Abstract
Herein, we describe an efficient and high-yielding method to synthesize hyaluronan oligosaccharide–lipid conjugates. This strategy is based on first covalently attaching diphytanoyl glycerophosphatidylethanolamine (DiPhPE) to commercially available high molecular weight hyaluronic acid (HA), via the carboxylate group of the glucuronic acid using carbodiimide chemistry. The HA-lipid conjugate mixture is then digested with bovine testicular hyaluronidase to yield HA-DiPhPE conjugates that have a narrow distribution of moderately sized HA oligosaccharides. These HA-lipid conjugates can be incorporated into liposomes or micelles to selectively target CD44 that is overexpressed on many cancer or cancer initiating cells.
Introduction
Hyaluronic acid (HA) is a high molecular weight linear polysaccharide, composed of a repeating disaccharide unit of d-glucuronic acid and N-acetyl-d-glucosamine linked through an α-1,3 glycosidic bond (Figure 1). The disaccharides are in turn linked to each other through an α-1,4 glycosidic bond. HA is found in the extracellular matrix and is the main ligand for CD44, a type 1 transmembrane glycoprotein that is overexpressed in many cancers.1
Figure 1.
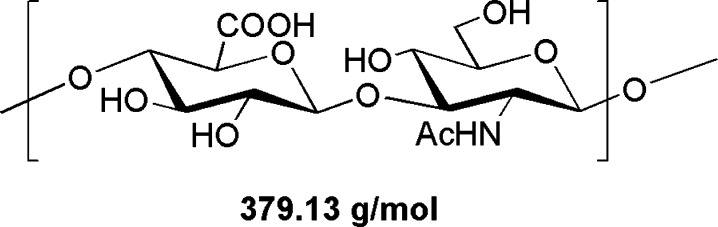
Disaccharide repeat unit of HA.
HA has been extensively used as a drug carrier2 and a ligand on liposomes/nanoparticles3−12 to target anticancer drugs to CD44 overexpressing cells. Luo et al. found that HA-drug conjugates are internalized via CD44 receptors and the drug is released primarily by intracellular enzymatic hydrolysis.11,12 Drugs, prodrugs, proteins, or lipids can be potentially attached to HA via the carboxylate group on the glucuronic acid residue, the primary hydroxyl on the N-acetylglucosamine moiety, or via reductive amination chemistry through the reducing end of HA. Liposomes, bearing HA conjugated to a phosphatidylethanolamine lipid, have been used for targeting CD44 receptors.5−8 In an earlier report to prepare bioadhesive liposomes, high molecular weight HA was coupled to phosphatidylethanolamine via the glucuronic carboxylate group in preformed liposomes.13 This approach resulted in multipoint attachment of HA to liposomes, but the size distribution of the HA oligosaccharides was uncharacterized. In an alternate conjugation method, the phosphatidylethanolamine was attached to the oligosaccharide HA via reductive amination to the reducing end of HA, but the precise composition of the oligosaccharide mixture coupled to the lipid was not specified.5
The molecular weight of the HA oligomers used for conjugation is critical for the resulting interaction of HA with CD44. Journo-Gershfeld et al.14 have evaluated the correlation between the lengths of HA oligomers and their binding affinity to CD44 receptor. They demonstrated that polymer conjugates, bearing HA oligomers containing 10 monosaccharides or more, bound strongly to CD44-overexpressing ovarian cancer cells, and internalized to a greater extent relative to HA-polymer conjugates of 8 oligomers or less. Moreover, the conjugate synthesized with HA34 was 50 times more cytotoxic to cells relative to the control. HA oligomers (containing 12–28 monosaccharides) also inhibit melanoma cell proliferation in vitro as well as formation of tumors from subcutaneously injected cells in vivo.15
Our ongoing work16 to target CD44 overexpressing cells requires an efficient route to synthesize well-defined HA-lipid conjugates for their incorporation into liposomes. The current approach involves the digestion of high molecular weight (HMW) HA with hyaluronidase, followed by separation of well-defined populations of oligomers by running a size exclusion column. These oligomers are subsequently coupled to lipids, and the HA-lipid conjugates are then purified. This conventional approach is low yielding, both in the production of the appropriate length HA oligomers and in the coupling of the lipid to the reducing end of the HA oligomers, and hence is inefficient. To improve upon the yields, we designed an alternate chemoenzymatic synthesis of HA-lipids.
