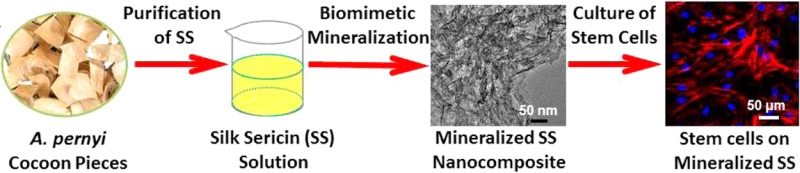
Abstract
Biomacromolecules have been used as templates to grow hydroxyapatite crystals (HAps) by biomineralization to fabricate mineralized materials for potential application in bone tissue engineering. Silk sericin is a protein with features desirable as a biomaterial, such as increased hydrophilicity and biodegradation. Mineralization of the silk sericin from Antheraea pernyi (A. pernyi) silkworm has rarely been reported. Here, for the first time, nucleation of HAps on A. pernyi silk sericin (AS) was attempted through a wet precipitation method and consequently the cell viability and osteogenic differentiation of BMSCs on mineralized AS were investigated. It was found that AS mediated the nucleation of HAps in the form of nanoneedles while self-assembling into β-sheet conformation, leading to the formation of a biomineralized protein based biomaterial. The cell viability assay of BMSCs showed that the mineralization of AS stimulated cell adhesion and proliferation, showing that the resultant AS biomaterial is biocompatible. The differentiation assay confirmed that the mineralized AS significantly promoted the osteogenic differentiation of BMSCs when compared to nonmineralized AS as well as other types of sericin (B. mori sericin), suggesting that the resultant mineralized AS biomaterial has potential in promoting bone formation. This result represented the first work proving the osteogenic differentiation of BMSCs directed by silk sericin. Therefore, the biomineralization of A. pernyi silk sericin coupled with seeding BMSCs on the resultant mineralized biomaterials is a useful strategy to develop the potential application of this unexplored silk sericin in the field of bone tissue engineering. This study lays the foundation for the use of A. pernyi silk sericin as a potential scaffold for tissue engineering.
1. Introduction
Bone is formed by a series of complex events involving mineralization with calcium phosphate in the form of hydroxyapatite crystals (HAps) on extracellular matrix.1−3 Therefore, as a biomimetic strategy, many macromolecular materials have been used as templates to grow HAps to form mineralized materials that can be used as a building block for bone implant fabrication, such as collagen, phage, and silk fibroin.4−10 HAps-coated silk fibroin promotes osteogenic differentiation of BMSCs,11,12 which provides an appropriate osteoconductive environment for BMSCs to regenerate sufficient new bone tissue.13 However, unlike silk fibroin, another silk-derived protein, silk sericin, has not been studied regarding how its HAps mineralization can affect the osteogenic differentiation of BMSCs .
Silk sericin is a global protein synthesized in the middle silk gland of silkworm, which is coated on the fibroin fiber when silkworm spins cocoon. In comparison to silk fibroin, silk sericin has its unique characteristics including hydrophilicity, oxidation resistance, ultraviolet resistance, and biodegradation.14−18 The silk sericin from Bombyx mori (B. mori) silkworm, a well-known domesticated silkworm, can be mineralized, leading to improvement of the cell viability.19,20 Thus, HAps-coated B. mori sericin (BS) has been proposed to form potential scaffolds for bone tissue engineering. Meanwhile, another silk sericin can be produced by Antheraea pernyi (A. pernyi) silkworm, a large species of wild silkworm, in large quantity. The amino acid composition of A. pernyi sericin (AS) is different from that of BS with AS having a lower percentage of serine and tyrosine (Table S1). However, there is no report on the mineralization of AS and its potential application as a building block to build bone implants and scaffolds for bone tissue engineering. Therefore, the mineralization of AS needs to be investigated to fill this gap.
Hence, this study aimed to investigate AS-mediated nucleation of HAps to form mineralized AS and the impact of mineralization of AS on the osteogenic differentiation of BMSCs. Figure 1 shows our strategy to achieve this goal. We first extracted aqueous AS from A. pernyi cocoon (Figure 1A). The amino acid analysis (Table S1) indicated that AS contains the acidic amino acid such as Glu and Asp, which are considered as the sites for triggering HAps nucleation on silk fibroin and BS.20−23 Thus we anticipated that AS could control the nucleation of HAps in the presence of Ca2+ and PO43–. As described in Figure 1B, the anionic side-chains of AS first bind Ca2+ through electrostatic attraction, which further attracts PO43– to initiate the nucleation of HAps and in turn promotes the assembly of AS and HAps into clusters (Figure 1C). It was found that HAps could promote the osteogenic differentiation of BMSCs,8,9 so we hypothesized that mineralized AS would improve cell viability and osteogenic differentiation due to the presence of bone minerals in the resultant materials. To test this hypothesis, we studied the effect of mineralization of AS on the cell viability and on the osteogenic differentiation of the human bone marrow derived mesenchymal stem cells (BMSCs; Figure 1D).
Figure 1.
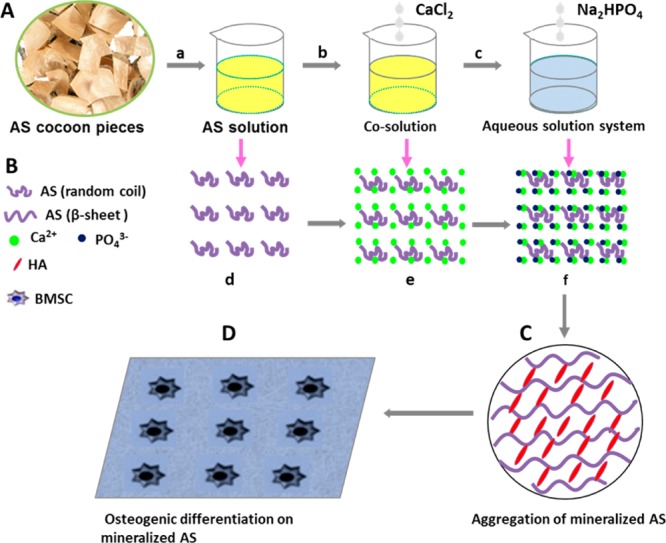
Mineralization of AS and its biological properties. (A) Schematic representing preparation of AS solution and its biomineralization; (B) Proposed schematic describing the nucleation of HAps mediated by AS; (C) The assembly structure of mineralized AS with β-sheet conformation; (D) The osteogenic differentiation of BMSCs on mineralized AS; (a) A. pernyi cocoons were heated in deionized water at 120 °C for 30 min and the AS solution was extracted; (b) CaCl2 solution was first added into AS solution; (c) Na2HPO4 solution was added into cosolution; (d) AS was originally in the random coil conformation; (e) Calcium ions were bound to the anionic side-chains of AS; (f) The nucleation of HAps was initiated after addition of Na2HPO4 solution.
