Abstract
The interactions between the immune and nervous systems are very complex, and yet our understanding of these interactions is still relatively limited. The neuroinflammatory reaction that can accompany HIV infection occurs because of a cascade of events that appears to require the migration of HIV-infected cells across the blood–brain barrier. In susceptible individuals, this leads to inflammatory processes which can include substantial changes in neuronal function. It is possible to consider the inflammatory events to be composed of two essential processes. The first process is cellular traffic, and the second, is the expression and recognition of the various pro-inflammatory and/or toxic mediators. The added complication of drug abuse adds complexity to the traffic and mediator release events, and depending on the specific drug being abused, the disease can be exacerbated in these individuals. An understanding of the fine details of these mediator and traffic processes should provide useful targets for therapeutic intervention to attenuate disease associated with HIV infection.
Keywords: Neuroimmune axis, Drugs of abuse, AIDS, HIV
Introduction
This review is intended to provide a framework for instruction in the area of neuroimmune pharmacology. This overview is not intended as an in depth discussion of each area of the immune system that might be involved in the pharmacology of the interactions of the nervous and immune systems. Rather, the intention is to provide a description of selected critical areas of immunology that are particularly relevant to the field of neuroimmune pharmacology. A set of references is provided at the end of this review which should allow for more depth into the various aspects of immunity that are discussed. The author of this review is engaged in studies on the function of the immune system as it intersects with the nervous system, and as such, he is also a “student” of this area. Students at every level will hopefully find this discussion of selected areas of immunology to be helpful in either teaching, or additional learning, in the field of neuroimmune pharmacology (Ikezu and Gendelman 2008; Gendelman et al. 2005).
The field of immunology is very broad, and it is important to touch on subject areas that are relevant to this sub-field. It is important to keep in mind that this sub-field is rapidly expanding and becoming more diverse. Consequently, the subject matter that will be discussed will almost certainly fall short of the field as it grows over time. Boyle’s law indicates that a body cools as it expands, and yet it appears certain that the field of neuroimmune pharmacology is becoming very “hot” (at least in terms of the research that is being carried out). In order to obey Boyle’s law, the number of investigators working in this area must be growing (increasing the volume of research), in order for the temperature to continue to rise.
The immune response has both “innate” and “acquired” components, and it is becoming apparent that these components have considerable overlap. The innate defense system is composed of phagocytic cells (e.g., neutrophils and monocytes in the blood), anatomic barriers to infection (e.g., skin and mucous membranes), and physiological or chemical barriers (low pH, lysozyme, and body temperature). One of the consequences of the innate immune system is the inflammatory response. Inflammation is a complex process involving most of the cells of the immune system, mediators released from these cells, the participation of structural cells including endothelial cells and epithelial cells, and chemoattractant molecules produced by various cells (Segal 2005; Soehnlein and Lindbom 2010; Luo et al. 2007; Barton and Kagan 2009; Watts 2005; Sims and Smith 2010; Dinarello 2009). The inflammatory response also involves the activation of leukocytes through one or more pattern recognition receptors, and the most critical of these proteins are the toll receptors (Barton and Kagan 2009). These receptors typically recognize a particular chemical structure that is a microbial product, and the activation of toll receptors leads to the induction of leukocyte function. In an inflammatory response induced by microbial infection, the activation of leukocyte functional activity often leads to a protective immune response.
