Abstract
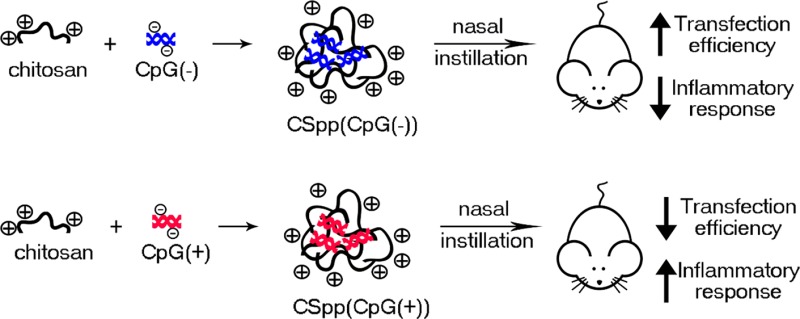
Chitosan polyplexes containing plasmid DNA (pDNA) have significant potential for pulmonary gene delivery applications. However, prior to using chitosan/pDNA polyplexes (CSpp) in clinical applications, their potential cytotoxicity needs to be investigated. In this study, we formulated 200–400 nm CSpp with amine to phosphate (N/P) ratios that ranged from 1 to 100. We compared two types of plasmids within CSpp: pDNA that was free of CpG sequences (CpG(−)) and pDNA that contained CpG sequences (CpG(+)). Both forms of CSpp showed low cytotoxicity when cultured with A549 and HEK293 cell lines in vitro. CSpp(CpG(−)) generated higher luciferase expression both in vitro, for A549 cells, and in vivo, compared with CSpp(CpG(+)). In addition, CSpp(CpG(−)) elicited milder inflammatory responses in mice one day subsequent to nasal instillation, as determined by proinflammatory cytokine levels within the bronchoalveolar lavage fluid. Our findings suggest that to achieve optimal gene expression with minimal cytotoxicity, inflammation, and oxidative stress, the N/P ratios and CpG sequences in the pDNA of CSpp need to be considered. These findings will inform the preclinical safety assessments of CSpp in pulmonary gene delivery systems.
Keywords: chitosan, plasmid DNA, polyplexes, nanoparticles, gene delivery, lung inflammation, transfection, luciferase, CpG
1. Introduction
Chitosan is an abundant linear polysaccharide derived from crustacean shells and is composed of random β-(1–4)-linked d-glucosamines (deacetylated units) and N-acetyl-d-glucosamines (acetylated units). It is a biodegradable and biocompatible polymer that has been studied intensively for its therapeutic applications as a drug and gene delivery system.1−4 Chitosan has garnered interest as a cationic nonviral gene delivery vector because of its (i) relative ease of preparation; (ii) low toxicity; and (iii) ability to condense DNA which provides protection against nuclease degradation.5,6
Previous studies have shown that chitosan/pDNA polyplexes (CSpp) are particularly promising as gene delivery formulations for treatment of pulmonary pathologies such as cystic fibrosis,7,8 asthma,9 and lung cancer.10 Although chitosan causes minimal cytotoxicity and is generally noninflammatory, a drawback is its low transfection efficiency when compared to viral vectors and nonviral synthetic vectors such as polyethylenimine (PEI). Thus, most of the studies involving CSpp delivery have been focused on strategies to improve transfection efficiency such as optimizing the molecular weight and the degree of deacetylation of chitosan,11−13 as well as establishing appropriate amine to phosphate ratios (N/P) of chitosan/DNA polyplexes.13 Mixing chitosan with other cationic gene vectors14,15 and/or modifying the structure of chitosan has also resulted in enhanced transfection efficiencies.16,17
The toxicity of pure or chemically modified chitosan has been investigated in its free form as well as in the form of micro- and nanoparticles. Pure chitosan is well-known to be nontoxic.18 Culturing chitosan nanoparticles, prepared by ionotropic gelation with pentasodium tripolyphosphate (TPP), with a cell line derived from human conjuntival epithelium, had no effect on cell viability compared to untreated cells.19 In addition, it has been reported that CSpp have little or no significant impact on cell viability in vitro for a range of cell types.20−22 This is in contrast to commonly used transfection reagents such as LipofectAMINE 2000 which can be highly cytotoxic.23 Despite showing promise in cytotoxicity assays in vitro, a thorough characterization of the cytotoxicity and inflammation induced by CSpp in vivo, particularly with respect to pulmonary delivery, needs further investigation.
Aside from chitosan, pDNA itself may also play a critical role in the cytotoxicity and inflammation imparted by CSpp in vivo. Unmethylated 5′-cytosine-guanosine-3′ dinucleotide (CpG) sequences in pDNA vectors can potentially induce inflammatory responses through the production of proinflammatory cytokines, which, in turn, may result in lowered transfection efficiencies.24 Yew et al. reported that nasal instillation of complexes composed of cationic lipids and CpG-depleted pDNA resulted in lower proinflammatory cytokine levels in mouse bronchoalveolar lavage fluid (BAL) when compared to the complexes comprising CpG-rich pDNA.25 Additionally, Hyde et al. compared pulmonary inflammatory responses to complexes comprising cationic liposomes and various plasmids containing varying numbers of unmethylated CpG sequences per plasmid. It was found that intranasal administration of complexes made with CpG-free plasmids produced no inflammatory responses while the presence of one unmethylated CpG sequence per plasmid was enough to trigger inflammatory responses.26 Lesina et al. showed that PEI complexes with a CpG-free plasmid administered in aerosolized form resulted in 60 times higher luciferase expression in the lung than that of PEI complexes containing plasmids with unmethylated CpG sequences.27 Although both types of PEI complexes increased cytokine levels at 1 h after administration, the cytokine levels of mice treated with PEI complexes with CpG-free plasmids reverted back to normal after 24 h while the others remained elevated.28
The main goals of this study were to characterize CSpp, evaluate their cytotoxicity in vitro, and determine the degree of pulmonary inflammation following nasal delivery to mouse lungs. CpG(−) and CpG(+) pDNA were compared to determine whether unmethylated CpG sequences in plasmid DNA influence transfection efficiency and inflammation.
2. Materials and Methods
2.1. Characterization of Chitosan/pDNA Polyplexes (CSpp)
2.1.1. Chitosan Purification
Low molecular weight chitosan was purchased from Sigma-Aldrich, Co. (St. Louis, MO). The deacetylation degree was 96.1%. The viscosity of 1% chitosan in 1% acetic acid was 35 cps. Chitosan was purified according to a previously published method with some modifications.29 Briefly, 1% (w/v) chitosan was dissolved in 1% (v/v) acetic acid and filtered with Whatman 541 filter paper (GE Healthcare, Amersham Place Little Chalfont, Buckinghamshire, U.K.). The supernatant was titrated with 1 N NaOH to pH ∼8.5 and then filtered to collect precipitated chitosan. Insoluble chitosan was redispersed in 0.1 M sodium bicarbonate solution, pH 8.3. Deproteinization and demetallization were performed by adding 0.5% (w/v) sodium dodecyl sulfate (SDS) and 20 mM ethylenediaminetetraacetic acid (EDTA) disodium salt. Insoluble chitosan was filtered, dialyzed (SnakeSkin dialysis tubing; 10K MWCO, Thermo Fisher Scientific Inc., Rockford, IL), and lyophilized. Purified chitosan tested negative (<LLOD, 0.024 EU/mL) for endotoxin using a modification of the kinetic chromogenic Limulus Amebocyte Lysate (LAL) assay (Lonza, Walkersville, MD).30
2.1.2. Plasmid Amplification and Purification
Two types of plasmids used in this study were pCpG-Luc (CpG(−)) (InvivoGen, San Diego, CA) and VR1255 (CpG(+)) (Vical, San Diego, CA). CpG(−) is completely devoid of CpG motifs (4696 bps). The CpG(+) plasmid contains CpG motifs (6413 bps). Both plasmids encode for luciferase. All plasmids were purified using a GenElute HP Endotoxin-Free Plasmid Maxiprep Kit (Sigma-Aldrich), according to the manufacturer’s protocol. pDNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA).
