Abstract
Objective.
Bidirectional interactions between granulocyte-macrophage colony-stimulating factor–positive (GM-CSF+) T cell and interferon regulatory factor 5–positive (IRF-5+) macrophages play a major role in autoimmunity. In the absence of SH2 domain-containing phosphatase 1 (SHP-1), GM-CSF–stimulated cells are resistant to death receptor (DR)–mediated apoptosis. The objective of this study was to determine whether TRA-8, an anti-DR5 agonistic antibody, can eliminate inflammatory macrophages and CD4 T cells in the SHP-1–defective condition.
Methods.
Ubiquitous Cre (Ubc.Cre) human/mouse-chimeric DR5-transgenic mice were crossed with viable SHP-1–defective motheaten (mev/mev) mice. TRA-8 was administered weekly for up to 4 weeks. The clinical scores, histopathologic severity, and macrophage and CD4 T cell phenotypes were evaluated. The role of TRA-8 in depleting inflammatory macrophages and CD4 T cells was also evaluated, using synovial fluid obtained from patients with rheumatoid arthritis (RA).
Results.
The levels of Inflammatory macrophages (interleukine-23–positive [IL-23+] IRF5+) and CD4 T (IL-17+GM-CSF+) cells were elevated in mev/mev mice. In DR5-transgenic mev/mev mice, DR5 expression was up-regulated in these 2 cell populations. TRA-8 treatment depleted these cells and resulted in a significant reduction of inflammation and in the titers of autoantibodies. In synovial cells from patients with RA, the expression of IRF5 and DR5 was negatively correlated with the expression of PTPN6. TRA-8, but not TRAIL, suppressed RA inflammatory macrophages and Th17 cells under conditions in which the expression of SHP-1is low.
Conclusion.
In contrast with TRAIL, which lacks the capability to counteract the survival signal in the absence of SHP-1, TRA-8 eliminated both IRF5+ IL-23+ M1 macrophages and pathogenic GM-CSF+ IL-17+ CD4 T cells in a SHP-1-independent manner. The results of the current study suggest that TRA-8 can deplete inflammatory cell populations that result from a hyperactive GM-CSF/IRF-5 axis.
The bidirectional inflammatory macrophage –lymphocyte interaction is a key element in development of autoimmunity and inflammation (1). An increased frequency of pro-inflammatory M1 macrophages with high levels of IL-23 has been observed in human RA (2, 3). Recently, it has been reported that the production of GM-CSF by Th17 cells is stimulated by IL-23 through the retinoic acid-related orphan receptor gamma t (RORγt) pathway (4-6). On the other hand, GM-CSF promotes the polarization of M1 macrophages, serving a non-redundant function in the initiation of autoimmune inflammation. The importance of GM-CSF in the development of RA is suggested by the report by a recent report that GM-CSF is up-regulated before the onset of disease in RA (7). GM-CSF exacerbates collagen II-induced arthritis (CIA) (8) and its production by bone marrow (BM)-derived cells has been shown to be required for the development of CIA (9, 10).
Interferon regulatory factor 5 (IRF-5) is the key transcription factor mediating the GM-CSF inflammatory signal, which determines the commitment to M1 macrophages differentiation (1, 11). It is interesting that IRF5 not only mediates cell survival and differentiation but was also found to upregulate DR5, Fas, and TRAIL and exhibits the ability to sensitize tumor cells to death receptor-mediated apoptosis (12). However, it remains unclear whether regulation of survival pathways or apoptosis pathways by IRF5 is central to the development of inflammatory diseases, which might contribute to the strategies of developing therapeutics to induce apoptosis of apoptosis-resistant inflammatory cells. One compelling question is why the endogenous TRAIL–DR5 apoptosis machinery is not properly functional cells with high GM-CSF/IRF5/DR5 in inflammatory conditions. This defective endogenous TRAIL-DR5 apoptosis machinery also underscores the need to need to search for a therapeutic reagent that can promote DR-mediated cell destruction of IRF5+ pathogenic cells in order to regain immune homeostasis.
One candidate protein that governs death receptor-mediated apoptosis signaling is the hematopoietic protein tyrosine phosphatase src homology 2 (SH2) domain–containing phosphatase 1 (SHP-1; PTPN6). Daigle et al previously showed that the SH2-containing phosphatases (SHP-1, SHP-2, and src homology 2 [SH2] domain – containing inositol phosphatase) bound to a phosphotyrosine motif situated in the cytoplasmic death domain of tumor necrosis factor (TNF) receptor type I, Fas, and DR5. Interestingly, recruitment of SHP-1 is needed to enable the apoptosis-counteracting signal to negatively regulate the GM-CSF-mediated survival signal of neutrophils (13). Under the SHP-1-deficient condition derived from heterozygous motheaten mice, anti-Fas cannot effectively counteract GM-CSF-mediated survival in neutrophils (13). These results are consistent with observations that the increased production of pathogenic Mϕs in viable motheaten (B6-mev/mev) mice is due in part to increased GM-CSF activity, resulting in increased production of M1 macrophages that produce high levels of IL-6 and TNFα (14).
We and other investigators previously demonstrated that an anti-human DR5 antibody, TRA-8, which is in phase III clinical trials for cancer treatment, can induce apoptosis of RA synovial fibroblasts (15, 16). Due to the limited apoptosis-inducing function of the available anti-mouse DR5 antibody (17), establishment of the hu/mo DR5 Tg mouse, which expresses the extracellular domain of human DR5 and the intracellular domain of mouse DR5 driven by the mouse DR5 3-kb promoter, further enabled us to clarify the detailed targets and mechanisms underlying TRA-8-mediated apoptosis in vivo using a mouse CIA model (18). However, the macrophage-depleting effect of TRA-8 has not been tested in other disease models, and it is not known whether TRA-8 can directly target CD4 T cells. It is also critical to verify these observations using cells from subjects with autoimmunity.
In the current study, we analyzed the expression of hu/mo chimeric DR5 on macrophages and CD4 T cells of Ubc.Cre DR5 Tg mev/mev mice and the ability of TRA-8 to eliminate these cells in vivo. We also analyzed the expression pattern of DR5 in cell populations isolated from synovial fluid of patients with RA. In synovial lining tissues, a negative correlation between PTPN6 and IRF5/DR5 was found. Consistent with these results, administration of SHP-1 inhibitor, sodium stibogluconate (SSG), to cells from TRA-8-resistant subjects increased the inflammatory macrophages and CD4 T cells and their DR5 expression. SSG treatment also restores the susceptibility of synovial fluid M1 macrophages and Th17 cells to TRA-8–induced apoptosis but not TRAI–induced apoptosis. These results show that PTPN6 deficiency in both mice and humans results in increased production of M1-inflammatory macrophages and IL-17+ GM-CSF+ CD4 T cells with high DR5 expression, which are resistant to TRAI–induced apoptosis, but can be eliminated by an anti-DR5 antibody, TRA-8.
MATERIALS AND METHODS
Mice
B6 (C57BL/6)-mev/mev, and Tg (UBC-cre/ESR1)1Ejb/J (Ubc.Cre) mice were obtained from the Jackson Laboratory. Ubc.Cre DR5 Tg mice were generated as we previously described (18). Ubc.Cre DR5 Tg B6-mev/mev mice, referred to as DR5 Tg mev/mev mice, were obtained by crossing Ubc.Cre DR5 Tg B6-mev/+ mice with B6-mev/+ mice. Cre expression was induced as described previously (18). All animal procedures were approved by The University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee.