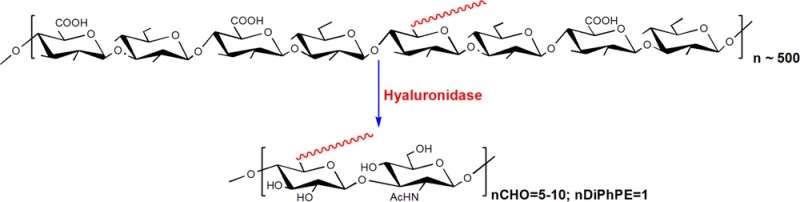
To the best of our knowledge, this is the first report on such an approach to synthesize phospholipo-oligosaccharides. This strategy is initiated by a carbodiimide-mediated coupling of diphytanoyl glycerophosphatidylethanolamine (DiPhPE) to commercially available HMW HA, followed by digestion of the mixture with hyaluronidase and then a final purification of the HAn-DiPhPE from both the free HA oligomers generated (during digestion) and the unreacted lipid. This strategy is efficient and high yielding, since we bypass the size exclusion column step, shorten the overall synthetic route, and obtain a narrow oligosaccharide distribution (HA monosaccharides ranging from 10 to 20) for binding to the CD44 receptor, on the resulting lipo-oligosaccharide.
A 10–20 oligomer HA length is long enough to bind to the CD44 receptor but shorter than the HA length required to tightly bind to the HARE receptor, found in liver endothelial cells. Binding constants of HA oligomers to liver endothelial cells17 or the HARE receptor18 are in the low micromolar range for HA oligomers composed of 10–20 monosaccharides, but binding constants rapidly increase to subnanomolar levels as the HA length grows longer.17,18 The consequence of having a facile chemoenzymatic route to HA conjugates in the size range reported herein is that it enables the synthesis of targeting ligands that will preferentially bind to CD44 but not to HARE; longer HA length ligands are rapidly eliminated by HA receptors found in the liver. This rapid removal of high molecular weight HA-drug conjugates has previously been reported,19 and this is a limitation for their application to target drugs to CD44 expressing cells.
Results and Discussion
Conventionally, HA conjugates of defined range of sizes are prepared by first enzymatically digesting commercially available HMW HA, followed by separation of the oligomers using either size exclusion or anion exchange chromatography. These oligomers are then conjugated to lipids, proteins, or drugs. This multistep approach is cumbersome, low yielding,5,16 and difficult to scale up. To circumvent these issues, we designed a chemoenzymatic synthetic route to prepare HA conjugates. In this approach we first conjugated the high molecular weight HA with a lipid, with the hope that the presence of a lipid moiety in a semi-random fashion might restrict the digestion of the conjugate by the enzyme so that a mixture of HA-lipid conjugates encompassing a narrow range of moderately sized HA oligomers (containing monosaccharides in the range of 10–20) would be formed.
To test our hypothesis, we coupled DiPhPE to commercially available HMW HA. Also, to overcome the challenge of reacting two molecules of very different solubilities: high molecular weight HA and lipid, we converted the HA to its tetrabutylammonium (TBA) salt which had enhanced solubility in solvents like (CH3)2SO, and switched to phospholipid with branched fatty acyl chains, DiPhPE. DiPhPE has a much lower transition temperature than other commonly used phosphoethanolamines such as DPPE and DSPE with linear acyl chains. The diphytanyl phospholipids are also less susceptible to oxidation than the low transition temperature unsaturated PE’s. We calculated the molecular weight of the starting HA based on the molecular weight of a decasaccharide, and then added the same equivalents of DiPhPE so that, on an average, every 10 sugar residues contained one DiPhPE molecule. To some extent the coupling is random and uncontrollable. The coupled product was then subjected to hyaluronidase digestion for different time intervals (5 h, 12 h, 18 h, 24 h, and 48 h). The above samples were individually dialyzed (to remove unmodified HA) and lyophilized. The products were then analyzed by MALDI-TOF (Figure S1) and no significant difference was observed in the mass profiles of the four samples, indicating that the presence of the lipid tail on HA had indeed restricted the enzyme digestion, and longer digestion times did not further reduce the extent of digestion of the HAn-DiPhPE conjugates. After passing the above material through a C18 Sep Pak cartridge (to separate pure conjugate from the unreacted DiPhPE) the overall yields were in the range of ∼60–65%.
