Abstract
2-Hydroxypropylmercapturic acid (2-HPMA) is a urinary biomarker of exposure to propylene oxide, a mutagen and carcinogen to which humans are exposed through inhalation of cigarette smoke as well as in certain environmental and occupational settings. 2-HPMA is the final product of a detoxification pathway in which propylene oxide is conjugated with glutathione, and the resulting conjugate is further metabolized and excreted. We have developed and validated a liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometric (LC-APCI-MS/MS) method for the rapid quantitation of 2-HPMA in human urine. The method was applied to an analysis of urine samples from 40 smokers and 40 nonsmokers as well as from a group of 15 subjects who quit smoking. The results demonstrate that smokers have significantly (P < 0.001) higher levels of urinary 2-HPMA (median = 480 pmol/mg creatinine) than do nonsmokers (208 pmol/mg). Similarly, subjects who quit smoking for four weeks exhibited a significant (P < 0.001) 52% median decrease in urinary 2-HPMA upon cessation. Approximately 5% of all urine samples had unusually high levels of 2-HPMA (> 10 times higher than the median), apparently unrelated to tobacco smoke exposure or available demographic data. The method presented here can be used to rapidly quantify an individual’s exposure to propylene oxide via tobacco smoke or other sources.
Keywords: propylene oxide, mercapturic acid, cigarette smoke, smoking cessation
1. Introduction
Propylene oxide (PO; 2-methyloxirane) is a colorless, volatile liquid and strong irritant. PO has shown clear evidence of carcinogenicity in rats, some evidence of carcinogenicity in mice [1], and is “reasonably anticipated to be a human carcinogen” by the National Toxicology Program [2]. The International Agency for Research on Cancer evaluated PO as “possibly carcinogenic to humans” (Group 2B) [3].
Cigarette mainstream smoke contains PO at levels reported as 0.65–0.93 μg/cigarette [4]. It has been listed by the U.S. Food and Drug Administration as one of the “harmful and potentially harmful constituents” of cigarette smoke [5]. Nonsmokers can also be exposed to PO. It is used as a soil fumigant, herbicide, insecticide, and fungicide; and also as a means of sterilization for packaged foods [2,6]. Certain household products, such as carpet cleaners and automobile lubricants, contain PO [7]. PO is used industrially as an intermediate in making polyurethane foams and propylene glycol resins, and workers in these industries can be exposed via inhalation or dermal contact. Another potential source of PO exposure is metabolism of propylene, but this seems to play a relatively minor role [3,8].
PO may contribute to the toxic and carcinogenic effects of cigarette smoking, but there is relatively little information available. One way of monitoring PO uptake in smokers and nonsmokers is by quantifying its metabolites. PO is conjugated with glutathione, leading ultimately to excretion of 2-hydroxypropylmercapturic acid (2-HPMA) in urine (Scheme 1). There are only 3 previous reports of 2-HPMA levels in the urine of smokers and nonsmokers [9–11]. They all indicated high levels in smokers. We are not aware of any reports on the effect of smoking cessation on urinary 2-HPMA levels. In the study reported here, we have developed an accurate and precise liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry (LC-APCI-MS/MS) method for analysis of 2-HPMA in human urine and have applied it to urine samples from smokers, nonsmokers, and smokers who quit smoking.
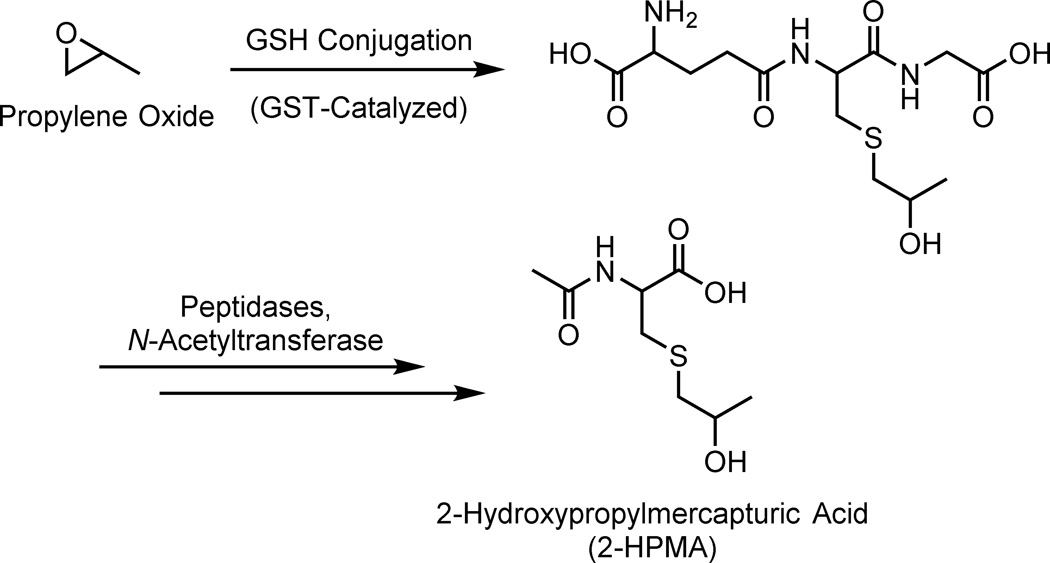
Scheme 1.
Formation of 2-HPMA from propylene oxide. Propylene oxide reacts with glutathione, which can be catalyzed by GSTs. The initial glutathione conjugate is further metabolized to 2-HPMA and excreted in the urine.
