Abstract
Background
Limited understanding of the interpretability of patient-reported pain scores may impact pain management. The current study assessed the minimal clinically significant improvement in pain and pain scores signifying patient-reported need for medication and treatment satisfaction in patients with sickle cell disease (SCD).
Procedure
Patients, 8–18-years-old, with SCD were recruited while receiving treatment for pain. Patients completed initial pain severity ratings using the Visual Analog Scale (VAS) and the Numeric Rating Scale (NRS). Serial assessments of pain severity, pain relief, perceived need for medication, and treatment satisfaction were completed every 30 minutes within the emergency department and 3 times daily throughout the hospitalization. Data were used to calculate the minimal clinically significant improvement in pain and pain scores associated with perceived need for pain medication and treatment satisfaction.
Results
Twenty-eight patients completed 305 assessments during 37 total visits. A decrease in pain severity score of 0.97cm for the VAS and 0.9 for the NRS was found to be the minimum clinically significant improvement in pain. Pain scores greater than 7.45cm on the VAS or 7.5 on the NRS were suggestive of patient-reported need for pain medication. Pain scores less than 7.35cm on the VAS or 8.5 on the NRS were suggestive of patient-reported treatment satisfaction discrimination.
Conclusions
The minimal clinical significant improvement was defined for the VAS and NRS and both scales were able to discriminate between important clinical findings including pain relief, need for pain medication, and treatment satisfaction. Collectively, this study provides data designed to improve our understanding of pain severity ratings of pediatric patients with SCD.
Keywords: pain assessment, sickle cell disease, pain, pediatrics
INTRODUCTION
Sickle cell disease (SCD) is an inherited hemoglobinopathy affecting over 100,000 individuals within the United States.1 Vaso-occlusive pain events (VOE), the hallmark of SCD, occur unpredictably and vary in severity and frequency. Although many episodes are managed at home, individuals frequently require stronger analgesics warranting emergency department care or hospitalization. Overall, pain accounts for up to 91% of emergency department visits and 68% of hospital admissions in SCD.2
Guidelines for the assessment and management of acute pain, such as VOE, recommend that pain severity be assessed before initiating treatment and routinely throughout treatment to guide medical decision making.3–5 Given the importance of pain assessment during the treatment process, attempts to standardize routine pain assessment have occurred. Despite these efforts, research has not consistently found improvement in pain outcomes, such as pain severity or treatment satisfaction, secondary to changes in pain assessment and documentation.6–9
The lack of an association between routine pain assessment and improved pain outcomes may be related to poor understanding of the clinical significance or interpretability of pain scores thus limiting their usefulness in the medical decision making process.10–12 Several studies have attempted to improve the understanding of self-reported pain scores by comparing these scores to more clinically relevant measures. For example, studies have compared patient-reported pain scores to patient-reported perceptions of pain relief, need for medication, and treatment satisfaction in adults experiencing acute or chronic pain.13–20 To date, relatively few of these studies have been completed in pediatric patients undergoing acute pain management21–23 and no studies have included pediatric patients with SCD, whom experience recurrent pain events. Ultimately, further work focused on understanding pain scores could be used clinically to guide medical decision making and serve as treatment outcomes in future pain research.
As noted, guidelines for pain management in SCD recommend that pain be assessed routinely to guide treatment of pain.4,5 Nevertheless, patients with SCD often report suboptimal treatment of their pain, which may be attributed to limited understanding of patient-reported pain by medical providers.24,25 Therefore, we completed the current study to address the limited understanding of patient-reported pain scores in children with SCD. Specifically, the aim of the current study was to determine in pediatric patients with SCD undergoing treatment for uncomplicated VOE: 1) the change in pain scores over time that would signify the minimal clinically significant improvement in pain, 2) the pain score signifying patient-reported need for pain medication, and 3) the pain score signifying patient-reported treatment satisfaction.
METHODS
Participants
Patients were recruited from the emergency department at a children’s hospital in the Midwest. Patients were eligible for participation if they met all of the following criteria: 1) between 8 and 18 years of age; 2) English as their primary language, 3) documented diagnosis of any genotype of SCD; and 4) receiving analgesic treatment for an uncomplicated VOE (i.e., no presence of acute chest, stroke, or priapism symptoms). Patients underwent routine pain management utilzing the institutions standardized treatment protocol. This protocol incorporates immediate intravenous access followed by provision of intravenous morphine or dilaudid. If pain does not improve after two doses of intravenous medication, patient controlled analgesia is used and the patient is admitted for continued pain management. While admitted, patients remain on continued patient controlled analgesia until they can tolerate oral pain medications.