In order to analyze the mass of these relatively large lipo-oligosaccharides, the laser power used in the MALDI-TOF had to be increased, which possibly led to fragmentation of the lipid. This fragmentation, coupled with the various different adducts formed, made it difficult to conclusively determine the size distribution of HA oligomers that were conjugated to DiPhPE. Some HA molecules may have more than one lipid molecule attached to them, further complicating the analysis.
Therefore, to obtain an estimate of the DiPhPE/HAn ratio we digested a small aliquot of HAn-DiPhPE with phospholipase D (Figure 2) and analyzed the aqueous layer obtained after extraction. The mass results (Figure S2) confirmed that the MW of the HA oligomers ranged from 10 monosaccharides to 18 monosaccharides, with principally the monosubstituted DiPhPE and a very small fraction of disubstituted DiPhPE lipid residues.
Figure 2.
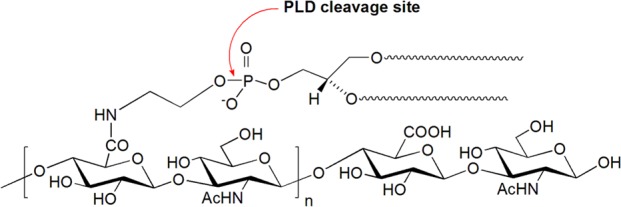
Digestion of HAn-DiPhPE with Phospholipase D.
The lipid to carbohydrate ratio determined from the phosphate analysis (1 phospholipid per 9.8 monosaccharides) confirmed the MALDI analysis on the digested lipo-oligosaccharide, if we assume that the sample by mass was a mixture of X HA disaccharide units (379.13 g/M) Figure 1 and the lipid DiPhPE (804 g/M).
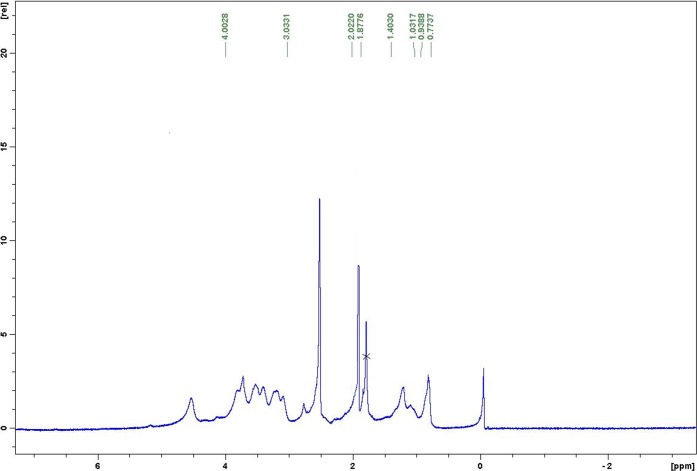
Purified HAn-DiPhPE (obtained after elution from Sep Pak cartridge) was characterized by 1H NMR (Figure 3). The NMR showed characteristic peaks for both the sugar and lipid: δ 4.00–3.03 (sugar protons), δ 1.87 (sugar N-acetate protons), δ 1.40–1.03 (methylene protons of DiPhPE), and δ 0.93–0.77 (methyl protons of DiPhPE). Comparing the ratio of the methyl protons of DiPhPE to the N-acetyl protons of HA gave us a value of 1.76. This would correspond to 13 monosaccharides per lipid molecule. The spectra were slightly broadened, so precise integration was not possible; the value obtained is consistent with both the oligosaccharide size distribution and the phosphate/carbohydrate ratio.