Acquired or “adaptive” immunity is a response that is specific for the antigen, and requires the interactions of antigen-presenting cells (e.g., dendritic cells) and T cells. This interaction typically leads to the activation of T cells, and once activated, a sub-population of T cells is able to “help” B cells differentiate into antibody-producing plasma cells. There are multiple sub-types of T cells, and these can be partially defined on the basis of the proteins that each population express on their cell surface (e.g., CD4 or CD8), or the cytokines that each sub-type produces (Castellino and Germain 2006; Zhu et al. 2010). The T cells which are initially stimulated by antigen-presenting cells express CD4, and produce cytokines that assist or “help” B cells become activated antibody-producing cells. These CD4-expressing T cells also help CD8-expressing T cells to become activated, and differentiate into T cells which have cytotoxic or “killer” or “cytotoxic” activity (Castellino and Germain 2006). Finally, the CD4 cells are actually composed of multiple sub-populations which differ on the basis of the collection of cytokines that these cells produce (Zhu et al. 2010; Belkaid and Tarbell 2009; Sakaguchi et al. 2010). For example, “TH1” cells have pro-inflammatory activity, and produce cytokines including interferon-γ (IFNγ) and interleukin (IL)-2. However, the strongest pro-inflammatory CD4 T cell sub-population is referred to as “TH17” because these cells produce IL-17, along with several other pro-inflammatory cytokines. These sub-populations are activated in certain physiological situations, and have the potential to play a substantial role in the immune response.
An analysis of the literature suggests that the growth in interest in the area of neuroimmune pharmacology is due, in large part, to increased activity by investigators who are involved in studies in one (or more) of three areas: first, investigators interested in the effects of drugs of abuse on the immune system; second, those interested in the neurological consequences of HIV infection (or other diseases which have a component of neurodegeneration); and third, those interested in the role of the immune response in the etiology of pain. An important underlying consideration for these areas of research is the nature of the interactions between the immune and nervous systems. These interactions primarily occur at two fundamental levels; first, through the production of various mediators (cytokines, chemokines, neuropeptides, neurotransmitters, etc.), and their regulation of the functions of both immune and neuronal cells (Randolph et al. 2008; Woodland and Kohlmeier 2009; Auffray et al. 2009; Martinez et al. 2009; Pollard 2009; Geissmann et al. 2010; Ma et al. 2006; Theofilopoulos et al. 2005; Cyster 2005; Allen et al. 2007; Zhang and Oppenheim 2005); and second, through the ability of cells of the immune system to traffic in and out of the central nervous system (Roberts et al. 2010; Banks and Erickson 2010; Persidsky and Poluektova 2006; Persidsky et al. 2006). In other words: “Mediators and Traffic.” Of course, there are a number of additional issues that contribute to the interplay of neuronal cells and leukocytes, but these events tend to be at least somewhat secondary.
Mediators
There are literally hundreds of mediators that contribute to the function of the immune system. It has become very clear that receptors for many of these “immune” mediators also are expressed by neuronal cells. The expression of these receptors on both neuronal and immune cells provides the basis for the ability of the immune system to influence the function of the nervous system. In addition, we now know that mediators previously thought to be primarily neuronal, are recognized by receptors expressed on one or more types of immune cells. This includes receptors for the μ-, κ-, and δ-opioids, and there is now a substantial literature which documents the function of opioid receptors in the function of the immune system (Finley et al. 2008; Bidlack et al. 2006; Eisenstein et al. 2006; Machelska and Stein 2006; Molina 2006). As such, these receptors are prime targets for the effects of the opioid drugs of abuse, and the capacity of these receptors to modulate immune and neuronal cell function is an important considerations for the study of drugs of abuse (Cabral and Griffin-Thomas 2009; Klein and Cabral 2006; Ferris et al. 2008; Ugen and Nyland 2006; Achur et al. 2010; Szabo and Mandrekar 2009).
It is helpful to classify these mediators in various ways in order to associate the activities of the mediators with a particular aspect of immune or neuronal function. For example, a number of immune mediators are participants in (or promote) inflammatory reactions, and as such, are classified as “pro-inflammatory.” Several of the receptors for the pro-inflammatory mediators are expressed by neuronal cells, including both IL-1 and TNFα, and as one might expect, these mediators are critical participants in neuroinflammatory disease states (Watts 2005; Sims and Smith 2010; Dinarello 2009). Moreover, we are now learning that these mediators can participate in the regulation of “normal” neuronal function.