2. Methods
2.1 Materials
2-HPMA dicyclohexylammonium salt and [D3]2-HPMA dicyclohexylammonium salt were purchased from Toronto Research Chemicals. Oasis MAX solid-phase extraction 96-well plates (60 mg, 60 μm, 2 mL reservoir) were acquired from Waters Corporation. Square 2 mL 96-well plates with TrueTaper™ 100 μL tapered reservoirs were purchased from Analytical Sales and Services. Sealing mats for square 96-well plates and a 50 × 3.0 mm Synergi C12, 2.5 μm, Max-RP, 100 Å HPLC column were obtained from Phenomenex. All other materials and chemicals were purchased from U-Stores, University of Minnesota. All solutions and buffers were prepared freshly on the same day as the assay.
2.2 Urine samples
These studies were approved by the University of Minnesota Institutional Review Board. The levels of 2-HPMA were measured in a set of 40 smokers’ and 40 nonsmokers’ urine, obtained through the University of Minnesota Tobacco Programs Biorepository, which was established to collect biological samples for tobacco-related biomarker analysis and development. Demographic information (gender, age, and cigarettes smoked per day) was collected from each of the subjects. Pooled smokers’ and nonsmokers’ urine samples used for method validation were also obtained from this biorepository and from voluntary subjects, respectively.
2-HPMA levels were also analyzed in a smoking cessation study, the details of which have been previously reported [12]. Briefly, cigarette smokers who wanted to quit smoking were recruited from the local Twin Cities area via advertisements on the radio, cable TV, the internet, and in brochures in doctors’ offices. Inclusion criteria were 18–70 years of age, smoked ≥ 10 cigarettes per day for ≥ 1 year, generally in good physical and mental health, and possible candidates for nicotine replacement therapy. To aid in cessation, the subjects were offered a nicotine patch, gum, lozenge, or combination thereof, as needed. Subjects were paid in increasing amounts as they abstained for the duration of the study. Twenty-four-hour urine samples were collected seven days before cessation to establish baseline biomarker levels and collected again four weeks after cessation.
2.3 Analysis of 2-HPMA in urine
Urine samples (0.4 mL) were transferred into a 96-well plate and 0.1 mL internal standard solution [100 ng (0.45 nmol) of [D3]2-HPMA dicyclohexylammonium salt] was added. Mixed-mode anion exchange solid phase extraction cartridges (Oasis MAX) were preconditioned with CH3OH (0.7 mL) and 2% aq NH4OH (0.7 mL, pH = 11). The urine samples were applied, and the cartridges were washed with 2% aq NH4OH (0.7 mL) and CH3OH (0.7 mL) and then dried for 5 min with a stream of N2. The cartridges were washed with 2% aq HCOOH (0.7 mL, pH = 1), and then the analyte was eluted with 30% CH3OH in 2% aq HCOOH (0.7 mL). This fraction was concentrated to dryness in vacuo and reconstituted in 20% CH3OH (100 μL) for LC-APCI-MS/MS analysis.
For the chromatographic conditions, the aqueous phase consisted of 15 mM NH4OAc (pH 6.8, unadjusted) and the organic phase was CH3OH. The gradient program (% aqueous:% organic) was as follows: 98:2 from 0–4 min, ramp for 0.5 min and held at 30:70 from 4.5–6.5 min, ramp for 0.5 min and held at 98:2 from 7–12 min. The flow rate was 400 μL/min, the column temperature was 40 °C and the injection volume was 3 μL. LC-APCI-MS/MS analysis was conducted on a TSQ Quantum Discovery Max instrument (Thermo Scientific) with conditions as follows: ionization source, negative mode APCI; collision energy, 13 V; peak width parameters, Q1 = 0.7, Q3 = 0.7; scan width, 0.4 m/z; scan time, 0.1 s; and selected reaction monitoring (SRM), m/z 220.07 → 91.0 ± 0.2 for 2-HPMA and m/z 223.09 → 91.0 ± 0.2 for [D3]2-HPMA.
Quantitation of 2-HPMA was based on a linear calibration curve, constructed in water from five standard solutions of 2-HPMA (0.4, 2, 10, 50, and 100 ng/μL), each with the same concentration of [D3]2-HPMA (1.1 ng/μL). The measured area ratio of 2-HPMA:[D3]2-HPMA was plotted against the known concentration ratio. The slope of the calibration curve was 1.01 (range 0.98–1.03), and the calibration covered the range of observed 2-HPMA values in urine samples. The same stock solution of [D3]2-HPMA was used to construct the calibration curve as was added to each sample as the internal standard. 2-HPMA in the samples was calculated by relating the measured area ratio of 2-HPMA:[D3]2-HPMA to the unknown 2-HPMA concentration via the slope of the calibration curve and the known concentration of [D3]2-HPMA. Four samples of pooled smokers’ urine were included with each set of urine to monitor assay performance.
2.4 Analysis of nicotine, cotinine, and creatinine
Urinary nicotine and cotinine levels were analyzed as described [13]. Briefly, 0.1 mL urine was added to 0.9 mL 50% aq K2CO3 (pH 12), and to this was added 10 μL of [CD3]nicotine and [CD3]cotinine internal standard mixture (47.6 ng and 17 ng, respectively). The mixture was extracted into 1 mL CH2Cl2, and the organic layer was concentrated and analyzed by gas chromatography-mass spectrometry. Urinary creatinine was analyzed by an established colorimetric assay using a Creatinine Microplate Assay from Eagle Biosciences (Boston, MA) [14].