Figure 3.
1H NMR of Han-DiPhPE recorded in DMSO.
The HA-lipid conjugates were evaluated for their ability to bind to either COS7 cultured cells or CD44 expressing COS7 cells using both a FACS assay to quantify the level of binding of liposomes to the cells and a fluorescent confocal imaging assay. The imaging analysis was used to determine if both CD44 and liposomes were present on the target cells but absent on the control cells. In the binding assay, liposomes that incorporated the HA-DiPhPE conjugates on their surface were preferentially bound to cells that expressed CD44 receptors, compared to the control liposomes. Binding and uptake of liposomes was quantified by FACS as shown in Figure 4.
Figure 4.
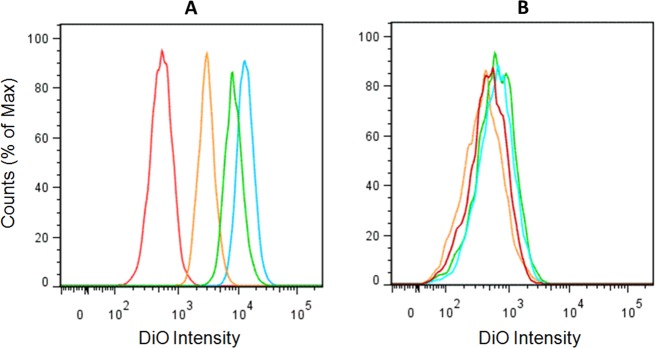
Uptake of liposomes as determined by FACS in (A) COS 7 cells overexpressing CD44 receptors and (B) COS 7 cells. DMPG control (red), 0.3 mol % undigested conjugate HA-DiPhPE 2 (blue), 0.3 mol % digested conjugate HAn-DiPhPE 3 (green), and 0.03 mol % digested conjugate HAn-DiPhPE 3 (orange).
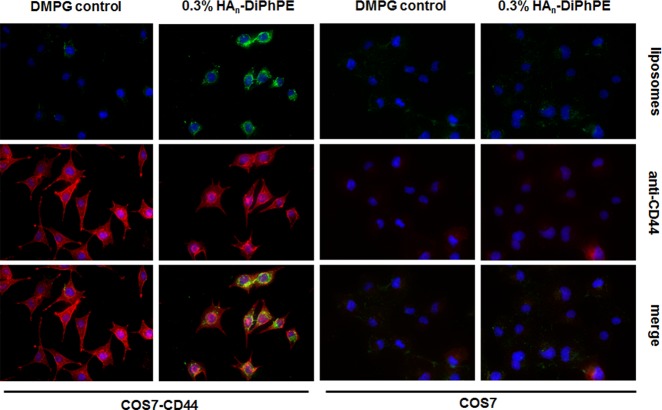
In the fluorescent confocal imaging analysis, HA-liposomes were internalized into CD44 expressing cells and colocalized with intracellular compartments that stained positive for the CD44 receptor (Figure 5), as we had previously reported for liposomes modified with a HA-conjugate prepared by reductive amination of the reducing end of HA oligomers.5 Control liposomes did not appreciably bind to the cultured cells nor were they internalized.
Figure 5.
Internalization of liposomes as visualized by imaging experiments in COS 7 cells overexpressing CD44 receptors (left two columns) and COS 7 cells lacking CD44 (right two columns). The first row consists of images of liposomes containing DiO tracker dye (green fluorescence). In the second row, CD44 receptors are detected with an anti-CD44 antibody (red fluorescence). The third row is a merged image of rows 1 and 2, respectively, illustrating the co-localization of HA-liposomes with compartments that stained positive for CD44 receptors. Under these incubation conditions there is little binding of control liposomes to either CD44 expressing or cells that did not express CD44.
In summary, we have designed a facile chemoenzymatic synthetic route to prepare HA-lipid conjugates that encompass a population of moderately sized HA oligomers, that may be useful for targeting liposomes, solid lipid particles, micelles, emulsions, or other nanoparticles to CD44 receptors. This approach can be extended to the conjugation of other drug molecules, imaging agents, and proteins to generate HA-conjugates containing moderately sized oligomers that may be useful for CD44 targeted imaging or drug delivery.