2.1.3. Preparation of CSpp
The CSpp were prepared by ionic interaction between positively charged chitosan and negatively charged pDNA molecules.20 In this study, dextran sulfate (sodium) (5 kDa, Sigma-Aldrich, St. Louis, MO) was introduced into the formulation to weaken the complexation of chitosan and DNA in order to limit excessive retention of DNA and thus ensure higher transgene expression.
CSpp were formulated at different ratios of primary amine groups in chitosan to phosphate groups in pDNA (N/P ratios). Chitosan solutions were prepared by dissolving the lyophilized purified chitosan in 1% (v/v) acetic acid solution, then adjusting to pH 5.5–5.7 using 1 N NaOH. Then 50 mM acetate buffer (pH 5.5) was added to the prepared solution to achieve a final concentration of 5 mg/mL. Dextran sulfate stock solution (1 mg/mL) was prepared in UltraPure DNase/RNase-Free distilled water (Invitrogen, Grand Island, NY). Dextran sulfate:chitosan (1:10 (w/w)) was used in this study. Plasmid DNA (CpG(−) or CpG(+)) in UltraPure DNase/RNase-free distilled water (200 μg/mL) was heated in a water bath to 50–55 °C prior to mixing. All solutions were sterilized by filtering with a 0.22 μm syringe filter (Millex-GS, Millipore Corporation, Billerica, MA). An equal volume of the desired concentration of cationic solution was pipetted into the anionic solution and then vortexed immediately for 20–30 s. The polyplex suspensions were incubated at room temperature for 30 min before use. The final concentrations of pDNA after polyplex formation were 2.5 μg/50 μL (in vitro) and 12.5 μg/50 μL (in vivo).
As a positive control in the in vitro transfection experiments, branched PEI, MW 25 kDa (Sigma-Aldrich), was used to make polyplexes at an N/P ratio of 10. These types of PEI/pDNA polyplexes (PEIpp) have previously displayed strong transfection efficiencies generating high luciferase activity in HEK293, COS7, and HeLa cell lines.31 To prepare PEI/pDNA polyplexes, a stock solution (1 mg/mL) of PEI in water was diluted to the desired concentration and mixed with pDNA in the same manner as CSpp.
2.1.4. Determination of Size and Zeta Potential of Polyplexes
Particle size and zeta potential of CSpp (with varying N/P ratios) were measured using a Zetasizer Nano ZS particle analyzer (Malvern Instrument Ltd., Southborough, MA). Size and zeta potential measurements were performed on polyplexes that were dispersed in acetate buffer solution, pH 5.46, at 25 °C.
2.1.5. Investigation of the Ability of Chitosan to Bind with pDNA
CpG(+) plasmid and the CSpp(CpG(+)) at N/P ratios of 1, 5, 10, 20, 60, and 100 were mixed with 2× BlueJuice gel loading buffer (Invitrogen). The final solutions/suspensions were loaded into the wells of a 1% (w/v) agarose gel containing 0.5 μg/mL of ethidium bromide in 1× Tris-acetate-EDTA (TAE) buffer. Gels were exposed to a constant current of 100 mA for 2 h. DNA migration was then visualized with a UV transilluminator (Spectroline, Westbury, NY).
2.2. Evaluating of the Cytotoxicity of Chitosan/pDNA Polyplexes in Vitro
2.2.1. Cell Lines and Cell Culture
Adenocarcinomic human alveolar basal epithelial cells (A549) were maintained in RPMI-1640 medium (Gibco, Life technologies, Grand Island, NY). Human embryonic kidney 293 (HEK293) cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD) and were maintained in Dulbecco’s modified Eagle medium (DMEM) (Gibco). All of the media were supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 10 mM HEPES (Gibco), 50 μg/mL gentamycin sulfate (Cellgro, Manassas, VA), 1 mM sodium pyruvate (Gibco), and 1 mM Glutamax (Gibco). Cells were incubated at 37 °C and 5% CO2.
2.2.2. Evaluation of the Cytotoxicity of CSpp
Twenty-four hours prior to treatment, cells were seeded (1 × 104/well) into a 96-well plate. Treatments (CSpp at various N/P ratios, PEI/pDNA polyplexes, and pDNA alone) were added in a volume of 100 μL/well (equivalent to 1 μg of pDNA) of serum-free medium. Without aspiration, 100 μL of fresh complete medium was added after 4 h of incubation. After 48 h, the treatment was aspirated and replaced with 100 μL of fresh complete medium and 20 μL of the MTS tetrazolium compound or CellTiter 96 Aqueous One Solution reagent (Promega Corporation, Madison, WI). The plate was incubated at 37 °C at 5% CO2 and incubated for 1–4 h, and then the absorbance was recorded at 490 nm using a Spectra Max plus 384 Microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). Relative cell viability values are expressed as the percentage of UV absorbance from wells containing treated cells compared to the control wells containing live cells treated with phosphate buffered saline (PBS).
2.2.3. Estimation of Intracellular Superoxide Levels
A549 or HEK293 cells were plated in 60 mm2 dishes (2 × 104 cells/dish) 24 h prior to treatments. Cells were treated with different polyplexes containing N/P 10, chitosan solution, PEI solution, or naked pDNA. After 48 h, cells were harvested by trypsinizing the monolayer cultures. Cells were subsequently washed with PBS containing 5 mM pyruvate and incubated in a solution of PBS containing 5 mM pyruvate with 10 μM dihydroethidium (DHE) for 40 min at 37 °C and 5% CO2. The control tubes received 0.1% dimethyl sulfoxide (DMSO), and the electron transport chain blocker antimycin A (Ant A) was used as a positive control. Following the incubation the cells were filtered through 35 μm mesh, transferred to flow tubes, and placed on ice to stop the reaction. Samples were analyzed using a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, Inc., San Jose, CA) using an excitation wavelength of 488 nm and emission of 585 nm. The mean fluorescence intensity (MFI) of 10,000 cells was recorded for each sample, and the mean of three samples was calculated for each treatment group. The background fluorescence was subtracted from each sample to generate the net MFI. Each sample was normalized to the control group to yield the normalized MFI (NMFI).
2.3. Evaluating Transfection Efficiencies of CSpp in Vitro
Cells (HEK293 and A549 cell lines) were seeded (8 × 104 cells/well) into a 24-well plate. After 24 h, 500 μL of serum-free medium and 100 μL of the treatments (pDNA solution, the CSpp and PEI/pDNA polyplexes) were added. Each treatment contained 5 μg of pDNA. Additional complete medium (500 μL) was added 4 h after the treatment. The medium was then replenished every 24 h. At 48 h, the cells were treated with reporter lysis buffer (RLB) (Promega Corporation) followed by two freeze–thaw cycles (frozen at −80 °C for 20 min then thawed at room temperature for 30 min). Cell suspensions were then transferred to microcentrifuge tubes and centrifuged at 16100g for 5 min, after which the supernatants were collected. Luciferase expression was measured using a luciferase assay system according to the company protocol (Promega Corporation). Briefly, 20 μL of each supernatant was added to 100 μL of the luciferase assay reagent and luciferase activity was measured for 10 s using a Lumat LB 9507 luminometer (EG&G Berthold, Wildbad, Germany). The remaining supernatant was analyzed for protein concentration using a Micro BCA protein assay kit (Pierce Biotechnology Inc., Rockford, IL). The results are expressed as relative light units (RLU)/mg of total protein.