All procedures were approved by the Institutional Review Board. Written informed consent from parents and guardians and written assent from children/adolescents were obtained prior to participation in the study. Patients and parents/guardians were compensated for their time with gift cards to a local store upon study completion. Patients were allowed to participate twice in this study.
Procedure
Patients completed initial ratings of pain severity using both the Visual Analog Scale (VAS) and the Numerical Rating Scale (NRS) within 1 hour of arriving to the emergency department. Thereafter, patients completed serial assessments of pain severity using both the VAS and NRS simultaneously every 30 minutes while in the emergency department and 3 times daily (0800–1000, 1400–1600, 2000–2200) while inpatient. Patients also completed ratings of perceived pain relief, need for pain medication, and treatment satisfaction during each serial assessment. Patients were blinded to their responses from previous assessments. Parents completed a demographic/health questionnaire.
Measures
Demographic/Health
Parents/guardians provided information regarding the patient’s age and gender. Parents/guardians also reported the patient’s sickle cell genotype, medical co-morbidities, and the number of hospital admissions for pain within the past 12 months, which were verified against the patient’s electronic medical record.
Pain Severity
Patient-reported pain severity was assessed using both the VAS and the NRS. The VAS and NRS are frequently used within the clinical setting and in pediatric research and have been used to assess pain in children with SCD.26–29 The VAS is a 10cm, non-hatched line anchored with one end as “no pain” and the other end as “worst pain possible.” Patients were instructed to “Please mark on the line your level of pain. One end is no pain and the other end is the worst pain possible. Place a mark to tell us your current level of pain.” VAS scores were calculated by measuring the distance, in centimeters, between the “no pain” anchor and the patient’s mark indicating their level of pain resulting in a pain severity score ranging from 0mm to 10cm. The NRS is an 11-point scale anchored with “no pain” and “worst pain possible.” Patients were instructed to “Please tell me your current level of pain on a scale from 0 to 10 where 0 means no pain and 10 means the worst pain possible. Write the number that relates to your current level of pain.”
Pain Relief
A global assessment of how a patient’s pain may have changed since the last assessment (i.e., current assessment minus previous assessment) was used to anchor the changes noted on the NRS and VAS scales. Patients reported pain relief in response to the question: “Compared to the last time you marked your pain, tell us how much your pain has changed.” Patients could respond that their pain was “a lot worse,” “a little worse,” “the same,” “a little better,” or “a lot better.”
Need for Pain Medication
Patient-reported perception of the need for pain medication was assessed as a “yes” or “no” response to the question, “Do you feel like you need more pain medication right now?”
Treatment Satisfaction
Patient-reported treatment satisfaction was assessed using the question, “Given how much pain you have right now, how satisfied (happy) are you with your pain medicine?” Patients could respond with “not at all,” “somewhat satisfied (happy),” “very satisfied (happy),” or “do not know.”
Data Analysis
Data were analyzed using SAS 9.2 (SAS Institute, Cary, NC) and SPSS 20.0 (SPSS Inc., Chicago, IL) statistical software and described as proportions, mean ± SD, median and interquartile range (IQR), and 95% confidence interval (CI), as applicable. Given the potential for different administration of medications between follow-up assessments, morphine equivalents and the presence of non-steroidal anti-inflamatory durgs were determined for each follow-up assessment. This information was used to adjustment for differences in pain medication during follow-up analyses. The minimal clinically significant improvement in pain was calculated using the mean change in pain severity scores associated with the global report of “a little better” pain relief. Mann-Whitney U tests were used to compare pain severity scores between patients in the emergency department and hospitalized patients. A Wilcoxon Signed-rank test was used to compare pain severity scores in patients at baseline relative to discharge. In order to account for repeated follow-ups per patient, a Generalized Estimating Equation model with gamma distribution and log link function was used to determine the relationships between pain severity scores and the need for pain medication and treatment satisfaction. Receiver operating characteristic (ROC) curve analyses were used to assess the ability of the VAS and NRS to discriminate the patient’s reported need for pain medication and treatment satisfaction. Areas under the curve (AUC) of 0.70 and 0.80 were considered fair and 0.80 to 1.00 as good to excellent measures of accuracy/discrimination. The sensitivity and specificity of the derived pain severity cut-points related to need for pain medication and treatment satisfaction (i.e., “somewhat satisfied” or “very satisfied”) were also analyzed.