2. Materials and Methods
2.1. Materials
A. pernyi silkworm cocoons were purchased from Shandong Academy of Sericulture, China. CaCl2, Na2HPO4, NaHCO3, and other reagents of analytical grade were purchased from Sinopharm Chemical Reagents Co. Ltd., China. Deionized water was used throughout the experiment. Fetal bovine serum (FBS) and 0.25% trypsin were purchased from Invitrogen. Dulbecco’s modified Eagle’s medium (DMEM) and 1% penicillin–streptomycin were purchased from Gibco.
2.2. Preparation of AS Solution
The aqueous AS solution was prepared from A. pernyi silkworm cocoons according to the reported procedure.24A. pernyi cocoons were cut into small pieces and heated in deionized water at 120 °C for 30 min in an autoclave as described in Figure 1A. After that, the solution was centrifuged at 6000 rpm for 10 min. The aqueous AS solution was obtained by collecting the supernatant. The final concentration of AS solution was calculated by weighing the remaining solid after drying. The concentrations of aqueous AS solutions were varied from 0.5 to 8 mg/mL for the following experiments.
2.3. Biomineralization of AS Solution
Nucleation of HAps mediated by AS solution was performed by a wet precipitation method described in the previous paper.17 In order to investigate the effect of AS concentration and mineralization time on the nucleation of HAps, the concentrations of AS were controlled to be 0.5, 2, and 8 mg/mL, and the mineralization time was set at 2, 6, 12, and 24 h, respectively. To investigate the role of AS concentration in HAps nucleation, CaCl2 was first added into 100 mL of AS solution with various concentrations of 0.5, 2, and 8 mg/mL, respectively, and mixed completely. The concentration of CaCl2 was controlled at 20 mM. Subsequently, 100 mL of 12 mM Na2HPO4 was added into above cosolution at a rate of 5 mL/min controlled by a constant flow pump. This aqueous solution system was stirred to allow the HAps nucleation on AS. The mineralization time was controlled at 24 h. The pH value of this aqueous solution system was adjusted to a constant value of 9.5 by adding 0.1 M NaOH solution. After the mineralization was finished, the aqueous solution system was centrifuged at 6000 rpm for 10 min. The mineralized AS was collected by gathering the precipitation followed by washing it with deionized water three times. In addition, to find out whether mineralization of AS is dependent on time, the same procedure was performed on AS with concentration of 2 mg/mL by controlling the mineralization time at 2, 6, 12, and 24 h, respectively. The resultant mineralized AS (MAS) was named as MAS2, MAS6, MAS12, and MAS24, respectively.
2.4. Characterization of HAps Nucleation Mediated by AS
TEM was used to observe the nucleation of HAps under different concentrations of AS and mineralization times. The aqueous solution system was sonicated for 10 min and 10 μL of this solution was dropped onto the copper grids. After dried in air, the sample was washed by deionized water twice to remove any soluble salts. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM) were carried out on JEOL JEM-1200EX and FEI Tecnai G2F30, respectively. In order to only observe the morphology of HAps in the mineralized composites, the composites were not stained before TEM imaging. X-ray diffraction (XRD) and Fourier transform infrared (FT-IR) spectroscopic measurements were performed to confirm the formation of HAps. For XRD, X-ray diffraction patterns of AS and mineralized AS were measured by X′ Pert PRO diffractometer. The powder samples were scanned in the range of 10° to 60°. FT-IR spectra were recorded using a Fourier transform infrared spectrometer (FTIR-8400S, Shimadzu, Japan). A total of 2 mg AS or mineralized AS were mixed with 200 mg KBr and then pressed into discs, respectively. The measurements were performed with the wavenumber ranging from 400 to 4000 cm–1.
2.5. Assembly Structure of Mineralized AS
The assembly structure of mineralized AS was observed with atomic force microscopy (AFM, MultiMode, VEECO, U.S.A.) in tapping mode. For AFM, the concentration of aqueous solution system was diluted to be 1 × 10–2 mg/mL by deionized water. Four μL of this diluted solution was deposited on freshly cleaved mica and air-dried, followed by washing with 20 μL deionized water. The images were taken and processed by software (NanoScope Image). In addition, the secondary structure of AS before and after mineralization was observed by circular dichroism (CD) with the concentration of 0.1 mg/mL at room temperature. The CD measurement was performed between 190 and 250 nm with a MOS-450 spectrometer (Biologic, France) using a quartz cell with a path length of 1 mm. The reported CD pattern represents an average of three consecutive scans measured at the rate of 0.5 nm/s.
2.6. Cell Viability Assay on AS and Mineralized AS
We used human BMSCs as testing cells. Human BMSCs were isolated as described in our previous work.25,24 Cells at the fourth to sixth passage were used for experiment. BMSCs were cultured in DMEM with 10% FBS and 1% penicillin–streptomycin. Medium was changed every 3 days. MAS24 was used as MAS for cell viability test. The empty well and BS were used as control, respectively. The BS, AS, and MAS solutions (100 μL) were added to 96-well microplates, respectively, and then desiccated under vacuum for 24 h. This process allowed the BS, AS, and MAS to form a film on the well of microplates. Each well was sterilized by 75% (V/V) ethanol, and washed with physiological saline three times. BMSCs (104 cells/cm2) were seeded on the BS, AS, and MAS films, respectively, and cultured in DMEM supplemented with 10% fetal bovine serum in a 5% CO2 incubator at 37 °C.