2.4. Evaluating Transgene Expression and Inflammatory Responses Induced by CSpp in Mouse Lungs
2.4.1. Animals
Mice (C57Bl/6, males, 6 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were kept in a vivarium in polypropylene, fiber-covered cages in HEPA-filtered Thoren caging units (Hazelton, PA, USA) in the Pulmonary Toxicology Facility at the University of Iowa. Animals were acclimatized in the vivarium for 10 days prior to the instillation exposures. Food (sterile Teklad 5% stock diet, Harlan, Madison, WI) and water (via an automated watering system) was provided ad libitum, and a 12 h light–dark cycle was maintained in the animal room. All animal protocols used in these studies were approved by the Institutional Animal Care and Use Committee and complied with NIH Guidelines.
2.4.2. Nasal Instillation
Mice were exposed to CSpp by nasal instillation (dose 12.5 μg of pDNA/50 μL) twice with a 1 h interval between each dose. The mice were anesthetized with isoflurane (3%) by inhalation using a precision Fortec vaporizer (Cyprane, Keighley, U.K.). There were three control groups in these studies: (1) mice without exposure; (2) mice exposed to chitosan solution; and (3) mice exposed to CpG(+) alone. Mice were euthanized 24 h after nasal instillation, bronchoalveolar lavage (BAL) fluid was collected and lungs were harvested as previously described.32
2.4.3. Measuring Transgene Expression in Mouse Lungs after Nasal Instillation of CSpp
The method used for tissue preparation was adapted from Mohammadi et al.,20 Manthorpe et al.,33 and Stammberger et al.34 Lungs from euthanized mice were washed with ice-cold PBS, weighed, and homogenized using a Tissue Tearor (Biospec Products, Inc., Bartlesville, OK) in ice-cold reporter lysis buffer (4 μL/mg of lung tissue) for 1 min. Lysates were then passed through three freeze–thaw cycles (frozen at −80 °C for 20 min and thawed in a 37 °C water bath for 5 min). Lysates were then centrifuged at 16100g for 5 min. Supernatants were analyzed for luciferase expression in the same manner as described in section 2.3.
2.4.4. Evaluating Pulmonary Inflammatory Responses
The lungs from each euthanized mouse (n = 6) were lavaged via cannulated trachea three times, in situ, with approximately 1 mL/wash of sterile saline (0.9% (w/v) sodium chloride solution). Cells were collected from BAL fluid by centrifugation at 800g for 5 min at 4 °C. The cell pellets were resuspended in Hanks’ balanced salt solution, and the total white cells were counted using a hemocytometer. The remaining supernatants were stored at −80 °C and used for measuring total protein, LDH activity, and inflammatory cytokine/chemokine concentrations. Different cell types were counted after the BAL cells were placed on microscope slides, spun using Cytospin 4 (Thermo Shandon, Thermo Scientific, Waltham, MA) at 800g for 3 min, and stained using a HEMA 3 stain set (Fisher Scientific Company LLC, Midland, MI). A total of 400 cells (macrophages, neutrophils, lymphocytes, and eosinophils) per mouse were counted.
The concentration of total protein in BAL fluid supernatants was determined using a Bradford protein assay (Bio-Rad Laboratories, Inc., Hercules, CA). The activity of lactate dehydrogenase enzyme (LDH) in BAL fluid supernatants was measured using a commercially available cytotoxicity detection kit (LDH) (Roche Diagnostics, Mannheim, Germany). Inflammatory cytokine/chemokine (tumor necrosis factor [TNF]-α, interferon [IFN]-γ, interleukin [IL]-6, IL-12 (p40), keratinocyte-derived cytokine [KC], and macrophage inflammatory protein [MIP]-1α) levels in the BAL fluid were determined using a multiplexed fluorescent bead-based immunoassay (Bio-Rad Laboratories, Inc.). Values for data falling below the lower limit of detection (LLOD) for each cytokine were imputed with LLOD/√2.35
2.5. Statistical Analysis
Data are expressed as mean ± SD. Statistical significance was determined using a one-way ANOVA (analysis of variance) with Bonferroni’s multiple comparison test. A p-value less than 0.05 was considered significant. Statistical analyses were performed using GraphPad Prism version 5.02 for Windows (Graphpad Software, Inc., San Diego, CA).
3. Results and Discussion
3.1. Characterization of CSpp by Size and Zeta Potential
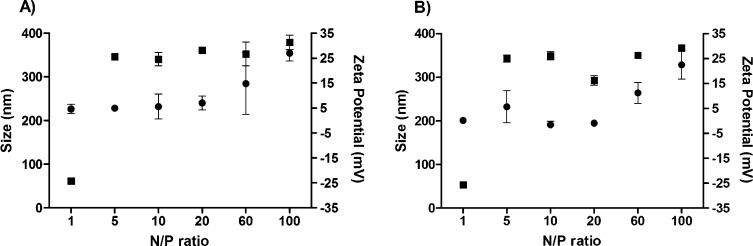
Sizes of the CSpp prepared for the in vitro study (2.5 μg of pDNA/50 μL) ranged between 200 and 400 nm for all N/P ratios tested (Figure 1). The sizes of the polyplexes at N/P ratios ranging from 1 to 20 were similar (∼230 nm) for both CSpp(CpG(−)) and CSpp(CpG(+)) while polyplexes progressively increased in size at N/P 60 (250–300 nm) and N/P 100 (300–350 nm). Both types of polyplexes had narrow size distributions with an average polydispersity index (PdI) equal to 0.136. At N/P 1, the cationic charge contributed by chitosan was lower than the negative charge from pDNA. Thus, the zeta potential values of both types of polyplex were negative (∼-25 mV). However, when the concentration of chitosan increased (at N/P ratios of 5 to 100), all polyplexes tested were positively charged (25–35 mV).
Figure 1.
Particle sizes and zeta potentials of CSpp(CpG(−)) (A) and CSpp(CpG(+)) (B) at N/P 1–100 as measured by Zetasizer Nano ZS in acetate buffer, pH 5.46, at 25 °C. Data are expressed as mean ± SD (n = 3–5). ● = size measurements. ■ = zeta potential measurements.
Zetasizer Nano ZS measurements showed that particle size increased when pDNA concentrations were increased above 2.5 μg/50 μL (data not shown). In contrast, concentrations below 2.5 μg of pDNA/50 μL have previously been shown to have no effect on the size of CSpp.36 For the in vitro experiments (sections 3.3 and 3.4) polyplexes were used at a concentration of 2.5 μg of pDNA/50 μL. For the in vivo experiments (sections 3.5 and 3.6) the concentration was increased to 12.5 μg of pDNA/50 μL in order to overcome the limitation of delivery volume. This increase in pDNA concentration resulted in an increase in polyplex size as well as a small amount of aggregation of the polyplexes and was consistent with findings from other groups.37,38 A bimodal distribution was observed in particle size measurements for CSpp with a pDNA concentration of 12.5 μg/50 μL, N/P 10 (523.0 ± 3.8 nm, 94.6 ± 2.3% by volume and 4888.0 ± 355.3 nm, 5.4 ± 2.3% by volume). Concentrations of greater than 12.5 μg/mL pDNA resulted in substantial precipitation of the polyplexes, rendering them unsuitable for pulmonary delivery.
