 cm. The fixation guide had a diameter of 0.44°.
For the orientation task, individual disks were composed of random phase sine-wave gratings with a spatial frequency of 3.4 cycles per degree presented at 50% contrast in a circular hard-edged mask with a diameter of 0.44° (Supplementary Fig. S1A). For the size task, individual disks had the same characteristics as for orientation but varied in size and were randomly oriented (Supplementary Fig. S1B). The spatial frequency of the grating was scaled to the diameter of the disk such that the number of cycles presented remained constant across changes in size. In addition, for the size task, the contrast of individual disks was randomly jittered in the range of 25% to 75% (sampled from a flat distribution) in order to minimize the availability of contrast cues. For the motion tasks, white dots with a diameter of 0.44° were used instead of windowed gratings (Fig. 1B). Individual dots had a lifetime of 300 ms, were spatially updated every 50 ms, moved at 3°/s, and were presented at 50% contrast.
Data Transformation and Filtration
All variables, with the exception of age and age of migraine onset, were log transformed as this typically reduced skew and kurtosis. Following this transformation, the distribution of variables did not differ significantly from normal (P values > 0.05; one-sample Kolmogorov-Smirnoff tests). Data were then filtered (separately for CON, MO, and VA groups) so that extreme outliers with respect to parameter estimates and associated confidence intervals (>2.58 z scores from the group mean) were excluded from analysis. This led to the exclusion of 5.42% of the data, which represented outliers that were seemingly randomly distributed across the different groups (migraine [1.75%], control [3.67%]); tasks (motion coherence [0.87%], motion EN [1.05%], orientation EN [1.22%], and size EN [2.27%]); and individual participants.
Results
None of the variables of interest differed significantly between migraine subgroups (MO and VA) (independent t-tests, P values > 0.05); consequently, MO and VA data were pooled for all subsequent analyses. The percentage of catch trials answered correctly was at ceiling and did not differ between groups or across tasks (ANOVA, P values > 0.05).
Motion Coherence Thresholds
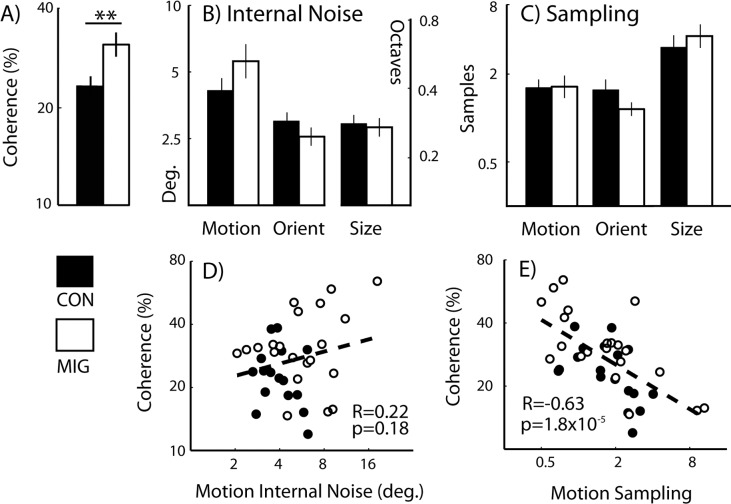
To determine whether performance on the motion coherence task differed between migraine and control groups (Fig. 2A), coherence thresholds were analyzed using an independent t-test (Table 2). A 1-tailed test was employed since there are multiple reports of elevated coherence thresholds in migraine (see Introduction section). Motion coherence thresholds were elevated in the migraine group (32% ± 3.3%) relative to the control group (24% ± 1.8%) (t(37) = −2.37, P = 0.01, Cohen's d = 0.78), requiring a higher proportion of signal-to-noise dots to reliably classify the direction of signal motion.
Figure 2.