As we move ahead, it is important to consider the broader activities of mediators, and understand that these important molecules have functional activities outside of a single specific interest area. The study of mediators is an essential element of both immunology and neuroscience, and the study of the mechanisms of action of the mediators is important in both fields. Moreover, the biochemical and molecular basis for mediator action in leukocytes can provide valuable insights for the mechanisms of action in neuronal cells, and vice versa. It is common to organize mediators in a systematic manner in order to ease the learning experience in the classroom. The following organization might be considered as a part of the classification of mediator activity.
Proinflammatory. Examples: IL-1, TNF-α, IL-6, and IL-17.
Anti-inflammatory. Examples: IL-10 and TGF-β.
Chemoattractants. Examples: CCL2, CCL5, CXCL10, and CX3CL1, and their critical receptors CCR2, CCR5, CXCR3, and CX3CR1.
Cellular activators. Examples, IFN-γ and IL-2.
Cell and tissue homeostasis or development. Examples, CXCL12 and IL-7.
It should be understood that there is considerable overlap among the mediators with this organization (Watts 2005; Sims and Smith 2010; Dinarello 2009; Cyster 2005; Allen et al. 2007; Zhang and Oppenheim 2005). For example, CCL2 is a chemoattractant, and like IL-8, is very pro-inflammatory. Moreover, several mediators can express distinct activities depending on the cell population. A good example is TGF-β, an extremely pleotropic cytokine. One might consider including some discussion of why mediators have a given set of activities in one particular set of cells, but not others. This is particularly relevant to an understanding of the diversity of actions of mediators in the CNS when compared with the peripheral immune system. For example, the chemokine CXCL12 is a potent chemoattractant for leukocytes and regulates the movement of immature leukocytes out of the bone marrow. However, in the brain, this chemokine can regulate the inflammatory response to certain HIV products.
It is important to provide a discussion of mediators to students in a digestible form. In a discussion of mediators which participate in neuroinflammatory disease states it may be a mistake to attempt to exhaustively discuss every pro-inflammatory mediator. An alternative approach would be to discuss the major participants in certain disease states, and allow for the fact that inevitably a number of other molecules are almost certain to make an additional contribution (either positive or negative). It is particularly important to include a discussion of the essential attributes of immune mediators. These include synergy (the ability of two or more mediators to induce an effect which is much greater than the additive effects of the individual mediators alone), pleiotropy (the ability of a single mediator to act on multiple cellular targets to induce distinct actions), and redundancy (the ability of two or more mediators to induce a given activity on a target cell).
Perhaps the most important lesson in a discussion of mediators is the fact that in nature, mediators are never alone. Even though researchers typically study one mediator at a time, mediators always are present in a complex mixture of other mediators (plus other biologically active “stuff”). How a mediator works when it is present without other mediators in a laboratory setting is informative, but it falls short of recapitulating the true biologically relevant condition. In addition, the functional activity of a given chemoattractant can be affected by the local anatomical environment. For example, the capacity of a leukocyte to migrate is dependent on the presence of adhesion molecules, in addition to other chemoattractants that are also present (Randolph et al. 2008; Woodland and Kohlmeier 2009). This results in a great deal of diversity when comparing the migration of a leukoctye out of blood vessels, as opposed to migration within the brain (or the brain when infected with HIV, or exposed to drugs of abuse, etc.).
Traffic
The immune system is dependent on cellular traffic for normal functional activity. In fact, it is unlikely that an acquired immune response is possible in the absence of leukocyte traffic (Belkaid and Tarbell 2009; Sakaguchi et al. 2010). This requirement for cellular mobilization appears to be a critical distinction with the functional activity of the nervous system. The generation of a neuroinflammatory response typically requires the transit of leukocytes across the blood–brain barrier (BBB). The cellular and biochemical nature of the function of the BBB is an area of intense study at this time, because of the obvious role of this structure in the development of neuroinflammatory diseases such as multiple sclerosis and AIDS encephalitis (Roberts et al. 2010; Banks and Erickson 2010; Persidsky and Poluektova 2006; Persidsky et al. 2006). The capacity of cells to pass the BBB is based on several factors, but most prominently, the function of chemoattractant mediators and the expression and activation of adhesion molecules on the surfaces of leukocytes and endothelial cells.