After 1, 3, and 7 days culture, cell morphology observation was performed by fixing specimens in 4% formalin solution for 15 min and 1% Triton X-100 in PBS for 10 min. The cytoskeletons were visualized by actin (F-actin was detected using TRITC-conjugated Phalloidin; Millipore), and cell nuclei were visualized by DAPI (Beyotime Institute of Biotechnology, China). The cells were viewed under confocal microscope system (Olympus FV1000, Japan). In addition, the cell viability on AS and mineralized AS was determined by using Cell Counting Kit-8 (CCK-8, Dojindo). After culture of 1, 3, and 7 days, the cells were incubated in 10% CCK-8 solution in a 5% CO2 incubator at 37 °C for 2 h. The intense orange-colored formazan derivative formed by cell metabolism is soluble in the culture medium. The absorbance of the culture medium was detected at 450 nm using a microplate reader (Bio-Rad 680, U.S.A.). Cell number was correlated to optical density (OD).
2.7. Osteogenic Differentiation of BMSCs on AS and Mineralized AS
Human BMSCs culture was described above. Cells at the fourth to sixth passage were used for experiment. BS was used as a control. Films were cast from aqueous solutions of AS, MAS, and BS. BMSCs (104/cm2) were seeded onto the films and cultured in osteogenic induction medium. After 2 weeks, the levels of mRNA for osteogenic specific genes (Smad 8, Runx2, and OCN), integrin (integrin α2), and adipose marker (PPARγ) of BMSCs cultured on the AS, MAS, and BS films in the osteogenic medium were assessed using real-time PCR. Total cellular RNA was extracted by lysis in trizol (Invitrogen). PCR was performed using Brilliant SYBR Green QPCR Master Mix (TakaRa) with a Light Cycler apparatus (ABI 7900HT). The PCR cycling consisted of 40 cycles of amplification of the template DNA with primer annealing at 60 °C. The relative level of expression of each target gene was then calculated using the 2–ΔΔCt method.26 The amplification efficiencies of primer pairs were validated to enable quantitative comparison of gene expression. All primers (Invitrogen) were designed using primer 5.0 software and are listed in Table 1. Each real-time PCR was performed on four different experimental samples and representative results are shown as target gene expression normalized to reference gene GAPDH.27
Table 1. Primer Sequences Used for Quantitative Reverse Transcription-Polymerase Chain Reaction Gene Expression Analysis.
| genes | 5′-3′ | primers | product size (bp) |
|---|---|---|---|
| Smad8 | forward | CACGGCTTTGAAGTCGTGTAT | 198 |
| reverse | TGAAGAAATGGGGTTATGTGGA | ||
| Runx2 | forward | GTGATAAATTCAGAAGGGAGG | 118 |
| reverse | CTTTTGCTAATGCTTCGTGT | ||
| osteocalcin | forward | AGGGCAGCGAGGTAGTGAAGA | 181 |
| reverse | TAGACCGGGCCGTAGAAGC | ||
| integrin a2 | forward | CCGACAGGGGTTATCATAGGCA | 224 |
| reverse | CATTCACCACACCAGCGAGC | ||
| PPARγ | forward | TTCTCCTATTGACCCAGAAAGC | 307 |
| reverse | CTCCACTTTGATTGCACTTTGG |
Alkaline phosphatase (ALP) and alizarin red staining were also performed to investigate BMSCs differentiation. BMSCs (104/cm2) were seeded onto the AS and MAS film and cultured in osteogenic induction medium. After 2 weeks, ALP activity was assayed using a BCIP/NBT alkaline phosphatase color development kit (Beyotime Institute of Biotechnology). The ALP staining was observed under an optical microscope (Olympus IX71, Japan). Calcium deposits were detected by staining with 2% alizarin red S (pH 4.2; Sigma). To quantify the stained nodules, the stain was solubilized with 0.5 mL 5% sodium dodecyl sulfate (SDS) in 0.5N HCl for 30 min at room temperature. Solubilized stain was transferred to wells of a 96-well plate, and absorbance was measured at 405 nm.28 The final absorbance was obtained by subtracting the absorbance of corresponding wells containing the same scaffolds but without cells seeded in order to remove the contribution from the minerals in the mineralized scaffolds.
2.8. Statistical Analysis
Data were presented as mean values ± standard deviation (SD), n = 4. Statistical differences among the samples were determined by two tailed, unpaired student t test. Differences between groups were considered statistically significant at p < 0.05 and highly significant at p ≤ 0.01.
3. Results and Discussion
3.1. Nucleation of HAps Mediated by AS
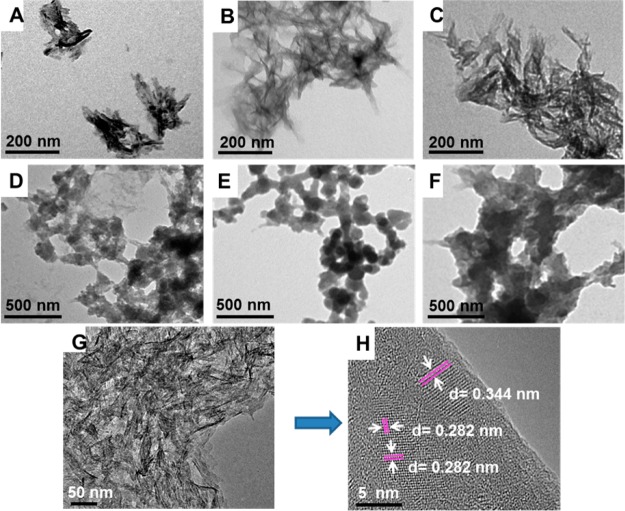
The nucleation of HAps on B. mori fibroin and sericin is triggered by anionic side-chains that bind Ca2+.9,17,29 This implies that the amount of acidic amino acid of AS can affect the nucleation HAps. Therefore, we expected that the concentration of AS can impact the nucleation of HAps because the amount of acidic amino acids is dependent on the concentration. We used three different AS concentrations (0.5, 2, and 8 mg/mL) to investigate the effect of AS concentration on the nucleation of HAps. The TEM images show that AS concentration can mediate the morphology of HAps (Figure 2A–C). HAps were grown into nanoneedle-like morphology when the AS concentration was 0.5 mg/mL (Figure 2A). When the AS concentration was increased to 2 mg/mL, the length of the nanoneedles was increased (Figure 2B). Aggregation of HAps were clearly observed at the AS concentration of 8 mg/mL (Figure 2C). This result confirmed that concentration indeed plays an important role in the nucleation of HAps, implying that a higher concentration of AS was in favor of nanoneedle-like crystal formation.