RESULTS
Twenty-eight patients with SCD undergoing treatment for uncomplicated VOE were recruited. Nine patients participated twice resulting in 37 total visits. Three hundred and five total assessments were collected during the 37 visits. Eighty-two percent of the patients utilized pain medication at home prior to seeking treatment within the emergency department. Of these patients, pain medication type was equally distributed with one-third of the patients using non-steroidal anti-inflammatory drugs (NSAID) only, narcotic medications only, or both NSAID and narcotic medications combined, respectively. Twenty four of the initial pain assessments were completed before pain medication was administered within the emergency department. On 18 of the 37 visits, patients were admitted for continued pain management with a mean length of stay of 3.1 days (SD=0.96 days). Sixty percent of the follow-up assessments were completed within the emergency department. No significant differences in pain severity were found between patients receiving care within the emergency department relative to patients admitted for pain management on the VAS (p=0.12) or the NRS (p=0.77). Demographic and medical information is described in Table 1.
Table I.
Patient demographic and disease characteristics (n=28)
| Variable | n (%)* |
|---|---|
| Age, mean years ± SD | 14.65 ± 3.12 |
| Females | 14 (50) |
| Genotype | |
| Hb SS | 16 (57) |
| Hb SC | 9 (32) |
| Hb Sβ0 thalassemia | 3 (11) |
| Hospital admissions in past 12 months, median (IQR) | 1 (0–4) |
| Initial Pain Severity, median (IQR) | |
| VAS | 7.5cm (6.1–8.7cm) |
| NRS | 8 (6–10) |
| Median Pain Severity during Follow-ups (IQR) | |
| Emergency Department VAS | 7.5cm (4.4–8.6cm) |
| Inpatient VAS | 7.95cm (6.3–8.9cm) |
| Emergency Department NRS | 8 (5–10) |
| Inpatient NRS | 8 (7–9) |
Data are given as the number of children/caregivers (%) except as indicated.
Pain Severity
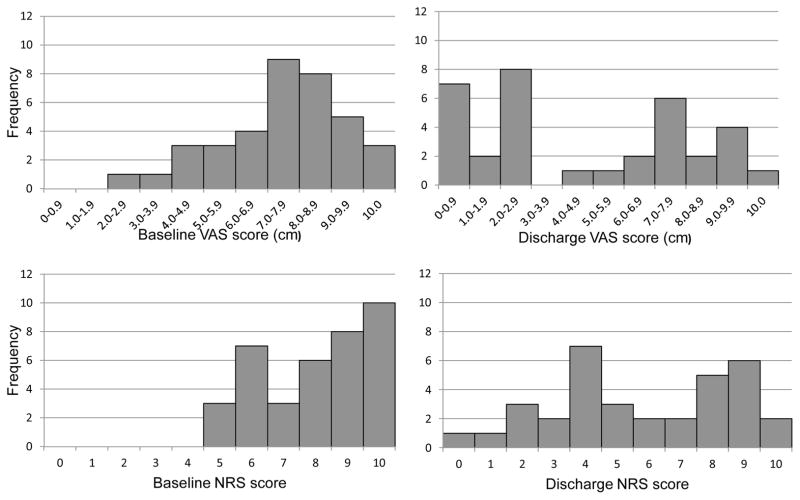
Figure 1 shows the distribution of VAS and NRS scores, respectively, at baseline and at discharge assessments. At baseline, the distribution skewed towards higher scores with a median pain severity score of 7.5cm (IQR: 6.1–8.7cm) on the VAS and 8 (IQR: 6–10) on the NRS. At discharge, scores were statistically lower with median pain severity scores of 4.95cm (IQR: 1.95–8.05cm) (p<0.001) on the VAS and 6 (IQR: 4–8.5) on the NRS (p<0.001).
Figure 1.
Frequency distribution of baseline and discharge VAS and NRS scores.