Experimental Procedures
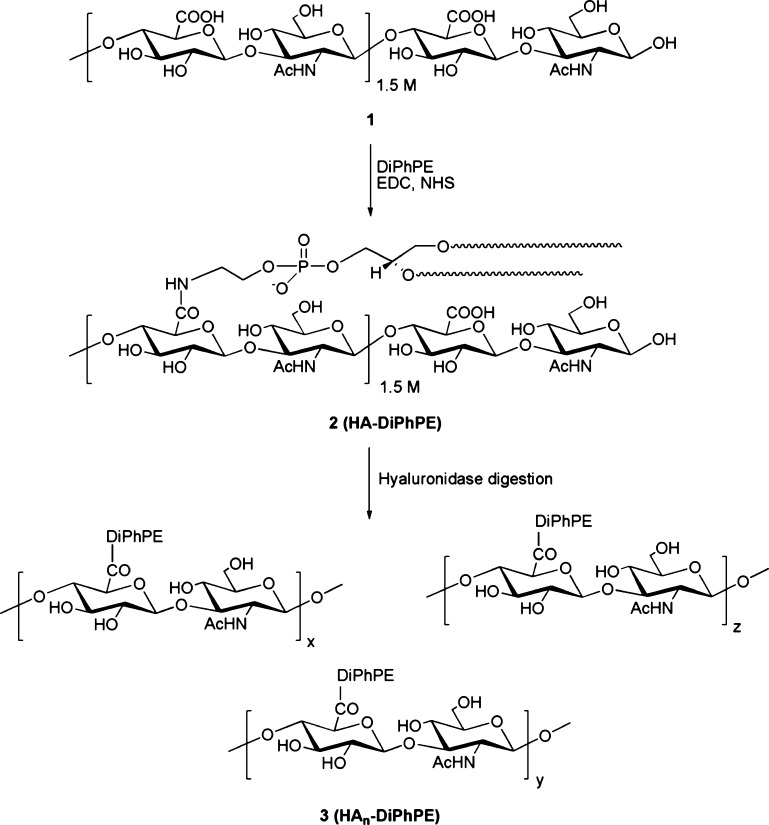
Synthesis of the Lipooligosaccharide 2 (Scheme 1)
Scheme 1. Chemoenzymatic Synthesis of HAn-DiPhPE Conjugates.
HMW sodium hyaluronate, molecular weight 1.5 MDa (Lifecore Biomedical, Chaska, MN), was converted to its tetrabutylammonium (TBA) salt using Dowex 50WX8–400 cation exchange resin as described previously.20 All solvents were anhydrous and the HA-TBA salt was co-dried with toluene prior to use. HA-TBA was dissolved in a (CH3)2SO:CH3OH solvent mixture (3:2) ratio v/v at a 6 mg/mL concentration to prepare an opalescent mixture. To this solution was added 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS), and the mixture was allowed to stir at 60 °C for 2 h, followed by addition of required equivalents of DiPhPE (Avanti Polar Lipids, Alabaster, AL) prepared in a 1:1 v/v solution of toluene and CH3OH. Another batch of EDC and NHS was added after 24 h, and the reaction was allowed to stir at 60 °C for 48 h to yield the corresponding HA-DiPhPE conjugate, with DiPhPE attached to every tenth monosaccharide via the carboxylate group on an average.
A typical preparation started with 300 mg of HA-TBA in 50 mL of (CH3)2SO:CH3OH (3:2 v/v) mixture. To this was added a solution of EDC (90 mgs) and NHS (90 mgs) in 3 mL of CH3OH. The mixture was stirred for 1.5 h at 60 °C, followed by the addition of a solution of DiPhPE (300 mg) prepared in 6 mL of 1:1 v/v toluene:CH3OH mixture. After 24 h, another batch of EDC and NHS (same amount as described above) was added and stirring was continued for an additional 24 h. The final reaction mixture was poured into acetone and centrifuged at 3800 rpm for 20 min. Acetone was decanted off and the precipitate was collected.