2.5 Statistical analysis
There were 78 urine samples from 39 smokers and 39 nonsmokers with complete 2-HPMA data and two samples below the limit of detection. Our goal was to compare smokers versus nonsmokers, but any difference between these two groups might be confounded by a number of factors (age, gender, and creatinine level). We applied multiple regression analysis, with 2-HPMA as the dependent variable in order to adjust for the potential confounders. Data for 2-HPMA were highly skewed to the right, so we performed the analysis on the regular scale and the log scale. The analysis proved that use of the log scale was justified; the R-squared value was much higher on the log scale than on the regular scale (0.241 versus 0.068). The data from the smoking cessation study were analyzed by the one-sample (or paired) Student’s t-test. Values were analyzed both on the regular and log scale. A P-value < 0.05 was considered statistically significant.
3. Results
3.1 Method Validation
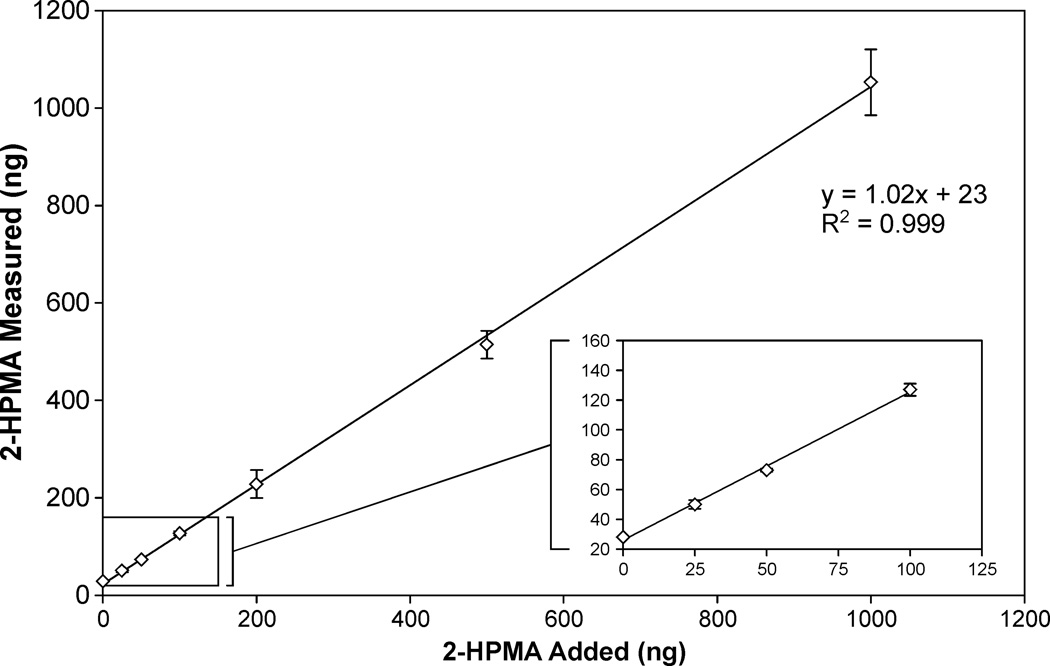
The LC-APCI-MS/MS method for quantitation of 2-HPMA was validated using pooled urine samples from smokers and nonsmokers. Accuracy was assessed by spiking triplicate 0.4 mL samples of pooled nonsmokers’ urine with 25, 50, 100, 200, 500, or 1000 ng of 2-HPMA (MW = 221.27 g/mol). The results are shown in Figure 1; the y-intercept (23 ng) agreed with the average amount of 2-HPMA in 0.4 mL pooled non-smokers’ urine (19 ng). The analysis had excellent linearity over this range (slope = 1.02, R2 > 0.99), and the accuracy was 98.1% (range 94–102%). Intra-day precision was determined by analyzing 2 sets of 7 replicates, each from pooled urine samples. The value for the pooled nonsmokers’ urine set was (mean ± S.D.) 48 ± 3.3 ng/mL [coefficient of variation (CV) = 6.8%]. The value for the pooled smokers’ urine set was 346 ± 30 ng/mL urine (CV = 8.7%). Inter-day precision was assessed from 32 pooled smokers’ urine positive control samples, analyzed over the course of four months, and was found to be 333 ± 40 ng/mL (CV = 12.0%). The on-column limit of detection (LOD) was 50 pg 2-HPMA, which corresponds to a detection limit in urine samples of approximately 4.5 pmol/mL urine. The limit of quantitation (LOQ) for the assay was approximately 20 pmol/mL urine. These data are summarized in Table 1.
Figure 1.
Accuracy curve plotting measured 2-HPMA values against 2-HPMA standard added to triplicate 0.4 mL pooled nonsmokers’ urine samples. The lower 2-HPMA levels are shown in more detail in the inset.
Table 1.
Validation parameters for analysis of 2-HPMA in urine. Precision, accuracy, and limits of detection are based on analysis of 0.4 mL urine samples.