Our current understanding of neuroinflammation suggests that neuronal cells are a source of chemokines such as CCL2 and IL-8, and these chemoattractants promote the traffic of leukocytes across the BBB. Once present within the brain, these leukocytes may take up residence within brain tissue, and then serve as an additional source of chemokines and other pro-inflammatory mediators (Rivest 2009; Gonzalez-Scarano and Martin-Garcia 2005; McMichael et al. 2010; Hauser et al. 2007). This can serve as a self-perpetuating inflammatory stimulus, and in addition, increase the potency and complexity of the mediator mixture. In addition, these leukocytes also serve as a potential source of mediators which can influence the function of neuronal cells. This is a particularly important consideration because these influences can be very complex. For example, the release of pro-inflammatory cytokines from brain-resident leukocytes can influence the function of microglial cells and promote the release of additional pro-inflammatory mediators (Rivest 2009). Moreover, neurons can respond directly to cytokines such as IL-1 and TNFα, and neuronal function may be altered by this change in the microenvironment. Recent research suggests that the release of chemokines within the brain can result in substantial alterations in the activity of neurons (Rivest 2009; Hauser et al. 2007).
A discussion of traffic should include mention of the important immunological paradigms related to T cell function. Specifically, it is increasingly apparent that both TH1 and TH17 immunity are important considerations for neurodegenerative diseases. Here again, the relevant mediators that characterize these two types of T cell function should be included in any discussion of the intersection of the immune and nervous systems (Roberts et al. 2010; Banks and Erickson 2010). Much more needs to be learned regarding the combination of chemoattractants and adhesion molecules which specifically direct the traffic of TH1 and TH17 cells across the BBB or other sites of inflammation. An examination of these issues should prove to be an exciting area of future research.
Issues related to HIV infection
There are a number of immunological issues which are relevant to a meaningful discussion of the interactions between the immune and nervous systems in the context of HIV infection. Here again, many of these issues can be considered issues of “Mediators and Traffic.” First, the attachment of HIV-1 to monocytes/macrophages and T cells is dependent on the expression of the viral receptor CD4, as well as one or more co-receptor which is most often either CCR5 or CXCR4. Of course the latter proteins also are chemokine receptors, and the fact that the immune system and the virus both use these receptors presents interesting issues for the response to the infection (Gonzalez-Scarano and Martin-Garcia 2005; McMichael et al. 2010). On the one hand, the capacity of leukocytes to produce chemokine ligands for the co-receptors presents a double-edged sword for the virus. Leukocytes at sites of inflammation produce substantially increased levels of chemokines such as CCL2, CCL3, and CCL5, and these are all ligands for CCR5. The production of these chemokines could inhibit virus infection of monocytes and macrophages by blocking viral attachment to CCR5. It is particularly interesting that HIV-1 infection itself induces the expression of these (and other) chemokines (Gonzalez-Scarano and Marin-Garcia 2005). On the other hand, the production of these chemokines at sites of infection will likely attract uninfected cells that also express CCR5 and are susceptible to infection. This may be a means by which the virus has evolved a strategy for spread of the infection by actively attracting uninfected susceptible target cells to anatomical sites where the virus is being produced.
