Abstract
Ruthenium(II) tris(2-pyridylmethyl)amine (TPA) is an effective caging group for nitriles that provides high levels of control over the enzyme activity with light. Two caged nitriles were prepared, [Ru(TPA)(MeCN)2](PF6)2 (1) and [Ru(TPA)(3)2](PF6)2 (2), where 3 is the cathepsin K inhibitor Cbz-Leu-NHCH2CN, and characterized by various spectroscopic techniques and mass spectrometry. Both 1 and 2 show the release of a single nitrile within 20 min of irradiation with 365 nm light. Complex 2 acts as a potent, photoactivated inhibitor of human cathepsin K. IC50 values were determined for 2 and 3. Enzyme inhibition for 2 was enhanced by a factor of 89 upon exposure to light, with IC50 values of 63 nM (light) and 5.6 μM (dark).
Short abstract
The photochemical release of nitriles from the caging fragment ruthenium tris(2-pyridylmethyl)amine (TPA) was studied. Caged complexes of the general formula [Ru(TPA)(RCN)2]2+ are stable in the dark but release a single nitrile upon irradiation with 365 nm light. Photoactivated inhibition of cathepsin K was demonstrated with a caged inhibitor complex.
Caging molecules with photolabile protecting groups has revolutionized our ability to interrogate the spatial and temporal aspects of biological activity.1−4 The caging approach involves the bonding of biologically active molecules to organic or metal-based protecting groups5 that are cleaved with light. To date, the most widely used inorganic protecting group for photocaging has been Ru(bpy)2 (bpy = 2,2′-bipyridine). Pioneering work demonstrated that Ru(bpy)2 can be used to cage neurotransmitters;6,7 later examples were applied to anticancer agents8 and enzyme inhibitors.9 By and large, the development of ruthenium-based caging groups has focused on planar, chelating heteroaromatic ligands similar to bpy.10 In this Communication, we report that ruthenium(II) tris(2-pyridylmethyl)amine (TPA), distinct from the established Ru(bpy)2 class, is an effective caging group for nitriles that provides high levels of control over the enzyme activity with light.
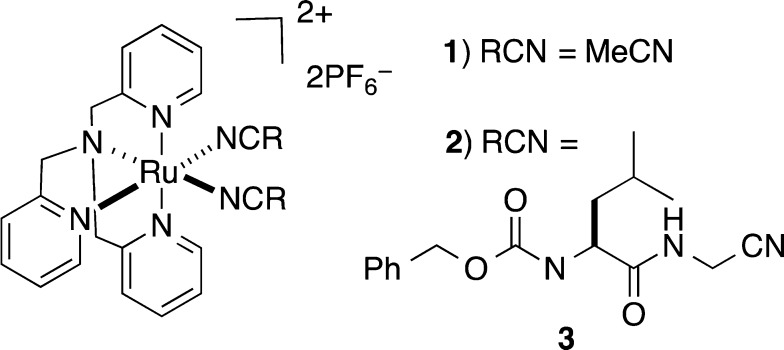
Two caged nitriles of the general formula [Ru(TPA)(RCN)2](PF6)2 were prepared for this study (Figure 1). The complex [Ru(TPA)(MeCN)2](PF6)2 (1) contains two caged MeCN ligands, whereas the complex [Ru(TPA)(3)2](PF6)2 (2) contains 2 equiv of the cysteine protease inhibitor Cbz-Leu-NHCH2CN (3), a potent and selective inhibitor of human cathepsin K.11 Complex 1 was prepared as a yellow solid by heating [Ru(TPA)Cl(Me2SO)]Cl12,13 in 1:1 H2O/MeCN, followed by precipitation with NH4PF6. Complex 2 was prepared by heating [Ru(TPA)(H2O)2](OTf)214 in the presence of 5 equiv of the protease inhibitor 3 in EtOH. Concentration, aqueous workup, and precipitation as a hexafluorophosphate salt from 1:1 H2O/MeOH furnished 2 as a pale-yellow solid.
Figure 1.
Structures of caged nitriles 1 and 2.
Complexes 1 and 2 were characterized by a suite of methods, including UV–vis, NMR and IR spectroscopies, and electrospray ionization mass spectrometry. UV–vis spectra for 1 and 2 show maxima at 380 nm (ε = 11200 M–1 cm–1) and 375 nm (ε = 12000 M–1 cm–1), respectively (Figure S1 in the Supporting Information, SI). 1H NMR spectroscopic analysis of 1 indicated the presence of two distinct MeCN ligands, with singlets at 2.88 and 2.47 ppm, consistent with the expected structure with one MeCN ligand trans to the basic nitrogen donor of TPA and one in the cis position (Figure S2 in the SI). Likewise, the NMR spectrum of 2 showed two multiplets, at approximately 4.9 and 4.5 ppm, assigned to the α-CN methylene unit of ligand 3, which were separated by approximately 0.5 ppm (Figure S3 in the SI). IR spectra for 1 and 2 (Figures S4 and S5 in the SI) showed stretches for νCN at 2276 and 2269 cm–1, respectively, consistent with nitrile binding to ruthenium(II).15 Mass spectra of 1 and 2 showed prominent ion clusters with major peaks at m/z 619.1 and 1143, along with suitable isotopic distributions, which match those expected for the cations [Ru(TPA)(MeCN)2](PF6)+ and [Ru(TPA)(3)2](PF6)+ (Figures S6 and S7 in the SI).