3.2. Ability of Chitosan To Complex with pDNA
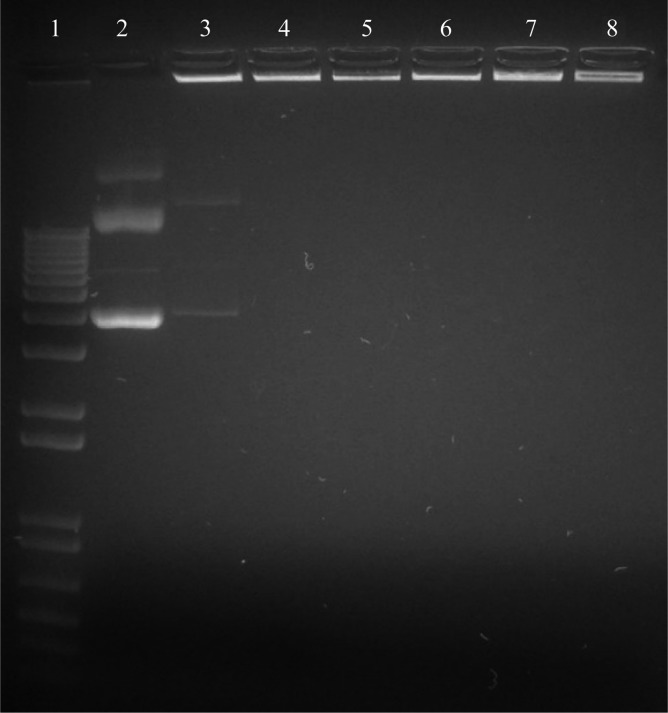
Plasmid DNA complexed with chitosan did not migrate into an agarose matrix (1% (w/v)) when an electric field was applied. The pDNA from CSpp remained in the loading wells at N/P ratios >1 (Figure 2). This finding is consistent with the zeta potential data showing a negative charge for CSpp N/P = 1 and a positive charge for CSpp N/P ≥ 5 suggesting that, at N/P ratios ≥5, chitosan binds to all free pDNA molecules, creating polyplexes.
Figure 2.
Gel retardation assay in 1% agarose gel containing ethidium bromide: lane 1, DNA ladder; lane 2, naked CpG(+) pDNA; lanes 3–8, CSpp(CpG(+)) with N/P ratios of 1, 5, 10, 20, 60 and 100, respectively.
These results may vary depending on the characteristics of the chitosan, since the binding strength between chitosan and DNA has been shown to be affected by the degree of deacetylation and the molecular weight of the chitosan. Chitosan with a high molecular weight and high degree of deacetylation binds to DNA more strongly than chitosan with a relatively low molecular weight and low degree of deacetylation.12,39
3.3. Evaluation of in Vitro Cytotoxicity
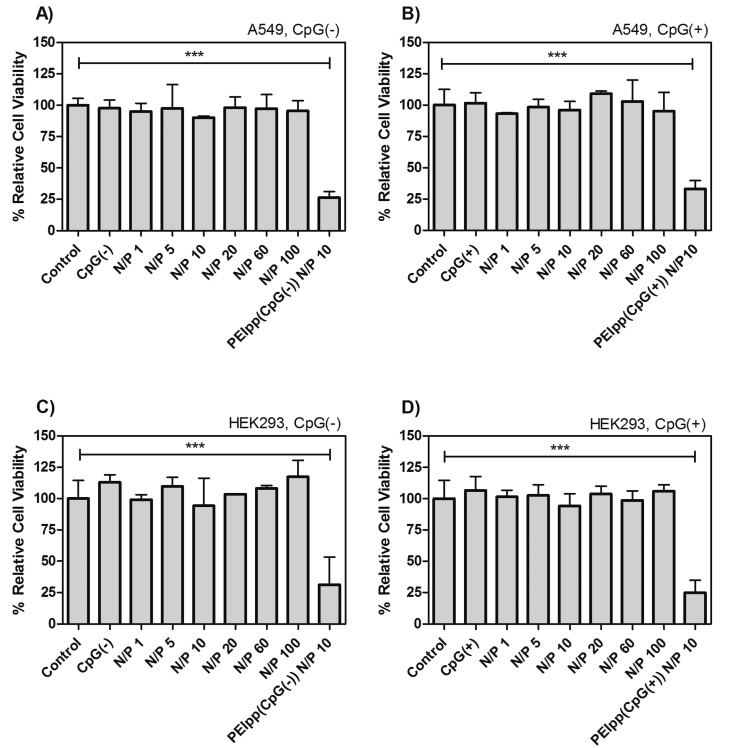
Cell viability assays (see section 2.2.2) involving A549, a human lung epithelial cell line, and HEK293, a human embryonic kidney cell line, commonly used in transfection studies, were used to test CSpp at N/P ratios of 1–100 using both types of pDNA (CpG(−) or CpG(+)). All formulations of CSpp tested resulted in at least 85–90% cell viability in A549 (Figures 3A and 3B) and HEK293 (Figures 3C and 3D), with no significant differences compared to PBS-treated control cells. In contrast, PEIpp at N/P 10 showed significantly lower cell viabilities in both A549 (Figures 3A and 3B) and HEK293 (Figures 3C and 3D) (∼25%, p-value <0.001).
Figure 3.
Cell viability of A549 (A, B) and HEK293 (C, D) cell lines treated with CSpp(CpG(−)) (A, C) and CSpp(CpG(+)) (B, D) was determined by MTS assay. Cells were treated with CSpp containing N/P ratios ranging from 1 to 100. CpG(−) represents soluble CpG-free plasmid DNA while CpG(+) represents soluble CpG-containing plasmid DNA. PEIss(CpG(−)) or PEIpp(CpG(+)) were PEI complexes with pDNA at N/P 10. Control = cells treated with PBS. The cells were seeded into a 96-well plate at a density of 1 × 104 cells per well one day prior the experiment and exposed to the treatments for 48 h. Each treatment contained 1 μg of pDNA per well. Data are expressed as mean ± SD (n = 3). Percent relative cell viability compared to cells treated with PBS. *** p < 0.001.
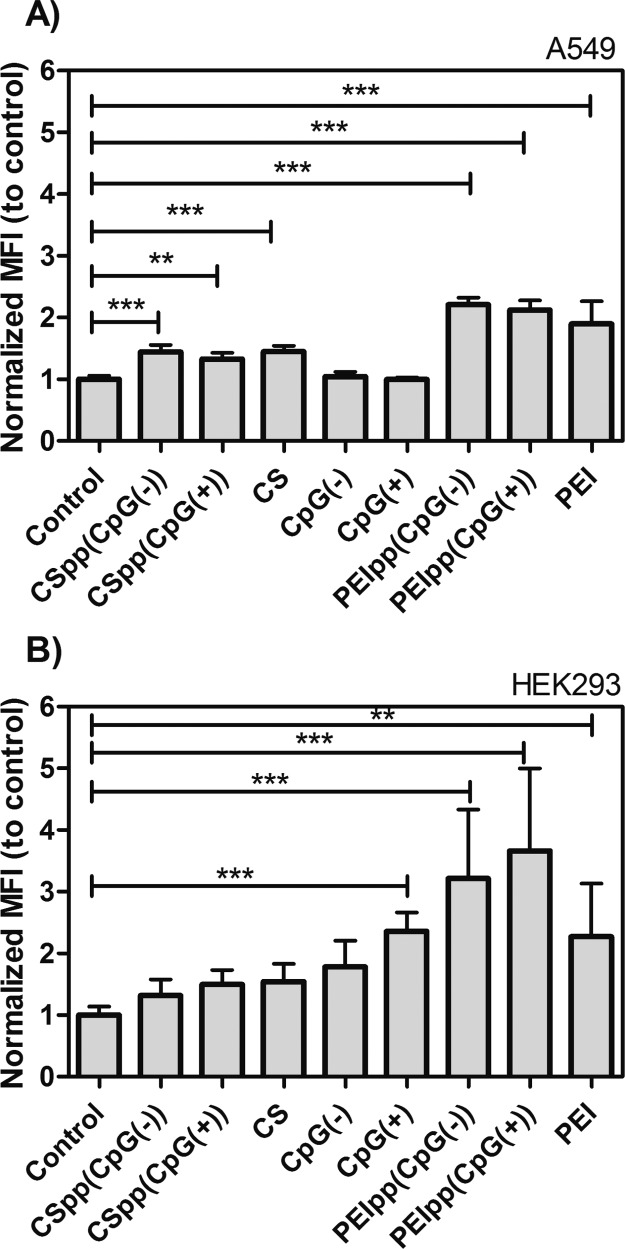
In order to determine if treatment with the polyplexes affects intracellular reactive oxygen species (ROS) levels, superoxide levels were estimated using the oxidation sensitive fluorescent dye, dihydroethidium (DHE). The results indicated that A549 cells treated with CSpp, PEI polyplexes (PEIpp), chitosan solution, or PEI solution showed significant increases (1.5–2.0-fold) in DHE oxidation relative to the control group (p-value <0.001 and <0.01) (Figure 4A). Treatment of HEK293 cells with the CSpp or the chitosan solution did not significantly affect the DHE oxidation levels compared to the control group. However, treatment with PEIpp, CpG(+) alone, or PEI solution showed significant increases (1.5–3.0-fold) in DHE oxidation (p-value <0.001 and <0.01) (Figure 4B). Both A549 and HEK293 cells treated with CSpp containing either CpG(+) or CpG(−) had lower DHE oxidation when compared with cells treated with PEIpp and PEI solution.