Coherence and equivalent noise plots. Group mean (A) coherence thresholds, (B) levels of internal noise, and (C) sampling are shown for control and migraine participants. Scatterplots show correlations between motion coherence thresholds and (D) motion internal noise and (E) motion sampling. Error bars denote the standard error of the mean. Deg., degrees. Note: data have been log-transformed; however, for ease of interpretation, axis tick marks denote equivalent untransformed values.
Table 2.
Comparing Group Performance on Motion Coherence and Equivalent Noise Tasks
|
t |
df* |
PValue† |
Cohen'sd |
||
| Coherence | Th | −2.37 | 37 | 0.01‡ | 0.78 |
| Motion | σint | −2.33 | 33.02 | 0.03 | 0.71 |
| nsamp | −0.04 | 41 | 0.97 | 0.02 | |
| Orientation | σint | 1.21 | 41 | 0.23 | 0.38 |
| nsamp | 1.82 | 32.56 | 0.08 | 0.59 | |
| Size | σint | 0.22 | 39 | 0.83 | 0.07 |
| nsamp | −0.67 | 38 | 0.51 | 0.22 |
Migraine and control group performance were compared using independent t-tests. Bonferroni corrections were made for three multiple comparisons in the analysis of equivalent noise measures, reflecting the three different visual dimensions tested (corrected α = 0.0167). t, t-statistic; Cohen's d, effect size; Th, motion coherence threshold; σint, internal noise; nsamp, sampling.
Appropriate corrections were made to df, where equal variances could not be assumed.
P values reported are for 2-tailed tests, with the exception of the analysis of motion coherence thresholds, for which a single-tailed test was used (corrected α = 0.1) (see Motion Coherence Thresholds section for further details).
Significant effect at the stated α level.
Internal Noise and Sampling
To determine whether there was a general trend for group differences in internal noise, a multivariate ANOVA (MANOVA) was undertaken with one between-participants factor (group at two levels: migraine and control) and three dependent variables (orientation, motion, and size internal noise) (Fig. 2B). This revealed no main effect of group for internal noise (Wilks' λ = 0.85, F(3,34) = 2, P = 0.14, and partial-η2 = 0.15). A similar analysis revealed no effect of group on sampling (Wilks' λ = 0.86, F(3,33) = 1.83, P = 0.16, and partial-η2 = 0.14; Fig. 2C).
To determine whether group differences existed on a subset of EN tasks, levels of internal noise and sampling were exposed to a series of post hoc independent t-tests comparing migraine and control group performances (Table 2). Since analyses were undertaken for all visual dimensions tested (orientation, motion, and size), Bonferroni corrections were made for three multiple comparisons (corrected α level = 0.0167). The analyses revealed no significant differences in levels of internal noise or sampling between migraine and control groups for any of the EN tasks.
Predicting Coherence Thresholds From Internal Noise and Sampling
To determine how motion coherence thresholds related to EN performance, bivariate correlations were undertaken (Figs. 2D, 2E). Motion sampling was found to be highly negatively correlated with motion coherence thresholds (R = −0.63, P = 1.8 × 10−5). Participants who were good at global pooling of information in the EN task needed fewer signals dots in the coherence task to correctly classify the direction of signal motion (Fig. 2E). In contrast, motion internal noise did not correlate with motion coherence thresholds (R = 0.22, P = 0.18; Fig. 2D).
Next, a regression analysis was undertaken. This tested the extent to which the three predictor variables (group [migraine or control], motion internal noise, and motion sampling) predicted variance in motion coherence thresholds (the outcome variable) (Table 3). The resulting model was highly significant (F(3,34) = 13.3, P = 7 × 10−6) and accounted for 54% of the variance in coherence thresholds (R = 0.74). Both group (6.6%) and motion sampling (39.44%) variables were found to predict a significant proportion of unique variance in coherence thresholds, whereas internal noise did not (2.4%). These findings indicate that even when differences in levels of internal noise and sampling were factored out, group membership (migraine versus control) accounted for a significant proportion of variance in coherence thresholds.
Table 3.