Pain Relief
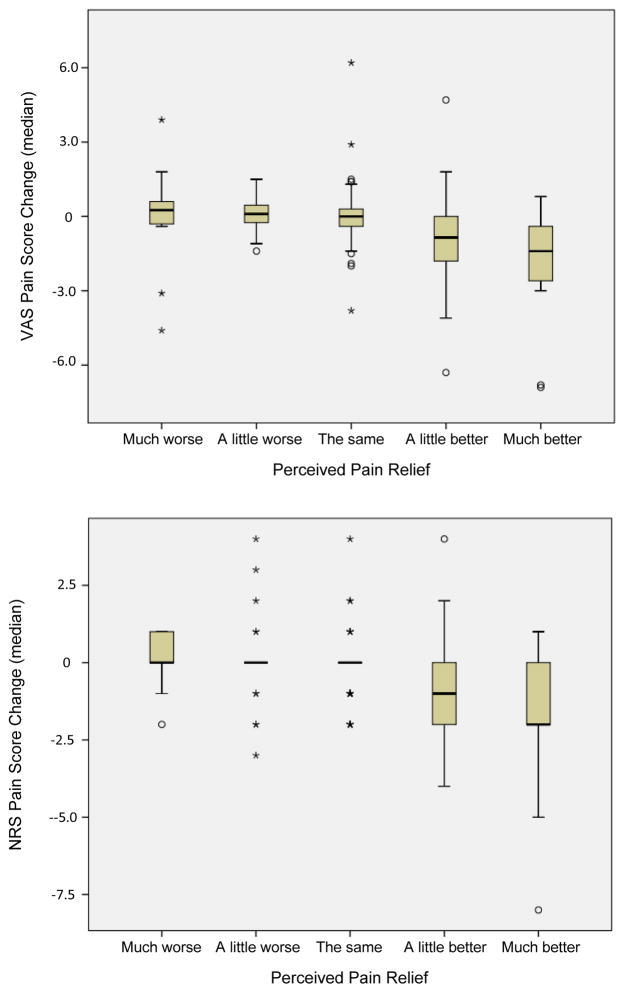
Figure 2 displays the change in VAS and NRS scores associated with patient global perception of pain relief. The mean change in pain score associated with “a little better” pain (minimal clinically significant improvement) was found to be 0.97cm (SE=0.27cm) for the VAS and 0.9 (SE=0.24) for the NRS.
Figure 2.
Change in VAS (cm) (top panel) and NRS pain scores (bottom panel) related to perceived pain relief
Need for Pain Medication
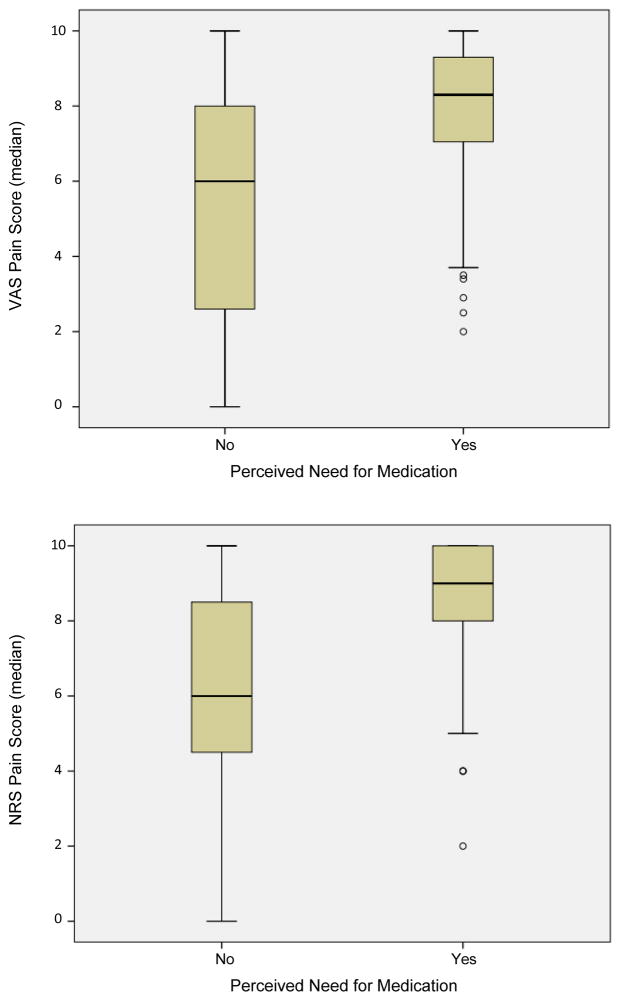
Patients endorsed a need for pain medications on 126 (58%) of the follow-up assessments. Figure 3 displays the distribution of VAS and NRS scores associated with patient perceived need for medication. Pain scores associated with the perceived need for medication were significantly higher than were scores associated with “no” need in this sample (VAS: median 8.3cm vs. 6.0cm, p<0.001; NRS: median 9 vs. 6, p<0.001). ROC curves were calculated for both the VAS and NRS scores comparing “yes” versus “no” need for pain medication. The AUC was 0.77 (95% CI: 0.71–0.83) for VAS scores and 0.75 (95% CI: 0.68–0.82) for NRS scores reflecting a “fair” ability of both VAS and NRS scores to discriminate a patient’s need for medication. Pain scores greater than 7.45cm (sensitivity 0.73, specificity 0.67) on the VAS and greater than 7.5 (sensitivity 0.82, specificity 0.59) on the NRS were able to maximize discrimination of need for medication.