Cells that are either infected by HIV, or are activated as a part of the immune response against the virus, can produce a number of mediators in addition to the CCR5 ligands just mentioned. These mediators include other chemokines, pro-inflammatory cytokines, and products of oxidative metabolism. The release of these mediators at sites of infection can promote tissue damage, as in the case of HIV-induced encephalitis (Gonzalez-Scarano and Martin-Garcia 2005; McMichael et al. 2010). These mediators can also induce the production of additional pro-inflammatory mediators and the potentially self-perpetuating response as described previously. Moreover, among the mediators that are produced during HIV infection is IFNγ, a cytokine with potent anti-viral activity. HIV has evolved multiple biochemical mechanisms to prevent the antiviral activity of IFN, and this is important when considering the nature of the immune response during HIV infection.
Finally, the development of neuroinflammation and degeneration associated with HIV infection is related directly to the process of leukocyte trafficking. As mentioned previously, the production of chemokines such as CCL2, largely by microglial cells, promotes the transit of HIV-infected monocytes across the BBB (Roberts et al. 2010). These monocytes rapidly differentiate into macrophages and take up residence in the perivascular space. These cells release the virus, which spreads to susceptible microglial cells, and this provides an additional source of infectious virus. The release of viral products, including gp120 and tat, influences the function of neuronal cells directly, leading to altered function and/or damage of neurons, activation of microglia and astrocytes, and the subsequent release of mediators which can further damage neuronal tissue. This neurodegeneration demonstrates the cooperation between the virus, the cells of the peripheral immune system, and neuronal cells. Each of the cells in this process are “activated” by both viral products and cellular mediators, and each cell in turn actively extends the cascade by releasing additional mediators. The process eventually leads to alterations in the function of neurons, potentially leading to altered cognitive ability (Kraft-Terry 2010).
Drugs of abuse
A substantial portion of HIV-infected individuals also abuse drugs, and the complex interplay between the virus, and the immune and nervous systems is complicated in these individuals by the added influences of these drugs. Moreover, in almost all cases, the abuse involves more than a single drug, making the situation much more complicated. Let us consider the situation which occurs with opioid abuse. The opioid drugs are well documented to manifest a broad array of effects on the functional activities of the cells of the immune system (Finley et al. 2008; Bidlack et al. 2006; Eisenstein et al. 2006; Machelska and Stein 2006; Molina 2006). For example, the μ-opioids (including morphine) are documented to induce the expression of both CCR5 and CXCR4 on both monocytes and T cells. In addition, these opioids also stimulate the production of a number of chemokines, including those that are ligands for CCR5. (The potential influence of the production of these chemokines on the response to HIV was discussed above.) Studies are currently ongoing to determine whether the opioid modulation of chemokine and chemokine receptor expression influences the progression of disease following HIV infection. It will be very interesting to determine whether there is a change in the development of neuroinflammation and neurodegeneration following HIV infection in those individuals with opioid abuse.
Conclusions
This overview is intended to provide a discussion of the nature of the interactions between the immune and nervous systems that are relevant during HIV infection, and the added complications that arise with drug abuse. It is suggested that two basic phenomena, mediators and traffic, are critical for the intersection between the immune system, the virus, the nervous system, and drugs of abuse. As one formulates an educational program that is relevant to these issues, it is recommended that several fundamental events should be points of emphasis. These events are summarized in Fig. 1, and a series of lectures might be organized to cover these events as a prelude to more specific and detailed lectures (or more advanced coursework) that cover additional topics of neuroimmune pharmacology.
Fig. 1.