Predicting Motion Coherence Thresholds
|
Predictor |
β |
βst |
t |
PValue |
| Motion σint | 0.15 | 0.17 | 1.34 | 0.19 |
| Motion nsamp | −0.36 | −0.63 | −5.40 | 5.2 × 10−6* |
| Group | 0.1 | 0.28 | 2.20 | 0.03* |
A regression analysis showing the prediction of motion coherence thresholds from variance in three predictor variables (motion internal noise, motion sampling, and group [migraine or control]). All variables were added to the model simultaneously (i.e., nonhierarchically). β, β coefficient; βst, standardized β coefficient.
Predicts a significant proportion of unique variance in the outcome variable.
Finally, none of the psychophysical measures recorded (coherence thresholds, internal noise, or sampling) correlated with migraine characteristics (Supplementary Table S1). However, we note that the migraine characteristics included were based on self-report (e.g., migraine frequency, duration, severity) and, hence, were highly subjective and prone to recall bias. Also, they do not capture the fact that the nature of participants' migraines may have changed with time.
Discussion
In support of previous findings, motion coherence thresholds were elevated in the migraine group relative to the control group. However, this difference could not be attributed to deficits in either local or global processing. Equivalent noise analysis generated statistically indistinguishable estimates of internal noise (local processing) and sampling (global processing) for migraine and control groups across all three judgment types (orientation, motion, and size). Further, regression analysis indicated that group membership (migraine or control) predicted a significant proportion of the variance in coherence thresholds, even once levels of internal noise and sampling were controlled for. As discussed below, these findings are consistent with a relative inability to exclude visual noise in migraine.
The finding of elevated motion coherence thresholds in the migraine group is consistent with a number of previous reports. Whilst basic judgments of local position14 and motion11 do not differ between migraine and control groups, repeated studies have shown impaired performance on global form and global motion coherence tasks in which participants must detect global structure embedded in noise.6,9,10,12–14 However, it has been argued that so-called “global” coherence paradigms of this kind do not rely exclusively on global integration processes; instead, performance may also be limited by local processing (i.e., internal noise15) or the ability to exclude external noise.16 Consequently, EN analysis was undertaken so that independent estimates of local and global processing limits could be obtained.
The EN analysis undertaken here showed that levels of internal noise did not differ between migraine and control groups across any of the dimensions tested (orientation, motion, or size). This is consistent with a number of previous studies. For example, a technique known as the N-pass method,23–25 which measures the consistency in a participant's responses to sequential presentations of identical signal-plus-noise stimuli, has been used to estimate levels of internal noise in migraine.26–28 The principle underlying the technique is that internal noise reflects the level of random firing in a cell population that is sensitive to the dimension of interest (e.g., the direction of motion). As a result, a participant that is characterized by high internal noise will show poor consistency in responses across sequential presentations, since intrinsic variability in cellular responses, which is independent of the stimulus, will limit performance and drive random responses. Studies using this technique have shown that for global motion28 and for two out of three global form tasks tested,26–28 levels of internal noise levels of internal noise in participants with migraine are indistinguishable from those of control participants.
The EN analyses undertaken here also indicated normal global integration in migraine: levels of sampling were indistinguishable from control participants' for judgments of orientation, motion, and size. Although EN analysis has been applied to the study of migraine previously, it has not been used to characterize visuospatial performance; instead, previous studies have incorporated judgments of visual contrast. Thus, the findings are not directly comparable to our own: contrast EN analysis is different from spatial and motion versions of the task, most pertinently, with respect to the nature of the external noise added to the stimulus.29 Consequently, performance is captured by a more complex model that includes additional free parameters including a multiplicative noise term.30,31 Nonetheless, two independent studies using contrast EN analysis have reported indistinguishable levels of sampling in participants with and without migraine.27,32 Further, they showed that levels of additive internal noise (equivalent to the local noise parameter in the EN model used here) also did not differ between groups. This suggests that the findings we report (i.e., normal local and global processing in migraine) may extend to other (nonspatial) visual dimensions.