Subjects
Synovial tissue samples from 12 patients with RA (11 females and 1 male), and 11 patients with osteoarthritis (OA, 9 females and 2 males) were obtained from the UAB Tissue Procurement Center as described previously (19). For analysis of peripheral blood mononuclear cells (PBMCs) and synovial fluids, a total of 10 patients with RA (7 females and 3 males; 7 Caucasians and 3 African-Americans) were recruited by sequential selection from the UAB Rheumatology Clinic (mean age = 53 years, ranging from 38 to 79 years old; mean duration of disease of 14 years, ranging from 6 to 22 years). Eight out of the 10 patients were being treated with methotrexate, and 6 were being treated with a TNF inhibitor at the time of sample acquisition. All RA patients met the American College of Rheumatology 1987 revised criteria for RA. These studies were conducted in compliance with the Helsinki Declaration and approved by the institutional review board at UAB. All participants provided informed consent.
Preparation of PBMCs and isolation of human synovial fluid-derived mononuclear cells and lining fragments
PBMCs from RA subjects were isolated using lymphocyte separation medium (Cellgro) according to the manufacturer’s instruction. Synovial effusions from RA and OA subjects described above were aspirated as clinically indicated using a standard sterile procedure. The synovial fluid was centrifuged at 200–400 × g (based on the viscosity of the samples) for 10 min. Pellets including synovial lining fragments and mononuclear cells were resuspended in RPMI-1640 medium (Invitrogen) containing 10% fetal bovine serum, and these two components were further separated by low-speed centrifugation (20–30 × g) or, alternatively, by using lymphocyte-separation medium (Cellgro).
Flow cytometric analysis
For mouse experiments, single-cell suspensions were stained using mouse-specific Abs, including FITC–anti-CD11b (BD Biosciences) and rabbit anti-IRF5 (Abcam) followed by Alexa 647–donkey anti-rabbit IgG (Invitrogen), Alexa 647–anti-IL-23p19 (eBioscience), PE–anti-TNF-α, PE–anti-IL-6, PE–anti-IFN-γ, PE–anti-CD80, PE/Cy7–anti-Ly6C, APC-Ly6G, PE/Cy7-F4/80, FITC–anti-CD4, PE/Cy7–anti-Thy1.2, FITC–anti-IFN-γ, APC–anti-IFN-γ, PE–anti-IL-17, and PE–anti-GM-CSF. Tg chimeric DR5 was stained with biotin–anti-human DR5 followed by Streptavidin eFluor 450 (eBioscience). Prior to staining, Fc receptors were blocked by anti-mouse CD16/32 (Biolegend). Intracellular and intranuclear staining was performed as described previously (18). For macrophage analysis, the following strategies were applied to increase the specificity of CD11b: 1) FSClow SSChigh cells were gated out, which included neutrophils and large granular lymphocytes (NK cells). 2) Ly6G+ granulocyte cells were gated out; iii) F4/80 was also included in analysis; iv) transcription factor and cytokine staining were combined with CD11b staining.
Human synovial fluid cells or PBMC were stained using human-specific Abs, including FITC–anti-CD68, eFluor660–anti-IL-23p19 (eBioscience), and rabbit anti-IRF5 (Abcam) followed by Alexa 647–donkey anti-rabbit IgG (Invitrogen), PE–anti-CD80, PE/Cy7–anti-CD4, FITC–anti-IFN-γ, and Alexa 647-anti-IL-17A. Unless specified, all reagents used for FACS analysis were purchased from Biolegend (San Diego, CA). Data were acquired on a BD LSRII flow cytometer and analyzed using FlowJo software (Tree Star, Inc.).
Cell sorting
Human synovial fluid mononuclear cells were stained with FITC–anti-CD68, PE–anti-CD80, PE/Cy7–anti-CD4, PE–anti-CD45RA, PerCP/Cy5.5–anti-CCR2, PE/Cy7–anti-CCR4, Alexa 700–anti-CCR5, FITC–anti-CCR6, Pacific Blue–anti-CXCR3, and PE–anti-CD161 Abs (all Biolegend) and sorted into CD68+CD80+ (M1 macrophages), CD68+CD80− (M2 macrophages), CD4+CXCR3+CCR6− (Th1) (20), CD4+CXCR3−CCR4+CCR6+CD161+ (Th17) (20) and CD4+CXCR3+CCR6+ (Th1/17) (20) with purities of > 96%. FACS sorting was performed on a FacsAria II cell sorter (BD Biosciences). Total CD4 T cells for TRA-8 in vitro treatment were purified using CD4 T cells isolation kit II (Miltenyi Biotec).
Quantitative reverse transcription PCR (qRT-PCR)
RNA isolation, first-strand cDNA synthesis, and qRT-PCR were carried out as described previously (18). All primers used in the present study are described in Supplementary Table 1, which is available at the Arthritis & Rheumatism Web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38057/abstract.
TRA-8 treatment of DR5 Tg mev/mev mice
TRA-8 treatment (Daiichi-Sankyo) dissolved in phosphate buffered saline, 0.2 mg per mouse, or IgG1 isotype control was administrated intraperitoneally weekly, beginning at the age 3 weeks and continuing for 3-4 weeks or until the mice either died or were killed.
Immunohistochemical and immunofluorescence staining
All mouse tissue were processed and stained as described previously (18, 21, 22). Synovial lining fragments were isolated as described above and were fixed in 4% formaldehyde for 15 min. Macrophages in the fragments were visualized by Alexa 555– or Alexa 488–anti-human CD68 Ab (Biolegend). DR5 was recognized by staining with biotin–anti-hDR5 (Biolegend) followed by Streptavidin-Alexa 488 (Invitrogen). Apoptosis was detected by using Annexin V-EnzoGold (Enzo Life Sciences). Fluorescence imagines were captured and analyzed using an LSM710 laser scan confocal microscope (Zeiss) with Zen software.
Enzyme-linked immunosorbent assay (ELISA)
Cytokine levels were measured by ELISA according to the manufacturer’s manual (Biolegend). Anti-histone and anti-DNA titers in the mouse serum were determined by ELISA as previously described (23).
In vitro SSG treatment
Isolated synovial fluid mononuclear cells or PBMC from RA patients were seeded in 96-well plates at a density of 2×106/ml, 200 μl/well in the presence of 2 μg/ml anti-CD3 Ab, 2 μg/ml anti-CD28 Ab, 20 ng/ml human GM-CSF (R&D Systems), and 20 ng/ml human IL-23 (R&D Systems), with or without the presence of 10 μg/ml SSG (Calbiochem). Three days later, TRA-8 (1 μg/ml) or human recombinant TRAIL (Enzo Life Sciences, 1 μg/ml) were added. After incubation for an additional 2 days, FACS analysis was performed as described above.
Statistics
Statistical analyses were performed using the two-tailed Student’s t test, one-way ANOVA, and bivariate correlation analysis. P values <0.05 were considered statistically significant.