Figure 4.
DHE oxidation levels indicated as normalized mean fluorescence intensity (Normalized MFI) showing estimates of relative intracellular superoxide levels in A549 cells (A) and HEK293 cells (B) treated with indicated polyplexes. Cells were treated with CSpp(CpG(−)) or CSpp(CpG(+)) containing N/P 10. CS is chitosan solution without pDNA. PEIss(CpG(−)) or PEIpp(CpG(+)) are PEI complexes with pDNA at N/P 10. PEI is PEI solution without pDNA. Control = cells treated with PBS. Data are expressed as mean ± SD (n = 6–9). *** p < 0.001, ** p < 0.01.
The results from both cell lines showed CSpp had a negligible impact on cell viability and cells treated with CSpp trended toward lower DHE oxidation when compared to cells treated with PEIpp. Malich et al. studied the sensitivity and specificity of the MTS assay with human cell lines. They found that results from the in vitro toxicity assay did not necessarily correlate well with in vivo results.40 The in vitro models might not be capable of elucidating the inflammatory potency. Thus, the toxicity, oxidative stress levels, and inflammatory response in an animal model needed to be assessed before CSpp delivery systems can be used for clinical applications (see section 3.5).
3.4. In Vitro Transfection Efficiencies of CSpp: Presence versus Absence of CpG Sequences
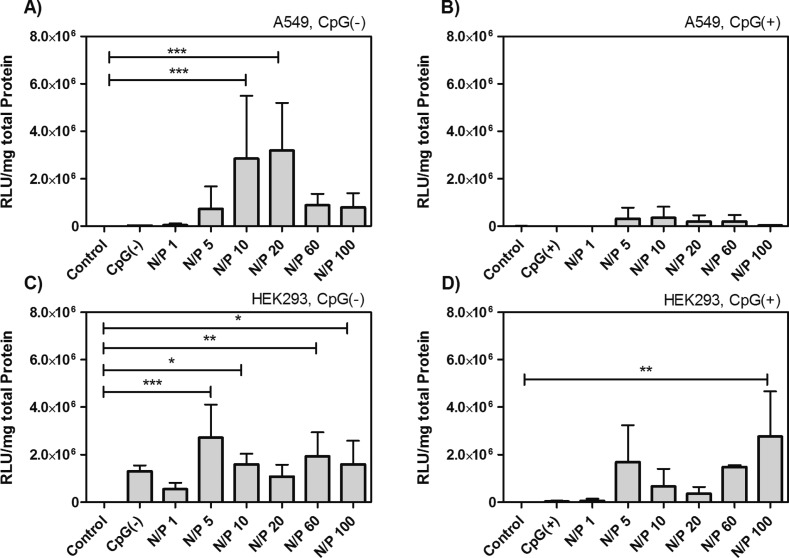
The effect of the presence of CpG sequences within CSpp on transfection efficiencies was examined initially in vitro using A549 and HEK293 cell lines. The transfection efficiency of CSpp(CpG(−)) at an N/P ratio of 10 and 20 was significantly higher than that of the control groups for A549 as shown in Figure 5A. In contrast, relatively low transgene expression was observed when CSpp(CpG(+)) were used to transfect A549 cells (Figure 5B). In the HEK293 cell line, the luciferase expression pattern was similar for both CSpp(CpG(−)) and CSpp(CpG(+)) (Figures 5C and 5D). It has been reported that A549 cells express the Toll-like receptor 9 (TLR-9), which recognizes CpG sequences in pDNA, and we speculate that this may have had a detrimental effect on CSpp(CpG(+))-mediated transfection of A549 cells.41 Finally, A549 cells gave higher luciferase expression than HEK293 cells when treated with CSpp(CpG(−)). Mao et al., in studies investigating transfection efficiencies of CSpp in cell lines of different lineages, reported differences in luciferase expression levels indicating that the transfection efficiency when using CSpp may be dependent on cell-type.42
Figure 5.
Transfection efficiency of A549 (A, B) and HEK293 (C, D) cells treated with CSpp(CpG(−)) (A, C) or CSpp(CpG(+)) (B, D). Varying N/P ratios are indicated. Control = cells treated with PBS. Each treatment contained 5 μg of pDNA/well. Data are expressed as mean ± SD (n = 3–7). * p < 0.05, ** p < 0.01, *** p < 0.001. PEIpp N/P 10 was used as a positive control for transfection (not shown in graphs). The luciferase expression of PEIpp-transfected A549 cells was equal to 2.32 × 1010 ± 7.45 × 109 RLU/mg (total protein) (for PEIpp(CpG(−)) and 2.54 × 1010 ± 1.62 × 1010 RLU/mg (for PEIpp(CpG(+)). The luciferase expression in HEK293 cells was 5.38 × 1010 ± 3.96 × 109 RLU/mg (for PEIpp(CpG(−)) and 4.23 × 1010 ± 2.92 × 1010 RLU/mg (for PEIpp(CpG(+)).
3.5. In Vivo Transfection Efficiencies in Mouse Lungs: Effect of Presence versus Absence of CpG Sequences in CSpp
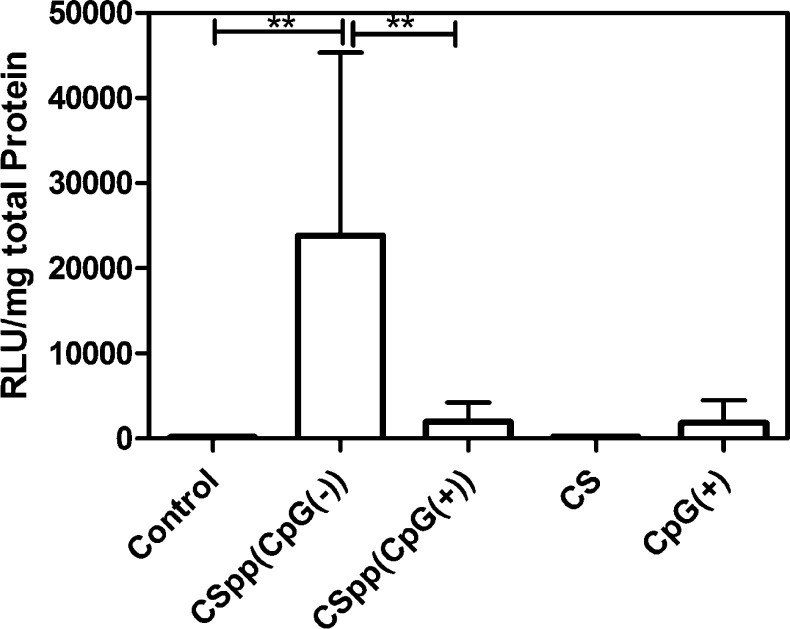
The supernatant obtained from homogenized lungs of mice treated with CSpp(CpG(−)) (at an N/P ratio of 10) demonstrated significantly (p < 0.01) higher luciferase expression (mean, 23846 RLU/mg of total protein; median, 23043) than mice treated with CSpp(CpG(+)) (mean, 1969 RLU/mg of total protein; median, 777) (Figure 6). In addition, only the group in which mice were treated with CSpp(CpG(−)) expressed significantly higher luciferase than the controls (p < 0.01). These results are consistent with our in vitro data using A549 cells. As such, a possible explanation for our in vivo transgene expression results is that CpG sequences stimulate inflammatory responses that deleteriously affect transfection. We subsequently performed experiments to investigate this possibility.