Taken together with previous studies, the data reported here can be reconciled with a simple model of visual processing in migraine that posits normal local and global processing, coupled with a low tolerance to external noise. Thus, performance is seemingly unaffected on tasks that only require integration of the signal (e.g., spatial and motion EN tasks) but is impaired on judgments that first require segregation of the signal from noise (e.g., form and motion coherence tasks). It is noteworthy that a selective deficit in the mechanisms of external noise exclusion has previously been demonstrated in another clinical group characterized by visuocortical dysfunction.16 Thus, in amblyopia, performance is reportedly normal on EN tasks that involve judgments of global form33,34 and motion,35 but impaired on related form coherence36 and motion coherence tasks.37–40 Although speculative, the similarity in the pattern of these findings in migraine and amblyopia, coupled with their widely differing etiologies, raises the possibility that the mechanisms involved in external noise exclusion are particularly vulnerable following cortical damage or cortical reorganization.
A number of cortical models of migraine have already been suggested in the literature. The majority of these are based on the notion of abnormal levels of cortical excitation,4,41 (i.e., hypo-excitability [reduced neural activity] or more commonly, hyperexcitability [elevated neural activity] relative to healthy controls [see Ref. 5 for review]). Thus, strengthened excitatory connections,42,43 impaired mechanisms of inhibition,44,45 and abnormal pre-activation levels46 have all been posited in migraine. However, these models are often poorly specified, such that precise behavioral predictions cannot be made on their basis. For example, hyperexcitability could imply elevated levels of stimulus-driven (i.e., spiking) activity, a specific elevation in baseline firing rates, or else a generalized increase in activity, all of which would lead to different predicted effects on the signal-to-noise ratio, and hence, visual psychophysical performance.5
With respect to the current study, the data reported are clearly inconsistent with versions of both the hyper- and hypo-excitability models that posit an abnormal level of baseline firing rates, since these would predict an elevation or reduction (respectively) in internal noise. Instead, we report normal levels of internal noise in migraine across all three visual dimensions tested (coupled with a selective elevation in motion coherence thresholds). An alternative version of the hyperexcitability model, which is broadly consistent with these data, is one in which stimulus-driven (spiking) activity is elevated, whilst baseline firing rates are unaffected. Let us assume that a predominant direction of motion is selected by the observer once a threshold firing rate is exceeded within a population of appropriately tuned direction-sensitive neurons: if a single direction of motion is presented, hyperexcitability will increase the likelihood that activity associated with the target direction will reach threshold, and hence be reported. However, for a noisy (e.g., motion coherence) stimulus, a state of hyperexcitability will also increase the probability that activity driven by the noise will reach threshold and hence compete with representations of the signal.
Consistent with this model of (stimulus-driven) cortical hyperexcitability, Antal et al.9 demonstrated superior motion discrimination performance in migraine (relative to controls) for a stimulus composed of a single direction of motion (100% coherence), coupled with impoverished (relative) performance once the coherence of the stimulus was decreased (i.e., noise was increased). In an earlier study, Antal et al.47 showed that a similar dissociation could also be induced in healthy control participants: following an experimental reduction in the excitability of cortical area MT, the discrimination of intermediate coherence motion was enhanced, whilst the discrimination of 100% coherent motion was impaired. Although we did not find superior classification performance in migraine for a stimulus composed of a single direction of motion (remember that these trials were interleaved with a high noise staircase in the EN task, potentially making the task harder), we did find a selective impairment in the processing of a noisy (motion coherence) stimulus. Taken together, these data suggest that a dissociation in the processing of motion coherence stimuli and stimuli composed of a single direction of motion (as reported) may be a signature of cortical (stimulus-driven) hyperexcitability.