| Limit of Detection (pmol/mL urine) |
Limit of Quantitation (pmol/mL urine) |
Precision (CV, %) |
Accuracy (%) |
||
|---|---|---|---|---|---|
| Intraday | Interday | ||||
| 2-HPMA | 4.5 | 20 | 6.8 | 12.0 | 98.1 |
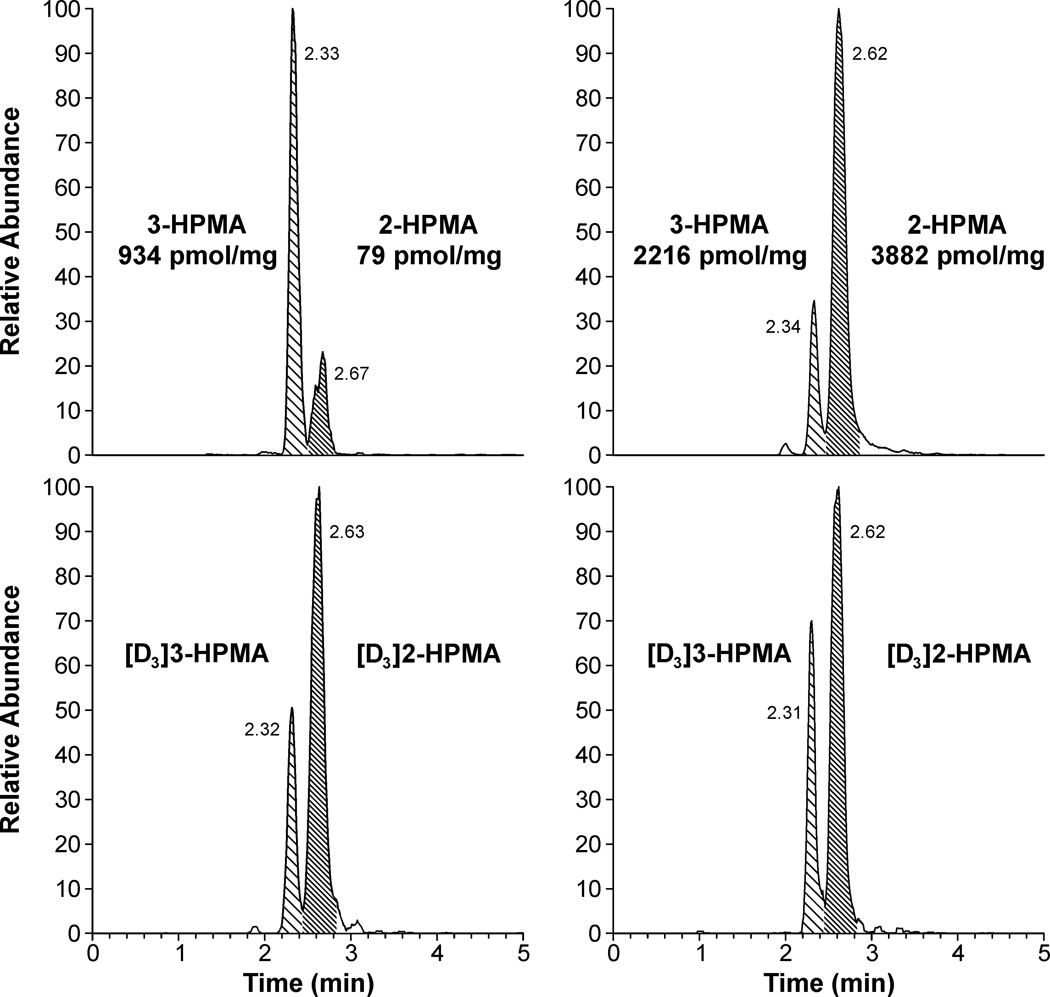
3-Hydroxypropylmercapturic acid (3-HPMA) is a urinary metabolite of acrolein [15]. This structural isomer of 2-HPMA has the same SRM mass transition so must be separated by chromatography. The LC conditions were designed to obtain optimal separation and simultaneous quantitation of 2-HPMA and 3-HPMA. The separation was achieved using a Synergi column under elevated temperature and isocratic conditions, and the method includes a column wash cycle to decrease buildup of chemical noise. These conditions yielded near-baseline resolution (R ≈ 1.2) with retention times of 2.3 min for 3-HPMA and 2.6 min for 2-HPMA (Figure 2). We were not able to separate the diastereomers of 2-HPMA under these conditions.
Figure 2.
Chromatograms obtained upon analysis of human urine for 2-HPMA (retention time 2.6 min). 3-HPMA is observed at 2.3 min. Top traces, analytes; bottom traces, internal standards. These chromatograms represent the broad range of observed 2-HPMA levels in human urine. Values shown are in pmol analyte per mg creatinine.