Figure 6.
Luciferase expression in mouse lungs after nasal instillation of indicated CSpp formulations. CS = chitosan solution; control = no treatment. Each treatment, aside from control and CS, contained 25 μg of pDNA (12.5 μg pDNA/50 μg, 2× nasal instillations). Data are expressed as mean ± SD (n = 6). ** p < 0.01.
3.6. Inflammatory Responses Measured from BAL Fluids
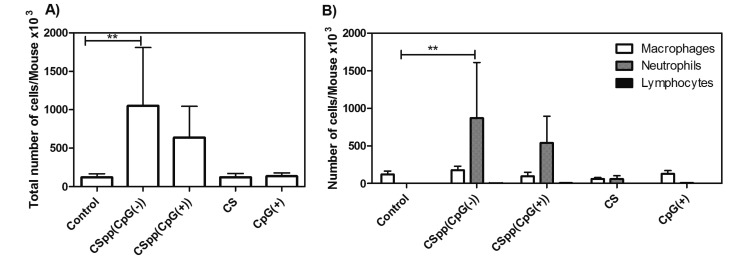
The inflammatory response to CSpp was investigated by analyzing BAL fluids from variously treated mice. Acute inflammatory responses were assessed by enumeration of white blood cell types (macrophages, neutrophils, and lymphocytes) 24 h subsequent to nasal instillation of CSpp formulations. CSpp(CpG(−)) caused a significant 9-fold increase in the total number of white blood cells in the BAL fluid compared to control treatment, while CSpp(CpG(+)) caused a 7-fold increase (albeit not statistically significant) over controls (Figure 7A). The majority of these infiltrating white cells proved to be neutrophils (Figure 7B), which represents a classical inflammatory response.
Figure 7.
BAL cell counts: total number of cells (A) and the number of macrophages, neutrophils, and lymphocytes (B) in the BAL fluid/mouse after nasal instillation of indicated CSpp formulations. CS = chitosan solution, CpG(+) = naked CpG(+) pDNA solution. Control = no treatment. Each treatment (aside from control and CS) contained 25 μg of pDNA (12.5 μg of pDNA/50 μg, 2× nasal instillations). Data are expressed as mean ± SD (n = 6). ** p < 0.01.
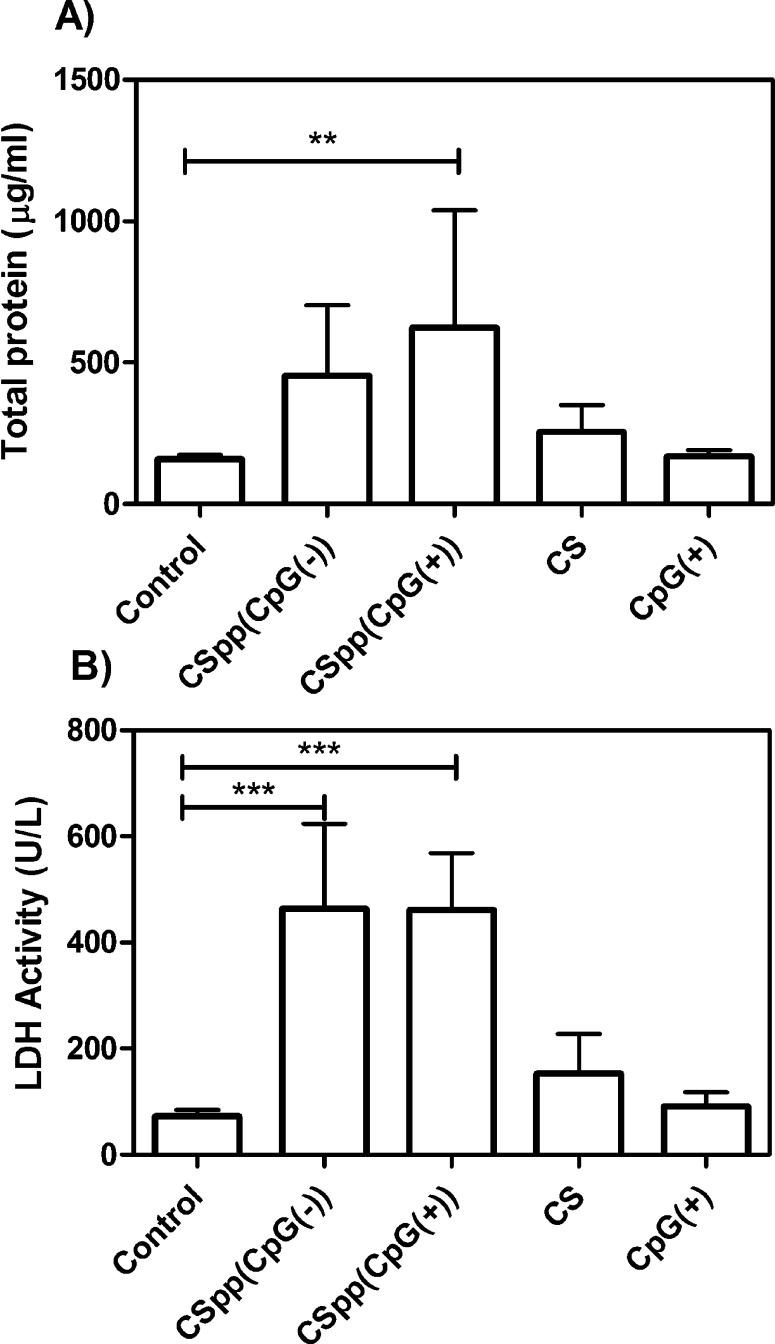
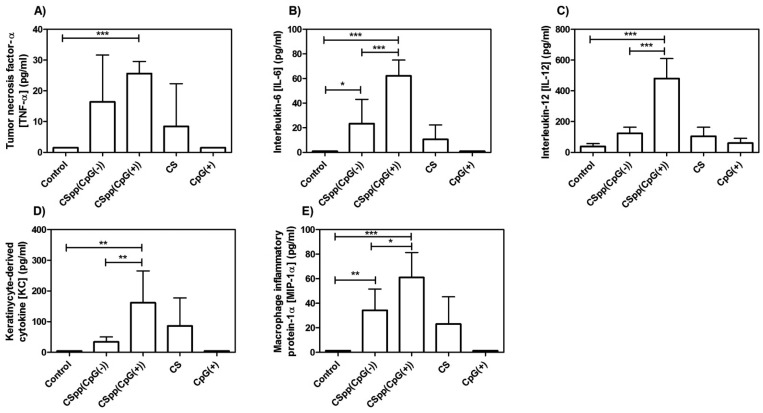
Concentrations of total protein in BAL fluid (an indicator of the increased permeability of the alveolar capillary membrane) was significantly higher (compared to control) in mice treated with CSpp(CpG(+)). Total protein concentration was also enhanced (but not significantly) in mice receiving CSpp(CpG(−)) (Figure 8A). Activity of LDH, which denotes cell membrane damage, was not statistically different in BAL fluid from mice treated with either CSpp(CpG(−)) or CSpp(CpG(+)) (Figure 8B). There were no statistically significant differences for both total protein levels and LDH activity between mice treated with CSpp(CpG(−)) and with CSpp(CpG(+)). Finally, levels of cytokines (IL-6, IL-12, KC, and MIP-1α) were consistently significantly higher in the BAL fluids from mice treated with CSpp(CpG(+)) compared to CSpp(CpG(−)) treated mice (Figure 9). Of particular distinction were IL-12 and KC levels. TNF-α levels were also higher in the mice receiving CSpp(CpG(+)), however, not significantly. These observed increases in inflammatory cytokines could be due to the presence of CpG since it is well established that triggering TLR-9 can result in a MyD88-dependent, NF-kB-mediated upregulation of inflammatory cytokines, including IL-12.43 We also tested for INF-γ; however, levels were below the lowest limit of detection (0.95 pg/mL) in all samples analyzed (data not shown).