Figure 2.
TEM images showing the nucleation of HAps mediated by AS with different concentrations and mineralization times. (A–C) The mineralization time was 24 h, but the concentration of AS was set at (A) 0.5, (B) 2, and (C) 8 mg/mL; (D–F) The concentration of AS was fixed at 2 mg/mL, but the mineralization time was chosen at (D) 2, (E) 6, and (F) 12 h; (G) HRTEM image showing the nanoneedles of HAps mediated by AS with the concentration of 2 mg/mL after mineralization for 24 h; and (H) is a high magnification image of (G). White arrows show the lattice spacings of 0.344 and 0.282 nm, which are corresponding to the d-spacings of (002) and (211) lattice planes, respectively.
The effect of mineralization time on the nucleation of HAps was described in Figure 2D–F. On MAS2, a clump of starfish-like crystals was randomly scattered, indicating that AS provided nucleation sites for crystal growth (Figure 2D). On MAS6, spherical crystals were formed and aggregated (Figure 2E). When the mineralization time was increased to 12 h (MAS12), spindly crystals were heavily aggregated (Figure 2F). HRTEM image of MAS24 (Figure 2G) indicated that nanoneedle crystals were grown after mineralization for 24h (Figure 2G). The size of nanoneedle crystals was approximately 20 to 40 nm in length and 3 to 5 nm in width. The lattice spacing of 0.344 and 0.282 nm in Figure 2H proved the nucleation of HAps as they are corresponding to the d-spacing of (002) and (211) lattice planes of HAps, respectively.30,31 These results proved that the nucleation of HAps mediated by AS is a gradual process, which is in agreement with our proposed mechanism on HAps nucleation as described in Figure 1B.
In addition, the nucleation of HAps was verified by XRD and FT-IR measurements. The sharp peaks at 25.9° and 32.1° assigned to the planes (002) and (211) of HAps were observed in Figure S1. The intensity of these two peaks was increased with increase in the mineralization time, indicating the mineralization time can affect the growth of HAps. The peaks of 561, 602, and 1030 cm–1 at FT-IR spectra (Figure S2) proved the nucleation of HAps. The peaks of 561 and 602 cm–1 are corresponding to the O–P–O stretching vibration while the peak at 1030 cm–1 can be assigned to the P–O stretching vibration of HAps.6 XRD and FT-IR results further confirm the formation of HAP observed by TEM. In addition, the peak for the amide II in the FT-IR spectra is shifted from 1540 to 1525 cm–1, indicating that AS are assembled into β-sheet with the nucleation of HAps.
3.2. Assembly Structure of Mineralized AS
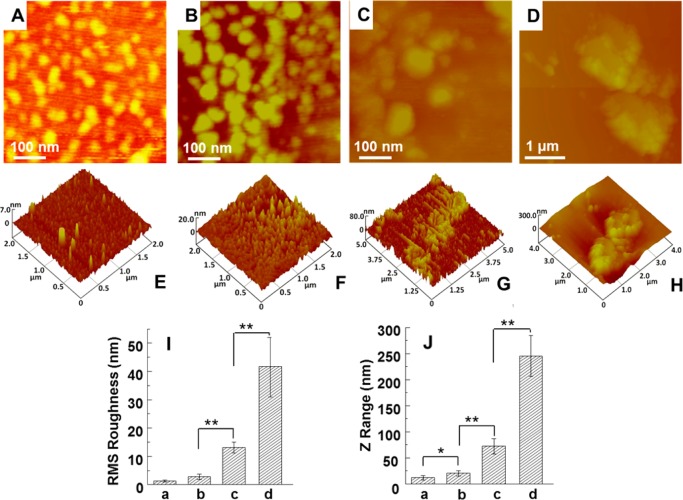
We hypothesized that the Ca2+ chelated AS would attract PO43– to initiate the nucleation of HAps and simultaneously to drive β-sheet assembly of AS due to hydrogen bond interaction. TEM observation shows that HAps are nucleated and their morphologies are changed from spindly to nanoneedle-like (Figure 2). AFM was also used to observe the assembly structure of AS during HAps growth. Figure 3A–D are the topographies of AS, MAS2, MAS6, and MAS24, respectively. Figure 3E–H are the 3D AFM images corresponding to Figure 3A–D, respectively. The results of statistical analysis of RMS roughness and Z range of AS, MAS2, MAS6, and MAS24 are indicated in Figure 3I and J, respectively.
Figure 3.
Topographies of AFM images and corresponding section analysis of AS and mineralized AS (MAS): (A) AS, (B) MAS2, (C) MAS6, (D) MAS24; (E–H) three-dimensional images corresponding to (A–D), respectively; (I, J) histograms of RMS roughness and z range; (a–d) in I and J are the samples corresponding to (A–D), respectively. MAS2, MAS6, and MAS24 represent AS after mineralized for 2, 6, and 24 h, respectively.
Prior to mineralization, AS appeared to be individual globules (Figure 3A), similar to the morphology of BS.32 AS was homogeneously distributed on the surface of mica substrate due to the Brownian motion (Figure 3E).33 Its RMS roughness was 1.5 ± 0.3 nm (Figure 3I-a), and the z range was 11.5 ± 3.2 nm (Figure 3J-a), suggesting that AS formed a flat surface. After mineralization of 2 h, the individual globules were enlarged and the height was increased (Figure 3B,F). The cluster patterns tended to be aggregated in the case of MAS6. With the mineralization time increased to 24 h, larger aggregation was formed with a diameter of 2 μm. Correspondingly, the RMS roughness was significantly increased to 41.7 ± 11.2 nm (Figure 3I), and the z range to 245.2 ± 35.2 nm, respectively (Figure 3J). AFM images clearly demonstrate that AS and HAps are assembled into globules, verifying the self-assembly of AS during mineralization as anticipated in Figure 1B,C.