Complex 1 was characterized further by X-ray crystallography. Diffusion of Et2O into a solution of 1 in MeCN furnished small yellow blocks of 1 suitable for X-ray crystallographic analysis. Select data for 1 are described in Figure 2; full tables can be found in the SI. The Ru1–N1 and Ru1–N6 bond distances are identical within error. The structural parameters for 1 are similar to those reported recently for [Ru(TPA)(MeCN)2](SbF6)2.12
Figure 2.
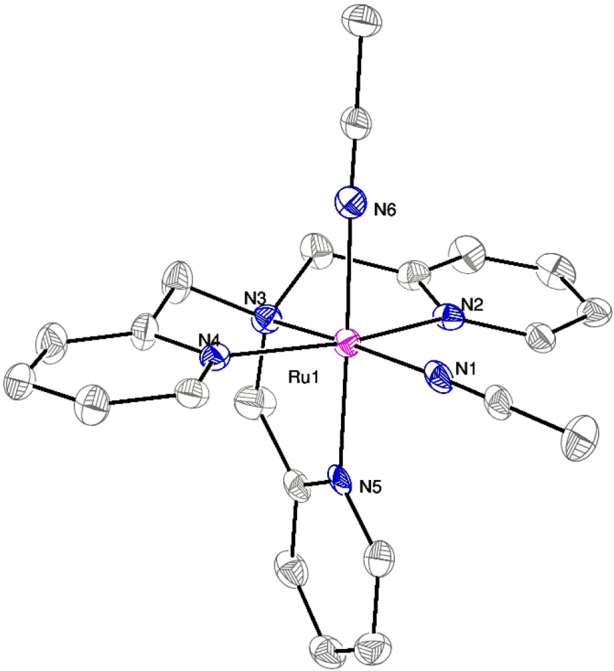
ORTEP diagram of the dication [Ru(TPA)(MeCN)2]2+. Thermal ellipsoids are shown at 50% probability. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (deg): Ru–N1, 2.031(5); Ru–N2, 2.062(4); Ru–N3, 2.053(4); Ru–N4, 2.071(4); Ru–N5, 2.056(4); Ru–N6, 2.037(5); N1–Ru–N6, 88.8(2).
Complexes 1 and 2 show the release of a single nitrile upon relatively short irradiation times with 365 nm light.16 A decrease in the absorption peaks at 370 and 365 nm for 1 and 2, respectively, tentatively assigned as metal-to-ligand charge-transfer bands,13 is observed within 10–15 min of irradiation with λ > 345 nm in H2O solutions (2% acetone), with the concomitant appearance of a new band at 397 and 390 nm, respectively (Figure 3). The quantum yields for decomposition of 1 and 2 are 0.012(1) and 0.011(1), respectively (λirr = 350 nm). When the same photochemical reactions are followed in deuterated solvents by 1H NMR spectroscopy, data indicate that nitrile-based ligands are released from 1 and 2. However, only one of two possible nitriles is exchanged with the solvent. The intensities of downfield resonances, assigned to methyl and methylene protons α to the nitrile of 1 and 2, decrease as the peaks associated with free MeCN (2.05 ppm) and free 3 (4.16 ppm) increase (Figures S8 and S9 in the SI). Released nitriles are assigned as cis to the basic nitrogen of the TPA ligand, based on COSY and NOESY 1H NMR data (for analysis, see Figures S10 and S11 in the SI). This structural assignment is further supported by the fact that downfield shifts for resonances of α-CN protons in 1 and 2 would be expected because of shielding by two cis-pyridine rings of the TPA ligand, whose π systems are orthogonal to the Ru–N vector of the nitrile that is released upon photolysis.
Figure 3.
