Abstract
Spermines are naturally abundant polyamines that partially condense nucleic acids and exhibit the proton-sponge effect in an acidic environment. However, spermines show a limited efficiency for transfecting nucleic acids because of their low molecular weight. Therefore, spermines need to be modified to be used as nonviral vectors for nucleic acids. Here, we synthesized linear bisspermine as well as a linear and dendritic tetraspermine with different molecular architectures. These oligospermines were self-assembled into polyplexes with siRNA. The structure–activity relationship of the oligospermines was evaluated in terms of their efficiency for delivering siRNA into a nonsmall cell lung carcinoma cell line. Oligospermines displayed minimal cytotoxicity but efficient siRNA condensation and showed better stability against polyanions than polyethylenimine. The morphology of the polyplexes was strongly affected by the oligospermine architecture. Linear tetraspermine/siRNA polyplexes showed the best gene-silencing efficiency among the oligospermines tested at both the mRNA and protein expression levels, indicating the most favorable structure for siRNA delivery.
Introduction
RNA interference (RNAi) is a post-transcriptional gene-silencing mechanism (PTGS) that occurs naturally in the cell in a sequence-specific manner to break down double-stranded RNA (dsRNA) and to regulate RNA expression.1 RNAi-based therapeutics have rapidly progressed from basic research to clinical trials. In 1998, RNAi was discovered in Caenorhabditis elegans worms by Fire and Mello, for which they received the Nobel Prize in Physiology and Medicine in 2006.2 Small interfering RNA (siRNA) is an intermediate in the RNAi process and comprises double-stranded RNA of 21–25 nucleotides in length. Synthetic siRNA can be used to achieve RNAi and to downregulate overexpressed genes.3,4 In 2001, siRNA was reported to induce RNAi in mammalian cells.5 At present, only a few human clinical trials for siRNA therapeutics are ongoing; among which, two therapeutics are targeting the lung (i.e., ALN-RSV01 and ExcellairTM).6
The primary challenge of siRNA therapeutics, however, is the hurdle of intracellular delivery. siRNA cannot cross a biological membrane because it is a hydrophilic, negatively charged macromolecule and is highly prone to nuclease degradation.4 Viral vectors achieve high transduction but are associated with many safety problems at the clinical level such as immune responses and carcinogensis.7 Therefore, safe and effective nonviral siRNA carriers are required for pulmonary delivery of siRNA.8
Cationic polymers interact with negatively charged oligonucleoutides via charge complexation to form polyelectrolyte complexes.9,10 Endogenous spermines (SPE) are safe, naturally occurring, small, linear tetraamines with two primary amines and two secondary amines. Spermines aid in packaging cellular DNA into a compact state, which is essential in cell growth processes in eukaryotic cells.11−15 The polyamine structure is required for stable DNA binding. The interaction between a single cationic amine and anionic phosphate groups of nucleic acids is relatively week and is further weakened by competition of salt binding under biological conditions.14 Exogenous spermine poorly condenses and can transfect nucleic acids into cells, which could be due to its low molecular weight (∼200 Da).16,17 In addition, spermines yield limited endosomal escape despite their good proton-buffering capacity.17 It is hypothesized that the maximum interaction of siRNA with cations consists of four carbon bridges. However, the low molecular weight of spermine limits its siRNA complexation ability.18 Therefore, it was necessary to modify the spermine units to increase their molecular weight, which in turn may lead to increased buffering capacity and enhanced endosomal escape and therefore enhanced transfection efficiency.16,19 One way of modifying spermine is via polymerization of spermine units. This suggests that polymerized spermines could be capable of condensing siRNA and of disassembling at the target site.20 Polyspermines have shown a high buffering capacity.21 Many studies have described the linkage of spermines through their amino groups by different cleavable linkers such as disulfide bonds or esters.16,21,22 When polyspermines are degraded to release spermine monomers, sometimes fragments of the linker are still attached to spermine monomers, which affects their transfection properties.16,21 Very recently, Du et al. compared three polymerized spermines and showed that linkage structures play an important role in the activity of the polyspermine-based nucleic acid carriers.10
Moreover, spermine polymerization allows for multistep intracellular degradation of a biocompatible polymeric platform.20 Several groups have studied spermine-based carriers for DNA,16,23,24 siRNA,16,25−28 and short RNA delivery,29,30 some of which specifically target lung cancer.29−31 Spermines have been incorporated in many delivery systems such as lipoplexes,26,32 conjugates,25 and nanoparticles33 for siRNA delivery to enhance transfection efficiency. Vijayanathan et al. synthesized a series of spermine homologues with different methylene chain lengths separating the secondary amino groups of the polyamines. The lower homologues, which contained shorter methylene chains, were more efficacious in DNA condensation than the higher analogues that contained longer methylene chains. These results showed the importance of the regiochemical distribution of the positive charge in the polyamines presented by the varying distance of methylene spacing, which affected the polyamine’s ability to provoke structural changes in the DNA and hence strongly affected the DNA condensation and size of the DNA complexes.34 Different structures of spermine oligopolymers were studied, for example, when spermine was used as surface groups of a dendron structure to target human breast carcinoma cells (MDA-MB-231) and murine myoblast cells (C2C12). Spermine-decorated dendrons were able to transfect DNA into cells only in the presence of chloroquine, which enables endosomol escape. Because blank spermine is completely protonated at physiological pH, it is possible that these dendritic structures have only a limited proton-sponge effect. It was concluded that dendritic spermine derivatives act more similar to polylysine and not like proton-sponge polymers such as polyamidoamine (PAMAM) or polyethylenimine (PEI).17
In this study, spermine units were polymerized to synthesize different chemical structures of oligospermines described as linear bisspermine, linear tetraspermine, and dendritic tetraspermine. These cationic polymers were used to condense siRNA molecules in the form of polyplexes. Oligospermine/siRNA polyplexes were characterized and evaluated as nonviral carriers for condensation, stability, and transfection of siRNA and gene knockdown in H1299 human nonsmall cell lung carcinoma cells. The aim of this study was to identify a suitable oligospermine architecture for siRNA delivery.
Experimental Section
Materials
Linear bisspermine (MW 1299.40), linear tetraspermine (MW 2581.82), and dendritic tetratspermine (MW 2625.87) were synthesized as described below. Lipofectamine 2000 (LF), SYBR Gold dye, and (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Life Technologies (Grand Island, NY). Polyethylenimine (PEI, MW 5 kDa) was obtained from BASF (Lupasol, Cologne, Germany). Dicer substrate double-stranded siRNA (DsiRNA) targeting the firefly luciferase gene (FLUC siRNA, 25/27-mer), human glyceraldehyde 3-phosphate dehydrogenase (hGAPDH) gene, and nonspecific control (siNegCon) DsiRNA as well as Alexa Fluor-488 labeled siRNA were purchased from Integrated DNA Technologies (IDT, Coralville, IA). RPMI-1640 medium (1×) with 2.05 mM l-glutamine, HyClone trypsin, penicillin/streptomycin, 4-(2(hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES), and SurePrep TrueTotal RNA purification kits were purchased from Thermo Fisher Scientific (Waltham, MA). Dulbecco’s phosphate-buffered saline (PBS), heat-inactivated fetal bovine serum (FBS), d-(+)-glucose, sodium bicarbonate, sodium pyruvate, 2-mercaptoethanol, dimethyl sulfoxide Hybri-Max (DMSO, ≥99.7%), ethylenediaminetetraacetic acid (EDTA, 99.4–100.06%), trypan blue (0.4%, sterile filtered), and luciferin solution were purchased from Sigma-Aldrich (St. Louis, MO). Hs_GAPDH_primers and Hs_β-actin-primers were purchased from Qiagen (Valencia, CA). Brilliant III SYBR green qRT-PCR master mix was purchased from Agilent (Santa Clara, CA), and DNase I reaction buffer and DNase/RNase-free water were purchased from Zymo Research (Irvine, CA).
Synthesis of Oligospermines
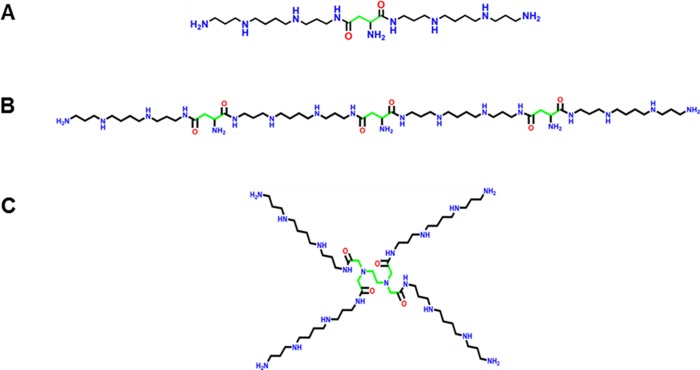
Three different polycatonic-based oligospermines, namely, linear bisspermines, linear tetraspermines, and dendritic tetraspermines (Figure 1), were successfully synthesized as described previously.35 Briefly, the process involved (1) the synthesis of MPBBSP (monoprotected bis-boc spermine) monomer I, (2) the synthesis of the reactive intermediates of the 2- and 4-arm linkers, and (3) the conjugation of monomer I to the linkers to obtain the respective protected oligospermines. Deprotection of the boc groups yielded oligospermines as salts of trifluoroacetic acid that were used for biological characterization. All compounds synthesized were characterized using NMR, MS/MALDI, and HPLC to confirm their identity and purity.