RESULTS
Increased number of inflammatory M1 macrophages in B6-mev/mev mice
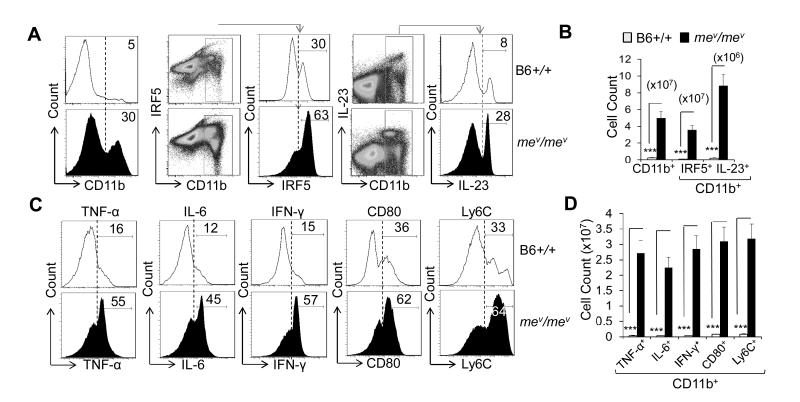
B6-mev/mev mice exhibit increased development of macrophages, in part due to increased levels of GM-CSF, increased signaling through its receptor (14), and decreased susceptibility to apoptosis (24-27). A 6-fold increase in the frequency of CD11b+ macrophages in B6-mev/mev mice compared with B6-+/+ mice was observed in the spleen (Figure 1A). Similar results were obtained when F4/80 was used as a macrophage marker (data not shown). Within the CD11b+ population, there was a statistically significant increase in the IRF5+ and IL-23+ macrophages in B6-mev/mev mice compared with B6 mice (Figure 1A, B). Similar results were obtained when TNFα, IL-6, IFN-γ, CD80, and Ly6C were used to characterize the M1 macrophages (Figure 1C, D).
Figure 1.
Increased inflammatory macrophage counts in B6-mev/mev mice. A, Percentage of CD11b+ (left), CD11b+ interferon regulatory factor 5–positive (IRF-5+) (middle), and CD11b+ interleukin-23–positive (IL-23+) (right) macrophages in a single-cell suspension of spleen from B6-+/+ and B6-mev/mev mice as determined by fluorescence-activated cell sorting (FACS) analysis. B, Cell counts of the indicated populations in the spleens of B6-+/+ and B6-mev/mev mice. C, Percentage of intracellular tumor necrosis factor α (TNF-α), IL-6, interferon-γ (IFN-γ), and surface CD80 and lymphocyte antigen (Ly6C) expression on the CD11b+ subpopulation of spleen cells, as determined by FACS analysis. D, Total cell count of CD11b+ macrophages that express the indicated intracellular or surface molecules, as determined by multiplying the total single-cell suspension count by the percentage in the gated population. Values are the mean (± SEM) (n = 5 mice per group). *** = P < 0.001.
DR5 marked CD11b+ IRF5+, IL-23+, and TNF-α+ M1 macrophages in DR5–transgenic B6-mev/mev mice and rendered them susceptible to TRA-8-mediated cell depletion
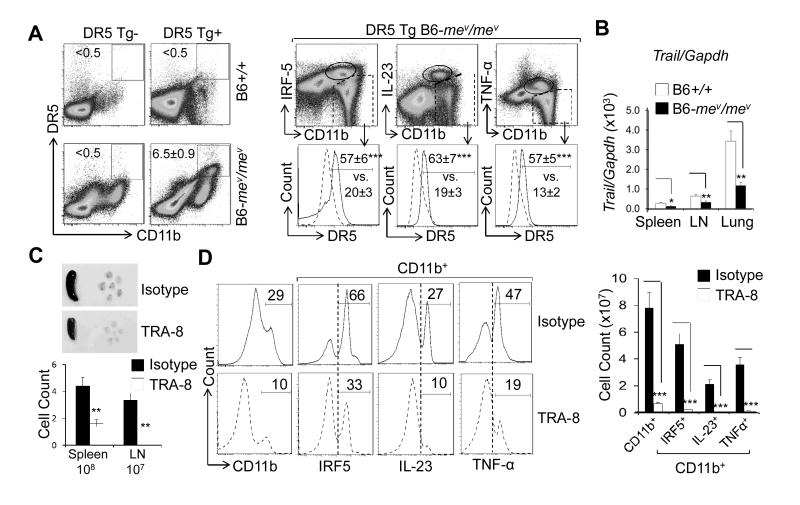
It has been reported that IRF5 up-regulates DR5 in tumor cells (12). We examined whether DR5 expression is up-regulated in macrophages, especially in IRF-5+ M1 macrophages. Transgenic DR5 expression was significantly up-regulated in CD11b+ cells from DR5 -transgenic B6- mev/mev mice (Figure 2A), but was undetectable in CD11b+ cells from non-transgenic B6-+/+ and mev/mev mice. Very low levels were observed in DR5-transgenic B6-+/+ mice (Figure 2A, left). Importantly, the upregulation of transgenic DR5 expression was higher in IRF5+, IL-23+, and TNFα+ M1 macrophages (Figure 2A, right). A similar pattern of endogenous mouse DR5 expression was also observed in these cells (data not shown). These data strongly indicate that DR5 is selectively up-regulated in IRF5+ inflammatory M1 macrophages. Although the expression of DR5 was increased, the expression of murine Trail in the spleen, lymph nodes (LNs), and lungs of B6-mev/mev mice was significantly reduced compared with that in B6-+/+ mice (Figure 2B). Therefore, defects in both TRAIL expression and SHP-1 signaling exaggerate the inflammatory M1 macrophage phenotypes.
Figure 2.
Death receptor 5 (DR5) expression on inflammatory macrophages and TRA-8 –induced depletion of IRF-5+, IL-23+, and TNFα+ macrophages in DR5-transgenic (Tg) B6-mev/mev mice. A, Percentage of transgenic DR5+ cells in CD11b+ cells from the indicated mice (left panels), as determined by FACS analysis. CD11b+ cells from spleen of DR5-transgenic B6-mev/mev mice were further separated into subsets based on their expression levels of IRF-5, IL-23, and TNF-α (top right panels). Transgenic DR5 expression was then analyzed (bottom right panels). B, TRAIL expression in the spleen, lymph nodes (LNs), and lung of viable motheaten and wild-type mice, as determined by quantitative reverse transcription-polymerase chain reaction. C, Top panels, Images of representative spleen and LNs (cervical, axillary, and inguinal) from DR5-transgenic B6-mev/mev mice treated with TRA-8 or isotype control antibodies. Bottom panel, Cell counts in the spleen and LNs of the mice treated with TRA-8 or isotype control antibodies. D, Left panels, Percentage of CD11b+, CD11b+IRF-5+, CD11b+IL-23+, and CD11b+TNF-α+ macrophages after TRA-8 or isotype control treatments, as determined by FACS analysis. Right panel, Cell counts of the indicated populations. Values are the mean ± (SEM) (n = 5 mice per group). * = P < 0.05, ** = P < 0.01, *** = P < 0.001. See Figure1 for other definitions.