In conclusion, the findings reported here are inconsistent with local or global processing deficits in migraine but, instead, implicate impaired mechanisms of visual noise exclusion. This hypothesis has the potential to integrate a wide range of findings from the existing literature and open up novel avenues for investigation. Specifically, it predicts that relative to control participants, people with migraine will be impaired on any visual discrimination or detection task for which signal and external noise must be segregated prior to an integration stage, provided that sufficient external noise is added to the stimulus. Future studies should focus on the mechanisms involved in visual noise exclusion, since little is known about this process. One possibility that has been raised is that impaired noise exclusion reflects a state of (stimulus-driven) cortical hyperexcitability, which increases competition between representations of the signal and the noise. An alternative possibility, which is equally speculative, however, is that representations of the noise compete with the signal to a greater extent in migraine because of a failure in endogenous attentional control (i.e., an inability to selectively monitor channels of interest that are most likely to carry the signal).48,49 To begin to tease these possibilities apart, it is clear that sophisticated psychophysical techniques must be employed in conjunction with clearly specified models of cortical function so that highly specific predictions can be tested. We believe that the efficient version of the EN paradigm, which can be adapted to test across multiple sensory dimensions and modalities, represents an invaluable tool in this approach.
Acknowledgments
Supported by The Wellcome Trust, the National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital and University College London (UCL) Institute of Ophthalmology, and Birkbeck College.
Disclosure: M.S. Tibber, None; M.G. Kelly, None; A. Jansari, None; S.C. Dakin, None; A.J. Shepherd, None
References
- 1. Woodhouse A, Drummond PD. Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia. 1993; 13: 417–421 [DOI] [PubMed] [Google Scholar]
- 2. Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J. Classification of primary headaches. Neurology. 2004; 63: 427–435 [DOI] [PubMed] [Google Scholar]
- 3. Hay KM, Mortimer MJ, Barker DC, Debney LM, Good PA. 1044 women with migraine: the effect of environmental stimuli. Headache. 1994; 34: 166–168 [DOI] [PubMed] [Google Scholar]
- 4. Chronicle EP, Mulleners WM. Visual system dysfunction in migraine: a review of clinical and psychophysical findings. Cephalalgia. 1996; 16: 525–535, discussion 523 [DOI] [PubMed] [Google Scholar]
- 5. Shepherd AJ. Models of cortical function in migraine: can psychophysical studies distinguish between them? A review of the evidence for interictal cortical hyper- and hypo-excitability. In: Clarke LB. ed Migraine Disorders Research Trends. New York, NY: Nova Science Publishers; 2007: 145–164 [Google Scholar]
- 6. Shepherd AJ, Beaumont HM, Hine TJ. Motion processing deficits in migraine are related to contrast sensitivity. Cephalalgia. 2012; 32: 554–570 [DOI] [PubMed] [Google Scholar]
- 7. Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962; 160: 106–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braddick O. Segmentation versus integration in visual motion processing. Trends Neurosci. 1993; 16: 263–268 [DOI] [PubMed] [Google Scholar]