Figure 1.
Chemical structures of (A) linear bisspermine, (B) linear tetraspermine, and (C) dendritic tetraspermine.
Preparation of Oligospermines–siRNA Polyplexes
The ratio between the polymer amine groups (N) and the siRNA phosphate groups (P) in a polyplex is defined as the N/P ratio. The N/P ratio obtained after complexation was calculated on the basis of the molecular weight, the number of protonatable units of the oligospermines, and the number of base pairs in the siRNA duplexes. Polymer stock solutions (1 mg/mL) were diluted with a 5% glucose solution, and siRNA stock solutions (100 μM) were diluted with RNase-free water. All solutions used were filtered with 0.2 μm pore size syringe filters (Fisher Scientific). The amount of oligospermines required to prepare polyplexes with a specific amount of siRNA and at a specific N/P ratio was calculated as follows
| 1 |
where m is the mass of the polymer needed and n is the amount of siRNA used per well. The total number of nucleotides in DsiRNA is 52. N/P is the ratio between polymer amine groups and siRNA phosphate groups.
Equal volumes of polymer and siRNA solutions were mixed to obtain the appropriate N/P ratio, vortexed for 30 s, and incubated at room temperature for 20 min.
Size and Zeta (ζ)-Potential Analysis
Sizes of the polyplexes were evaluated by dynamic light-scattering (DLS) analysis. Polyplexes were prepared with 40 pmol of FLUC siRNA at N/P ratios of 2 and 10, as described above, in a total volume of 350 μL. Measurements were performed with a Zetasizer Nano ZS (Malvern Instruments Inc., Westborough, MA) in quadruplicate at 25 °C using disposable cuvettes (low volume 70 μL, Brookhaven Instruments Corporation, Holtsville, NY) for size measurements. Measurements were set up at a 173° backscatter angle with 15 runs per measurement. For data analysis, the viscosity (0.88 mPa s) and the refractive index (1.33) of water at 25 °C were used. Results are given as Z average in nanometers ± standard deviation. Polyplexes were then diluted to 700 μL with a 5% glucose solution before ζ-potential measurements were performed in disposable capillary cells (Malvern Instruments Inc.). Results are given in millivolts ± standard deviation.
Size and Morphology: Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM)
For transmission electron microscopy (TEM), polyplexes were prepared as described above at a N/P ratio of 2 with 40 pmol of FLUC siRNA in a total volume of 20 μL. A drop of particle suspension was dispensed on a copper-coated grid (200 mesh) and left to dry before imaging with a transmission electron microscope (JEOL 2010 TEM). Several representative images were taken for each sample at different magnifications. Atomic force microscopy (AFM) was performed using a Pico LE atomic force microscope (Molecular Imaging, Agilent Technologies). Polyplex suspensions were freshly prepared as described above. A drop was incubated on a freshly cleaved mica surface for 5 min and rinsed with deionized water to remove excess liquid. Samples were allowed to dry at room temperature and imaged in contact mode using a Si3Ni4 V-shaped cantilever.
siRNA Condensation Efficiency and Stability against Polyanions under Neutral and Acidic Conditions: SYBR Gold Dye Binding Assays and Heparin Competition Assays
SYBR Gold assays were used to evaluate the capacity of the oligospermines to condense siRNA at various N/P ratios (0–20). SYBR Gold dye intercalates only with free and accessible siRNA and does not fluoresce if the siRNA is condensed and protected by a polycation. In a FluoroNunc 96-well white polystyrene plate (Nunc, Thermo Fisher Scientific), 50 pmol of FLUC siRNA per well in 50 μL was complexed with the appropriate amount of oligospermine in the same volume to obtain the corresponding N/P ratios in a total volume of 100 μL of a 5% glucose solution. PEI (5 kDa) was used for comparison. Formulations were incubated at room temperature for 20 min. A 4× SYBR Gold solution (30 μL) was added to each well, and plates were incubated in the dark for 10 min. Fluorescence was measured at 495 and 537 nm excitation and emission wavelengths, respectively, on a Synergy 2 multi-mode microplate reader (BioTek Instruments, Winooski, VT). For heparin assays, polyplexes were prepared at a N/P ratio of 2 as described above. In addition, experiments were performed in the presence of two different media to compare the stability of the polyplexes at different pH and ionic strengths. The media used were a 5% glucose solution (pH 7.4) and sodium acetate buffer (pH 4.5). For the heparin assays, a master solution of heparin was prepared (0.1 IU/μL). Serial dilutions of heparin were then prepared (0–1 IU/well) and added to the wells (10 μL/well). Subsequently, a 4× SYBR Gold solution (30 μL/well) was added, and plates were incubated for 10 min. After different incubation times with heparin (20 min and 1, 2, and 3 h) at 25 °C, fluorescence was measured on a Synergy 2 multi-mode microplate reader (BioTek Instruments) at 495 and 537 nm excitation and emission wavelengths, respectively. Measurements were performed in triplicate. The relative stability of the polyplexes was determined by normalizing the fluorescence intensity of the intercalating SYBR Gold dye to SYBR gold only (0%) and to SYBR gold with free siRNA (100%). Results are shown as mean values ± standard deviation and analyzed by GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA).
Cell Culture
NCI-H1299/LUC cells are derived from a human nonsmall cell lung carcinoma cell line (ATCC) that was transfected to stably express the reporter gene luciferase.36 H1299/LUC represents an established model for gene knockdown studies, as shown previously.36,37 Cells were cultured and grown in RPMI-1640 cell culture medium (Thermo Scientific Hyclone, Pittsburgh, PA) supplemented with sodium pyruvate (1 mM), HEPES (10 mM), 10% fetal bovine serum (Thermo Scientific Hyclone), and 1% penicillin/streptomycin. Cells were grown in 75 cm2 cell culture flasks (Thermo Scientific) at 37 °C and 5% CO2 and subcultured until approximately 90% confluence, with fresh culture medium changes occurring every 2 to 3 days.
Cytotoxicity of Polyplexes: MTT Assay
H1299/LUC cells were seeded in a 96-well plate (Thermo Scientific) with 10 000 cells per well in 100 μL of growth medium and incubated for 24 h at 37 °C and 5% CO2 in a HERAcell 150i CO2 incubator (Thermo Scientific). Oligospermines with varying concentrations (2–1000 μg/mL) were added to the cells in fresh media, and plates were incubated for 24 h at 37 °C and 5% CO2. A sterile-filtered MTT solution (5 mg/mL) was added to the cells (10 μL/well), and plates were incubated for 4 h at 37 °C and 5% CO2. Water-soluble MTT is enzymatically converted to insoluble formazan particles by metabolically active mitochondria.38 Subsequently, the cell culture media was removed, DMSO (200 μL/well) was added, and platest were incubated at room temperature for 10 min to solubilize the formazan particles. The optical absorbance was measured at 540 nm on a Synergy 2 multi-mode microplate reader (BioTek Instruments). The percentage of cell viability and proliferation is measured as the ratio between the absorbance of a sample and the untreated control cells. Results are shown as the mean value ± standard deviation of triplicate samples.
Quantification of Cellular Uptake by Flow Cytomtery
H1299/LUC cells were seeded in 24-well plates (Corning Incorporated, Corning, NY) at a density of 200 000 cells/well, and plates were incubated for 24 h at 37 °C and 5% CO2. Polyplexes were freshly prepared as described above with 40 pmol of Alexa Fluor 488-labeled siRNA at N/P ratios of 2 and 10. Negative controls included untreated control cells. PEI (5 kDa) was used as a positive control for comparison. Cells were transfected for 4 h with 100 μL of cell culture medium and 100 μL of polyplexes, after which growth medium was added to a total volume of 500 μL, and cells were incubated for another 20 h. Trypan blue quenching was used to extinguish the extracellular fluorescence caused by polyplex binding and to confirm the internalization of siRNA in the cells. Trypan blue 0.4% (100 μL per well), a dye that quenches the extracellular fluorescence,39,40 was added to the samples for 5 min before trypsinizing the cells. Results were compared to those obtained with cells that did not undergo trypan blue staining. Cells were rinsed with 1× PBS supplemented with 2 mM EDTA, treated with trypsin, and incubated at 37 °C and 5% CO2 for 3 to 4 min to detach the cells. Fresh medium (400 μL) was added to each well to deactivate the trypsin. Samples were transferred to microcentrifuge tubes (Seal-Rite, USA Scientific, Orlando, FL) and centrifuged at 400g for 5 min. Samples were washed twice with 1× PBS supplemented with 2 mM EDTA. Fluorescence was quantified by flow cytometry on an LSR II (BD Biosciences, San Jose, CA) after staining with 4′,6-diamidino-2-phenylindole (DAPI) for dead cells. Cell fluorescence was measured with excitation at 488 nm, and a 530/30 band-passfilter set was used to detect emission. Cell gating and data analysis were performed using FACSDiva (BD Biosciences) software. Measurements were performed in triplicate; for each measurement, 10 000 viable cells were gated and analyzed. Mean fluorescence intensity (MFI) results are given as the mean value of three independent measurements. Data analysis was performed by GraphPad Prism 5.0 software.