Figure 3.
Distribution of VAS (cm) (top panel) and NRS (bottom panel) pain scores related to perceived need for medication
Treatment Satisfaction
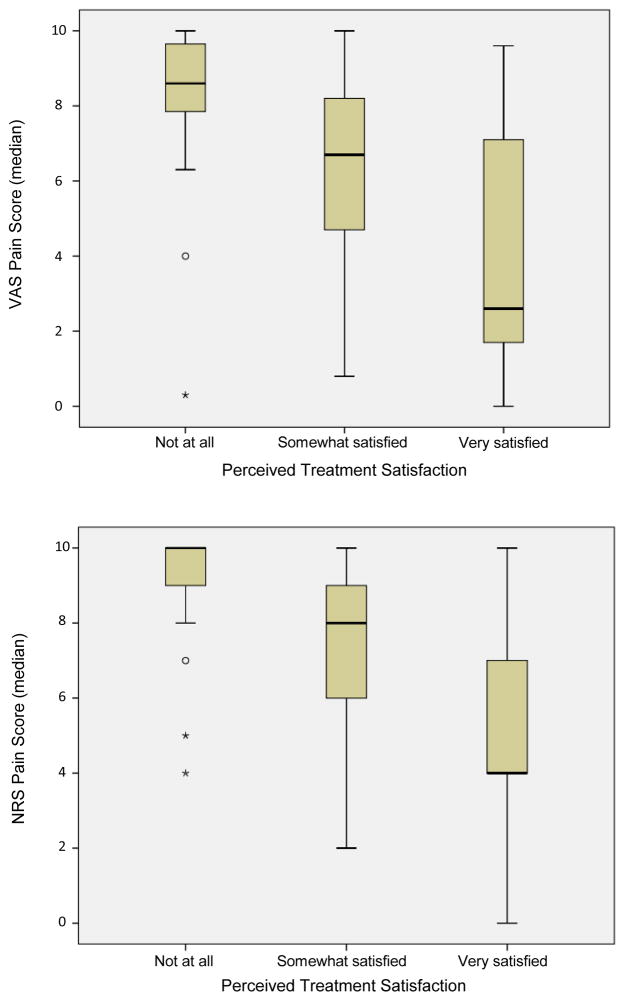
Patients stated they were “somewhat” or “very satisfied” with their pain treatment during 121 (68%) of follow-up assessments and “not satisfied” during 58 (32%) of the assessments. Figure 4 demonstrates the distribution of VAS and NRS scores across satisfaction levels. Pain scores associated with treatment satisfaction (i.e., somewhat satisfied or very satisfied) were significantly lower than pain scores associated with no satisfaction (VAS: median 6.0cm vs. 8.6cm, p<0.001; NRS: median 7 vs. 10, p<0.001). ROC curves were calculated for both the VAS and NRS scores comparing satisfaction (i.e., somewhat satisfied or very satisfied) versus no satisfaction (i.e., not at all satisfied). The AUC was 0.81 (95% CI: 0.75–0.88) for VAS scores and 0.82 (95% CI: 0.76–0.88) for NRS scores reflecting a “good” ability of both VAS and NRS scores to discriminate a patient’s treatment satisfaction. Pain scores less than 7.35cm (sensitivity 0.64, specificity 0.89) on the VAS and less than 8.5 (sensitivity 0.72, specificity 0.81) on the NRS were able to maximize discrimination of treatment satisfaction.
Figure 4.