3.2 Smokers’ and Nonsmokers’ Urine
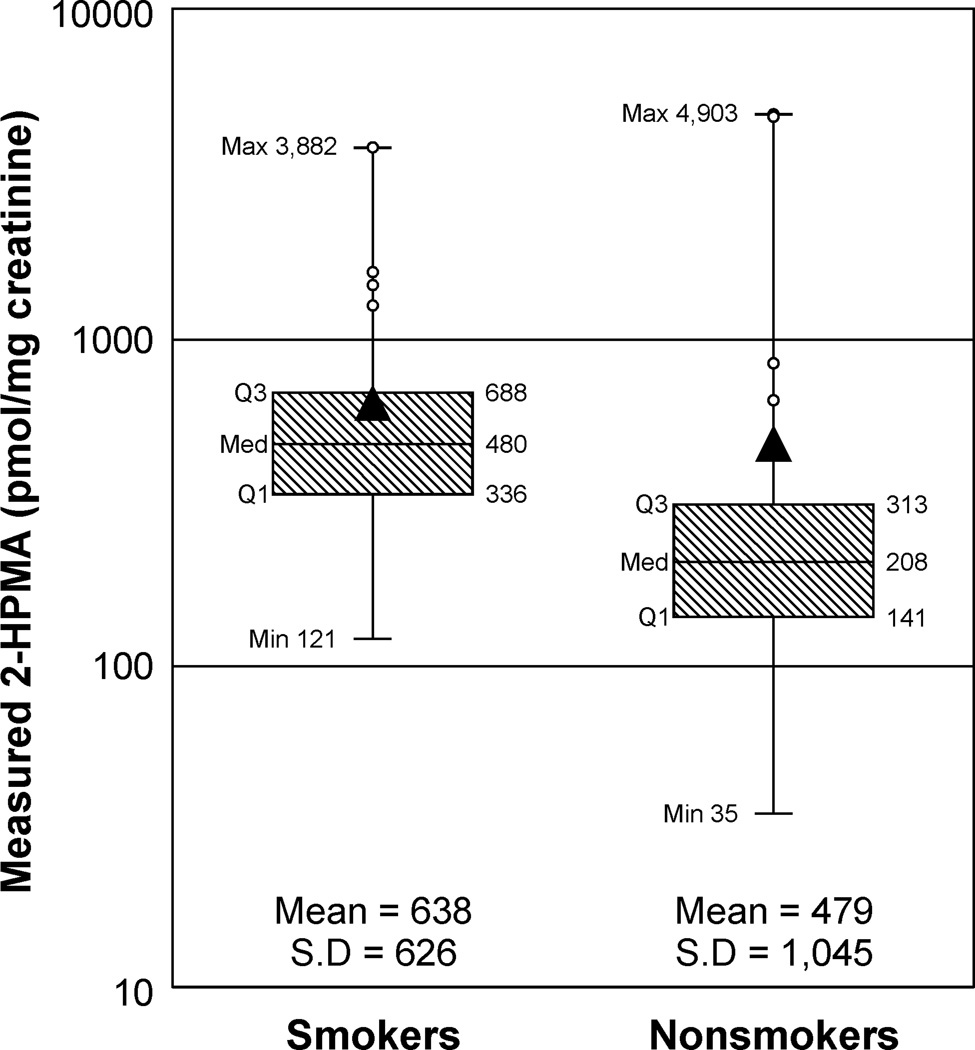
A set of 80 urine samples – 40 each from smokers and nonsmokers – was analyzed. There were 23 females in each group, and the mean ± S.D. age was 34.4 ± 9.4 years (range 19–50) for smokers and 34.2 ± 9.1 years (range 19–50) for nonsmokers. The number of cigarettes smoked per day was 18 ± 6 (range 10–35). Mean and median levels of 2-HPMA were higher in smokers than in nonsmokers (Figure 3). Two urine samples (one smoker and one nonsmoker) were below the limit of detection for 2-HPMA. A comprehensive list of demographic and 2-HPMA data can be found in the supplemental information (SI Table 1).
Figure 3.
Box plot of 2-HPMA levels measured in 39 smokers’ and 39 nonsmokers’ urine. Two samples below LOD are not included. The boxes represent the first quartile, median, and third quartile; and the whiskers mark the maximum and minimum. The triangle denotes the mean values, and the highest 10% of samples (8 individuals) are marked with an O. The data are plotted on a log scale to account for the high-end skew.
Three subjects had 2-HPMA values > 3,500 pmol/mg creatinine, which was more than ten times greater than the median value. Because these three samples highly skew the data set to the right, there was no statistical difference between smokers and nonsmokers on the regular scale (P = 0.4). With 2-HPMA on the log scale, smoking status was statistically highly significant (P = 0.00023). No statistically significant relationships of 2-HPMA to age, gender, or creatinine level were observed. Even with adjustment for these potential confounders, a simple application of the two-sample t-test to the 2-HPMA levels on the log scale to compare smokers versus nonsmokers showed a similar significant result (P < 0.001). Excluding the three samples noted above, levels of 2-HPMA did not correlate with age or cigarettes per day, but there was a moderate correlation with urinary nicotine and cotinine levels (r = 0.55 for nicotine, r = 0.46 for cotinine) and a good correlation with 3-HPMA levels (r = 0.79). The three subjects with high 2-HPMA levels were not remarkable in terms of age, gender, smoking status, or urinary levels of nicotine, cotinine, or 3-HPMA.
3.3 Smoking Cessation
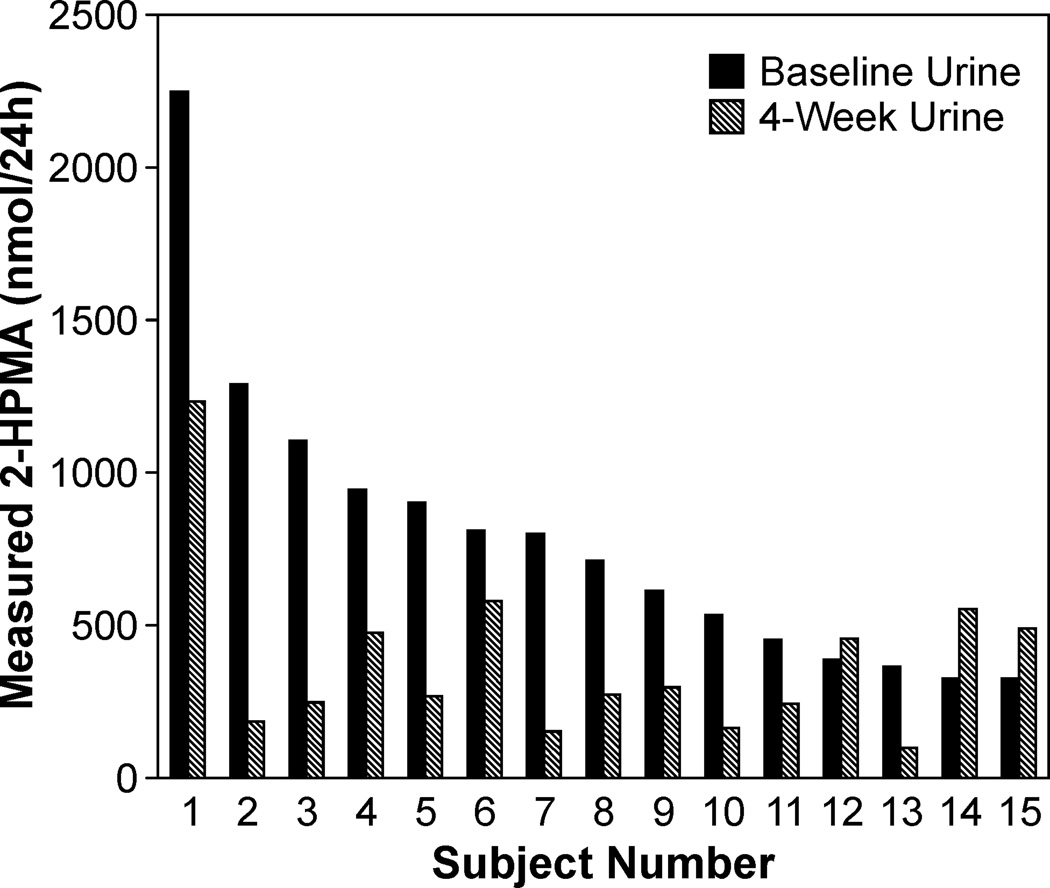
A group of 15 smokers provided baseline 24-h urine samples and abstained from smoking for 4 weeks. The number of cigarettes smoked per day at baseline was 17 ± 6 (range 7–27). There was a decrease in mean urinary 2-HPMA levels upon smoking cessation from 787 nmol/24 h to 380 nmol/24 h, a 52% reduction. This was significant whether the analysis was performed on the regular scale (P = 0.0007) or the log scale (P = 0.0005). The results are consistent with the data above which demonstrate that the median 2-HPMA value for nonsmokers was 57% lower than that of smokers. There were three subjects with increased levels of 2-HPMA after cessation (Figure 4). These subjects had relatively low levels of 2-HPMA in both their initial baseline samples and their 4-week samples.
Figure 4.
Levels of 2-HPMA in smokers who abstained from smoking cigarettes for four weeks. The subjects were sorted by baseline 2-HPMA levels (black), and the majority of the subjects experienced a decrease in urinary 2-HPMA levels after four weeks (gray). Values are reported in nmol 2-HPMA/24-h urine.