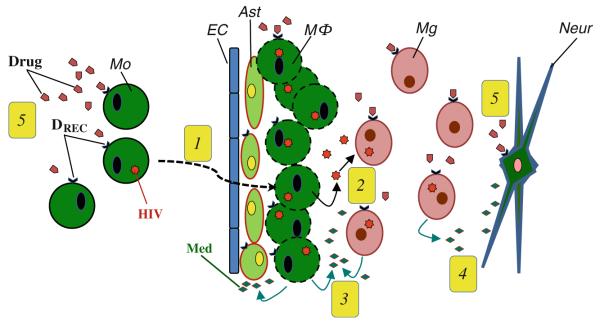
Critical event sets in neuroinflammation that are associated with HIV-1 infection and drug abuse. 1 Peripheral blood HIV-infected monocytes express chemokine receptors which permit the migration of these cells across the blood–brain barrier. This process involves a series of changes in the functional status of the monocytes, endothelial cells, astrocytes, and microglial cells. 2 The immigrated HIV-infected monocytes accumulate as perivascular macrophages and release HIV which infects microglial cells. 3 Infected macrophages, microglia, and astrocytes release a complex mixture of mediators including chemoattractant factors, proinflammatory cytokines, and toxic factors. 4 Mediator release also influences neurons leading to changes in neuron function. 5 Drugs of abuse alter the functional activity of HIV-infected blood monocytes, macrophages, astrocytes, microglial cells, and neurons. The complex of mediators and drugs of abuse result in complicated changes in the dynamic among these cell populations. Ast astrocytes, DREC drug receptor, EC endothelial cells, Mo monocyte, Mg microglial cell, MΦ macrophage, Med mediator, Neur neuron
It should be apparent that the interactions of the immune and nervous systems are very complex, and we are only now at an early point in an appreciation of the extent of these interactions. Our current understanding of how these systems interact has historically been guided largely by studies of certain disease states where the two systems are important. This has included research using models of multiple sclerosis in rodents, as well as studies using neurotropic viral infections in experimental animals. Research into the neuroinflammatory response following HIV infection will almost certainly continue to contribute a great deal to our understanding of the potential for interactions between these systems.
Acknowledgments
Source of Support Supported, in part, by NIH grants DA-14230, DA-25532, PO1DA-23860, and P30DA-13429.
Footnotes
Conflict of Interest Disclosure The author has neither a financial nor a personal relationship which might bias this work.
References
General Readings: The following references provide sections with a general discussion of the area of neuroimmune pharmacology. These texts are well referenced and can be used as a starting point for more extensive research.
- Gendelman HE, Grant I, Everall IP, Lipton SA, Swindells S, editors. The neurology of AIDS. 2nd ed. Oxford University Press; 2005. ISBN: 0-19-852610-5. [Google Scholar]
- Ikezu T, Gendelman HE, editors. An overview: neuroimmune pharmacology. Springer Science; New York: 2008. Reviews of Specific Areas: A. Innate immunity and inflammation. The following references provide a discussion of cells of the innate immune system, the soluble mediators which are important for the inflammatory response, and the receptors that are critical for the evolution of the inflammatory response.
- Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. The following review describes the basic biology of the cell considered the first line of defense against microbial infectious agents. It includes a discussion of mechanisms of phagocytosis and the biochemistry of microbicidal activity.
- Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. The following is an excellent review of the cellular interactions involved in the traffic of phagocytic cells to sites of inflammation, and the mediators which control these interactions. An outstanding discussion of the phases of inflammatory response.
- Barton GM, Kagan JC. A cell biological view of toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Luo B-H, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. B. Adaptive Immunity. The papersin this section review many of the cell populations that are responsible for acquired immunity, the receptors expressed by these cells, and the soluble mediators produced by these diverse leukocytes. CD4-expressing T cells provide “help” to both B cells and CD8+ T cells. The following review describes the complexities that are a part of the interactions between these two T cell populations.
- Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. An excellent overview of the TH1, TH2, and TH17 cell populations, and the molecular basis for the expression of cytokines in these individual sub-populations. The following review also describes a population of “inducible” regulatory T cells (“iTreg”) that contribute to the down-regulation of effector T cell activity.
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. The following is an excellent review of the functional activity of “natural” (or “non-inducible”) regulatory T cells and the contribution of these cells during the immune response to certain infectious agents.
- Belkaid Y, Tarbell K. Regulatory T cells in the control of host–microorganism interactions. Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FoxP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. References Randolph et al (2008) and Woodland and Kohlmeier (2009) describe the trafficking of dendritic and T cell populations that occurs as a part of the adapative immune response. These excellent reviews point out the critical role of cell mobility for the immune response.