Distribution of VAS (cm) (top panel) and NRS (bottom panel) pain scores related to perceived treatment satisfaction
DISCUSSION
In this study, we were able to define the minimal clinically significant improvement in pain using two widely accepted pain measures. Specifically, we found that mean reductions in pain scores of 0.97cm on the VAS and 0.9 units on the NRS were found to represent minimal clinically significant improvements in pain based on patient-report. We found that VAS and NRS pain scores were significantly higher in patients endorsing a need for pain medication and significantly lower in patients reporting any satisfaction with their current pain treatment, as expected. Finally, the VAS and NRS scores were found to be “fair” discriminators of patient-reported need for medication, whereas VAS and NRS scores were “good” discriminators of patient-reported treatment satisfaction. Ultimately, this provides significant information to address our limited clinical understanding of pain scores within the population.
The pain severity scores reported in this study were similar to prior studies that examined pain over time in children hospitalized for VOE.30–32 The minimal clinically significant improvement in acute pain that others have reported in non-SCD patients range between 0.9 to 1.35cm on the VAS and 1 on the NRS. Within adults with SCD, Lopez and colleagues identified a 13.5mm reduction in VAS score as the minimal clinically significant improvement in pain.17 Our study found 0.97cm on the VAS and 0.9 units on the NRS to be the minimal clinically significant improvement in pain severity and is the first to identify this in pediatric patients with SCD. Interestingly, out of all instances in which pain relief was endorsed, 25% of the VAS pain severity ratings and 25% of the NRS pain severity ratings were associated with actual increases in pain severity. These contradictory findings are likley due to the fact that patients were not allowed to view previous pain severity ratings.
Relatively few studies have examined the relationship between pain severity ratings and perceived need for medication, often with variable findings. For instance, Blumstein and Moore14 described poor sensitivity and specificity for the pain severity ratings in predicting the need for pain medications in adults experiencing acute pain within the emergency department. Conversely, Voepel-Lewis et al. 23 found that pain severity ratings were “good” at discriminating patient-reported need for medication in pediatric patients experiencing post-operative pain. Both the VAS and NRS within our study were found to be “fair” in their ability to discriminate the need for pain medication. When examining pain severity scores associated with perceived need for pain medication, Voepel-Lewis et al.23 found that scores greater than 4 on the NRS were suggestive of pediatric post-operative patients endorsing a need for medication; whereas our study found much higher pain severity levels of 7.45cm on the VAS and 7.5 on the NRS were suggestive of a need for medication in patients with SCD. Such findings may be due to the recurrent and chronic nature of SCD in that previous pain experiences within this population may result in increased tolerance with pain and a correspondingly higher level of pain severity before pain medication is requested. Conversely, these findings may reflect a higher propensity of patients to report more severe pain such that medical providers will respond to their pain severity report with sufficient medication dosing.
Several adult studies have demonstrated poor to fair relationships between pain severity ratings and treatment satisfaction.33,34 Within pediatrics, Voepel-Lewis and colleagues23 again found that pain severity scores were “good” at discriminating patient-reported treatment satisfaction in patients experiencing acute post-operative pain. Similar to Voepel-Lewis and colleagues23, our study found that both the VAS and NRS pain scores were “good” at discriminating treatment satisfaction in pediatric patients with SCD. When examining pain severity scores associated with perceived treatment satisfaction, Voepel-Lewis et al.23 found that scores less than 6 on the NRS were suggestive of pediatric post-operative patients endorsing positive treatment satisfaction; whereas our study found much higher pain severity levels of 7.35cm on the VAS and 8.5 on the NRS were suggestive of for treatment satisfaction in patients with SCD. Again, such findings may be due to the recurrent and chronic nature of SCD in that previous pain experiences within this population may result in increased tolerance with pain and thus, patients with SCD may be satisfied with treatment at higher pain severity levels than patients with less pain tolerance.
Collectively, data from this study improves our understanding of pain severity relative to other clinically relevant patient-reported outcomes. Identifying the minimal clinically significant improvement in pain within pediatric patients with SCD allows providers to better interpret changes in pain relative to absolute pain values. In addition, defining the minimal clinically significant improvement in pain allows researchers to use this metric as an outcome variable for future pain research in SCD. Further, identification of pain scores suggestive of the need for medication and treatment satisfaction provides thresholds as outcomes for clinical management of pain or pain research.