4. Discussion
We have developed an accurate and precise method for the rapid quantitation of urinary 2-HPMA, a biomarker of exposure to PO. Cigarette smokers have significantly higher levels of 2-HPMA than do nonsmokers, and individuals who quit smoking exhibit a significant decrease in their urinary 2-HPMA within 4 weeks. These data demonstrate that cigarette smoke is an important source of exposure to PO, even though mainstream cigarette smoke contains < 1 μg/cigarette PO.
We are aware of only three previously published methods for the analysis of 2-HPMA in human urine [9–11]. These methods all utilize negative-mode ESI, but in our hands, negative APCI was a superior ionization technique for detection of 2-HPMA. The first method, published by Schettgen et al. in 2008, requires that 2 mL of urine be acidified to pH 2.5 and purified on a nonpolar ENV+ SPE cartridge [9]. We instead chose to use mixed-mode anion exchange SPE cartridges to afford a more specific purification of the mercapturic acids. Schettgen’s method had variable accuracy, though, based on their low-concentration (Qlow, spiked with 40 ng/mL) and high-concentration (Qhigh, spiked with 400 ng/mL) quality control pooled urine samples: Qlow: 70% accuracy (range 64–78%) and Qhigh: 114% (range 104–129%). This is possibly because 2-HPMA was quantified based on [D3]3-HPMA, which had a retention time difference of 0.9 min in their method. In our assay, we experienced similar inconsistency when quantitating 2-HPMA based on [D3]3-HPMA (accuracy was 133% and CV was 32%), so we determined that the appropriate internal standards must be used for both 2-HPMA and 3-HPMA. Even after an off-line SPE cleanup, the urinary matrix can cause significant fluctuations in signal suppression during the chromatographic run, so internal standards for quantitation must be as close as possible to the same retention time as the analyte.
The second method was published by Eckert et al. in 2010 and then applied to smokers and nonsmokers in 2011 [10,16]. This method also required that 2 mL urine be acidified and purified on an ENV+ cartridge. The original publication used [D3]3-HPMA to quantitate 2-HPMA, and the accuracy for the assay was 60% (range 24–83%). For this reason, they synthesized [13C2]2-HPMA and greatly improved the reliability of the method (accuracy was 95–106% and intraday and interday precision were < 10% CV).
The final method was from Alwis et al. in 2012 [11]. This method required only 50 μL urine and employed a “dilute-and-shoot” methodology without any off-line cleanup. Using [D3]2-HPMA for quantitation, the accuracy was 105% (range 101–109%) and interday precision was 6.6–8.5%. They were able to achieve a chromatographic run of only 9 min including re-equilibration time by utilizing a UPLC system. Eckert’s and Schettgen’s LC methods had run times of 22 min and 26 min, respectively. Our method allowed us to elute the analytes of interest within 3 min, then wash and re-equilibrate the column for a total of 12 min per sample. Alwis’ method had an LOD of 5.9 pmol/mL urine, which was comparable to our 4.5 pmol/mL and slightly better than Schettgen’s and Eckert’s 23 pmol/mL.