To determine whether the anti-human DR5 antibody TRA-8 can circumvent impaired TRAIL apoptosis pathway and effectively reduce the number of M1 macrophages in the setting of a SHP-1 deficiency condition, DR5-transgenic B6-mev/mev mice were treated with either TRA-8 or isotype control antibody from 4 to 8 weeks of age. Both the size and cell counts of the spleen and LNs were significantly decreased in TRA-8-treated B6-mev/mev mice, compared with isotype-treated mice (Figure 2C). TRA-8 treatment also resulted in a significant decrease in the percentage and total numbers of CD11b+ macrophages as well as IRF-5+, IL-23+, and TNFα+ M1 macrophages in the spleen (Figure 2D).
TRA-8– induced reduction of IL-17+IFNγ+ and GM-CSF+ inflammatory CD4 T cells with increased DR5 expression
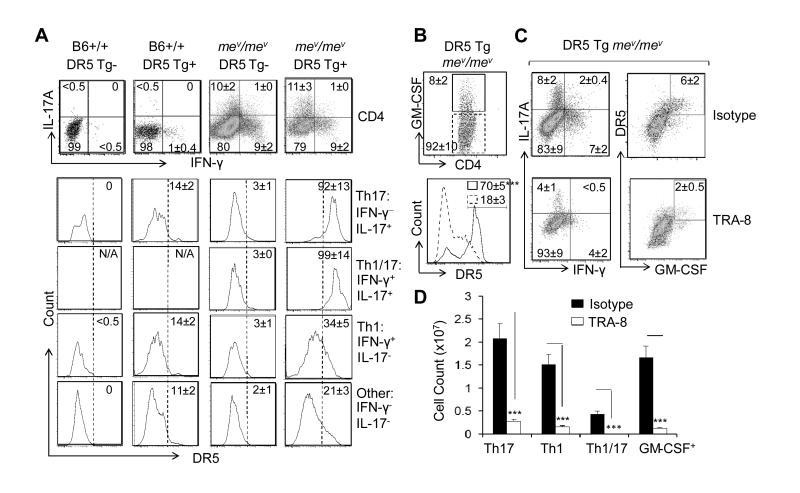
SHP-1 was recently shown to be an important regulator of Th17 development (28). Consistently, an increased percentage of Th1/17(CD4+, IL-17A+IFN-γ+), and Th17 (CD4+, IL-17A+IFN-γ−) T cells was observed in the spleens of B6-mev/mev compared with the spleens of B6-+/+ mice (Figure 3A). Deficiency of SHP-1 is also known to be associated with increased p53 (24, 29), which can subsequently upregulate DR5 (30). Similar to what was observed in M1 macrophages, defective SHP-1 resulted in increased expression of transgenic DR5 in the CD4+ T cells, with a pattern of Th1/17> Th17 > Th1 > other CD4 T cells (Figure 3A).
Figure 3.
Transgenic death receptor 5 (DR5) expression on CD4 T cells and TRA-8–induced depletion of IL-17+ IFN-γ+ and granulocyte-macrophage colony-stimulating factor-positive (GM-CSF+) inflammatory CD4 T cells in DR5-transgenic B6-mev/mev mice. A. Top panels, Percentage of Th1 (CD4+, IFN-γ+, IL-17A−), Th1/17 (CD4+, IFN-γ+, IL-17A+), and Th17 (CD4+, IFN-γ−, IL-17A+) T cells in the spleen of B6-+/+ and B6-mev/mev mice with and without transgenic DR5, as determined by FACS analysis. Bottom panels, Expression of transgenic DR5 on CD4 T cells that were divided into 4 subpopulations based on their expression of IFN-γ and IL-17A. B, Top, Percentage of GM-CSF+ and GM-CSF− CD4 T cells from the spleen of DR5-transgenic B6-mev/mev mice. Bottom, Expression of transgenic DR5 on cells in each subset. C, Effect of TRA-8 and isotype control treatment on the percentage of Th1, Th1/17, Th17, and GM-CSF+ CD4 T cells in spleen of DR5-transgenic B6-mev/mev mice. D. Total number of each subset of CD4 T cells after treatment with isotype control or TRA-8 was determined by multiplying the total cell count by the percentage of each indicated CD4 T cell subpopulation. Values are the mean ± SEM (n = 5 mice per group). *** = P < 0.001. NA = not applicable (see Figure 1 for other definitions).
GM-CSF was recently shown to be the major pathogenic inflammatory cytokine produced by IL-17+ CD4 cells, especially IL-17A+IFN-γ+ cells (4-6). Strikingly, transgenic DR5 expression was predominantly observed in the GM-CSF+ CD4 T cells of DR5 transgenic B6-mev/mev mouse spleen (Figure 3B). TRA-8 treatment resulted in a decrease in the number and percentage of IL-17+IFNγ+, IL-17+, and IFN-γ+ CD4 T cells as well as GM-CSF+ CD4 T cells in DR5 transgenic B6–mev/mev mice compared with isotype-treated cells (Figure 3C, 3D). These results suggest that anti-DR5 treatment eliminated both M1 macrophages and inflammatory CD4 T cells in vivo.
TRA-8 treatment attenuates tissue inflammation and increases the life span of DR5-transgenic B6-mev/mev mice
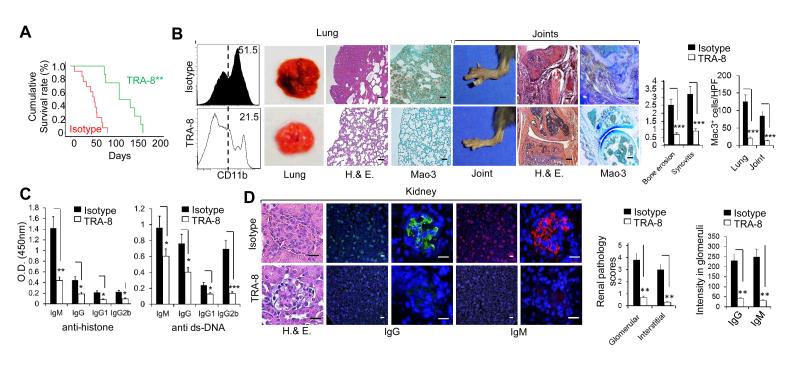
Consistent with the dramatic reduction in the number of inflammatory macrophages and CD4 T cells associated with anti-DR5 treatment, TRA-8 treatment also resulted in a > 2-fold increase in the survival of DR5-transgenic B6-mev/mev mice, as indicated by the Kaplan–Meier survival curve (mean survival 50 versus 120 days) (Figure 4A). After 4 weeks of TRA-8 treatment, the number of CD11b+ cells in the lungs of DR5-transgenic B6-mev/mev mice was significantly decreased, along with decreased hemorrhagic necrosis, decreased inflammatory cell infiltration, and decreased Mac-3+ macrophages infiltration in the lung of DR5-transgenic mev/mev mice 4 weeks after TRA-8 treatment (Figure 4B). Findings were similar in the joints of TRA-8–treated DR5-transgenic B6-mev/mev mice. Joint swelling, inflammation, bone erosion and macrophage infiltration were dramatically reduced in the joints of TRA-8–treated mice compared with isotype control–treated DR5-transgenic B6-mev/mev mice (Figure 4B). A significant decrease in anti-histone and anti-dsDNA IgM, IgG, IgG1, and IgG2b isotypes was observed in TRA-8 treated mice (Figure 4C). There was also a decrease in glomerulonephritis and immune complex deposition of both IgG and IgM isotypes in TRA-8-treated mice compared with isotype-treated mice (Figure 4D). Taken together, these results suggest that TRA-8 can inhibit both cellular and humoral immune responses by depleting pathogenic macrophages and CD4 T cells, thereby overcoming the SHP-1 defect–induced systemic inflammation and autoimmunity.