RNA Knockdown Measured by qRT-PCR
In 6-well plates (Corning Incorporated), H1299/LUC cells were seeded at a density of 500 000 cells/well, and plates were incubated for 24 h at 37 °C and 5% CO2. Polyplexes were prepared with 200 pmol of hGAPDH siRNA at a N/P ratio of 2 in a total volume of 100 μL and were added to 1 mL of cell culture medium per well. LF (0.5 μL/10 pmol of siRNA) was used as a positive transfection control. Cells were transfected with samples in fresh medium and incubated for 4 h. After 4 h of incubation, medium was added to a total volume of 3 mL, and cells were allowed to incubate for an additional 20 h. Subsequently, cells were washed with 1× PBS and lysed with lysis buffer (SurePrep True Total RNA Purification Kit (Fisher BioReagents, Fisher Scientific). Total RNA was then isolated from cells according to the manufacturer’s protocol with supplementary DNase I digestion, reverse transcribed to cDNA, and cDNA was amplified in a one-step protocol using Brilliant III SYBR green qRT-PCR master mix. Hs_GAPDH primers were used to quantify the gene expression of hGAPDH. Hs_β-actin primers were employed as a standard to evaluate the relative gene expression of the two genes. Serial dilutions of total RNA from untreated cells were performed to plot calibration curves for GAPDH and β-actin mRNA levels. Measurements were performed on a Stratagene Mx 3005P (Agilent Technologies). Ct values were analyzed with MxPro software (Mx 3005P version). Results are shown as mean values ± standard deviation of triplicate measurements and were analyzed by GraphPad Prism 5.0 software.
Protein Knockdown Measured in Reporter Gene Assays
H1299/LUC cells were seeded at a density of 25 000 cells/well in 24-well plates (Corning Incorporated), and plates were incubated at 37 °C and 5% CO2 for 24 h before transfection. Cells were transfected with polyplexes with 40 pmol FLUC siRNA or nonspecific control DsiRNA at a N/P ratio of 2 and were allowed to incubate for 4 h. Commercially available LF 2000 was used as a positive control. After 4 h of incubation, medium was added to a total volume of 500 μL, and plates were incubated for an additional 44 h. Cells were washed with 1× PBS and lysed with cell culture lysis reagent (CCLR 1×, 100 μL/well, Promega, Fisher Scientific) for 10 min. Cell lysates were then transferred to microcentrifuge tubes and centrifuged at 15 000g for 5 min. Luciferase expression was quantified by mechanical injection of 50 μL of luciferase assay buffer containing 10 mM luciferin into each well containing 20 μL of cell lysate, and relative light units (RLU) were measured using a Synergy 2 multi-mode microplate reader (BioTek Instruments) as the mean value of gene expression relative to untreated cells with full expression (100%) ± standard deviation of triplicate measurements. Data was statistically analyzed using GraphPad Prism 5.0 software.
Results and Discussion
Synthesis of Oligospermines
Spermine monomers were covalently coupled to yield three different polymers with different amounts of spermine units and distinct geometrical structures. The three polymers tested here are linear bisspermine, linear tetraspermine, and dendritic tetraspermine. The nomenclature of the polymers is based on the structure and number of spermine monomers. Oligospermine polymers were characterized by NMR and purified by HPLC. Molecular weights of the polymers were obtained by mass spectrometry for linear bisspermine (MW 1299.40), linear tetraspermine (MW 2581.82), and dendritic tetraspermine (MW 2625.87). The different architectures of the oligospermines were chosen to obtain differences in the charge distribution over the different structures. Our aim was to compare the siRNA polyplex formation of linear and dendritic structures with different cationic charge densities. At the neutral pH (7.4) of the intra- and extracellular environment, it is expected that only the primary amines are protonated, whereas only a small portion of the secondary amines are protonated.11 The linear and dendritic tetraspermines both bear eight protonable secondary amines compared to the linear bisspermine, which carries only half of the amount of protonable amines. This suggests that the tetraspermines have the ability to act as proton-sponge polymers at the acidic pH of the endolysosomal compartment.41 The structure of linear tetraspermine possesses multiple spermine units in a linear arrangement, which enables cross-linking of single oligospermine molecules. Cross-linked polymers have been reported to interact better with negatively charged regions of nucleic acids and can therefore yield enhanced transfection efficiencies.42
Dendritic structures are also very attractive as gene and drug delivery systems because they can be flexible structures with a multitude of end groups. The latter can be exploited to attach ligands, which opens various opportunities for cell-specific targeting. Because of their structure, dendrimers are believed to be more accessible for electrostatic interaction with RNA.43 In flexible dendrimers, the amines located within the inner structure are accessible for protonation, which results in an increased proton-sponge effect44 and consequently a better transfection efficiency.43
Size and Zeta (ζ)-Potential Analysis
To achieve efficient transfection, polyplexes must be well-characterized and reproducible. Many of the physicochemical properties of polyplexes determine if they can overcome intracellular and extracellular barriers.45 Their size is an important factor for intracellular uptake and transfection. Some reports indicate that particles with a size below 150 nm are required for uptake in lung cells by endocytosis.46,47 However, other reports describe spermine-based delivery systems with a larger size that have a good transfection efficiency in vivo and are suitable for lung cancer gene therapy.29 The ability of oligospermines to condense siRNA and to form polyplexes with defined structures was therefore evaluated here. Polyplexes prepared with 40 pmol of FLUC siRNA at N/P ratios of 2, 5, and 10 were compared in terms of their hydrodynamic diameters and zeta potentials. The change in the size and zeta potential of the polyplexes as a function of the carrier/siRNA ratio was examined to determine a suitable N/P ratio for further investigations. All three oligospermines were able to condense siRNA into particles of sizes from 198.7 to 423.1 nm in diameter (Table 1). All polyplexes at a N/P ratio of 5 were at least slightly larger than those at a N/P ratio of 2, which is in line with an earlier report that described N/P ratio-dependent trends in the sizes of siRNA polyplexes.48 Interestingly, both linear oligospermines showed an increase in size with increasing N/P ratios. Apparently, these polymers wrapped around the siRNA efficiently at an N/P ratio as low as 2 and then formed further layers of polymer on the surface of the polyplex. Another indication that supports this hypothesis is the increase of the zeta potential for the linear tetraspermine polyplexes with increasing N/P ratio (Table 1). Although linear bisspermine/siRNA polyplexes did not show a significant change in the zeta potential when increasing the N/P ratio, the zeta potentials of linear tetraspermine polyplexes increased from 1.5 to 10.6 to 12.7 mV for N/P ratios of 2, 5, and 10, respectively.
Table 1. Hydrodynamic Diameters and Zeta-Potential Values of siRNA Polyplexes Made with Linear Bisspermine, Linear Tetraspermine, and Dendritic Tetraspermine at N/P Ratios of 2, 5, and 10 at Room Temperaturea.
| parameter | size (nm) |
zeta-potential (mV) |
||||
|---|---|---|---|---|---|---|
| polymer in polyplex/N/P ratio | linear bisspermine | linear tetraspermine | dendritic tetraspermine | linear bisspermine | linear tetraspermine | dendritic tetraspermine |
| N/P 2 | 253.4 ± 26.3 | 198.7 ± 22 | 311.5 ± 18.5 | 6.1 ± 0.6 | 1.5 ± 0.5 | 12 ± 0.8 |
| N/P 5 | 289.2 ± 30 | 339.6 ± 13.8 | 351 ± 16.5 | 7.2 ± 0.2 | 10.6 ± 1.1 | 13.6 ± 1.1 |
| N/P 10 | 423.1 ± 30.9 | 317.3 ± 22.5 | 225.4 ± 15.8 | 6.1 ± 0.5 | 12.7 ± 0.7 | 17.6 ± 1.8 |
A 5% glucose solution was used as the suspension medium.
The dendritic tetraspermine, however, formed polyplexes with decreased size at a N/P ratio of 10 (225.4 nm in diameter and 17.6 mV). This behavior can be explained by its intertwining structure that results in not all amines of the tetraspermine being available for electrostatic interaction with siRNA at low N/P ratios. The comparably high zeta potentials that these polyplexes bear also support the idea of positively charged dendrimer arms that are unable to be neutralized by the interaction with phosphates.49 However, these relatively high zeta potentials could possibly mediate cytotoxicity.50 Zeta potentials of dendritic tetraspermines polyplexes increased with increasing N/P ratios. Comparing polyplexes of linear and dendritic tetraspermines, the zeta potential of the linear tetraspermine polyplexes was lower than that of the dendritic tetraspermine polyplexes at all of the tested N/P ratios (2, 5, and 10; Table 1). The surface charge of polyplexes is a significant factor for the transfection efficiency of the polymer. Other studies have shown the ability of spermine-based polymers to neutralize the negative charge of nucleic acids to yield an overall neutralized to slightly positive charge suitable for interaction with the negatively charged cell membrane.19 In our study, all oligospermines polyplexes were positively charged (Table 1).