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowatt AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. References Auffray et al (2009), Martinez et al. (2009), Pollard (2009) and Geissmann et al. (2010) describe monocyte and macrophage biology and the role of these cells in the immune response. These papers also discuss the heterogeneity in the functional activity of these cells, and the importance of this diversity in certain inflammatory disease states.
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. C. Chemoattractants. These reviews describe some of the endogenous chemoattractants that are involved in both innate and adapative immune responses. It should be appreciated that the chemoattractants described in these papers represent only a small fraction of the total number of chemoattractants that are produced in a given tissue, particularly during an inflammatory response.
- Allen SJ, Crown SE, Handel TM. Chemokine:Receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Chemokines, Sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Zhang N, Oppenheim JJ. Crosstalk between chemokines and neuronal receptors bridges immune and nervous systems. J Leukoc Biol. 2005;78:1210–1214. doi: 10.1189/jlb.0405224. D. Blood–Brain Barrier and Leukocyte Traffic into the Brain. A critical element of neuroinflammatory reactions is the migration of cells of the immune response across the blood-brain barrier. This process involves the active participation of several cell populations, and requires several biochemical events on the vascular endothelial surface in order for leukocyte migration to occur.
- Banks WA, Erickson MA. The blood–brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Poluektova L. Immune privilege and HIV-1 persistence in the CNS. Immunol Rev. 2006;213:180–94. doi: 10.1111/j.1600-065X.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–36. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- Roberts TK, Buckner CM, Berman JW. Leukocyte transmigration across the blood–brain barrier: perspectives on neuroAIDS. Front Biosci. 2010;15:478–536. doi: 10.2741/3631. E. Drugs of Abuse and the Immune Response. Most of the drugs of abuse exert effects on the immune system, and the literature suggests that both innate and adaptive immunity is modulated by these drugs. Several excellent reviews have recently been published which describe the activities of opioids, cannabinoids, cocaine and alcohol on cells of the immune system.
- Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlack JM, Khimich M, Parkhill AL, Sumagin S, Sun B, Tipton CM. Opioid receptors and signaling on cells from the immune system. J Neuroimmune Pharmacol. 2006;1:260–269. doi: 10.1007/s11481-006-9026-2. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 2009;11:e3. doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK, Rahim RT, Feng P, Thingalaya NK, Meissler JJ. Effects of opioid tolerance and withdrawal on the immune system. J Neuroimmune Pharmacol. 2006;1:237–249. doi: 10.1007/s11481-006-9019-1. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol. 2008;252:146–54. doi: 10.1016/j.cellimm.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW, Cabral GA. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J Neuroimmune Pharmacol. 2006;1:50–64. doi: 10.1007/s11481-005-9007-x. [DOI] [PubMed] [Google Scholar]
- Machelska H, Stein C. Leukocyte-derived opioid peptides and inhibition of pain. J Neuroimmune Pharmacol. 2006;1:90–97. doi: 10.1007/s11481-005-9002-2. [DOI] [PubMed] [Google Scholar]
- Molina PE. Opioids and opiates: analgesia with cardiovascular, haemodynamic and immune implications in critical illness. J Int Med. 2006;259:138–154. doi: 10.1111/j.1365-2796.2005.01569.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugen KE, Nyland SB. Injecting drugs of abuse and immunity: implications for HIV vaccine testing and efficacy. Springer Semin Immunopathol. 2006;28:281–7. doi: 10.1007/s00281-006-0045-0. E. Neurodegeneration and AIDS. These reviews describe innate immune responses in the brain, and the inflammatory processes that occur in the brain that are responsible for the neurodegeneration that can be associated with HIV infection. These papers provide an overview of inflammation in the brain, and the cells and mediators that are particularly critical for inflammation at this specific tissue site.
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–578. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]