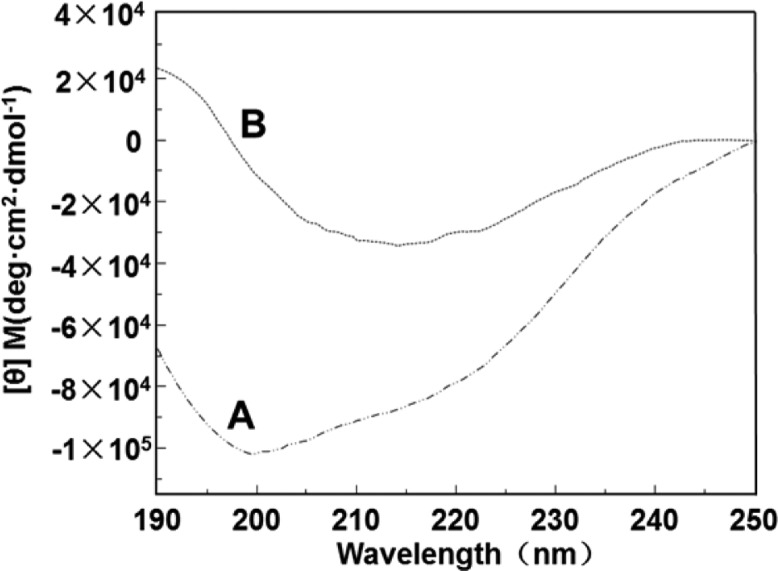
CD spectra proved the β-sheet assembly of AS through mineralization (Figure 4). AS adopts a random coil conformation according to a maximum degree of negative ellipticity at about 198 nm (Figure 4A). After mineralization, the appearance of the maximum degrees of positive ellipticity at 190 nm and the negative ellipticity and 216 nm (Figure 4B) proved the β-sheet structure of AS, indicating that AS can trigger the nucleation of HAps whereas the presence of Ca2+ and PO43– can induce β-sheet assembly of AS.
Figure 4.
CD spectra of AS and mineralized AS: (A) aqueous AS solution and (B) AS solution after mineralization for 24 h. CD spectra show that the structure of AS is transited from random coil to β-sheet after mineralization.
3.3. Cell Viability of Mineralized AS
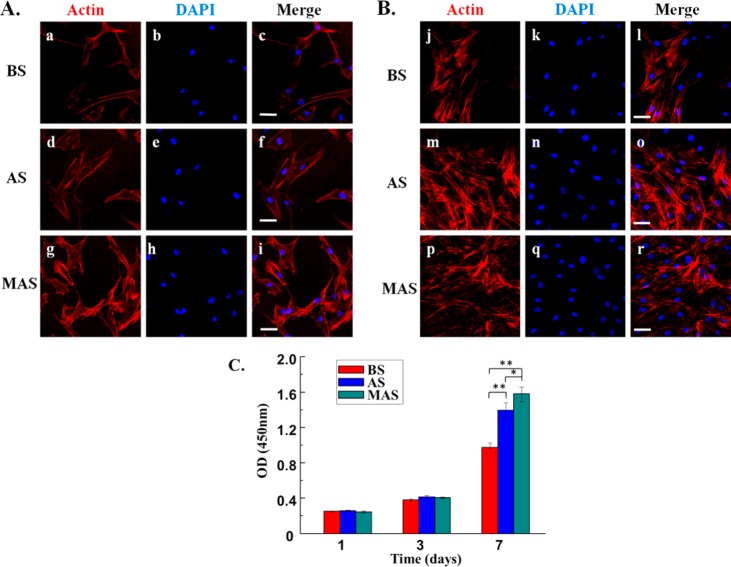
To determine the effect of the scaffolds on supporting cell growth, we seeded BMSCs on BS, AS and MAS, and then examined the morphology by immunofluorescence for 1 (Figure 5A), 3 (Figure S3), and 7 days (Figure 5B), and measured cell proliferation ability by CCK-8 assay (Figure 5C). The confocal micrographs indicated that BMSCs were of more spindle shape on BS and AS (Figure 5a,d,j,m), while most of BMSCs exhibited a more stellate-patterned phenotype on MAS (Figure 5g,p). It means cells spread widely on the MAS with distinct spread actin filaments compared to those on the AS and BS. This result illustrated that MAS could support cell adhesion and spreading. After 7 days culturing, there were plenty of BMSCs on AS and MAS, whereas fewer cells were observed on the BS (Figure 5B). Cell proliferation analysis by CCK-8 further confirmed that the proliferation rate on AS and MAS is higher than that on BS, and MAS shows higher proliferation rate than AS. Therefore, the CCK-8 assay results indicated that MAS promoted proliferation of BMSCs during the early period of cell culture, proving that mineralization of AS improves the cell viability of AS. It suggests that MAS can be used as a biocompatible biomaterial to support cell growth.
Figure 5.
Morphology and proliferation of human BMSCs on scaffolds. (A, B) Morphology of human BMSCs on BS, AS, and MAS after cultured for 1 day and 7 days; Cell skeleton staining with Actin (red); (a, d, g, j, m, p) Nuclear staining with DAPI (blue); (b, e, h, k, n, q) Merged images (c, f, i, l, o, r). The BMSCs were adherent and proliferated strongly on MAS and AS. (C) BMSCs proliferation on plate, BS, AS, and MAS. Scale bars, 50 μm; *P < 0.05, **P < 0.01. Blank means empty well without coating. BS, AS, and MAS denote well coated with B. mori sericin, A. pernyi sericin, and mineralized A. pernyi sericin, respectively.