Figure 4.
TRA-8 treatment reduced tissue inflammation and autoantibodies, and increased the lifespan of death receptor 5 (DR5)-transgenic B6-mev/mev mice. A, Kaplan–Meyer survival curve of TRA-8– and isotype control– treated DR5-transgenic B6-mev/mev mice (~15 mice/group). B, Left panels, Effect of TRA-8 treatment on the lung and joints of DR5-transgenic B6-mev/mev mice. Right panels, Quantification of bone erosion, synovitis and Mac-3–positive cells in the lungs and joints of mice treated with TRA-8 or isotype control. C, Serum levels of anti-histone and anti–double-stranded DNA (anti-dsDNA) antibodies in DR5-transgenic B6-mev/mev mice treated with TRA-8 or isotype control. D, Left panels, Effect of TRA-8 treatment on the kidneys of DR5-transgenic B6-mev/mev mice compared with the isotype control treatment. Right panels, Quantification of renal pathology scores and IgG and IgM deposits in the glomeruli of mice treated with TRA-8 or isotype control. Values are the mean (± SEM) (n = 5 mice per group except panel A). * = P < 0.05, ** = P < 0.01, *** = P <.001. Bars = 100μm. H&E = hematoxylin and eosin, hpf = high-power field.
TRA-8 eliminated inflammatory M1 macrophages and pathogenic CD4 T cells from RA patients with low PTPN6 expression
There was a significant increase in expression of the IL23 (P19), CSF2RA, IRF5, DR5 (M1-related, Figure 5A), IL-17A, and CSF2 (Th17-related, Figure 5A) in the synovial tissue of patients with RA compared with control patients with OA. These results indicated that RA is associated with increased M1 macrophages and Th17 activation.
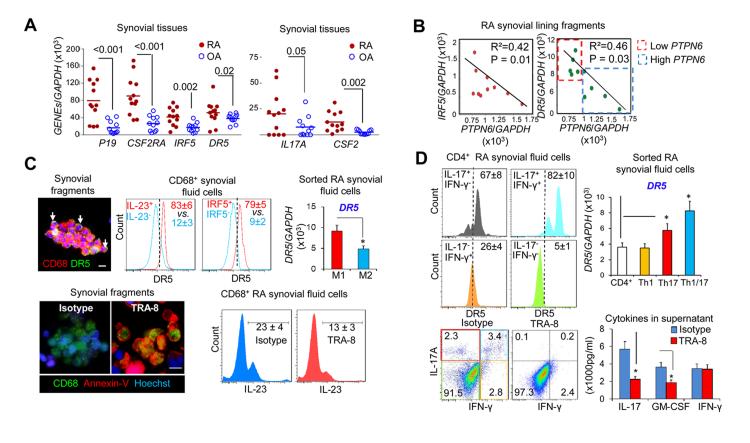
Figure 5.
TRA-8 eliminates inflammatory M1 macrophages and CD4 T cells in patients with rheumatoid arthritis (RA) with low PTPN6 levels. A. Expression of M1 macrophages and Th17, Th1/17 signature genes in synovial tissues from RA patients and OA patients. B. Correlation between PTPN6 and IRF5 or DR5 expression in RA synovial lining fragments. C. Top, Death receptor 5 (DR5) expression on CD68+ synovial fragment macrophages as visualized by immunofluorescence microscopy (left panel); in M1 (CD68+IL-23+, CD68+IRF5+) and M2 (CD68+IL-23−, CD68+IRF5−) macrophages from RA synovial fluid (middle panels) as determined by FACS analysis; and in sorted M1 (CD68+CD80+) and M2 (CD68+CD80−) macrophages from RA synovial fluid (right panel), as determined by real-time polymerase chain reaction (PCR). Bottom, TRA-8–induced apoptosis of CD68+ macrophages from synovial fragments, as indicated by Annexin-V (left panels) and TRA-8–induced depletion of CD68+IL-23+ macrophages from synovial fluid cells, as determined by FACS analysis (right panels). Bars = 20μm. D. Top, DR5 expression in subsets of CD4+ T cells, as determined by FACS analysis (left panels), and DR5 expression in sorted Th1, Th17, and Th1/17 cells, as determined by real-time PCR (right panel). Bottom, Depletion of Th17 and Th1/17 cells in sorted RA synovial fluid CD4 T cells incubated with TRA-8 or control. Cells were analyzed by FACS (left), and the indicated cytokines in the culture supernatant were quantified by enzyme-linked immunosorbent assay (ELISA) (right). Values are the mean (± SEM) (n = 5). * P < 0.05. GM-CSF = granulocyte-macrophage colony-stimulating factor (see Figure 1 for other definitions).
Dai et al previously identified synovial lining fragments in RA synovial fluid and used immunostaining to reveal the expression of CD68 in a subset of cells in a pattern characteristic of hyperplastic synovial lining (31). Based on our finding of the correlation of high IRF-5 and DR5 with low expression of PTPN6 (SHP-1) in mice, we hypothesized that such a correlation may exist in human RA synovial cells. Indeed, the expression of IRF5 and DR5 was significantly inversely correlated with the expression of PTPN6 (Figure 5B) in synovial lining fragments isolated from the synovial fluids of 10 patients with RA.
To further determine whether low PTPN6 expression is associated with susceptibility to TRA-8, TRA-8 mediated cell-killing studies were carried out using cells from the patients with RA. In ~50% of RA patients with lower PTPN6 expression, i.e., below the mean ± SD of 964.0±92.0, (Figure 5B), we observed a higher number of CD68+ DR5+ macrophages in synovial lining fragments from RA synovial fluid, as indicated by immunofluorescence analysis (Figure 5C, top left). DR5 expression was selectively up-regulated in the subpopulation of IL-23+ or IRF5+ inflammatory M1 macrophages compared with the IL-23− or IRF5− anti-inflammatory M2 macrophages (Figure 5C, top middle). Consistent with these findings, qRT-PCR analysis showed that DR5 expression was significantly higher in sorted CD68+CD80+ M1 macrophages than in sorted CD68+CD80− M2 macrophages using the freshly sorted macrophage subsets from RA synovial fluid (Figure 5C, top right). TRA-8 treatment of the RA synovial fragments leads to dramatic induction of apoptosis in CD68+ macrophages, as visualized by Annexin-V (Figure 5C, bottom left). Further analysis suggested that TRA-8 treatment dramatically reduced the percentage of IL-23+ M1 macrophages from synovial fluid mononuclear cell fraction (Figure 5C, bottom right).