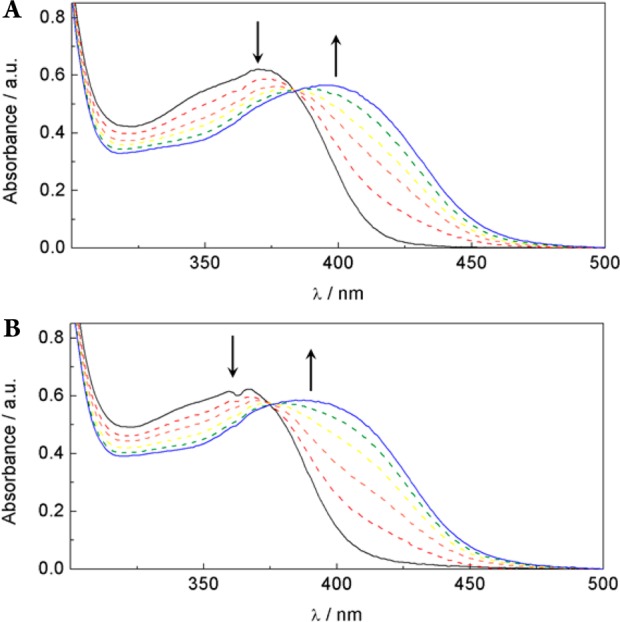
Changes in the electronic absorption spectra upon irradiation with λ > 350 nm in H2O (2% acetone) of 1 (A) for 0, 1, 2, 3, 5, and 10 min and 2 (B) for 0, 1, 3, 7, 10, and 15 min.
In addition to their photochemical release of nitriles, complexes 1 and 2 show excellent stability in solution in the dark. The decomposition rates for 1 and 2 in dimethyl sulfoxide (DMSO) and phosphate-buffered saline were determined spectrophotometrically. The rate constants were calculated from linear ln A versus t plots and ranged from 1.1(3) × 10–8 to 6(2) × 10–9 s–1 (Table S1 in the SI). These values correspond to half-lives of >730 days in solution, confirming that 1 and 2 are stable toward the release of their bound nitrile ligands in aqueous media.
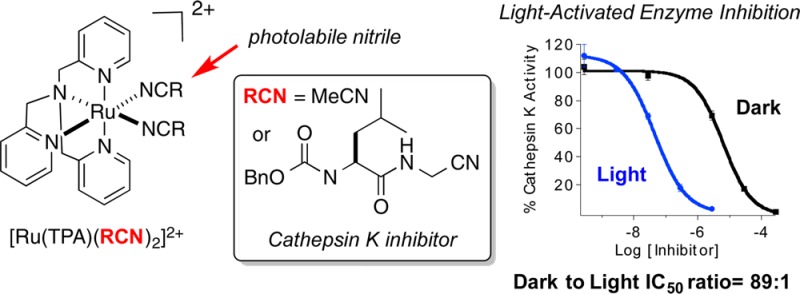
Complex 2 acts as a potent, photoactivated inhibitor of human cathepsin K. IC50 values were determined for 2 and 3 under dark conditions and upon irradiation with 365 nm light (Figure 4). Enzyme inhibition for 2 was enhanced by a factor of 89 upon exposure to light, with IC50 values of 63 nM and 5.6 μM, respectively. In contrast, inhibition by the free inhibitor 3 was identical within error under light and dark conditions, 27 nM and 34 nM, respectively, confirming that irradiation has no effect on inhibition under the assay conditions. Therefore, complex 2 is nearly as potent as 3 under light conditions. Control experiments with 1 showed no inhibition of cathepsin K under light and dark conditions at 500 μM, the highest concentration surveyed, confirming that neither the ruthenium complex nor its photochemical byproduct is responsible for the inhibition observed for 2 upon irradiation. Taken together, these data confirm that Ru(TPA) is an effective caging group.
Figure 4.
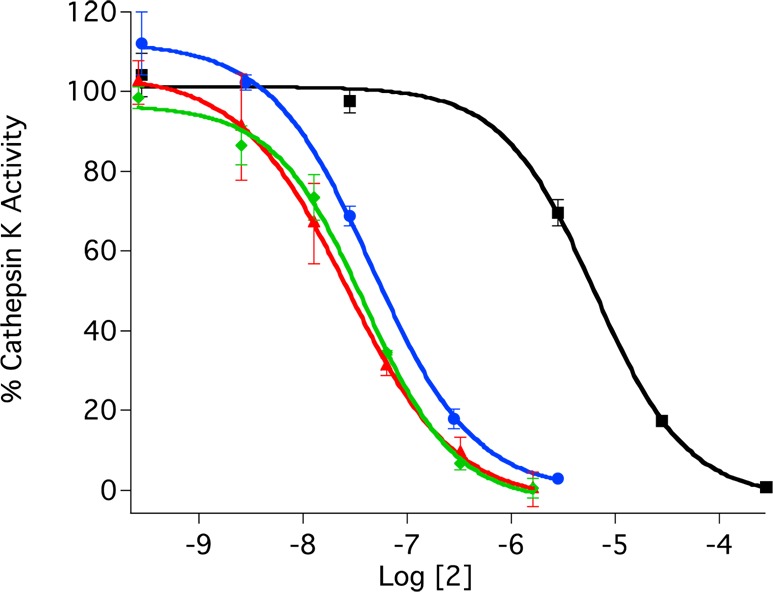
IC50 curves for ruthenium-caged inhibitor 2 (blue with irradiation and black without) and uncaged inhibitor 3 (red with irradiation and green without) against human cathepsin K. The enzyme activity was determined with the fluorogenic substrate Z-Gly-Pro-Arg-AMC and is expressed as a percentage, with 100% equal to the activity in the absence of inhibitor. Individual data points are the average of three wells, and the error bars are standard deviations. Data are representative of three independent experiments. Conditions: 0.4 M acetate buffer, pH 5.5, 1% DMSO, [cathepsin K] = 2 nM, [Z-Gly-Pro-Arg-AMC] = 100 μM, DTT = 8 mM, and 15 min of irradiation with a 365 nm light source (8 W).