Figure 8.
Total protein and LDH activity levels: the concentration of total protein (A) and LDH activity (B) in BAL fluids of mice subsequent to nasal instillation of indicated CSpp formulations. CS = chitosan solution, control = untreated. Each treatment, aside from control and CS, contained 25 μg of pDNA (12.5 μg of pDNA/50 μg, 2× nasal instillations). Data are expressed as mean ± SD (n = 6). *** p < 0.001, ** p < 0.01.
Figure 9.
The concentration of proinflammatory cytokines/chemokines detected in BAL fluid of mice subsequent to nasal instillation of indicated CSpp formulations. CS = chitosan solution; control = untreated. LLOD for TNF-α = 1.50 pg/mL (A), IL-6 = 0.96 pg/mL (B), IL-12 = 6.38 pg/mL (C), KC = 4.95 pg/mL (D), and MIP-1α = 1.3 pg/mL (E). Each treatment, aside from control and CS, contained 25 μg of pDNA (12.5 μg of pDNA/50 μg, 2× nasal instillations). Data are expressed as mean ± SD (n = 6). *** p < 0.001, ** p < 0.01, * p < 0.05.
The results gathered from the BAL fluids showed that both CSpp(CpG(−)) and CSpp(CpG(+)) induced inflammation, as determined by an increased influx of neutrophils as well as increases in total protein, LDH activity, and inflammatory cytokines. Mice treated with chitosan solution alone trended toward increased cytokine levels in their BAL fluid compared to untreated mice. These levels were further enhanced for all cytokines tested when chitosan was complexed with CpG(+) pDNA while CSpp(CpG(−)) treatment resulted in only marginally higher levels (TNF-α, IL-6, MP-1α). Thus, in terms of cytokine levels, chitosan polyplexes made from plasmids devoid of CpG sequences induced lower inflammatory responses than polyplexes harboring plasmids that contained CpG sequences.
4. Conclusions
The combination of plasmid DNA with chitosan results in polyplexes with the potential for use in pulmonary gene delivery systems. In this study, we formulated CSpp with 2 different plasmids (CpG(−) and CpG(+)) at N/P ratios of 1, 5, 10, 20, and 100. We found that CSpp(CpG(−)) generated superior transfection efficiencies both in vitro (using A549 cells) and in vivo (mouse nasal instillation) when compared with CSpp(CpG(+)). In addition, CSpp(CpG(−)) generated lower levels of proinflammatory cytokines (TNF-α, IL-6, IL-12, KC, and MIP-1α) in the BAL fluids. Our findings suggest that N/P ratios and plasmid CpG sequence content of CSpp are important factors to consider in achieving optimal gene expression with minimal toxicity and inflammation.
Acknowledgments
The authors gratefully acknowledge support from the Environmental Health Sciences Research Center (NIH P30 ES005605). Other sources of support include the American Cancer Society (RSG-09-015-01-CDD), the National Cancer Institute at the National Institutes of Health (1R21CA13345-01/1R21CA128414-01A2/UI Mayo Clinic Lymphoma SPORE) and the Lyle and Sharon Bighley Professorship. A.W. thanks Kristan L. S. Worthington, Katherine S. Walters, the Central Microscopy Research Facility at the University of Iowa and the Radiation and Free Radical Research Core Lab and P30-CA086862 for technical support in measuring DHE oxidation.
Glossary
Abbreviations Used
- BAL
bronchoalveolar lavage fluid
- CpG
unmethylated 5′-cytosine-gounosine-3′ dinucleotide
- CpG(−)
pDNA that is completely devoid of CpG motifs
- CpG(+)
pDNA that contains CpG motifs
- CSpp
chitosan/pDNA polyplexes
- CSpp(CpG(−))
chitosan/CpG(−) polyplexes
- CSpp(CpG(+))
chitosan/CpG(+) polyplexes
- N/P
amine to phosphate ratios
- pDNA
plasmid DNA
- PEI
polyethylenimine
- PEIpp
PEI/pDNA polyplexes
- PEIpp(CpG(−))
PEI/CpG(−) polyplexes
- PEIpp(CpG(+))
PEI/CpG(+) polyplexes
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Nagpal K.; Singh S. K.; Mishra D. N. Chitosan nanoparticles: a promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58111423–30. [DOI] [PubMed] [Google Scholar]
- Tong H.; Qin S.; Fernandes J. C.; Li L.; Dai K.; Zhang X. Progress and prospects of chitosan and its derivatives as non-viral gene vectors in gene therapy. Curr. Gene Ther. 2009, 96495–502. [DOI] [PubMed] [Google Scholar]
- Guang Liu W.; De Yao K. Chitosan and its derivatives--a promising non-viral vector for gene transfection. J. Controlled Release 2002, 8311–11. [DOI] [PubMed] [Google Scholar]
- Mansouri S.; Lavigne P.; Corsi K.; Benderdour M.; Beaumont E.; Fernandes J. C. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur. J. Pharm. Biopharm. 2004, 5711–8. [DOI] [PubMed] [Google Scholar]
- Saranya N.; Moorthi A.; Saravanan S.; Devi M. P.; Selvamurugan N. Chitosan and its derivatives for gene delivery. Int. J. Biol. Macromolecules 2011, 482234–8. [DOI] [PubMed] [Google Scholar]
- Lai W. F.; Lin M. C. Nucleic acid delivery with chitosan and its derivatives. J. Controlled Release 2009, 1343158–68. [DOI] [PubMed] [Google Scholar]
- Nydert P.; Dragomir A.; Hjelte L. Chitosan as a carrier for non-viral gene transfer in a cystic-fibrosis cell line. Biotechnol. Appl. Biochem. 2008, 51Part 4153–7. [DOI] [PubMed] [Google Scholar]
- Mohammadi Z.; Dorkoosh F. A.; Hosseinkhani S.; Gilani K.; Amini T.; Najafabadi A. R.; Tehrani M. R. In vivo transfection study of chitosan-DNA-FAP-B nanoparticles as a new non viral vector for gene delivery to the lung. Int. J. Pharm. 2011, 4211183–8. [DOI] [PubMed] [Google Scholar]
- Kong X.; Hellermann G. R.; Zhang W.; Jena P.; Kumar M.; Behera A.; Behera S.; Lockey R.; Mohapatra S. S. Chitosan Interferon-gamma Nanogene Therapy for Lung Disease: Modulation of T-Cell and Dendritic Cell Immune Responses. Allergy, Asthma, Clin. Immunol. 2008, 4395–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H.; Shiraki K.; Yasuda R.; Danjo K.; Watanabe Y. Chitosan-interferon-beta gene complex powder for inhalation treatment of lung metastasis in mice. J. Controlled Release 2011, 1502187–95. [DOI] [PubMed] [Google Scholar]
- Huang M.; Fong C. W.; Khor E.; Lim L. Y. Transfection efficiency of chitosan vectors: effect of polymer molecular weight and degree of deacetylation. J. Controlled Release 2005, 1063391–406. [DOI] [PubMed] [Google Scholar]
- Lavertu M.; Methot S.; Tran-Khanh N.; Buschmann M. D. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials 2006, 27274815–24. [DOI] [PubMed] [Google Scholar]
- Romoren K.; Pedersen S.; Smistad G.; Evensen O.; Thu B. J. The influence of formulation variables on in vitro transfection efficiency and physicochemical properties of chitosan-based polyplexes. Int. J. Pharm. 2003, 2611–2115–27. [DOI] [PubMed] [Google Scholar]