Enzymatic Digestion of HA-DiPhPE (2)
The above precipitate was suspended in digestion buffer (0.1 M sodium acetate, adjusted to pH 5.4) at a concentration of ∼100 mg/20 mL. To this was added 4000 U of bovine testicular hyaluronidase (Sigma-Aldrich Co., St. Louis, MO) and the solution was allowed to incubate at 37 °C for various times up to 48 h. The enzyme was then deactivated by immersing the test tube in boiling water for 10 min, and the contents were lyophilized.
Purification of HAn-DiPhPE Digest (3)
The lyophilized powder was dissolved in H2O and placed in a 25K cutoff dialysis membrane and dialyzed against 100 volumes of 1 M NaCl solution overnight, followed by H2O changed two times over a 2 day period. The dialyzed material was then lyophilized. The resulting white powder was dissolved in a 4:1 CH3OH:H2O solvent mixture and loaded onto a reverse phase C18 Sep Pak cartridge (Fisher Scientific, #11–131–8). HAn-DiPhPE was eluted out with 4:1 CH3OH and H2O, followed by elution of the unreacted DiPhPE using CH3OH and 3:2 CH3OH:CHCl3 successively. These solute mixtures contained 0.1% acetic acid to enable a cleaner separation. Each fraction was analyzed by MALDI-TOF (Microflex LT, Bruker Daltonics) using 2,4,6-trihydroxyacetone with sodium citrate as the matrix. The fractions were also spot tested with molybdenum stain for phosphate and ninhydrin reagent for amino functionality, respectively. NMR of the conjugate was recorded in (CD3)2SO on a 300 MHz Bruker instrument.
Phospholipase D Digestion of Purified HAn-DiPhPE
A minimum amount of purified HAn-DiPhPE was suspended in 0.1 M sodium acetate buffer pH 5.4, containing 20 mM CaCl2 to create an opalescent dispersion. To this was added 50 U of Phospholipase D (from white cabbage, Sigma-Aldrich Co., St. Louis, MO), and the mixture was allowed to incubate at 30 °C for 4 h. To this solution was added 1.1 volume CH3OH followed by 1.1 volume CHCl3 (to aqueous solution) and then transferred to a separating funnel. The contents were shaken well and allowed to form layers. The aqueous layer was collected and lyophilized, and analyzed by MALDI-TOF to determine the range of distribution of the HA oligosaccharides conjugated to the lipid. The control tube had everything except the enzyme and was processed in exactly the same manner as the experimental tube.
Analysis of Phosphate/Carbohydrate Ratio in the Conjugate
In order to experimentally determine an average ratio of the number of DiPhPE molecule conjugated to HA sugar units in the HAn-DiPhPE conjugate, we carried out a traditional phosphate colorimetric assay.21 We carefully weighed out a known amount of the conjugate and dissolved it in a known volume of water. The micromoles of phosphate present in the sample were determined from a phosphate standard curve. This value was converted to the micromoles of DiPhPE present in the sample. The ratio of lipid phosphate to HA carbohydrate was computed based upon the dried weight of the conjugate.
Liposome Preparation
Lipid films were prepared by drying 10 μmol of total lipid, including the HAn-DiPhPE conjugate on a rotary evaporator under vacuum. The lipid film was left to dry overnight under high vacuum. Liposomes (composed of POPC:Cholesterol:HAn-DiPhPE 50:40:0.03–0.3 mol %) containing 0.3 mol % of the fluorescent lipid tracer DiO (3,3′-dioctadecyloxacarbocyanine perchlorate) were prepared by rehydrating the above lipid film with 1 mL of 10 mM HEPES containing 10% sucrose (pH 7.4), followed by agitating the preparation using a vortex mixer for 1 min, and sonication at 40 °C for 20 min under argon. The liposomes were then extruded through a 0.08 μm polycarbonate membrane to produce liposomes of approximately 100 nm in size. Liposomes were stored at 4 °C under argon. Control liposomes were prepared at the same lipid concentration, but they contained dimyristoylphosphatidylglycerol (DMPG) at a mole ratio of 0.3 mol % in place of the HA lipid to provide a similar zeta potential to the control liposomes as used in the HA-containing liposomes.