Because TRA-8 was efficient in depletion of pathogenic IL-17+ and GM-CSF+ CD4 T cells in mice, we next determined whether increased expression of DR5 can be detected and whether TRA-8 exhibits the same effects to eliminate these cells derived from RA patients. Consistent with the DR5 expression identified in B6-mev/mev mice, in RA subjects with lower PTPN6 expression, DR5 expression in synovial fluid CD4 T cells followed a Th1/17 > Th17 > Th1 > other CD4 T cell pattern, as analyzed by FACS (Figure 5D, top left), which was further demonstrated at the messenger RNA level using freshly sorted cells from the synovial fluid (Figure 5D, top right). To determine whether TRA-8 can act directly on CD4+ T cells, purified CD4 T cells from RA synovial fluid was incubated with TRA-8 for 2 days, which resulted in significant depletion of both Th17 and Th1/17 cells, with less depletion of the Th1 cells (Figure 5D, bottom left). Consistent with this observation, the levels of IL-17 and GM-CSF, but not IFN-γ, were significantly reduced in the culture supernatant of TRA-8-treated cells compared with isotype-treated cells (Figure 5D, bottom right). Our data suggested that DR5 marks the highly pathogenic M1 macrophages, Th1/17 and Th17 cells, and thus TRA-8 can be used for targeted depletion of these cell populations, especially in patients with low PTPN6 expression.
SHP-1 inhibitor enhanced the susceptibility of inflammatory macrophages and CD4 T cells to TRA-8–, but not TRAIL–mediated cell apoptosis in RA subjects with high PTPN6 expression
To further demonstrate the causal relationship between SHP-1 and IRF5 or DR5, synovial fluid mononuclear cells from RA patients with higher PTPN6 expression, i.e., above the mean (Figure 5B) were pre-incubated with the specific SHP-1 inhibitor SSG for 3 days, followed by TRA-8 or TRAIL treatment. Consistent with the results of Figure 5B, functional inhibition of SHP-1 led to increased numbers of IRF5+IL-23+ M1 macrophages and DR5 expression on this cell subset (Figure 6A). More importantly, these macrophages also exhibited increased susceptibility to cell depletion mediated by TRA-8, but not TRAIL, after SHP-1 was inhibited (Figure 6A, right).
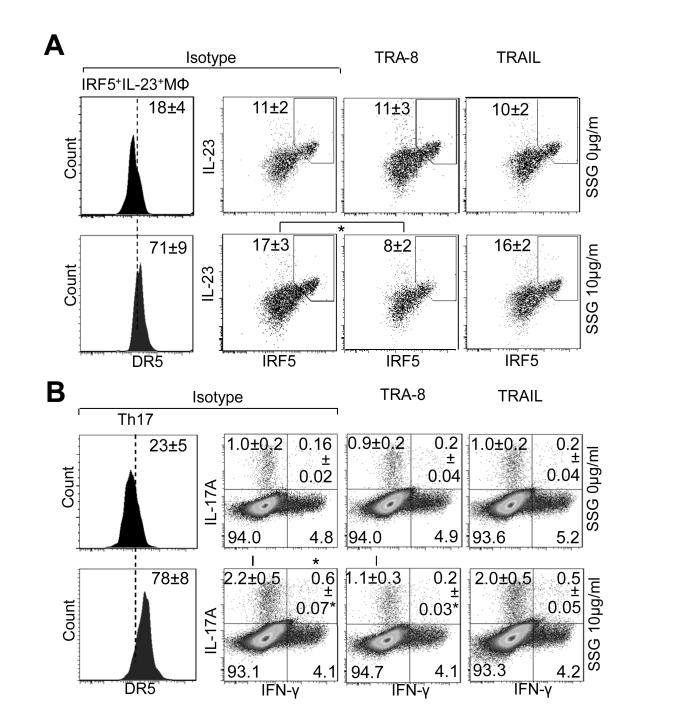
Figure 6.
Effects of SHP-1 inhibitor on the susceptibility of inflammatory macrophages and CD4 T cells to TRA-8–mediated and TRAIL–mediated cell apoptosis in rheumatoid arthritis patients with high PTPN6 expression. Mononuclear cells from synovial fluid (A) or peripheral blood (B) of patients with rheumatoid arthritis exhibiting high PTPN6 expression and resistance to TRA-8–induced apoptosis were pretreated with or without SSG for 3 days followed by TRA-8 or TRAIL treatment for 2 days. Cells were then analyzed by FACS. A. SSG increased the numbers of M1 macrophages with up-regulated death receptor 5 (DR5) expression, which could be depleted by TRA-8 but not TRAIL. B. SSG increased the numbers of Th1/17 and Th17 cells with up-regulated DR5 expression, which could be depleted by TRA-8 but not TRAIL. Values are the mean (± SEM) (n = 5 subjects per group). * = P < 0.05, TRA-8 treatment versus isotype control in the indicated cell populations. See Figure 1 for other definitions.
Similar to what was observed in macrophages, functional inhibition of SHP-1 led to increased Th1/17, Th17, and DR5 expression on these cell subsets isolated from PBMCs of patients with RA. These CD4 T cells also exhibited increased susceptibility to cell elimination induced by TRA-8, but not TRAIL, with SHP-1inhibition (Figure 6B, right). Taken together, these data demonstrate 1) the causal relationship between SHP-1 and DR5 expression/TRA-8 susceptibility in M1 macrophages and pathogenic CD4 T cells from patients with RA; 2) that DR5 is a marker for the highly pathogenic M1 macrophages, Th1/17 and Th17 cells, which can be used for targeted depletion of these populations by TRA-8; and 3) in contrast with TRAIL, TRA-8 can induce apoptosis effectively under condition of SHP-1 deficiency.
DISSCUSION
Hyperactive M1 macrophages exhibit extensive inflammatory properties, which stimulate both CD4 T cells and the development of autoreactive B cells (1,10,32). In parallel, inflammatory Th17 cells produce GM-CSF cytokine to further perpetuate the inflammatory properties of M1 macrophages (5). We previously showed that selective depletion of CD11bhighLy-6C+ macrophages in the human/mouse DR5-transgenic mice reduced the numbers of Th17 cells, increased the numbers of Treg cells, and prevented or effectively treated CIA (18). The present study demonstrated that TRA-8 not only effectively eliminated M1 macrophages but also directly targeted pathogenic CD4 T cells in SHP-1–defective mev/mev cells and synovial fluid cells from patients with RA.
An important question addressed in the present study is whether TRA-8 can be functional under conditions in which M1 macrophages are insensitive or refractory to physiologic apoptosis-inducing signals including TRAIL. To address this question, we tested the ability of TRA-8 to suppress the inflammatory macrophage– and CD4 T cell– positive feedback loop in SHP-1 defective mev/mev mice (33-35). Functional SHP-1 also limited IL-6– and IL-21– induced phosphorylation of STAT3 in primary CD4+ T cells, thus suppressing the generation of Th17 cells (28). Daigle et al (13) previously showed that recruitment of SHP-1 to the death domain blocks GM-CSF signaling-induced downregulation of pro-apoptotic Bax. In the absence of SHP-1, GM-CSF can promote JAK/STAT-mediated signaling and proliferation, making GM-CSF–stimulated cells resistant to TRAIL–, Fas ligand–, and TNF–mediated apoptosis (13).
Consistent with the previous reports that Fas is upregulated in SHP-1-deficient B6-mev/mev mice (26), the results of the current study show that DR5 expression is increased in both M1 macrophages and GM-CSF+ IL-17+CD4 T cells. We previously showed that macrophages and CD4 T from B6-mev/mev mice were resistant to apoptosis (24-26). Despite this apoptosis resistance, phosphorylation of p53 was increased in irradiated B6-mev/mev mouse cells compared with cells without SHP-1 defect (24). Because p53 enhances expression of murine DR5 (30) and also represses SHP-1 (29), this might be another mechanism associated with the up-regulation of DR5 in B6-mev/mev cells.