- 9. Antal A, Temme J, Nitsche MA, Varga ET, Lang N, Paulus W. Altered motion perception in migraineurs: evidence for interictal cortical hyperexcitability. Cephalalgia. 2005; 25: 788–794 [DOI] [PubMed] [Google Scholar]
- 10. McKendrick AM, Badcock DR. Motion processing deficits in migraine. Cephalalgia. 2004; 24: 363–372 [DOI] [PubMed] [Google Scholar]
- 11. McKendrick AM, Vingrys AJ, Badcock DR, Heywood JT. Visual dysfunction between migraine events. Invest Ophthalmol Vis Sci. 2001; 42: 626–633 [PubMed] [Google Scholar]
- 12. Braunitzer G, Rokszin A, Kobor J, Benedek G, Nagy A, Kincses ZT. Delayed development of visual motion processing in childhood migraine. Cephalalgia. 2012; 32: 492–496 [DOI] [PubMed] [Google Scholar]
- 13. McKendrick AM, Badcock DR, Badcock JC, Gurgone M. Motion perception in migraineurs: abnormalities are not related to attention. Cephalalgia. 2006; 26: 1131–1136 [DOI] [PubMed] [Google Scholar]
- 14. McKendrick AM, Badcock DR, Gurgone M. Vernier acuity is normal in migraine, whereas global form and global motion perception are not. Invest Ophthalmol Vis Sci. 2006; 47: 3213–3219 [DOI] [PubMed] [Google Scholar]
- 15. Dakin SC, Mareschal I, Bex PJ. Local and global limitations on direction integration assessed using equivalent noise analysis. Vision Res. 2005; 45: 3027–3049 [DOI] [PubMed] [Google Scholar]
- 16. Husk JS, Huang PC, Hess RF. Orientation coherence sensitivity. J Vis. 2012; 12 (6): 18 [DOI] [PubMed] [Google Scholar]
- 17. Barlow HB. Retinal noise and absolute threshold. J Opt Soc Am. 1956; 46: 634–639 [DOI] [PubMed] [Google Scholar]
- 18. Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983; 33: 113–120 [DOI] [PubMed] [Google Scholar]
- 19. Dakin SC. Information limit on the spatial integration of local orientation signals. J Opt Soc Am A Opt Image Sci Vis. 2001; 18: 1016–1026 [DOI] [PubMed] [Google Scholar]
- 20. Solomon JA, Morgan M, Chubb C. Efficiencies for the statistics of size discrimination. J Vis. 2011; 11 (12): 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997; 10: 433–436 [PubMed] [Google Scholar]
- 22. Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997; 10: 437–442 [PubMed] [Google Scholar]
- 23. Burgess AE, Colborne B. Visual signal detection. IV. Observer inconsistency. J Opt Soc Am A. 1988; 5: 617–627 [DOI] [PubMed] [Google Scholar]
- 24. Gold J, Bennett PJ, Sekuler AB. Signal but not noise changes with perceptual learning. Nature. 1999; 402: 176–178 [DOI] [PubMed] [Google Scholar]
- 25. Levi DM, Klein SA, Chen I. What is the signal in noise? Vision Res. 2005; 45: 1835–1846 [DOI] [PubMed] [Google Scholar]
- 26. Webster KE. Investigating Internal Noise in Migraine: A Possible Mechanism Underlying Perceptual Deficits [thesis]. Crawley, Australia: University of Western Australia; 2011. [Google Scholar]
- 27. Webster KE, Dickinson JE, Battista J, McKendrick AM, Badcock DR. Evidence for increased internal noise in migraineurs for contrast and shape processing. Cephalalgia. 2012; 32: 125–139 [DOI] [PubMed] [Google Scholar]
- 28. Webster KE. Edwin Dickinson J, Battista J, McKendrick AM, Badcock DR. Increased internal noise cannot account for motion coherence processing deficits in migraine. Cephalalgia. 2011; 31: 1199–1210 [DOI] [PubMed] [Google Scholar]
- 29. Pelli DG, Farell B. Why use noise? J Opt Soc Am A Opt Image Sci Vis. 1999; 16: 647–653 [DOI] [PubMed] [Google Scholar]
- 30. Lu ZL, Dosher BA. Characterizing human perceptual inefficiencies with equivalent internal noise. J Opt Soc Am A Opt Image Sci Vis. 1999; 16: 764–778 [DOI] [PubMed] [Google Scholar]