Another prerequisite for successful and reproducible transfection, especially in vivo, is a narrow size distribution of the polyplexes.49 The polydispersity, expressed as the polydispersity index (PDI), was low for polyplexes formed with the linear bisspermine (0.14 < PDI < 0.3) compared to those formulated with linear tetraspermine (0.26 < PDI < 0.34) and dendritic tetraspermine (0.22 < PDI < 0.36; Table SI 1). The broader size distribution of the polyplexes obtained with the tetraspermines can be interpreted as a result of coalescence of the polyplexes caused by interaction between one longer polycationic polymer with more than one siRNA molecule.49 Many physical and biological parameters, such as the molecular weight, play an important role in determining the efficiency of a polymer to condense and deliver siRNA.51 Linear tetraspermine (MW 2581.82) and dendritic tetraspermine (MW 2625.87) naturally have a higher molecular weight compared to linear bisspermine (MW 1299.40), which affects the ability of the polymers to interact with siRNA and to form polyplexes. As reflected by the size and zeta-potential data shown in Table 1, the structure of the polymer also plays a very important role in the ability of an oligospermine to interact electrostatically with siRNA.
On the basis of these results, the linear tetraspermine/siRNA polyplexes seemed to be the most favorable, with the smallest size at a N/P ratio of 2 (198.7 ± 22 nm) and a slightly positive zeta potential (1.54 mV). These characteristics are caused by (1) a favorable number of positively charged spermine units (four units) and (2) the linear structure, which seems to be important for efficient interaction with siRNA and yielding a low positive charge close to a neutral surface charge that facilitates the crossing of the particle across the negatively charged cell membrane barrier. Polyplexes at a N/P ratio of 2 with hydrodynamic diameters of 253.4 ± 26.3 nm for linear bisspermine polyplexes, 198.7 ± 22 nm for linear tetraspermine polyplexes, and 311.5 ± 18.5 nm for dendritic tetraspermine polyplexes were therefore selected for further experiments.
Size and Morphology: Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM)
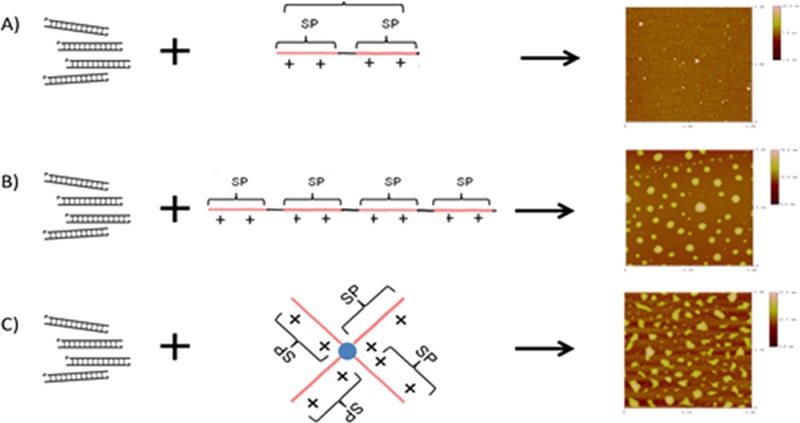
The morphologies and sizes of the different polyplexes at a N/P ratio of 2 were imaged by AFM (Figure 2). The sizes of the polyplexes estimated from the AFM images were 24–73 nm for linear bisspermine polyplexes, 101–348 nm for linear tetraspermine polyplexes, and 202–480 nm for polyplexes made with the dendritic tetraspermine. The differences between the sizes obtained by DLS compared to the AFM images can be explained by the different processes used to prepare the samples for DLS and AFM. The hydrodynamic diameters were determined in a suspension of the particles, whereas the particles were dried for AFM. It is possible that the polyplexes coalesced during the drying step. Additionally, the broad size distribution of the polyplexes shown by the imaging technique and confirmed by the polydispersity measurements (PDI) can explain why the Z average of the hydrodynamic diameters does not reflect the sizes measured by AFM. Most importantly, the AFM images showed different morphologies of oligospermine polyplexes as a result of the different chemical architectures of the polymers used. It is also possible that aggregation of particles occurred during the drying step. Both of the linear oligospermines formed spherical particles, whereas the dendritic tetraspermine complexes show a less defined morphology. These observations strengthen the hypothesis that linear oligospermines wrap around siRNA and condense it efficiently, whereas not all arms of the dendritic tetraspermine are involved in siRNA condensation, as shown in the fuzzy morphology of the polyplexes.
Figure 2.

AFM images of polyplexes at a N/P ratio of 2 with (A) linear bisspermine, (B) linear tetraspermine, and (C) dendritic tetraspermine showing different morphologies.
TEM showed electron-dense areas in the polyplexes, which could be the siRNA, and the presence of very small particles (about 40 nm) in all polyplex formulations besides larger particles of 440, 330, and 189 nm for linear bisspermine, linear tetraspermine, and dendritic tetraspermine polyplexes, respectively (Figure SI 1). AFM confirmed such small polyplexes (about 40 nm). The presence of small particles could explain the rather broad size distribution of the formulations.
siRNA Condensation Efficiency and Stability against Polyanions under Neutral and Acidic Conditions: SYBR Gold Dye Binding Assays and Heparin Competition Assays
Spermines have shown a higher DNA condensation and stabilization efficiency compared to other naturally occurring polyamines such as spermidine and putrescine.52 Spermine-based delivery systems condense DNA molecules by electrostatic interactions.53 Therefore, SYBR Gold assays were employed to compare the ability of different oligospermines to condense siRNA at various N/P ratios.54 In this assay, free or unbound siRNA is accessible to the intercalating dye, SYBR Gold, and is subsequently quantified on the basis of the fluorescence emitted. Results were compared to low-molecular-weight PEI (5 kDa) as a control. As expected, all of the polyplexes assayed were able to condense siRNA more efficiently as the N/P ratio increased (Figure 3). At higher N/P ratios, the net positive charge of the polyplexes was shown to increase, which is reflected by increasing zeta potentials (Table 1). With the rise of the zeta potential, the electrostatic interaction is enhanced, which is followed by higher condensation. All oligospermines were able to completely condense siRNA at N/P ratios of 2 and higher, whereas complete condensation of siRNA was achieved only at N/P ratios of 5 and higher for low-molecular-weight PEI. These results indicate that oligospermines tend to bind siRNA with higher affinity than PEI at low N/P ratios. Noticeably, linear bisspermine/siRNA polyplexes at a N/P ratio of 2 showed relatively low condensation of siRNA compared to the other two oligospermines. This observation can be explained by the low molecular weight and short chain length of bisspermine compared to the tetraspermines. The fact that the condensation efficiency did not increase for the bisspermine by increasing the N/P ratio additionally corroborates the observation of almost constant zeta potentials. Polyplexes with a N/P ratio of 2 were selected for further experiments based on their small size and overall good siRNA condensation ability.
Figure 3.
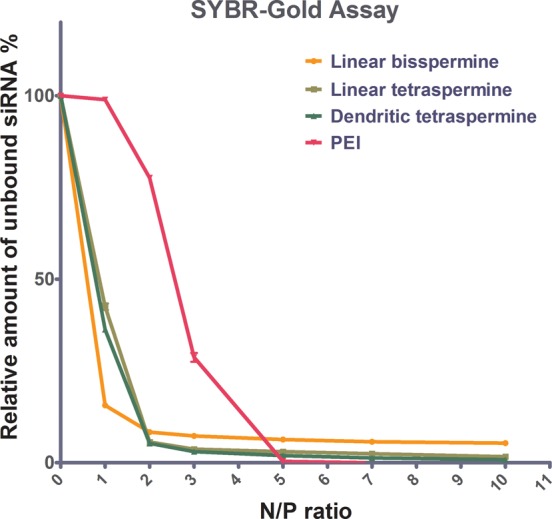
Condensation efficiency of oligospermines polyplexes measured by SYBR Gold intercalation of siRNA at increasing N/P ratios and compared to polyethylenimine (PEI 5 kDa). Results are given as the average of n = 3 ± SD.
The stability of cationic polyplexes is important for determining their efficiency as nonviral vectors. Their stability is influenced by the presence of competing anions55 in the cell membrane56 or in serum.36 Heparin assays were therefore performed to confirm the ability of oligospermines to protect the siRNA in the presence of polyanions under physiologically relevant conditions. Different pH conditions were chosen to mimic the neutral (7.4) or acidic (4.5) environment of the cytoplasm and endolysosome, respectively. The stability of all polyplexes decreased with increasing heparin concentration. The amount of siRNA released from the polyplexes increased rapidly as a function of heparin concentration. However, oligospermine polyplexes maintained higher stability profiles than PEI (5 kDa) polyplexes against heparin competition, especially at low concentrations of heparin (Figure 4). It is important to note that the release profile from low-molecular-weight PEI complexes needs to be evaluated in the context of its poor condensation at a N/P ratio of 2. As shown in Figure 3, at a N/P ratio of 2, 75% of the siRNA is not yet condensed by 5 kDa PEI. It is not surprising, therefore, that the same amount of siRNA (75%) is found to be accessible for intercalation even in the absence of heparin. The remaining 25% of the siRNA is consequently very easily released from the complexes, as shown in Figure 4.
Figure 4.