3.4. Osteogenic Differentiation of BMSCs on Mineralized AS
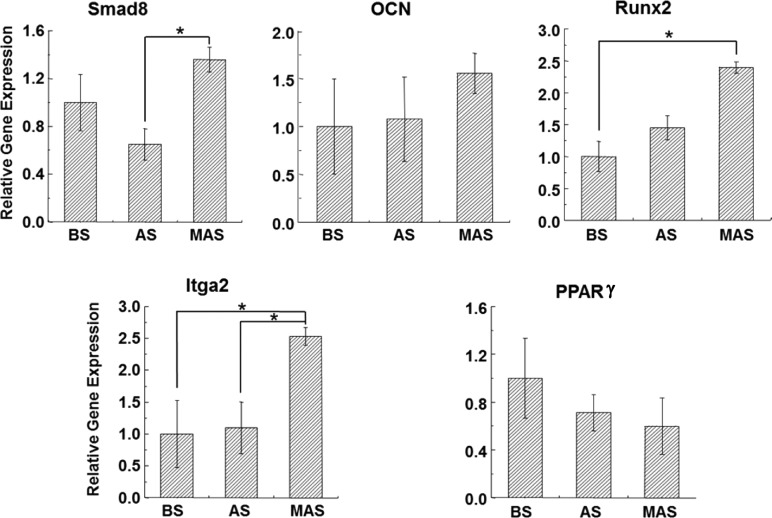
To examine the effect of MAS, AS, and BS on osteogenic differentiation of BMSCs, we examined gene expression profile of BMSCs in the osteogenic induction culture (Figure 6). The transcript levels of osteogenic marker genes including Smad8, OCN, and Runx2 were investigated. Expression of Smad8, OCN, and Runx2 was higher on MAS than that on AS and BS, suggesting MAS promoted the osteogenic differentiation of BMSCs. In addition, the expression of integrin, the major adhesion receptor that mediates cell adhesion and attachment,34 was compared among different substrates, according to its subunits, Integrin α2 (Itga2). The expression of Itga2 was remarkably up-regulated in BMSCs culture on MAS. Conversely, peroxisome proliferator-activated receptor γ (PPARγ), the adipose marker, was down-regulated when BMSCs were grown on MAS compared to that on AS and BS, indicating that BMSCs differentiation into adipogenic lineage was suppressed on MAS.
Figure 6.
Levels of mRNA for osteogenic specific genes (Smad8, OCN, and Rux2), integrin (Itga2), and adipose marker PPARγ of h-BMSCs cultured on BS, AS, and MAS in osteogenic medium for 2 weeks. The mRNA levels were quantified using real-time RT-PCR and are normalized to that of the reference gene GAPDH and compared to that of BS groups. *P < 0.05, data are presented as mean ± SD, n = 4. BS, AS and MAS mean B. mori sericin, A. pernyi sericin, and mineralized A. pernyi sericin, respectively.
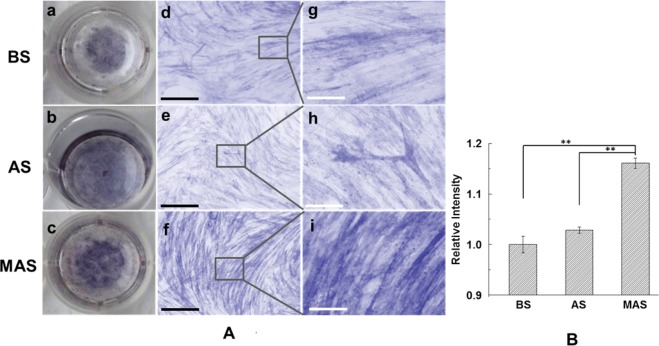
ALP staining and alizarin red staining further confirmed the real-time PCR results. Higher levels of ALP activities were also observed on MAS than on AS and BS from ALP staining images after 2 weeks of induction (Figure 7A). Quantitative analysis from alizarin red S staining shows the significant difference between MAS and AS (P < 0.01) as well as BS (P < 0.01; Figure 7B). Taken together, these data confirmed that MAS promoted the differentiation of BMSCs into osteoblasts in comparison to AS and BS.
Figure 7.
ALP staining and alizarin red staining of BMSCs cultured on BS, AS and MAS in osteogenic medium for 2 weeks: (A) ALP staining of BMSCs cultured on MAS; (B) The quantification result of alizarin red staining determined by absorbance at 405 nm; (a–c) are macroscopic view of ALP staining of BMSCs on BS, AS, and MAS, whereas (d–i) are microscopic view of ALP staining of BMSCs on (d, g) BS, (e, h) AS, and (f, i) MAS. Black scale bars = 500 μm, white scale bars = 200 μm. **P < 0.01, data are presented as mean ± SD, n = 4. BS, AS, and MAS were B. mori sericin, A. pernyi sericin, and mineralized A. pernyi sericin.
The fate of BMSCs could be directed by the compositions and structures of the substrates.35,36 In particular, the effect of HAps on osteogenic differentiation of BMSCs has been reported.37−39 It was found that HAps promoted osteogenic differentiation through early upregulation of osteopontin (OPN) and later upregulation of osteocalcin (OCN) and bone sialoprotein (BSP) in a 3D model system, relative to noncoated controls.37 Scaffolds impregnated with HAps increased in vitro bone nodule formation, as well as in vivo bone formation,38 providing an appropriate osteogenic environment for tissue engineering.39 The mineralization of silk fibroin scaffolds provides increased osteoconductive environment for BMSCs to construct tissue engineered bone to repair mandible defects in large animal.8−10 In this work, we confirmed that AS can mediate the nucleation of HAps according to Figures 2–4. The proliferation rate on AS is higher than that on BS, and MAS shows higher proliferation rate than AS (Figure 5). This might be due to their different composition. The results from Figures 6 and 7 also suggested that compared to AS and BS, MAS significantly enhanced osteogenic differentiation of BMSCs by activating integrin signaling pathway, but inhibited the adipogenic differentiation of BMSCs by repressing adipose marker gene. Therefore, we concluded that AS with incorporation of HAps promotes osteogenic differentiation of BMSCs, which provides an appropriate osteoconductive environment for BMSCs. Mineralization of AS is a useful biomimetic route to enhance the osteoconductivity conditions of AS for BMSCs. The mineralized AS would have potential application in the bone tissue engineering.