Several limitations of this study are worth mentioning. First, our population utilized pediatric patients with SCD presenting to the emergency department for pain management. As such, this population may have demonstrated higher pain severity than patients with SCD experiencing pain at home limiting the generalization of these findings to non-hospital settings. Second, the data collected was from a single institution limiting the ability to generalize findings to other hospitals with differing practice patterns. Thus future research should consider utilzing a multicenter approach to replicate these findings. Third, serial observations were completed every 30 minutes within the emergency department and three times daily once admitted. These assessments capture pain severity and associated pain outcomes at specific time intervals, but may not capture the variability in pain throughout the time interval. Fourth, factors besides pain severity that may have impacted clinical ratings, such as anxiety, depression, or coping factors were not evaluated. Finally, caution should be applied when using such data for individual treatment decisions given the variability in pain severity scores between patients as well as the degree of false negative and positives attributed with absolute cut-off scores.
Overall, the minimal clinically significant improvement was defined for the VAS and NRS in children and the scales were able to discriminate between important clinical findings including pain relief, need for pain medication, and treatment satisfaction. Collectively, these data improve our understanding of pain severity ratings of pediatric patients with SCD which is required to improve pain management within this population by providing a general guide for clinical practice and pain research development.
Acknowledgments
This work was supported by a grant through the Rebecca Slye Endowment Fund (Medical College of Wisconsin). The authors acknowledge the contributions of Robbie Kattappuram, Rebecca Farley, Maura Coffey, Paul Evans, Mark Nimmer, Jacquelyn Swietlik, Rachel Unteutsch, and Duke Wagner for patient recruitment and data collection and the patients and families for their time.
References
- 1.Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle cell disease in the United States: National and state estimates. Am J Hematol. 2010;85(1):77–78. doi: 10.1002/ajh.21570. [DOI] [PubMed] [Google Scholar]
- 2.Yang YM, Shah AK, Watson M, et al. Comparison of costs to the health sector of comprehensive and episodic health care for sickle cell disease patients. Public Health Rep. 1995;110(1):80–86. [PMC free article] [PubMed] [Google Scholar]
- 3.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists task force on acute pain management. Anesthesiology. 2012;116(2):248–273. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin LJ, Dampier CD, Jacox AK, et al. Guidelines for the management of acute and chronic pain in sickle-cell disease. Glenville, IL: American Pain Society; 1999. [Google Scholar]
- 5.Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol. 2003;120(5):744–752. doi: 10.1046/j.1365-2141.2003.04193.x. [DOI] [PubMed] [Google Scholar]
- 6.Boughton K, Blower C, Chartrand C, et al. Impact of research on pediatric pain assessment and outcomes. Pediatr Nurs. 1998;24(1):31–5. 62. [PubMed] [Google Scholar]
- 7.Franck LS, Allen A, Oulton K. Making pain assessment more accessible to children and parents: Can greater involvement improve the quality of care? Clin J Pain. 2007;23(4):331–338. doi: 10.1097/AJP.0b013e318032456f. [DOI] [PubMed] [Google Scholar]
- 8.Franck LS, Bruce E. Putting pain assessment into practice: Why is it so painful? Pain Res Manag. 2009;14(1):13–20. doi: 10.1155/2009/856587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrestha-Ranjit JM, Manias E. Pain assessment and management practices in children following surgery of the lower limb. J Clin Nurs. 2010;19(1–2):118–128. doi: 10.1111/j.1365-2702.2009.03068.x. [DOI] [PubMed] [Google Scholar]
- 10.Hodgins MJ. Interpreting the meaning of pain severity scores. Pain Res Manag. 2002;7(4):192–198. doi: 10.1155/2002/971935. [DOI] [PubMed] [Google Scholar]
- 11.Stevens B, Gibbins S. Clinical utility and clinical significance in the assessment and management of pain in vulnerable infants. Clin Perinatol. 2002;29(3):459–468. doi: 10.1016/s0095-5108(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 12.Voepel-Lewis T, Piscotty RJ, Jr, Annis A, et al. Empirical review supporting the application of the “pain assessment as a social transaction” model in pediatrics. J Pain Symptom Manage. 2012;44(3):446–457. doi: 10.1016/j.jpainsymman.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Bird SB, Dickson EW. Clinically significant changes in pain along the visual analog scale. Ann Emerg Med. 2001;38(6):639–643. doi: 10.1067/mem.2001.118012. [DOI] [PubMed] [Google Scholar]