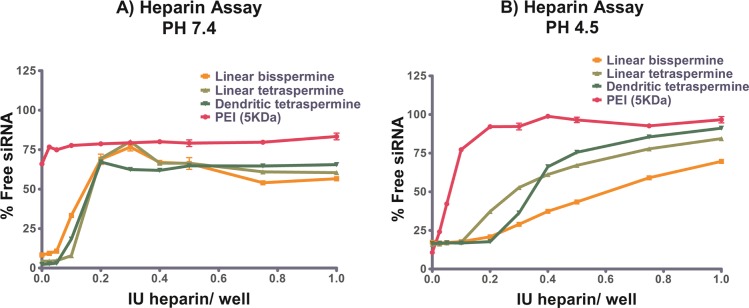
Release profiles of siRNA from oligospermines polyplexes (N/P ratio of 2; 50 pmol siRNA/well) compared to polyethylenimine (PEI) as a function of heparin concentration at pH (A) 7.4 and (B) 4.5. Results are given as mean normalized fluorescence (n = 3) ± SD.
At neutral pH, less than 75% of the siRNA was released from the oligospermine complexes even at the highest heparin concentration (Figure 4A). Because a balance between complexation and decomplexation is necessary to release siRNA in the cytosol for efficient incorporation to the RNA-induced silencing complex (RISC), the release profiles at lysosomal pH were tested also. At acidic pH, many amines, especially in PEI, which are not protonated at pH 7.4, were charged, leading to an increase of the complexation efficiency. However, siRNA was easily released from PEI complexes at comparably low heparin concentrations at acidic pH (Figure 4B). In comparison, oligospermine complexes displayed better stability again. In the acidic environment, the tetraspermine complexes released comparable amounts of siRNA as PEI at high heparin concentrations. Only bisspermine lacked efficient decomplexation properties.
To study the development of the polyplexes stability against heparin over time, heparin stability assays were performed for each polyplex after different incubation periods (20 min and 1, 2, and 3 h) with heparin at both pH 7.4 and 4.5 (Supporting Information). At pH 7.4, all polyplexes showed a slight increase of siRNA release over time. The strongest effects were observed for the linear spermine polyplexes. These differences can be explained by the structural differences of the oligospermines and their different interaction with siRNA. Although it is hypothesized that the linear oligospermines wrap around the siRNA and are efficiently neutralized, as reflected by rather low zeta potentials, not all amines of the dendritic oligospermine seem to be involved in the interaction with siRNA. It can therefore be understood that an excess of heparin binds to positively charged parts of the dendritic oligospermine before it displaces siRNA from a complex. Prolonged incubation of high concentrations of heparin with polyplexes made of linear spermines, however, results in more quantitative competition with siRNA and thus release of the latter.
Polymer Cytotoxicity: MTT Assay
The formulation of nonviral vectors composed of cationic polymers and anionic nucleic acids is constrained by the compromise of high transfection efficiency, which is oftentimes achieved only at the price of high cytotoxicity.57,58 Using cationic polymers with high molecular weight and charge density can protect the resulting polyplex from destabilization by natural cellular polyanions. The trade off, however, is that these positive charges can interact with cell membranes, inhibit crucial biological processes, and lead to cytotoxic effects.50 MTT assays were therefore used to evaluate the cytotoxic effect of the three cationic oligospermine polymers on H1299/LUC cells after 24 h of incubation with the polymers. Results are presented as the percentage of cell viability compared to untreated control cells. As expected, the cytotoxicity of oligospermines increased with increasing polymer concentration. Moreover, increasing the cationic charge of the polymer by increasing the number of spermine moieties also increased the cytotoxicity. Linear tetraspermine and dendritic tetraspermine showed a higher toxicity at higher concentrations when compared to linear bisspermine. This trend is due to the presence of a higher number of positively charged groups at neutral pH in the linear tetraspermine (13 positively charged groups) and dendritic tetraspermine (14 positively charged groups) compared to linear bisspermine (7 positively charged groups). The dendritic tetraspermine polymer was even more toxic than the linear tetraspermine at high concentrations (0.5–1 mg/mL), which may be due to its structure. On the basis of the obtained data points, the nonlinear fit for the dendritic spermine was not optimal. Nevertheless, the IC50 values reflect the trends of the toxicity of the materials very well. In conclusion, all oligospermines affected the cell viability significantly less than PEI (5 kDa, IC50 = 50.4 μg/mL) and commercially available LF 2000TM (IC50 = 45.7 μg/mL). At the corresponding polymer concentrations in the polyplexes used in the following experiments, the cell viability was at least 83% after treatment with linear bisspermine, 88% with linear tetraspermine, and 77.3% with dendritic tetraspermine (Figure 5). It is important to note that the positive charge of the polymers is neutralized after polyplex formation with siRNA, so the viability shown here after treatment with polymer only is the assumption of a worst-case scenario.
Figure 5.

Cytotoxicity profiles of oligospermine polymers obtained by MTT assays and compared to polyethylenimine (PEI) and lipofectamine (LF). Percentage of viability of H1299/LUC cells is shown as a function of increasing polymer concentration after 24 h of polymer incubation. The table shows the IC50 concentrations of the polymers in milligrams per milliliter.
Quantification of Cellular Uptake by Flow Cytomtery
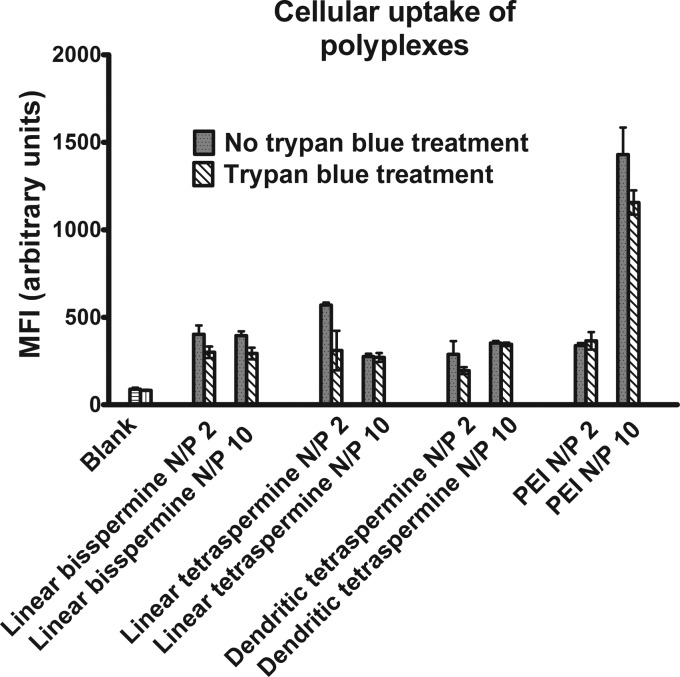
Cellular uptake was quantified by flow cytometry and compared to PEI (5 kDa) as a positive control and untreated cells as a negative control. Polyplexes with 40 pmol of Alexa Fluor 488-labeled siRNA at N/P ratios of 2 and 10 were compared. Additionally, 0.4% trypan blue was used on the cells to quench the extracellular fluorescence associated with polyplexes that bind to the surface but are not internalized. The results were compared to trypan blue-untreated cells. Overall, trypan blue-treated cells showed slightly lower mean fluorescence intensities compared to cells that did not undergo quenching of bound polyplexes. This indicates that a small fraction of the siRNA polyplexes was attached to the cell membrane but was not taken up intracellularly. Among the oligospermine polyplexes, the highest cellular uptake was achieved by polyplexes made of linear tetraspermine/Alexa Fluor 488-siRNA at a N/P ratio of 2 (no trypan blue treatment) (Figure 6). These results are surprising because linear tetraspermine polyplexes were almost neutral at a N/P ratio of 2 (1.54 ± 0.5 mV), whereas dendritic tetraspermine polyplexes had a more cationic zeta potential (12 ± 0.85 mV). However, these results can be interpreted as each molecule of the linear tetraspermine being able to interact with more siRNA molecules than the dendritic tetraspermine, resulting in better charge neutralization and a lower zeta potential but higher uptake of siRNA. In addition, the smaller size of linear tetraspermine polyplexes (198 ± 22 nm) compared to the dendritic tetraspermine polyplexes (311.5 ± 18.5) obtained by DLS could potentially enhance the cellular uptake. For polymers such as PEI, an increase of the zeta potential, which is obtained by increasing the N/P ratio, is expected to mediate stronger siRNA delivery. This trend was confirmed here. However, PEI polyplexes at high N/P ratios are known to cause toxicity and off-target effects in transfected cells.59 Although the siRNA delivery by oligospermine polyplexes was comparable to PEI at a N/P ratio of 2, an increase of the N/P ratio to 10 did not increase their efficiency. Linear tetraspermine polyplexes at a N/P ratio of 2 were found to have the smallest hydrodynamic diameters, however. It is possible that this parameter is favorable for uptake and crossing of the barrier of the cell membrane. Additionally, the spherical morphology of the linear oligospermine complexes compared to the fuzzy morphology of the dendritic tetraspermine polyplexes could have beneficially affected their internalization.
Figure 6.