4. Conclusions
To explore the biomaterials based on silk sericin extracted from wild silkworm, A. pernyi silkworm, this study investigated the mineralization of AS and its use in promoting osteogenic differentiation of BMSCs. It was found that AS mediated the mineralization in a wet precipitation method. The nucleation of HAps was controlled by AS concentration and mineralization time as evidenced by TEM, XRD, and FTIR results. HAps were nucleated into nanoneedles according to HRTEM images. AFM and CD observation proved that AS mediated HAps nucleation while self-assembling into β-sheets, and finally mineralized AS was assembled into globule aggregation. The mineralized AS could be cast into films to support cell growth and improve the cell attachment and proliferation due to incorporation of HAps. Most importantly, we found that mineralization of AS promoted osteogenic differentiation of BMSCs, representing the first report on the osteogenic differentiation of BMSCs on silk sericin. The mineralization of A. pernyi silk sericin coupled with its biocompatibility and capability in promoting osteogenic differentiation of BMSCs proved that A. pernyi silk sericin would have potential applications in the field of bone tissue engineering. Our work will lay foundation for the use of A. pernyi silk sericin as a potential scaffold material in tissue engineering.
Acknowledgments
M.Y. acknowledges the support of National High Technology Research and Development Program 863 (2013AA102507), Zhejiang Provincial Natural Science Foundation of China (LZ12C17001), National Natural Science Foundation of China (20804037 and 21172194), and Silkworm Industry Science and Technology Innovation Team (2011R50028). Y.Y. would like to thank the financial support from Projects of Zhejiang Provincial Science and Technology Plans (2012C12910). C.B.M. would like to thank the financial support from National Institutes of Health (EB015190 and HL092526), National Science Foundation (CBET-0854465, CMMI-1234957, CBET-0854414, and DMR-0847758), Department of Defense Peer Reviewed Medical Research Program (W81XWH-12-1-0384), Oklahoma Center for Adult Stem Cell Research (434003), and Oklahoma Center for the Advancement of Science and Technology (HR11-006).
Supporting Information Available
Figure S1, XRD patterns of samples; Figure S2, FTIR spectra of samples; Figure S3, EDX spectra of samples; Table S1: The amino acid composition analysis of AS. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
† These authors contributed equally to this work (M.Y., Y.S., and C.Z.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Denissen H.; De Groot K.; Makkes P. C.; Van den Hooff A.; Klopper P. Tissue response to dense apatite implants in rats. J. Biomed. Mater. Res. 1980, 146713–721. [DOI] [PubMed] [Google Scholar]
- Rai R. K.; Sinha N. Dehydration-induced structural changes in the collagen–hydroxyapatite interface in bone by high-resolution solid-state NMR spectroscopy. J. Phys. Chem. C 2011, 1152914219–14227. [Google Scholar]
- Shchukin D. G.; Sukhorukov G. B.; Möhwald H. Biomimetic fabrication of nanoengineered hydroxyapatite/polyelectrolyte composite shell. Chem. Mater. 2003, 15203947–3950. [Google Scholar]
- Rodrigues C.; Serricella P.; Linhares A.; Guerdes R.; Borojevic R.; Rossi M.; Duarte M.; Farina M. Characterization of a bovine collagen–hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials 2003, 24274987–4997. [DOI] [PubMed] [Google Scholar]
- Biswas A.; Bayer I. S.; Zhao H.; Wang T.; Watanabe F.; Biris A. S. Design and synthesis of biomimetic multicomponent all-bone-minerals bionanocomposites. Biomacromolecules 2010, 11102545–2549. [DOI] [PubMed] [Google Scholar]
- Liu L.; Liu J.; Wang M.; Min S.; Cai Y.; Zhu L.; Yao J. Preparation and characterization of nano-hydroxyapatite/silk fibroin porous scaffolds. J. Biomater. Sci. 2008, 193325–338. [DOI] [PubMed] [Google Scholar]
- Lin F.; Li Y.; Jin J.; Cai Y.; Wei K.; Yao J. Deposition behavior and properties of silk fibroin scaffolds soaked in simulated body fluid. Mater. Chem. Phys. 2008, 111192–97. [Google Scholar]
- He T.; Abbineni G.; Cao B.; Mao C. B. Nanofibrous bio-inorganic hybrid structures formed through self-assembly and oriented mineralization of genetically engineered phage nanofibers. Small 2010, 6, 2230–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.; Cao B.; Mao C. B. Bacteriophage bundles with pre-aligned Ca2+ initiate the oriented nucleation and growth of hydroxyapatite. Chem. Mater. 2010, 22, 3630–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.; Cao B.; George A.; Mao C. B. Controlled self-assembly and mineralization of genetically modifiable nanofibers driven by beta-structure formation. Biomacromolecules 2011, 12, 2193–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P.; Sahoo S.; Ng K. S.; Chen K.; Toh S. L.; Goh J. C. H. Enhanced osteoinductivity and osteoconductivity through hydroxyapatite coating of silk-based tissue-engineered ligament scaffold. J. Biomed. Mater. Res. 2013, A1012555–566. [DOI] [PubMed] [Google Scholar]
- He P.; Ng K. S.; Toh S. L.; Goh J. C. H. In vitro ligament–bone interface regeneration using a trilineage coculture system on a hybrid silk scaffold. Biomacromolecules 2012, 1392692–2703. [DOI] [PubMed] [Google Scholar]