- 14.Blumstein HA, Moore D. Visual analog pain scores do not define desire for analgesia in patients with acute pain. Acad Emerg Med. 2003;10(3):211–214. doi: 10.1111/j.1553-2712.2003.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 15.Cepeda MS, Africano JM, Polo R, et al. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105(1–2):151–157. doi: 10.1016/s0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs FM, Abraira V, Royuela A, et al. Minimal clinically important change for pain intensity and disability in patients with nonspecific low back pain. Spine (Phila Pa 1976) 2007;32(25):2915–2920. doi: 10.1097/BRS.0b013e31815b75ae. [DOI] [PubMed] [Google Scholar]
- 17.Lopez BL, Flenders P, Davis-Moon L, Corbin T, Ballas SK. Clinically significant differences in the visual analog pain scale in acute vasoocclusive sickle cell crisis. Hemoglobin. 2007;31(4):427–432. doi: 10.1080/03630260701587810. [DOI] [PubMed] [Google Scholar]
- 18.Salaffi F, Stancati A, Silvestri CA, et al. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–291. doi: 10.1016/j.ejpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Sloman R, Wruble AW, Rosen G, et al. Determination of clinically meaningful levels of pain reduction in patients experiencing acute postoperative pain. Pain Manag Nurs. 2006;7(4):153–158. doi: 10.1016/j.pmn.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Todd KH, Funk KG, Funk JP, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27(4):485–489. doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]
- 21.Bulloch B, Tenenbein M. Assessment of clinically significant changes in acute pain in children. Acad Emerg Med. 2002;9(3):199–202. doi: 10.1111/j.1553-2712.2002.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 22.Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med. 2001;37(1):28–31. doi: 10.1067/mem.2001.111517. [DOI] [PubMed] [Google Scholar]
- 23.Voepel-Lewis T, Burke CN, Jeffreys N, et al. Do 0–10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg. 2011;112(2):415–421. doi: 10.1213/ANE.0b013e318203f495. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro BS, Benjamin LJ, Payne R, et al. Sickle cell-related pain: Perceptions of medical practitioners. J Pain Symptom Manage. 1997;14(3):168–174. doi: 10.1016/S0885-3924(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 25.Stinson J, Naser B. Pain management in children with sickle cell disease. Paediatr Drugs. 2003;5(4):229–241. doi: 10.2165/00128072-200305040-00003. [DOI] [PubMed] [Google Scholar]
- 26.Koch J, Manworren R, Clark L, et al. Pilot study of continuous co-infusion of morphine and naloxone in children with sickle cell pain crisis. Am J Hematol. 2008;83(9):728–731. doi: 10.1002/ajh.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luffy R, Grove SK. Examining the validity, reliability, and preference of three pediatric pain measurement tools in African-American children. Pediatr Nurs. 2003;29(1):54–59. [PubMed] [Google Scholar]
- 28.Stinson JN, Kavanagh T, Yamada J, et al. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125(1–2):143–157. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Zempsky WT, Corsi JM, McKay K. Pain scores: Are they used in sickle cell pain? Pediatr Emerg Care. 2011;27(1):27–28. doi: 10.1097/PEC.0b013e318203ca03. [DOI] [PubMed] [Google Scholar]
- 30.Jacob E, Miaskowski C, Savedra M, et al. Changes in intensity, location, and quality of vaso-occlusive pain in children with sickle cell disease. Pain. 2003;102(1–2):187–193. doi: 10.1016/s0304-3959(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 31.Walco GA, Dampier CD. Pain in children and adolescents with sickle cell disease: A descriptive study. J Pediatr Psychol. 1990;15(5):643–658. doi: 10.1093/jpepsy/15.5.643. [DOI] [PubMed] [Google Scholar]
- 32.Zempsky WT, Loiselle KA, McKay K, et al. Retrospective evaluation of pain assessment and treatment for acute vasoocclusive episodes in children with sickle cell disease. Pediatr Blood Cancer. 2008;51(2):265–268. doi: 10.1002/pbc.21572. [DOI] [PubMed] [Google Scholar]
- 33.Dihle A, Helseth S, Paul SM, et al. The exploration of the establishment of cutpoints to categorize the severity of acute postoperative pain. Clin J Pain. 2006;22(7):617–624. doi: 10.1097/01.ajp.0000210905.57546.c1. [DOI] [PubMed] [Google Scholar]
- 34.Mendoza TR, Chen C, Brugger A, et al. Lessons learned from a multiple-dose postoperative analgesic trial. Pain. 2004;109(1–2):103–109. doi: 10.1016/j.pain.2004.01.015. [DOI] [PubMed] [Google Scholar]