- 31. Lu ZL, Dosher BA. Characterizing the spatial-frequency sensitivity of perceptual templates. J Opt Soc Am A Opt Image Sci Vis. 2001; 18: 2041–2053 [DOI] [PubMed] [Google Scholar]
- 32. Wagner D, Manahilov V, Loffler G, Gordon GE, Dutton GN. Visual noise selectively degrades vision in migraine. Invest Ophthalmol Vis Sci. 2010; 51: 2294–2299 [DOI] [PubMed] [Google Scholar]
- 33. Mansouri B, Allen HA, Hess RF. Detection, discrimination and integration of second-order orientation information in strabismic and anisometropic amblyopia. Vision Res. 2005; 45: 2449–2460 [DOI] [PubMed] [Google Scholar]
- 34. Mansouri B, Allen HA, Hess RF, Dakin SC, Ehrt O. Integration of orientation information in amblyopia. Vision Res. 2004; 44: 2955–2969 [DOI] [PubMed] [Google Scholar]
- 35. Hess RF, Mansouri B, Dakin SC, Allen HA. Integration of local motion is normal in amblyopia. J Opt Soc Am A Opt Image Sci Vis. 2006; 23: 986–992 [DOI] [PubMed] [Google Scholar]
- 36. Simmers AJ, Ledgeway T, Hess RF. The influences of visibility and anomalous integration processes on the perception of global spatial form versus motion in human amblyopia. Vision Res. 2005; 45: 449–460 [DOI] [PubMed] [Google Scholar]
- 37. Aaen-Stockdale C, Hess RF. The amblyopic deficit for global motion is spatial scale invariant. Vision Res. 2008; 48: 1965–1971 [DOI] [PubMed] [Google Scholar]
- 38. Aaen-Stockdale C, Ledgeway T, Hess RF. Second-order optic flow deficits in amblyopia. Invest Ophthalmol Vis Sci. 2007; 48: 5532–5538 [DOI] [PubMed] [Google Scholar]
- 39. Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Res. 2003; 43: 729–738 [DOI] [PubMed] [Google Scholar]
- 40. Simmers AJ, Ledgeway T, Mansouri B, Hutchinson CV, Hess RF. The extent of the dorsal extra-striate deficit in amblyopia. Vision Res. 2006; 46: 2571–2580 [DOI] [PubMed] [Google Scholar]
- 41. Wilkins A, Nimmo-Smith I, Tait A, et al. A neurological basis for visual discomfort. Brain. 1984; 107 (pt 4): 989–1017 [DOI] [PubMed] [Google Scholar]
- 42. Huang J, DeLano M, Cao Y. Visual cortical inhibitory function in migraine is not generally impaired: evidence from a combined psychophysical test with an fMRI study. Cephalalgia. 2006; 26: 554–560 [DOI] [PubMed] [Google Scholar]
- 43. Wilkinson F, Crotogino J. Orientation discrimination thresholds in migraine: a measure of visual cortical inhibition. Cephalalgia. 2000; 20: 57–66 [DOI] [PubMed] [Google Scholar]
- 44. Mulleners WM, Chronicle EP, Palmer JE, Koehler PJ, Vredeveld JW. Visual cortex excitability in migraine with and without aura. Headache. 2001; 41: 565–572 [DOI] [PubMed] [Google Scholar]
- 45. Palmer JE, Chronicle EP, Rolan P, Mulleners WM. Cortical hyperexcitability is cortical under-inhibition: evidence from a novel functional test of migraine patients. Cephalalgia. 2000; 20: 525–532 [DOI] [PubMed] [Google Scholar]
- 46. Ambrosini A, Rossi P, De Pasqua V, Pierelli F, Schoenen J. Lack of habituation causes high intensity dependence of auditory evoked cortical potentials in migraine. Brain. 2003; 126: 2009–2015 [DOI] [PubMed] [Google Scholar]
- 47. Antal A, Nitsche MA, Kruse W, Kincses TZ, Hoffmann KP, Paulus W. Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. J Cogn Neurosci. 2004; 16: 521–527 [DOI] [PubMed] [Google Scholar]
- 48. Lustig AG, Beck DM. Task-relevant and task-irrelevant dimensions are modulated independently at a task-irrelevant location. J Cogn Neurosci. 2012; 24: 1884–1895 [DOI] [PubMed] [Google Scholar]
- 49. Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nat Neurosci. 2002; 5: 631–632 [DOI] [PubMed] [Google Scholar]