- Wang G.; Yang H.; Li M.; Lu S.; Chen X.; Cai X. The use of silk fibroin/hydroxyapatite composite co-cultured with rabbit bone-marrow stromal cells in the healing of a segmental bone defect. J. Bone Joint Surg. 2010, 922320–325. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20291–100. [DOI] [PubMed] [Google Scholar]
- Zhaorigetu S.; Sasaki M.; Kato N. Consumption of sericin suppresses colon oxidative stress and aberrant crypt foci in 1,2-dimethylhydrazine-treated rats by colon undigested sericin. J. Nutr. Sci. Vitaminol. 2007, 533297–300. [DOI] [PubMed] [Google Scholar]
- Kundu S. C.; Dash B. C.; Dash R.; Kaplan D. L. Natural protective glue protein, sericin bioengineered by silkworms: potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 3310998–1012. [Google Scholar]
- Kaur J.; Rajkhowa R.; Tsuzuki T.; Millington K.; Zhang J.; Wang X.-G. Photo-protection by silk cocoons. Biomacromolecules 2013, 14103660–3667. [DOI] [PubMed] [Google Scholar]
- Nishida A.; Yamada M.; Kanazawa T.; Takashima Y.; Ouchi K.; Okada H. Sustained-release of protein from biodegradable sericin film, gel and sponge. Int. J. Pharm. 2011, 407144–52. [DOI] [PubMed] [Google Scholar]
- Cai Y.; Jin J.; Mei D.; Xia N.; Yao J. Effect of silk sericin on assembly of hydroxyapatite nanocrystals into enamel prism-like structure. J. Mater. Chem. 2009, 19325751–5758. [Google Scholar]
- Takeuchi A.; Ohtsuki C.; Miyazaki T.; Kamitakahara M.; Ogata S.-i.; Yamazaki M.; Furutani Y.; Kinoshita H.; Tanihara M. Heterogeneous nucleation of hydroxyapatite on protein: structural effect of silk sericin. J. R. Soc., Interface 2005, 24373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X.; Cui F.; Wang X.; Zhang M.; Zhang W. Silk fibroin regulated mineralization of hydroxyapatite nanocrystals. J. Cryst. Growth 2004, 2701197–202. [Google Scholar]
- Cheng C.; Shao Z.; Vollrath F. Silk fibroin regulated crystallization of calcium carbonate. Adv. Funct. Mater. 2008, 18152172–2179. [Google Scholar]
- Takeuchi A.; Ohtsuki C.; Miyazaki T.; Tanaka H.; Yamazaki M.; Tanihara M. Deposition of bone-like apatite on silk fiber in a solution that mimics extracellular fluid. J. Biomed. Mater. Res. 2003, A652283–289. [DOI] [PubMed] [Google Scholar]
- Padamwar M. N.; Pawar A. P.; Daithankar A. V.; Mahadik K. Silk sericin as a moisturizer: an in vivo study. J. Cosm. Derm. 2005, 44250–257. [DOI] [PubMed] [Google Scholar]
- Zou X. H.; Cai H. X.; Yin Z.; Chen X.; Jiang Y. Z.; Hu H.; Ouyang H. W. A novel strategy incorporated the power of mesenchymal stem cells to allografts for segmental bone tissue engineering. Cell Transplant. 2009, 184433–441. [DOI] [PubMed] [Google Scholar]
- Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-δδC) method. Methods 2001, 254402–408. [DOI] [PubMed] [Google Scholar]
- Ivey K. N.; Muth A.; Amold J.; King F. W.; Yeh R. F.; Fish J. E.; Hsiao E. C.; Schwartz R. J.; Conklin B. R.; Bernstein H. S.; Srivastava D. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell 2008, 23219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y. M.; Stuelten C. H.; Kilts T.; Wadhwa S.; Iozzo R. V.; Robey P. G.; Chen X. D.; Young M. F. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J. Biol. Chem. 2005, 2803430481–30489. [DOI] [PubMed] [Google Scholar]
- Yang M.; He W.; Shuai Y.; Min S.; Zhu L. Nucleation of hydroxyapatite crystals by self-assembled Bombyx mori silk fibroin. J. Polym. Sci. 2013, 519742–748. [Google Scholar]
- Li Z.; Lam W.; Yang C.; Xu B.; Ni G.; Abbah S.; Cheung K.; Luk K.; Lu W. Chemical composition, crystal size, and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials 2007, 2871452–1460. [DOI] [PubMed] [Google Scholar]
- Cai Y.; Jin J.; Mei D.; Xia N.; Yao J. Effect of silk sericin on assembly of hydroxyapatite nanocrystals into enamel prism-like structure. J. Mater. Chem. 2009, 19325751. [Google Scholar]
- Khire T. S.; Kundu J.; Kundu S. C.; Yadavalli V. K. The fractal self-assembly of the silk protein sericin. Soft Matter 2010, 692066–2071. [Google Scholar]
- Russel W. Brownian motion of small particles suspended in liquids. Ann. Rev. Fluid Mech. 1981, 131425–455. [Google Scholar]
- Ruoslahti E.; Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science 1987, 2384826491–497. [DOI] [PubMed] [Google Scholar]
- Wang J.; Wang L.; Li X.; Mao C. B. Virus activated artificial ECM induces the osteogenic differentiation of mesenchymal stem cells without osteogenic supplements. Sci. Rep. 2013, 3, 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H.; Cao B.; Zhen Z.; Laxmi A.; Li D.; Liu S.; Mao C. B. Controlled growth and differentiation of mesenchymal stem cells on grooved films assembled from monodisperse biological nanofibers with genetically tunable surface chemistries. Biomaterials 2011, 32, 4744–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y. F.; Dunn J. C.; Wu B. M. In vitro response of MC3T3 E1 preosteoblasts within three-dimensional apatite-coated PLGA scaffolds. J. Biomed. Mater. Res. 2005, B75181–90. [DOI] [PubMed] [Google Scholar]
- Bourgeois B.; Laboux O.; Obadia L.; Gauthier O.; Betti E.; Aguado E.; Daculsi G.; Bouler J. M. Calcium-deficient apatite: A first in vivo study concerning bone ingrowth. J. Biomed. Mater. Res. 2003, A653402–408. [DOI] [PubMed] [Google Scholar]
- Rosa A. L.; Beloti M. M.; Van Noort R.; Hatton P. V.; Devlin A. J. Surface topography of hydroxyapatite affects ROS17/2.8 cells response. Pesqui. Odontol. Bras. 2002, 163209–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.