Flow cytometry measurements showing the uptake of polyplexes made of Alexa Fluor 488-labeled siRNA and linear bisspermine and linear or dendritic tetraspermine compared to polyethylenimine (PEI). Mean fluorescence intensities were quantified in H1299/LUC cells after a 24 h incubation with polyplexes prepared at N/P ratios of 2 and 10. Trypan blue treatment was performed to quench the extracellular binding of siRNA polyplexes to the cell. Cells treated with trypan blue showed decreased mean fluorescence intensities.
RNA Knockdown Measured by qRT-PCR
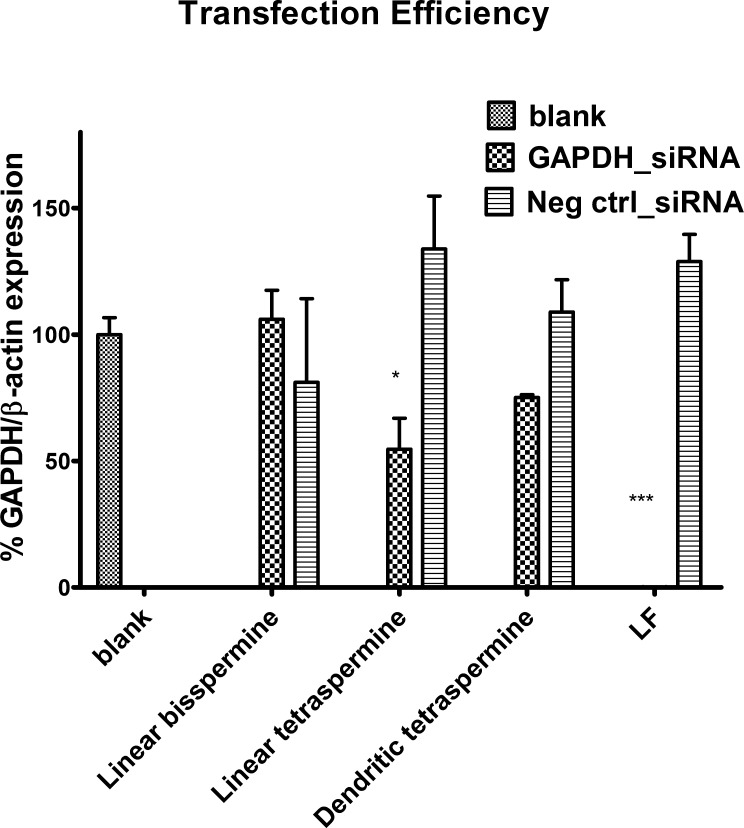
Real-time PCR was performed to quantify the knockdown of the mRNA level mediated by polyplexes made of GADPH siRNA (200 pmol/well) and oligospermines at a N/P ratio of 2. All oligospermines were used to form polyplexes with negative control siRNA (siNC) as well. Linear bisspermine/siRNA polyplexes did not show gene silencing. This can be attributed to the incomplete siRNA release from the polyplex in the endolysosomal compartment, as shown in Figure 4B. The most efficient oligospermine candidate was the linear tetraspermine, which is in line with the results of polyplexes size, zeta potentials, and flow cytometry. Linear tetraspermine polyplexes were shown to downregulate the RNA expression more effectively than dendritic tetraspermines (54.6 ± 17.3 vs 75.1 ± 1.5% residual GAPDH expression) (Figure 7). The dendritic structures showed less RNA knockdown compared to the linear tetraspermine structure, which could be explained by its less efficient uptake into the cells. Comparing the results of the three oligospermine polyplex formulations, we conclude that the difference in the architecture of the polymer strongly affected the efficiency of siRNA delivery into H1299/LUC cells. The linear tetraspermine structure is favored for successful siRNA delivery into lung cancer cells.
Figure 7.
Transfection efficiency in vitro (H1299/LUC cells) of oligospermines polyplexes at a N/P ratio of 2 on the mRNA level measured by qRT-PCR and compared to lipofectamine (LF). Hs_GAPDH primers were used to quantify hGAPDH gene expression. Hs_β-actin primers were employed as an internal standard to evaluate the relative gene expression of the two genes and to normalize the changes in hGAPDH expression. Calibration curves of hGAPDH and β-actin were plotted by serial dilution of cDNA from untreated cells. Polyplexes made of GADPH siRNA and linear tetraspermine showed the best knockdown compared to dendritic tetraspermine (54.6 vs 75.1% residual GAPDH expression) and linear bisspermine polyplexes (no knockdown).
Protein Knockdown Measured in Reporter Gene Assays
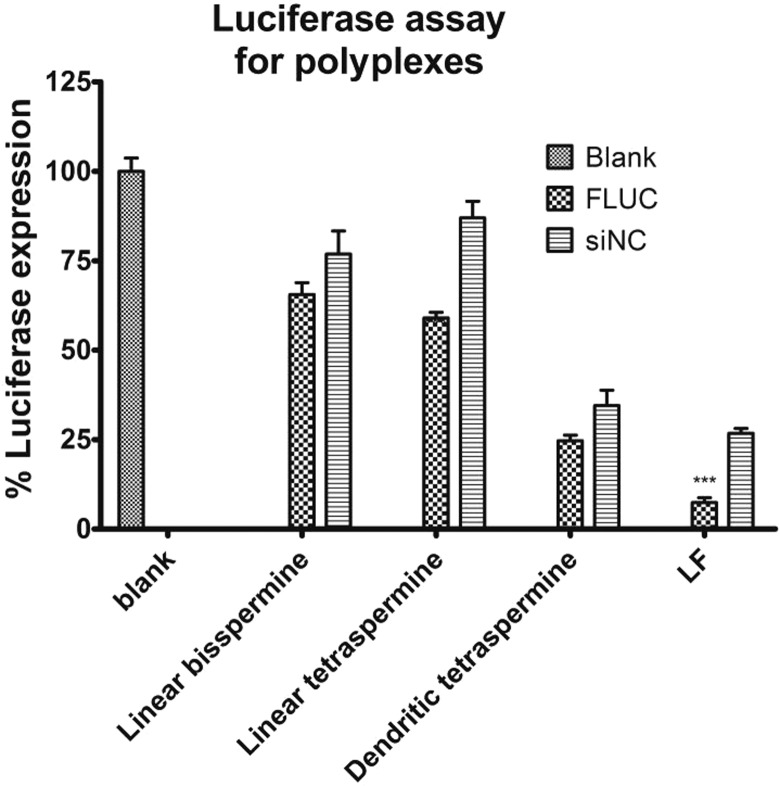
At pH 4.5, the secondary amines are protonated, leading to a strong buffering capacity inside the endosomes and thus a further influx of hydrochloric acid and water, leading eventually to endosomal rupture. This event is believed to release endocytosed polyplexes and to support their endosomal escape into the cytosol.11 The silencing efficiency on luciferase protein expression in H1299/LUC cells induced by oligospermines polyplexes at a N/P ratio of 2 was evaluated after transfection with 40 pmol of anti-LUC siRNA after 48 h of incubation. The results were normalized to the relative expression of untreated cells and compared to commercially available LF 2000. LF has been used in many studies as a positive control for siRNA-mediated knockdown efficiency.60 As in the mRNA knockdown experiments, negative control siRNA (siNC) was also used with all oligospermines and LF. Linear tetraspermine/siRNA polyplexes showed the best knockdown effect on luciferase expression compared to the other two oligospermines (Figure 8), which is in agreement with the qRT-PCR results. The dendritic tetraspermine and LF showed higher nonspecific gene knockdown with the control siRNA than the other polymers. This kind of off-target effect is normally understood as a cytotoxic effect, which can be explained by the cytotoxicity profiles shown in Figure 5. These results suggest that the oligospermine architecture not only affected the interaction of the protonated portions of the polymer with the phosphate groups of siRNA but also these different siRNA complexation behaviors also lead to different efficiencies of gene knockdown. Linear bisspermine polyplexes were taken up by the cell but showed neither knockdown at the mRNA nor the protein level. This is attributed to the lack of amines in the short chain length and the low molecular weight of the bisspermine structure, which does not condense siRNA as quantitatively as the tetraspermines (Figure 3) and also does not efficiently decomplex (Figure 4B). Our results are in line with other reports in which Eliyahu et al. compared two chemically modified spermine-based delivery systems for DNA delivery in terms of the number of spermine moieties and the distribution of charge density on the polymer backbone. In their study, a low- and a high-sperminated polymer were examined. The low-sperminated polymer showed 56% less spermine per weight and 28% less primary amines than the high-sperminated polymer. The low-sperminated polymer was less efficient in neutralizing the negative groups of the nucleic acids and hence showed a lower transfection efficiency compared to the high-sperminated polymer.11 Another study of cationic spermine conjugates with different polysaccharides showed efficient in vitro transfection with high spermine content (2000 nmol/mg).11 In vivo experiments showed that chemically modified dextran–spermine polyplexes successfully transfected mice with low toxicity and good tolerability when a combined intramuscular and intranasal administration was performed.42,61 However, for efficient transfection, a high positive zeta potential of the polyplex and large DNA doses were necessary.61 Dendritic structures have been described to be more accessible for electrostatic interaction with RNA.43 This is the case if the structurally inner amines are available for protonation, which then also enhances the proton-sponge effect,44 endosomal escape, and transfection efficiency.43 However, our results showed that the amines in the short dendritic structure are not all available for interaction with siRNA. In comparison with short linear structures, short dendrimers are more rigid. The protonated amines in the dendritic structure were thus not neutralized, which increased the cytotoxicity of the polyplexes. The polyplexes made with the dendritic structure did not show strong uptake or gene knockdown efficiency, which may be due to the larger sizes at a N/P ratio of 2 compared to the other two polymers or the less spherical morphology. Therefore, the structural architecture of dendritic tetraspermine was associated with increased cytotoxicity and decreased transfection efficiency.