All three previous studies demonstrated significantly higher levels of 2-HPMA in smokers than in nonsmokers (Table 2), confirming that cigarette smoke is an important source of exposure to propylene oxide. Our study is the first that we are aware of to investigate the effect of smoking cessation on urinary 2-HPMA. We have shown that, when a smoker quits smoking, their 2-HPMA excretion level reduces to approximately that of a nonsmoker.
Table 2.
Summary of the previous studies of 2-HPMA in the urine of smokers and nonsmokers. Qlow and Qhigh denote quality control pooled urine samples to which 40 and 400 ng/mL 2-HPMA were added, respectively. Dashes are used where data were not reported.
| Schettgen et al. (2008) Aachen, Germany |
Eckert et al. (2011) Erlangen, Germany |
Alwis et al. (2012) Atlanta, GA, U.S.A. |
||||
|---|---|---|---|---|---|---|
| Accuracy, % (Range) |
Qhigh: 114 (104–129) |
Qlow: 70 (64–78) |
— (95–106) |
105 (101–109) |
||
| Precision (CV, %) Intraday / Interday |
Qhigh: 7.3 / 13.4 |
Qlow: 4.9 / 22.2 |
< 10 / < 10 | — / (6.6–8.5) | ||
| LOD, pmol/mL | 23 | 23 | 5.9 | |||
| Smokers | Nonsmokers | Smokers | Nonsmokers | Smokers | Nonsmokers | |
| Subjects, n | 14 | 14 | 40 | 54 | 347 | 1,203 |
| Median 2-HPMA, pmol/mg creatinine (Range) |
170 (< 23–934) |
21 (< 23–333) |
209 (14–1235) |
55 (14–122) |
— | — |
| Mean 2-HPMA, pmol/mL (± S.D.) |
— | — | — | — | 837 (± 1063) |
367 (± 534) |
The data from these studies suggest that there may be differences in exposure to PO in different parts of the world. The two studies from Germany have comparable median values for smokers and nonsmokers, while our results are closer to those from Alwis’ study, which collected samples from anonymous, multi-ethnic, healthy participants. Detailed subject information was not published for the Alwis study, but it is likely that the urine came from people living in the United States. The data from our study and Alwis’ study suggest that 2-HPMA is approximately 2-fold higher in smokers, while Schettgen and Eckert found that smokers had 8-fold and 4-fold higher 2-HPMA than nonsmokers, respectively. Additionally, both studies in Germany found lower values overall in smokers and nonsmokers than did either of the two studies in the United States. It is possible, therefore, that residents of the Unites States have higher background exposures to PO.
We have now analyzed 2-HPMA in nearly 500 urine samples (data not shown), and we consistently find that a small percentage (~5%) of the samples have very high 2-HPMA levels (10-fold to 100-fold above the median). The data from Eckert’s study seem to reflect this same phenomenon. The 95th percentile for smokers in Eckert’s study was 887 pmol/mg, but the maximum was almost 50% higher (1,285 pmol/mg). This is similar to our smokers’ data with a 95th percentile of 1,482 pmol/mg and a maximum of 3,194 pmol/mg. However, our nonsmokers’ data also show a strong right-end skew, whereas the nonsmokers’ data in Eckert’s study do not. Ninety-fifth percentile data were not available from the other two studies, but the high standard deviation in Alwis’ study suggests a very broad range of excreted 2-HPMA, which we have also observed.
Nearly all human urine contains 2-HPMA. While cigarette smoke is an important source of PO, there are clearly other sources of exposure which have not yet been elucidated. The few subjects in this study with very high levels of 2-HPMA are not remarkable in any way with respect to age, gender, smoking status, cigarettes per day, or urinary levels of 3-HPMA, nicotine, or cotinine. PO can be formed as a product of incomplete combustion, so automobile and industrial exhaust may contribute to the exposure of the general population. Some household carpet cleaners and automobile lubricants also contain PO and can be a direct source of exposure. Low-level exposures could also result from ingestion of contaminated food items. PO is approved for use as a direct and indirect food additive by the U.S. Food and Drug Administration [17], but there are many limits defined in the code of federal regulations. According to the Federal Insecticide, Fungicide, and Rodenticide Act, tolerances for residual levels of PO must be below 300 ppm for dried garlic, dried herbs, and tree nuts; 200 ppm for cocoa powder; as low as 2 ppm for plums and prunes; and 1 ppm for grapes and raisins [2].
It is possible that there are significant interindividual differences in the activity of metabolic enzymes. Cytochrome P450 2E1 is the major isoform responsible for the conversion of propylene to propylene oxide [8,18]. If the P450-mediated conversion of propylene to PO is much more efficient in certain individuals, this would result in higher in vivo exposure to PO and thus higher 2-HPMA excretion. However, studies have shown that the levels of PO formation after exposure to propylene are not high enough to cause significant toxicity [8,18,19]. This is consistent with the fact that PO is carcinogenic while propylene is not. Cytochrome P450 epoxidation of propylene exhibits enzyme-saturation kinetics in vivo at high doses [20]. In humans, exposure to 23 ppm propylene resulted in only 0.9 nM concentrations of PO in the blood, thus relevant exposure levels to propylene are generally not considered to be a health risk.
We can speculate that there are sources of 2-HPMA other than the reaction between glutathione and PO. Alternative 3-carbon substrates may include 1-chloro-2-propanol or propylene glycol. 1-Chloro-2-propanol was investigated by the National Toxicology Program and found to have no evidence of carcinogenicity [21]. Displacement of chloride by glutathione could contribute to urinary 2-HPMA after further metabolism of the glutathione conjugate. Propylene glycol (propane-1,2-diol) is generally regarded as safe and is ubiquitous in manufactured products including pharmaceuticals, food preservatives, and electronic cigarettes [22]. It is also applied in antifreeze formulations and as a chemical intermediate for industrial syntheses. The 1-hydroxy group might be converted to a leaving group (e.g. via sulfation, acetylation or esterification in vivo), then displaced by glutathione and excreted as 2-HPMA. Presently, there have not been any studies performed to directly link 2-HPMA formation to either of these two compounds.