Cell Culture Growth and of Liposome Binding Assay
All cells were maintained in DMEM media supplemented with 10% fetal calf serum, 20 mM Hepes (pH 7.5), and 2 mM glutamine. For binding assays, cells were seeded 24 h prior to incubation with liposomes, in a 96 well plate at a density of 25,000 cells per well. Just before liposome addition the cells were washed twice with DMEM media without serum. Cells were then incubated at 37 °C for 3 h with liposomes at indicated concentrations in serum free DMEM supplemented with 20 mM Hepes, 2 mM glutamine, and 3% BSA. After the 3 h incubation, cells were washed twice with serum free media and twice with PBS. Cells were trypsinized to remove them from the culture plate, diluted 4-fold with PBS, and immediately analyzed by FACS (BD Biosciences, San Jose, CA).
Cell Imaging Experiments
COS 7 cells were grown on glass coverslips 24 h prior to incubation with liposomes in DMEM + 10% FCS. Liposomes (100 nmol of lipid) were incubated with cells at 37 °C for 3 h in serum free DMEM supplemented with 20 mM Hepes, pH = 7.5, containing 3% BSA and 2 mM glutamine. Cells were washed after liposome incubation with serum free media (2 times) and then with PBS (2 times). Cells were fixed with 4% formaldehyde in PBS at 4 °C for 15 min. Fixed cells were then washed and blocked with 1% normal goat serum, 1% BSA in PBS for 1 h at room temperature. Blocked cells were incubated overnight at 4 °C with anti-CD44 (clone IM7). On the following day, cells were washed 5 times with PBS followed by an 1 h room temperature incubation with Alexafluor 568 conjugated goat anti-rat secondary antibody and 5 PBS washes. Cells were mounted in DAPI containing Prolong Gold mounting media and visualized by a Nikon Ti-E epifluorescent imaging system. The cells were imaged using an air Plan Apo 40x objective with NA 0.95. The filter set is DAPI/FITC/TRITC/Cy5/Cy7-optimized Sedat type penta-band filter set from Semrock on the 6D epifluorescence microscope at the UCSF Nikon center.
Acknowledgments
We gratefully acknowledge the financial support from the National Institutes of Health (GM 061851). We would like to thank Dr. David Tran for his assistance in running the phosphate assay.
Supporting Information Available
MALDI-TOF results of HA-DiPhPE digested at different time intervals; MALDI-TOF results of the PLD digested HAn-DiPhPE. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare the following competing financial interest(s): F.C.S. discloses a conflict of interest. F.C.S. has a financial interest in a liposome drug delivery company and also has liposome related patents that are owned by the University of California.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Stern R., Ed. (2009) Hyaluronan in Cancer Biology, Academic Press, Inc., San Diego, CA: ISBN 978–0-12–374178–3. [Google Scholar]
- Platt V. M.; Szoka F. C. Jr. (2008) Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol. Pharm. 5, 474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyani T.; Prestwich G. D. (1994) Functionalized derivatives of hyaluronic acid oligosaccharides: drug carriers and novel biomaterials. Bioconjugate Chem. 5, 339–347. [DOI] [PubMed] [Google Scholar]
- Akima K.; Ito H.; Iwata Y.; Matsuo K.; Watari N.; Yanagi M.; Hagi H.; Oshima K.; Yagita A.; Atomi Y.; Tatekawa I. (1996) Evaluation of antitumor activities of hyaluronate binding antitumor drugs: synthesis, characterization and antitumor activity. J. Drug Targeting 4, 1–8. [DOI] [PubMed] [Google Scholar]
- Eliaz R. E.; Szoka F. C. Jr. (2001) (2001) Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 61, 2592–2601. [PubMed] [Google Scholar]