Figure 8.
Silencing efficiency of firefly luciferase expression in H1299/LUC cells by oligospermine polyplexes with FLUC siRNA or nonspecific control siRNA at a N/P ratio of 2 after 48 h of transfection compared to lipofectamine (LF). The relative gene silencing was normalized to blank untreated cells. Results are the mean value of triplicate measurements ± SD.
Conclusions
Here, we have highlighted the importance of the structure–activity relationship (SAR) of cationic oligospermines and its strong impact on siRNA-delivery efficiency. The complexation and decomplexation of siRNA and the carriers’ ability to escape the degradation in lysosomes are two main factors in determining the polymers’ transfection efficiency. The spatial availability of the positively charged amines in the polymer plays an important role in its electrostatic interaction with RNA and thus the shielding and protecting of siRNA. Therefore, the oligospermine architecture was shown to affect the transfection efficiency of polyplexes formed with siRNA. Consequently, an optimization of the polymer used is necessary. This can be achieved in many ways. Here, we investigated the effect of using different numbers of spermine monomers. In addition, we examined the effect of two different geometrical structures, namely, linear and dendritic oligospermines. We found that tetramers of spermine are required to provide the adequate positive charge for both uptake and the buffering effect for endosomal escape. From the comparison of linear bisspermines and linear tetraspermines, we found that increasing the number of spermines and charge density within the polymer enhanced the transfection efficiency with minimal toxicity. The linear structure is preferred over the dendritic structure because the former seems to interact more efficiently with siRNA, as not all amines of the latter are available for siRNA condensation, leading to a more positive surface charge. Showing more efficient charge neutralization, the linear tetraspermine polyplexes are less cytotoxic and were shown to be more efficiently transfected into lung carcinoma cells (H1299/LUC). Therefore, we conclude that linear tetraspermines are very promising siRNA-delivery systems. To enhance their intracellular uptake, coupling them with targeting ligands is currently being investigated.
Acknowledgments
We thank Daniel Feldmann (Cancer Biology Program, WSU) for critically reviewing this manuscript, Drs. Christine Chow and Andrew Feig (Department of Chemistry, WSU) for granting us access to their zetasizer, and Drs. Denise Conti and Sandro da Rocha (Department of Chemical Engineering, WSU) for help with the AFM imaging. The Wayne State Start-Up Grant to O.M.M. and the NIH Center grant P30CA22453 supporting the Wayne State Microscopy, Imaging, and Cytometry Resources (MICR) are gratefully acknowledged.
Supporting Information Available
Polydispersity values of oligospermines polyplexes at different N/P ratios; TEM images of polyplexes at N/P 2 with linear bisspermine, linear tetraspermine, and dendritic tetraspermine; and the temporal development of siRNA release at pH 4.5. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Zamore P. D.; Tuschl T.; Sharp P. A.; Bartel D. P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33. [DOI] [PubMed] [Google Scholar]
- Fire A.; Xu S.; Montgomery M. K.; Kostas S. A.; Driver S. E.; Mello C. C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [DOI] [PubMed] [Google Scholar]
- McManus M. T.; Sharp P. A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 2002, 3, 737–747. [DOI] [PubMed] [Google Scholar]
- Lam J. K.-W.; Liang W.; Chan H.-K. Pulmonary delivery of therapeutic siRNA. Adv. Drug Delivery Rev. 2012, 64, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir S. M.; Harborth J.; Lendeckel W.; Yalcin A.; Weber K.; Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [DOI] [PubMed] [Google Scholar]
- The U.S. National Institutes of Health ClinicalTrials.gov Home Page. http://www.clinicaltrials.gov/.
- Check E. Harmful potential of viral vectors fuels doubts over gene therapy. Nature 2003, 423, 573–574. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Shum K. T.; Burnett J. C.; Rossi J. J. Nanoparticle-based delivery of RNAi therapeutics: Progress and challenges. Pharmaceuticals 2013, 6, 85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr J. P.; Demeneix B.; Loeffler J. P.; Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc. Natl. Acad. Sci. U.S.A. 1989, 86, 6982–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z.; Chen M.; He Q.; Zhou Y.; Jin T. Polymerized spermine as a novel polycationic nucleic acid carrier system. Int. J. Pharm. 2012, 434, 437–443. [DOI] [PubMed] [Google Scholar]
- Eliyahu H.; Siani S.; Azzam T.; Domb A. J.; Barenholz Y. Relationships between chemical composition, physical properties and transfection efficiency of polysaccharide-spermine conjugates. Biomaterials 2006, 27, 1646–1655. [DOI] [PubMed] [Google Scholar]
- Jiang H. L.; Lim H. T.; Kim Y. K.; Arote R.; Shin J. Y.; Kwon J. T.; Kim J. E.; Kim J. H.; Kim D.; Chae C.; Nah J. W.; Choi Y. J.; Cho C. S.; Cho M. H. Chitosan-graft-spermine as a gene carrier in vitro and in vivo. Eur. J. Pharm. Biopharm. 2011, 77, 36–42. [DOI] [PubMed] [Google Scholar]
- Allen J. C. Biochemistry of the polyamines. Cell Biochem. Funct. 1983, 1, 131–140. [DOI] [PubMed] [Google Scholar]
- Tabor C. W.; Tabor H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [DOI] [PubMed] [Google Scholar]
- Igarashi K.; Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [DOI] [PubMed] [Google Scholar]
- Shim M. S.; Kwon Y. J. Dual mode polyspermine with tunable degradability for plasmid DNA and siRNA delivery. Biomaterials 2011, 32, 4009–4020. [DOI] [PubMed] [Google Scholar]
- Hardy J. G.; Kostiainen M. A.; Smith D. K.; Gabrielson N. P.; Pack D. W. Dendrons with spermine surface groups as potential building blocks for nonviral vectors in gene therapy. Bioconjugate Chem. 2006, 17, 172–178. [DOI] [PubMed] [Google Scholar]
- Fischer W.; Brissault B.; Prevost S.; Kopaczynska M.; Andreou I.; Janosch A.; Gradzielski M.; Haag R. Synthesis of linear polyamines with different amine spacings and their ability to form dsDNA/siRNA complexes suitable for transfection. Macromol. Biosci. 2010, 10, 1073–1083. [DOI] [PubMed] [Google Scholar]
- Duan S. Y.; Ge X. M.; Lu N.; Wu F.; Yuan W.; Jin T. Synthetic polyspermine imidazole-4,5-amide as an efficient and cytotoxicity-free gene delivery system. Int. J. Nanomed. 2012, 7, 3813–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y. J. Before and after endosomal escape: Roles of stimuli-converting siRNA/polymer interactions in determining gene silencing efficiency. Acc. Chem. Res. 2012, 45, 1077–1088. [DOI] [PubMed] [Google Scholar]
- Jere D.; Kim J. E.; Arote R.; Jiang H. L.; Kim Y. K.; Choi Y. J.; Yun C. H.; Cho M. H.; Cho C. S. Akt1 silencing efficiencies in lung cancer cells by sh/si/ssiRNA transfection using a reductable polyspermine carrier. Biomaterials 2009, 30, 1635–1647. [DOI] [PubMed] [Google Scholar]
- Jere D.; Kim T. H.; Arote R.; Jiang H. L.; Cho M. H.; Nah J. W.; Cho N. S. A poly(β-amino ester) of spermine and poly(ethylene glycol) diacrylate as a gene carrier. Key Eng. Mater. 2007, 342–343, 425–428. [Google Scholar]
- Blagbrough I. S.; Geall A. J.; Neal A. P. Polyamines and novel polyamine conjugates interact with DNA in ways that can be exploited in non-viral gene therapy. Biochem. Soc. Trans. 2003, 31, 397–406. [DOI] [PubMed] [Google Scholar]
- Chen Y.-Z.; Ruan G.-X.; Yao X.-L.; Li L.-M.; Hu Y.; Tabata Y.; Gao J.-Q. Co-transfection gene delivery of dendritic cells induced effective lymph node targeting and anti-tumor vaccination. Pharm. Res. 2013, 30, 1502–1512. [DOI] [PubMed] [Google Scholar]
- Maslov M. A.; Kabilova T. O.; Petukhov I. A.; Morozova N. G.; Serebrennikova G. A.; Vlassov V. V.; Zenkova M. A. Novel cholesterol spermine conjugates provide efficient cellular delivery of plasmid DNA and small interfering RNA. J. Controlled Release 2012, 160, 182–193. [DOI] [PubMed] [Google Scholar]