- Kim T. H.; Kim S. I.; Akaike T.; Cho C. S. Synergistic effect of poly(ethylenimine) on the transfection efficiency of galactosylated chitosan/DNA complexes. J. Controlled Release 2005, 1053354–66. [DOI] [PubMed] [Google Scholar]
- Zhao Q. Q.; Chen J. L.; Han M.; Liang W. Q.; Tabata Y.; Gao J. Q. Combination of poly(ethylenimine) and chitosan induces high gene transfection efficiency and low cytotoxicity. J. Biosci. Bioeng. 2008, 105165–8. [DOI] [PubMed] [Google Scholar]
- Jiang H. L.; Kim Y. K.; Arote R.; Jere D.; Quan J. S.; Yu J. H.; Choi Y. J.; Nah J. W.; Cho M. H.; Cho C. S. Mannosylated chitosan-graft-polyethylenimine as a gene carrier for Raw 264.7 cell targeting. Int. J. Pharm. 2009, 3751–2133–9. [DOI] [PubMed] [Google Scholar]
- Jiang H. L.; Kwon J. T.; Kim E. M.; Kim Y. K.; Arote R.; Jere D.; Jeong H. J.; Jang M. K.; Nah J. W.; Xu C. X.; Park I. K.; Cho M. H.; Cho C. S. Galactosylated poly(ethylene glycol)-chitosan-graft-polyethylenimine as a gene carrier for hepatocyte-targeting. J. Controlled Release 2008, 1312150–7. [DOI] [PubMed] [Google Scholar]
- Kean T.; Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Delivery Rev. 2010, 6213–11. [DOI] [PubMed] [Google Scholar]
- Enriquez de Salamanca A.; Diebold Y.; Calonge M.; Garcia-Vazquez C.; Callejo S.; Vila A.; Alonso M. J. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: toxicity, uptake mechanism and in vivo tolerance. Invest. Ophthalmol. Visual Sci. 2006, 4741416–25. [DOI] [PubMed] [Google Scholar]
- Mohammadi Z.; Abolhassani M.; Dorkoosh F. A.; Hosseinkhani S.; Gilani K.; Amini T.; Najafabadi A. R.; Tehrani M. R. Preparation and evaluation of chitosan-DNA-FAP-B nanoparticles as a novel non-viral vector for gene delivery to the lung epithelial cells. Int. J. Pharm. 2011, 4091–2307–13. [DOI] [PubMed] [Google Scholar]
- Sato T.; Ishii T.; Okahata Y. In vitro gene delivery mediated by chitosan. effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials 2001, 22152075–80. [DOI] [PubMed] [Google Scholar]
- Rajesh Kumar S.; Ishaq Ahmed V. P.; Parameswaran V.; Sudhakaran R.; Sarath Babu V.; Sahul Hameed A. S. Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from Vibrio (Listonella) anguillarum. Fish Shellfish Immunol. 2008, 251–247–56. [DOI] [PubMed] [Google Scholar]
- Corsi K.; Chellat F.; Yahia L.; Fernandes J. C. Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles. Biomaterials 2003, 2471255–64. [DOI] [PubMed] [Google Scholar]
- Scheule R. K. The role of CpG motifs in immunostimulation and gene therapy. Adv. Drug Delivery Rev. 2000, 442–3119–34. [DOI] [PubMed] [Google Scholar]
- Yew N. S.; Zhao H.; Wu I. H.; Song A.; Tousignant J. D.; Przybylska M.; Cheng S. H. Reduced inflammatory response to plasmid DNA vectors by elimination and inhibition of immunostimulatory CpG motifs. Mol. Ther. 2000, 13255–62. [DOI] [PubMed] [Google Scholar]
- Hyde S. C.; Pringle I. A.; Abdullah S.; Lawton A. E.; Davies L. A.; Varathalingam A.; Nunez-Alonso G.; Green A. M.; Bazzani R. P.; Sumner-Jones S. G.; Chan M.; Li H.; Yew N. S.; Cheng S. H.; Boyd A. C.; Davies J. C.; Griesenbach U.; Porteous D. J.; Sheppard D. N.; Munkonge F. M.; Alton E. W.; Gill D. R. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat. Biotechnol. 2008, 265549–51. [DOI] [PubMed] [Google Scholar]
- Lesina E.; Dames P.; Rudolph C. The effect of CpG motifs on gene expression and clearance kinetics of aerosol administered polyethylenimine (PEI)-plasmid DNA complexes in the lung. J. Controlled Release 2010, 1432243–50. [DOI] [PubMed] [Google Scholar]
- Lesina E.; Dames P.; Flemmer A.; Hajek K.; Kirchner T.; Bittmann I.; Rudolph C. CpG-free plasmid DNA prevents deterioration of pulmonary function in mice. Eur. J. Pharm. Biopharm. 2010, 743427–34. [DOI] [PubMed] [Google Scholar]
- Qian R. Q.; Glanville R. W.. Methods for purifying chitosan. US 2004/0118778 A1, 2005.
- Thorne P. S. Inhalation toxicology models of endotoxin- and bioaerosol-induced inflammation. Toxicology 2000, 1521–313–23. [DOI] [PubMed] [Google Scholar]
- Intra J.; Salem A. K. Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. J. Controlled Release 2008, 1302129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamcakova-Dodd A.; Stebounova L. V.; O’Shaughnessy P. T.; Kim J. S.; Grassian V. H.; Thorne P. S. Murine pulmonary responses after sub-chronic exposure to aluminum oxide-based nanowhiskers. Part. Fibre Toxicol. 2012, 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M.; Cornefert-Jensen F.; Hartikka J.; Felgner J.; Rundell A.; Margalith M.; Dwarki V. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum. Gene Ther. 1993, 44419–31. [DOI] [PubMed] [Google Scholar]
- Stammberger U.; Uduehi A. N.; Kubisa B.; Roth T.; Schmid R. A. Non-viral gene delivery to atelectatic and ventilated lungs. Ann. Thorac. Surg. 2002, 732432–6. [DOI] [PubMed] [Google Scholar]
- Hornung R. W.; Reed L. D. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990, 5146–51. [Google Scholar]
- Koping-Hoggard M.; Varum K. M.; Issa M.; Danielsen S.; Christensen B. E.; Stokke B. T.; Artursson P. Improved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomers. Gene Ther. 2004, 11191441–52. [DOI] [PubMed] [Google Scholar]
- Hsu E. C.; Fleet C.; Cheung A.; Gao J.. High concentration chitosan-nucleic acid polyplex compositions. US 2011/0039917 A1, 2011.
- Davies L. A.; McLachlan G.; Sumner-Jones S. G.; Ferguson D.; Baker A.; Tennant P.; Gordon C.; Vrettou C.; Baker E.; Zhu J.; Alton E. W.; Collie D. D.; Porteous D. J.; Hyde S. C.; Gill D. R. Enhanced lung gene expression after aerosol delivery of concentrated pDNA/PEI complexes. Mol. Ther. 2008, 1671283–90. [DOI] [PubMed] [Google Scholar]
- Kiang T.; Wen J.; Lim H. W.; Leong K. W. The effect of the degree of chitosan deacetylation on the efficiency of gene transfection. Biomaterials 2004, 25225293–301. [DOI] [PubMed] [Google Scholar]
- Malich G.; Markovic B.; Winder C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 1243179–92. [DOI] [PubMed] [Google Scholar]
- Droemann D.; Albrecht D.; Gerdes J.; Ulmer A. J.; Branscheid D.; Vollmer E.; Dalhoff K.; Zabel P.; Goldmann T. Human lung cancer cells express functionally active Toll-like receptor 9. Respir. Res. 2005, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H.-Q.; Roy K.; Troung-Le V. L.; Janes K. A.; Lin K. Y.; Wang Y.; August J. T.; Leong K. W. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J. Controlled Release 2001, 703399–421. [DOI] [PubMed] [Google Scholar]
- Kawai T.; Akira S. TLR signaling. Cell Death Differ. 2006, 135816–25. [DOI] [PubMed] [Google Scholar]