- Peer D.; Margalit R. (2004) Tumor-targeted hyaluronan nanoliposomes increase the antitumor activity of liposomal Doxorubicin in syngeneic and human xenograft mouse tumor models. Neoplasia 6, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D.; Margalit R. (2004) Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int. J. Cancer 108, 780–789. [DOI] [PubMed] [Google Scholar]
- Eliaz R. E.; Nir S.; Marty C.; Szoka F. C. Jr. (2004) Determination and modeling of kinetics of cancer killing by doxorubicin and doxorubicin encapsulated in targeted liposomes. Cancer Res. 64, 711–718. [DOI] [PubMed] [Google Scholar]
- Dufaÿ Wojcicki A.; Hillaireau H.; Nascimento T. L.; Arpicco S.; Taverna M.; Ribes S.; Bourge M.; Nicolas V.; Bochot A.; Vauthier C.; Tsapis N.; Fattal E. (2012) Hyaluronic acid-bearing lipoplexes: physico-chemical characterization and in vitro targeting of the CD44 receptor. J. Controlled Release 162, 545–552. [DOI] [PubMed] [Google Scholar]
- Arpicco S.; Lerda C.; Dalla Pozza E.; Costanzo C.; Tsapis N.; Stella B.; Donadelli M.; Dando I.; Fattal E.; Cattel L.; Palmieri M. (2013) Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur. J. Pharm. Biopharm. 85, 373–380. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Prestwich G. D. (1999) Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjugate Chem. 755–763. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Ziebell M. R.; Prestwich G. D. (2000) A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules 1, 208–218. [DOI] [PubMed] [Google Scholar]
- Yerushalmi N.; Arad A.; Margalit R. (1994) Molecular and cellular studies of hyaluronic acid-modified liposomes as bioadhesive carriers for topical drug delivery in wound healing. Arch. Biochem. Biophys. 313, 267–273. [DOI] [PubMed] [Google Scholar]
- Journo-Gershfeld G.; Kapp D.; Shamay Y.; Kopecek J.; David A. (2012) Hyaluronan oligomers-HPMA copolymer conjugates for targeting Paclitaxel to CD44-overexpressing ovarian carcinoma. Pharm. Res. 29, 1121–1133. [DOI] [PubMed] [Google Scholar]
- Zeng C.; Toole B. P.; Kinney S. D.; Kuo J. W.; Stamenkovic I. (1998) Inhibition of tumor growth in vivo by hyaluronan oligomers. Int. J. Cancer 77, 396–401. [DOI] [PubMed] [Google Scholar]
- Ruhela D.; Riviere K.; Szoka F. C. Jr. (2006) Efficient synthesis of an aldehyde functionalized hyaluronic acid and its application in the preparation of hyaluronan-lipid conjugates. Bioconjugate Chem. 17, 1360–1363. [DOI] [PubMed] [Google Scholar]
- Laurent T. C.; Fraser J. R. E.; Pertoft H.; Smersrød B. (1986) Binding of hyaluronate and chondroitin sulphate to liver endothelial cells. Biochem. J. 234, 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M. S.; Baggenstoss B. A.; Washburn J.; Harris E. N.; Weigel P. H. (2013) Glycobiology and Extracellular matrices: The hyaluronan receptor for endocytosis (HARE) activates NF κB mediated gene expression in response to 40–400 KDa, but not smaller or larger hyaluronans. J. Biol. Chem. 288, 14068–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara S.; Okuno S.; Yano T.; Hamana H.; Inoue K. (2001) Characteristics of tissue distribution of various polysaccharides as drug carriers: influence of molecular weight and anionic charge on tumor targeting. Biol. Pharm. Bull. 24, 535–543. [DOI] [PubMed] [Google Scholar]
- Oh E. J.; Park K.; Choi J. S.; Joo C. K.; Hanh S. K. (2009) Synthesis, characterization, and preliminary assessment of anti-Flt1 peptide-hyaluronate conjugate for the treatment of corneal neovascularization. Biomaterials 30, 6026–6034. [DOI] [PubMed] [Google Scholar]
- Bartlett G. R. (1959) Phosphorus assay in column chromatography. J. Biol. Chem. 234, 466–468. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.