Up-regulation of both DR5 and TRAIL can also be understood on the basis of increased GM-CSF signaling and up-regulation of IRF-5, which has been reported to upregulate both DR5 and TRAIL in tumor cells (12). However, the expression of TRAIL was lower in B6-mev/mev mice, which accounts, in part, for the failure of endogenous TRAIL to signal apoptosis through the murine TRAIL receptors, including DR5, which is up-regulated in B6-mev/mev mice. Although TRAIL-mediated apoptosis is not effective in the setting of SHP-1-defec, treatment with the more efficacious anti-human DR5 antibody, TRA-8, in DR5-transgenic B6-mev/mev mice can overcome apoptosis resistance. This unique ability to remove both types of inflammatory cells makes TRA-8 a potentially important therapeutic reagent for disrupting the inflammatory positive feedback loop between these 2 cell types. Consistent with the elimination of M1 macrophages and GM-CSF+ Th17 cells, TRA-8 treatment dramatically suppressed both systemic inflammation and autoantibody production in DR5-transgenic B6-mev/mev mice.
Increased GM-CSF and development of macrophages represent one of the earliest events in RA. GM-CSF has been shown to be up-regulated for months to years before the onset of RA, suggesting it may have important disease-promoting effects (7). Similar to observations in the B6-mev/mev mice, RA synovial fluid macrophages exhibited increased expression of IRF-5, IL-23, TNF-α, and DR5. Furthermore, RA synovial fluid Th1/17 CD4 T cells produce the highest levels of GM-CSF and may contribute to the development of M1 macrophages, which is consistent with previous reports that such IFNγ+ IL-17+ T cells are present in RA synovium, and that these may be the most pathogenic T cells (36, 37).
DR5 surface expression was upregulated in both inflammatory macrophages and CD4 T cells in RA synovial fluids. IRF5 was also inversely correlated with PTPN6 expression, suggesting that in different RA patients, variations in SHP-1 level may be related to macrophage pathogenesis, as observed in mev/mev mice. Inhibition of SHP-1 resulted in upregulation of IRF-5 and IL-23 in macrophages and up-regulation of IL-17 in CD4 T cells. Importantly, we further showed that macrophages or T cells with SHP-1 inhibition were resistant to TRAIL–induced apoptosis but remained sensitive to TRA-8–mediated apoptosis. These results suggest that TRA-8 treatment induces apoptosis in otherwise apoptosis-resistant inflammatory and pathogenic effector cells in vivo. Importantly, they further suggest that TRA-8 may be effective in a subgroup of patients who experience expansion of inflammatory cells as a result of defective SHP-1. Therefore, PTPN6 is potentially a novel biomarker that can identify subgroups of patients whose RA may be responsive to anti-DR5 therapy.
In summary, increased production of M1 macrophages and increased production of T1/17 or Th17 cells are 2 hallmarks of inflammatory autoimmune diseases in both mice (4, 5, 18, 38) and humans (1, 39, 40). Strategies have been developed to eliminate the inflammatory cells that persist due to decreased apoptosis (18, 41). The anti-DR5 antibody TRA-8 was screened and developed based on its ability to ligate DR5 in a novel fashion to overcome the TRAIL apoptosis resistance seen in some cancers (42, 43). Our data suggest that TRA-8 exhibits a unique ability to induce apoptosis of IRF-5+ IL-23+ inflammatory macrophages and GM-CSF+ Th17 cells that may be resistant to apoptosis mediated by TRAIL or, potentially, other TNF family molecules. In the present study, we demonstrate that DR5 marks the highly pathogenic IRF5+IL-23+ M1 macrophages and GM-CSF+IL-17+ CD4 T cells, rendering it an attractive target for autoimmune disease therapy.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mr. Larry Johnson and Mr. Jingju Zhang at the Rheumatic Diseases Core Center – Analytical Genomics and Transgenics Core for generation of the human/mouse DR5 transgenic mice (P30-AR-48311). We thank Ms. Enid Keyser of the UAB Rheumatic Diseases Core Center Comprehensive Flow Cytometry Core for operating the FACS instrument (P30-AR-48311 and P30-AI-027767). Confocal imaging was carried out at the UAB Arthritis and Musculoskeletal Disease Center Analytic Imaging and Immunoreagents Core (P30-AR-48311). Joint processing and sections were carried out at the University of Alabama at Birmingham, Center for Metabolic Bone Disease—Histomorphometry and Molecular Analysis Core Laboratory (NIH Grant P30-AR-46031). Dr. Paul Todd provided editorial assistance in the preparation of the manuscript.
This work was supported by the Arthritis Foundation (grant to Dr. Li), the Lupus Research Institute (grant to Dr. Hsu), the NIH (grants 1RO1-AI-083705 to Dr. Hsu and 1RO1-AI-071110 to Dr. Mountz), the Rheumatology Research Foundation (to Dr. Mountz), Daiichi Sankyo Company, Ltd. (to Dr. Mountz), and the Department of Veterans Affairs (Merit Review Grant 1I01BX000600-01 to Dr. Mountz). Portions of the study were performed at the flow cytometry facility at the University of Alabama at Birmingham Rheumatic Diseases Core Center (Analytical Genomics and Transgenics Core, Comprehensive Flow Cytometry Core, and Analytic Imaging and Immunoreagents Core), supported by NIH grant P30-AR-48311, and the University of Alabama at Birmingham Center for Metabolic Bone Disease–Histomorphometry and Molecular Analysis Core Laboratory, supported by NIH grant P30-AR-46031.
Footnotes
All authors claim to have no financial interests which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
AUTHOR CONTRIBUTIONS
J.L., H-C. H. and J.D.M. designed experiments, analyzed data, and wrote the manuscript. J.L. and PA. Y. paired, bred, and genotyped all mice used for this study. J.L., PA. Y., Q.W., H.L., and YN.D. carried out all in vivo and in vitro experiments. D.M.S. provided the human samples and clinical data and J.D.M. and H-C.H. directed all of the experiments.