- Metwally A. A.; Blagbrough I. S. Self-assembled lipoplexes of short interfering RNA (siRNA) using spermine-based fatty acid amide guanidines: Effect on gene silencing efficiency. Pharmaceutics 2011, 3, 406–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.; Wang X.; Lu Z.-R. Intracellular siRNA delivery with novel spermine based surfactants. Chin. Sci. Bull. 2012, 57, 3979–3984. [Google Scholar]
- Metwally A. A.; Reelfs O.; Pourzand C.; Blagbrough I. S. Efficient silencing of EGFP reporter gene with siRNA delivered by asymmetrical N(4),N(9)-diacyl spermines. Mol. Pharmaceutics 2012, 9, 1862–1876. [DOI] [PubMed] [Google Scholar]
- Jiang H.-L.; Hong S.-H.; Kim Y.-K.; Islam M. A.; Kim H.-J.; Choi Y.-J.; Nah J.-W.; Lee K.-H.; Han K.-W.; Chae C.; Cho C.-S.; Cho M.-H. Aerosol delivery of spermine-based poly(amino ester)/Akt1 shRNA complexes for lung cancer gene therapy. Int. J. Pharm. 2011, 420, 256–265. [DOI] [PubMed] [Google Scholar]
- Hong S. H.; Kim J. E.; Kim Y. K.; Minai-Tehrani A.; Shin J. Y.; Kang B.; Kim H. J.; Cho C. S.; Chae C.; Jiang H. L.; Cho M. H. Suppression of lung cancer progression by biocompatible glycerol triacrylate-spermine-mediated delivery of shAkt1. Int. J. Nanomed. 2012, 7, 2293–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. K.; Cho C. S.; Cho M. H.; Jiang H. L.. Spermine-alt-poly(ethylene glycol) polyspermine as a safe and efficient aerosol gene carrier for lung cancer therapy. J. Biomed. Mater. Res., Part A [Online early access]. DOI: 10.1002/jbm.a.34905. Published Online: Aug 13, 2013. [DOI] [PubMed] [Google Scholar]
- Soltan M.; Ghonaim H.; Sadek M.; Kull M. A.; El-aziz L.; Blagbrough I. Design and synthesis of N4,N9-disubstituted spermines for non-viral siRNA delivery – structure-activity relationship studies of siFection efficiency versus toxicity. Pharm. Res. 2009, 26, 286–295. [DOI] [PubMed] [Google Scholar]
- Duan S.; Yuan W.; Wu F.; Jin T. Polyspermine imidazole-4,5-imine, a chemically dynamic and biologically responsive carrier system for intracellular delivery of siRNA. Angew. Chem., Int. Ed. 2012, 51, 7938–7941. [DOI] [PubMed] [Google Scholar]
- Vijayanathan V.; Thomas T.; Shirahata A.; Thomas T. J. DNA condensation by polyamines: A laser light scattering study of structural effects. Biochemistry 2001, 40, 13644–13651. [DOI] [PubMed] [Google Scholar]
- Kolhatkhar R., to be submitted for publication.
- Merkel O. M.; Librizzi D.; Pfestroff A.; Schurrat T.; Buyens K.; Sanders N. N.; De Smedt S. C.; Behe M.; Kissel T. Stability of siRNA polyplexes from poly(ethylenimine) and poly(ethylenimine)-g-poly(ethylene glycol) under in vivo conditions: Effects on pharmacokinetics and biodistribution measured by fluorescence fluctuation spectroscopy and single photon emission computed tomography (SPECT) imaging. J. Controlled Release 2009, 138, 148–159. [DOI] [PubMed] [Google Scholar]
- Merkel O. M.; Beyerle A.; Beckmann B. M.; Zheng M.; Hartmann R. K.; Stoger T.; Kissel T. H. Polymer-related off-target effects in non-viral siRNA delivery. Biomaterials 2011, 32, 2388–2398. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Peterson D. A.; Kimura H.; Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997, 69, 581–593. [DOI] [PubMed] [Google Scholar]
- Germershaus O.; Mao S.; Sitterberg J.; Bakowsky U.; Kissel T. Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: Establishment of structure–activity relationships in vitro. J. Controlled Release 2008, 125, 145–154. [DOI] [PubMed] [Google Scholar]
- Innes N. P. T.; Ogden G. R. A technique for the study of endocytosis in human oral epithelial cells. Arch. Oral Biol. 1999, 44, 519–523. [DOI] [PubMed] [Google Scholar]
- Boussif O.; Lezoualc’h F.; Zanta M. A.; Mergny M. D.; Scherman D.; Demeneix B.; Behr J. P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. U.S.A. 1995, 92, 7297–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu H.; Joseph A.; Schillemans J. P.; Azzam T.; Domb A. J.; Barenholz Y. Characterization and in vivo performance of dextran–spermine polyplexes and DOTAP/cholesterol lipoplexes administered locally and systemically. Biomaterials 2007, 28, 2339–2349. [DOI] [PubMed] [Google Scholar]
- Posocco P.; Laurini E.; Dal Col V.; Marson D.; Karatasos K.; Fermeglia M.; Pricl S. Tell me something I do not know. Multiscale molecular modeling of dendrimer/dendron organization and self-assembly in gene therapy. Curr. Med. Chem. 2012, 19, 5062–5087. [DOI] [PubMed] [Google Scholar]
- Sonawane N. D.; Szoka F. C. Jr.; Verkman A. S. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem. 2003, 278, 44826–44831. [DOI] [PubMed] [Google Scholar]
- Wolff J. A.; Rozema D. B. Breaking the bonds: Non-viral vectors become chemically dynamic. Mol. Ther. 2008, 16, 8–15. [DOI] [PubMed] [Google Scholar]
- Lebhardt T.; Roesler S.; Beck-Broichsitter M.; Kissel T. Polymeric nanocarriers for drug delivery to the lung. J. Drug Delivery Sci. Technol. 2010, 20, 171–180. [Google Scholar]
- De Smedt S. C.; Demeester J.; Hennink W. E. Cationic polymer based gene delivery systems. Pharm. Res. 2000, 17, 113–126. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Pavan G. M.; Neeb M.; Schaper A. K.; Danani A.; Klebe G.; Merkel O. M.; Kissel T. Targeting the blind spot of polycationic nanocarrier-based siRNA delivery. ACS Nano 2012, 6, 9447–9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel O. M.; Zheng M.; Mintzer M. A.; Pavan G. M.; Librizzi D.; Maly M.; Hoffken H.; Danani A.; Simanek E. E.; Kissel T. Molecular modeling and in vivo imaging can identify successful flexible triazine dendrimer-based siRNA delivery systems. J. Controlled Release 2011, 153, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrobattista E.; Hennink W. E. Polymers for gene delivery: Charged for success. Nat. Mater. 2012, 11, 10–12. [DOI] [PubMed] [Google Scholar]
- Ahmed M.; Narain R. The effect of polymer architecture, composition, and molecular weight on the properties of glycopolymer-based non-viral gene delivery systems. Biomaterials 2011, 32, 5279–5290. [DOI] [PubMed] [Google Scholar]
- Wilson R. W.; Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. A light-scattering study. Biochemistry 1979, 18, 2192–2196. [DOI] [PubMed] [Google Scholar]
- Kurzbach D.; Velte C.; Arnold P.; Kizilsavas G.; Hinderberger D. DNA condensation with spermine dendrimers: Interactions in solution, charge inversion, and morphology control. Soft Matter 2011, 7, 6695–6704. [Google Scholar]
- Merkel O. M.; Beyerle A.; Librizzi D.; Pfestroff A.; Behr T. M.; Sproat B.; Barth P. J.; Kissel T. Nonviral siRNA delivery to the lung: Investigation of PEG-PEI polyplexes and their in vivo performance. Mol. Pharmaceutics 2009, 6, 1246–1260. [DOI] [PubMed] [Google Scholar]
- Izumrudov V. A.; Bronich T. K.; Novikova M. B.; Zezin A. B.; Kabanov V. A. Substitution reactions in ternary systems of macromolecules. Polym. Sci. U.S.S.R. 1982, 24, 367–378. [Google Scholar]
- Bolcato-Bellemin A. L.; Bonnet M. E.; Creusat G.; Erbacher P.; Behr J. P. Sticky overhangs enhance siRNA-mediated gene silencing. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. D.; Huang L. Non-viral is superior to viral gene delivery. J. Controlled Release 2007, 123, 181–183. [DOI] [PubMed] [Google Scholar]
- Mintzer M. A.; Simanek E. E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [DOI] [PubMed] [Google Scholar]
- D’Emanuele A.; Attwood D. Dendrimer-drug interactions. Adv. Drug Delivery Rev. 2005, 57, 2147–2162. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Samsonova O.; Sproat B.; Merkel O.; Kissel T. Biophysical characterization of hyper-branched polyethylenimine-graft-polycaprolactone-block-mono-methoxyl-poly(ethylene glycol) copolymers (hy-PEI-PCL-mPEG) for siRNA delivery. J. Controlled Release 2011, 153, 262–268. [DOI] [PubMed] [Google Scholar]
- Eliyahu H.; Joseph A.; Azzam T.; Barenholz Y.; Domb A. J. Dextran–spermine-based polyplexes–Evaluation of transgene expression and of local and systemic toxicity in mice. Biomaterials 2006, 27, 1636–1645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.