5. Conclusions
Propylene oxide is a carcinogenic environmental toxicant which is metabolized to the mercapturic acid 2-HPMA. This mercapturic acid is found in the urine of nearly all individuals, and is generally higher in smokers than in nonsmokers. We have developed and validated an accurate and precise method for the rapid quantitation of 2-HPMA in human urine, and we have applied this method to the urine of smokers and nonsmokers and in a smoking cessation study. We have shown that cigarette smoke is an important source of exposure to PO, but that some individuals are apparently exposed to high levels of PO from other sources. Further research is needed to clarify the source(s) of this exposure.
Supplementary Material
Highlights.
2-Hydroxypropylmercapuric acid (2-HPMA) is a biomarker of propylene oxide exposure
Cigarette smokers have significantly higher levels of urinary 2-HPMA
Smoking cessation results in a significant decrease of 2-HPMA excretion
Variation in 2-HPMA levels cannot be attributed solely to cigarette smoke
Acknowledgements
This study was supported by grant PO1 CA-138338 from the National Cancer Institute. Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, University of Minnesota, funded in part by Cancer Center Support Grant CA-77598. We thank Bob Carlson for editorial assistance.
Abbreviations
- PO
propylene oxide
- 2-HPMA
2-hydroxypropylmercapturic acid
- 3-HPMA
3-hydroxypropylmercapturic acid
- LOD
limit of detection
- LOQ
limit of quantitation
- CV
coefficient of variation
- LC-APCI-MS/MS
liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry
- SRM
selected reaction monitoring
- Qhigh
high-concentration quality control urine sample
- Qlow
low-concentration quality control urine sample
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Toxicology Program, Tech. Rep. Ser. 1985;267:1–168. [PubMed] [Google Scholar]
- 2.National Toxicology Program, 12th Report on Carcinogens. 2011:367–369. [Google Scholar]
- 3.International Agency for Research on Cancer, IARC Monogr. 1994;60:181–213. [Google Scholar]
- 4.Diekmann J, Douda M, Rustemeier K. J. Chromatogr. Sci. 2006;44:32–34. doi: 10.1093/chromsci/44.1.32. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration, Established List, Fed. Regist. 2012;77:20034–20037. [Google Scholar]
- 6.Hazardous Substances Data Bank. National Library of Medicine, http://toxnet.nlm.nih.gov, 8-3-2005. 9-20-2013.
- 7.Household Products Data Bank. National Library of Medicine, http://householdproducts.nlm.nih.gov, 1-31-2013. 9-20-2013.
- 8.Filser JG, Hutzler C, Rampf F, Kessler W, Faller TH, Leibold E, Putz C, Halbach S, Csanady GA. Toxicol. Sci. 2008;102:219–231. doi: 10.1093/toxsci/kfm311. [DOI] [PubMed] [Google Scholar]
- 9.Schettgen T, Musiol A, Kraus T. Rapid Commun. Mass Spectrom. 2008;22:2629–2638. doi: 10.1002/rcm.3659. [DOI] [PubMed] [Google Scholar]
- 10.Eckert E, Schmid K, Schaller B, Hiddemann-Koca K, Drexler H, Goen T. Int. J. Hyg. Environ. Health. 2011;214:196–204. doi: 10.1016/j.ijheh.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Anal. Chim. Acta. 2012;750:152–160. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Chem. Res. Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht SS, Carmella SG, Chen M, Koch JFD, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- 14.Heinegard D, Tiderstrom G. Clinica Chimica Acta. 1973;43:305–310. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- 15.Carmella SG, Chen M, Zarth A, Hecht SS. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2013;935:36–40. doi: 10.1016/j.jchromb.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert E, Drexler H, Goen T. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2010;878:2506–2514. doi: 10.1016/j.jchromb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Code of Federal Regulations, Part 172.892 Food Starch-Modified, Title 21[Subpart I - Multipurpose Additives]. 4-2-2001 [Google Scholar]
- 18.Pottenger LH, Malley LA, Bogdanffy MS, Donner EM, Upton PB, Li Y, Walker VE, Harkema JR, Banton MI, Swenberg JA. Toxicol. Sci. 2007;97:336–347. doi: 10.1093/toxsci/kfm038. [DOI] [PubMed] [Google Scholar]
- 19.Golka K, Peter H, Denk B, Filser JG. Arch. Toxicol. Suppl. 1989;13:240–242. doi: 10.1007/978-3-642-74117-3_40. [DOI] [PubMed] [Google Scholar]
- 20.Lee MS, Faller TH, Kreuzer PE, Kessler W, Csanady GA, Putz C, Rios-Blanco MN, Pottenger LH, Segerback D, Osterman-Golkar S, Swenberg JA, Filser JG. Toxicol. Sci. 2005;83:177–189. doi: 10.1093/toxsci/kfi006. [DOI] [PubMed] [Google Scholar]
- 21.National Toxicology Program, Tech. Rep. Ser. 1998;477:1–264. [PubMed] [Google Scholar]
- 22.Fowles JR, Banton MI, Pottenger LH. Crit Rev. Toxicol. 2013;43:363–390. doi: 10.3109/10408444.2013.792328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.