REFERENCES
- 1.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–8. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen TK, Andersen T, Hvid M, Hetland ML, Horslev-Petersen K, Stengaard-Pedersen K, et al. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J Rheumatol. 2010;37(10):2014–20. doi: 10.3899/jrheum.100259. [DOI] [PubMed] [Google Scholar]
- 3.Rong C, Hu W, Wu FR, Cao XJ, Chen FH. Interleukin-23 as a potential therapeutic target for rheumatoid arthritis. Mol Cell Biochem. 2012;361(1-2):243–8. doi: 10.1007/s11010-011-1109-6. [DOI] [PubMed] [Google Scholar]
- 4.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–7. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 5.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–75. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeachy MJ. GM-CSF: the secret weapon in the T(H)17 arsenal. Nat Immunol. 2011;12(6):521–2. doi: 10.1038/ni.2044. [DOI] [PubMed] [Google Scholar]
- 7.Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Rantapaa Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62(2):383–91. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 8.Campbell IK, Bendele A, Smith DA, Hamilton JA. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis. 1997;56(6):364–8. doi: 10.1136/ard.56.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook AD, Braine EL, Campbell IK, Rich MJ, Hamilton JA. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001;3(5):293–8. doi: 10.1186/ar318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook AD, Turner AL, Braine EL, Pobjoy J, Lenzo JC, Hamilton JA. Regulation of systemic and local myeloid cell subpopulations by bone marrow cell-derived granulocyte-macrophage colony-stimulating factor in experimental inflammatory arthritis. Arthritis Rheum. 2011;63(8):2340–51. doi: 10.1002/art.30354. [DOI] [PubMed] [Google Scholar]
- 11.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178(8):5245–52. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 12.Hu G, Barnes BJ. IRF-5 is a mediator of the death receptor-induced apoptotic signaling pathway. J Biol Chem. 2009;284(5):2767–77. doi: 10.1074/jbc.M804744200. [DOI] [PubMed] [Google Scholar]
- 13.Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat Med. 2002;8(1):61–7. doi: 10.1038/nm0102-61. [DOI] [PubMed] [Google Scholar]
- 14.Jiao H, Yang W, Berrada K, Tabrizi M, Shultz L, Yi T. Macrophages from motheaten and viable motheaten mutant mice show increased proliferative responses to GM-CSF: detection of potential HCP substrates in GM-CSF signal transduction. Exp Hematol. 1997;25(7):592–600. [PubMed] [Google Scholar]
- 15.Straughn JM, Jr., Oliver PG, Zhou T, Wang W, Alvarez RD, Grizzle WE, et al. Anti-tumor activity of TRA-8 anti-death receptor 5 (DR5) monoclonal antibody in combination with chemotherapy and radiation therapy in a cervical cancer model. Gynecol Oncol. 2006;101(1):46–54. doi: 10.1016/j.ygyno.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa K, Liu W, Fleck M, Zhang H, Zhao L, Ohtsuka T, et al. TRAIL-R2 (DR5) mediates apoptosis of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2003;171(2):1061–9. doi: 10.4049/jimmunol.171.2.1061. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Yamaguchi N, Akiba H, Kojima Y, Hayakawa Y, Tanner JE, et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199(4):437–48. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Hsu HC, Yang P, Wu Q, Li H, Edgington LE, et al. Treatment of arthritis by macrophage depletion and immunomodulation: testing an apoptosis-mediated therapy in a humanized death receptor mouse model. Arthritis Rheum. 2012;64(4):1098–109. doi: 10.1002/art.33423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Hsu HC, Chen J, Grizzle WE, Chatham WW, Stockard CR, et al. Increased expression of activation-induced cytidine deaminase is associated with anti-CCP and rheumatoid factor in rheumatoid arthritis. Scand J Immunol. 2009;70(3):309–16. doi: 10.1111/j.1365-3083.2009.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen CJ, Crome SQ, MacDonald KG, Dai EL, Mager DL, Levings MK. Human Th1 and Th17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. J Immunol. 187(11):5615–26. doi: 10.4049/jimmunol.1101058. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Liu J, Feng X, Yang P, Xu X, Hsu HC, et al. Synovial fibroblasts promote osteoclast formation by RANKL in a novel model of spontaneous erosive arthritis. Arthritis Rheum. 2005;52(10):3257–68. doi: 10.1002/art.21354. [DOI] [PubMed] [Google Scholar]
- 22.Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, et al. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci U S A. 107(7):3012–7. doi: 10.1073/pnas.0914902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu HC, Yang P, Wu Q, Wang JH, Job G, Guentert T, et al. Inhibition of the catalytic function of activation-induced cytidine deaminase promotes apoptosis of germinal center B cells in BXD2 mice. Arthritis Rheum. 2011;63(7):2038–48. doi: 10.1002/art.30257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu HC, Shultz LD, Su X, Shi J, Yang PA, Relyea MJ, et al. Mutation of the hematopoietic cell phosphatase (Hcph) gene is associated with resistance to gamma-irradiation-induced apoptosis in Src homology protein tyrosine phosphatase (SHP)-1-deficient "motheaten" mutant mice. J Immunol. 2001;166(2):772–80. doi: 10.4049/jimmunol.166.2.772. [DOI] [PubMed] [Google Scholar]
- 25.Su X, Zhou T, Wang Z, Yang P, Jope RS, Mountz JD. Defective expression of hematopoietic cell protein tyrosine phosphatase (HCP) in lymphoid cells blocks Fas-mediated apoptosis. Immunity. 1995;2(4):353–62. doi: 10.1016/1074-7613(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 26.Su X, Zhou T, Yang PA, Wang Z, Mountz JD. Hematopoietic cell protein-tyrosine phosphatase-deficient motheaten mice exhibit T cell apoptosis defect. J Immunol. 1996;156(11):4198–208. [PubMed] [Google Scholar]
- 27.Yousefi S, Simon HU. SHP-1: a regulator of neutrophil apoptosis. Semin Immunol. 2003;15(3):195–9. doi: 10.1016/s1044-5323(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 28.Mauldin IS, Tung KS, Lorenz UM. The tyrosine phosphatase SHP-1 dampens murine Th17 development. Blood. 2012;119(19):4419–29. doi: 10.1182/blood-2011-09-377069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montano X. Repression of SHP-1 expression by p53 leads to trkA tyrosine phosphorylation and suppression of breast cancer cell proliferation. Oncogene. 2009;28(43):3787–800. doi: 10.1038/onc.2009.143. [DOI] [PubMed] [Google Scholar]
- 30.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59(12):2770–5. [PubMed] [Google Scholar]
- 31.Dai L, Pessler F, Chen LX, Clayburne G, Schumacher HR. Detection and initial characterization of synovial lining fragments in synovial fluid. Rheumatology (Oxford) 2006;45(5):533–7. doi: 10.1093/rheumatology/kei206. [DOI] [PubMed] [Google Scholar]
- 32.Orme J, Mohan C. Macrophage subpopulations in systemic lupus erythematosus. Discov Med. 2012;13(69):151–8. [PubMed] [Google Scholar]
- 33.Kozlowski M, Mlinaric-Rascan I, Feng GS, Shen R, Pawson T, Siminovitch KA. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med. 1993;178(6):2157–63. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73(7):1445–54. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 35.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4(2):124–9. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 36.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165(11):6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 37.Axtell RC, Xu L, Barnum SR, Raman C. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J Immunol. 2006;177(12):8542–9. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum. 2009;60(5):1210–21. doi: 10.1002/art.24505. [DOI] [PubMed] [Google Scholar]
- 39.Hazlett J, Stamp LK, Merriman T, Highton J, Hessian PA. IL-23R rs11209026 polymorphism modulates IL-17A expression in patients with rheumatoid arthritis. Genes Immun. 2012;13(3):282–7. doi: 10.1038/gene.2011.80. [DOI] [PubMed] [Google Scholar]
- 40.Miossec P, Kolls JK. Targeting IL-17 and T(H)17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–76. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 41.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26(5):629–41. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7(8):954–60. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 43.Amm HM, Zhou T, Steg AD, Kuo H, Li Y, Buchsbaum DJ. Mechanisms of drug sensitization to TRA-8, an agonistic death receptor 5 antibody, involve modulation of the intrinsic apoptotic pathway in human breast cancer cells. Mol Cancer Res. 2011;9(4):403–17. doi: 10.1158/1541-7786.MCR-10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.