In conclusion, this study has established Ru(TPA) as a new caging group for bioactive nitriles. Efficient photoactivated enzyme inhibition against human cathepsin K was demonstrated with the caged inhibitor complex 2. Further studies to explore this new class of caging group are currently underway in our laboratory. Efforts are being directed toward understanding the photochemistry of nitrile release using steady-state and time-resolved techniques, lowering the energy of light required for efficient ligand exchange, and exploring biological applications.
Acknowledgments
We gratefully acknowledge the National Institutes of Health (Grant EB 016072) and Wayne State University for their generous support of this research.
Supporting Information Available
X-ray crystallographic data in CIF format, experimental procedure for the preparation of 1 and 2, characterization data for 1 and 2, and experimental procedures for photochemical and enzyme inhibition studies. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Lee H.-M.; Larson D. R.; Lawrence D. S. ACS Chem. Biol. 2009, 4, 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klán P.; Šolomek T.; Bochet C. G.; Blanc A.; Givens R.; Rubina M.; Popik V.; Kostikov A.; Wirz J. Chem. Rev. 2012, 113, 119–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiters A. ChemBioChem 2010, 11, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesienski K. L.; Franz K. J. Angew. Chem., Int. Ed. 2011, 50, 814–824. [DOI] [PubMed] [Google Scholar]
- Ford P. C. Coord. Chem. Rev. 1982, 44, 61–82. [Google Scholar]
- Zayat L.; Calero C.; Albores P.; Baraldo L.; Etchenique R. J. Am. Chem. Soc. 2003, 125, 882–883. [DOI] [PubMed] [Google Scholar]
- Filevich O.; Etchenique R. Ruthenium Prop., Prod. Appl. 2011, 269–291. [Google Scholar]
- Garner R. N.; Gallucci J. C.; Dunbar K. R.; Turro C. Inorg. Chem. 2011, 50, 9213–9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Respondek T.; Garner R. N.; Herroon M. K.; Podgorski I.; Turro C.; Kodanko J. J. J. Am. Chem. Soc. 2011, 133, 17164–17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambellone M. A.; David A.; Garner R. N.; Dunbar K. R.; Turro C. J. Am. Chem. Soc. 2013, 135, 11274–282. [DOI] [PubMed] [Google Scholar]
- Altmann E.; Aichholz R.; Betschart C.; Buhl T.; Green J.; Lattmann R.; Missbach M. Bioorg. Med. Chem. Lett. 2006, 16, 2549–2554. [DOI] [PubMed] [Google Scholar]
- The structural characerization of [Ru(TPA)(MeCN)2](SbF6)2 was reported while our work with compound 1 was in progress. See:Whiteoak C. J.; Nobbs J. D.; Kiryushchenkov E.; Pagano S.; White A. J. P.; Britovsek G. J. P. Inorg. Chem. 2013, 52, 7000–7009. [DOI] [PubMed] [Google Scholar]
- Kojima T.; Amano T.; Ishii Y.; Ohba M.; Okaue Y.; Matsuda Y. Inorg. Chem. 1998, 37, 4076–4085. [DOI] [PubMed] [Google Scholar]
- Radaram B.; Ivie J. A.; Singh W. M.; Grudzien R. M.; Reibenspies J. H.; Webster C. E.; Zhao X. Inorg. Chem. 2011, 50, 10564–10571. [DOI] [PubMed] [Google Scholar]
- Cruz A. J.; Kirgan R.; Siam K.; Heiland P.; Rillema D. P. Inorg. Chim. Acta 2010, 363, 2496–2505. [Google Scholar]
- Similar properties were recently reported for DMSO complexes of Ru(TPA). See:Weisser F.; Hohloch S.; Plebst S.; Schweinfurth D.; Sarkar B. Chem.—Eur. J. 2014, 20, 781–793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.