Abstract
Objective
It is well established that angiogenesis is a complex and coordinated multi-step process. However, there remains a lack of information about the genes that regulate individual stages of vessel formation. Here, we aimed to define the role of human interferon-induced transmembrane protein 1 (IFITM1) during blood vessel formation.
Approach and Results
We identified IFITM1 in a microarray screen for genes differentially regulated by endothelial cells (ECs) during an in vitro angiogenesis assay and found that IFITM1 expression was strongly induced as ECs sprouted and formed lumens. We showed by immunohistochemistry that human IFITM1 was expressed by stable blood vessels in multiple organs. siRNA-mediated knockdown of IFITM1 expression spared EC sprouting but completely disrupted lumen formation, both in vitro and in an in vivo xeno-transplant model. ECs lacking IFITM1 underwent early stages of lumenogenesis (i.e. intracellular vacuole formation) but failed to mature or expand lumens. Coimmunoprecipitation studies confirmed occludin as an IFITM1 binding partner in ECs and immunocytochemistry showed a lack of occludin at endothelial tight junctions in the absence of IFITM1. Finally, time-lapse video microscopy revealed that IFITM1 is required for the formation of stable cell-cell contacts during endothelial lumen formation.
Conclusions
IFITM1 is essential for the formation of functional blood vessels and stabilizes EC-EC interactions during endothelial lumen formation by regulating tight junction assembly.
Keywords: angiogenesis, blood vessel, vascular biology, endothelial cell, intercellular junction
INTRODUCTION
The formation of endothelial cell (EC)-lined blood vessels begins during embryonic development and continues in the adult, both under physiologic conditions (e.g. growth, wound healing) as well as in numerous disease states. Whether formed by vasculogenesis (de novo assembly) in the embryo or by angiogenesis (remodeling and sprouting from the preexisting vasculature), all new vessels must establish a lumen—the defining feature of the vasculature.1
The cellular mechanisms that coordinate EC lumen formation have been described using a variety of in vitro and in vivo models, and while common mechanisms do emerge, some controversy remains. For example, a cord hollowing and cellular rearrangement mechanism, in which the lumen forms de novo between ECs arranged in a cord, was observed in the retina and developing aorta in mice and in the intersegmental and two major axial vessels in zebrafish embryos.2-6 In contrast, other groups reported a cell hollowing mechanism, in which ECs form intracellular vacuoles that are exocytosed and fused between neighboring cells to form an intercellular lumen, again, in the developing aorta in mice and in the intersegmental vessels in zebrafish embryos, as well as in cultured ECs within 3D gels.7-11 Our data using an in vitro angiogenesis assay suggested that lumen formation might involve a combination of these two mechanisms.12 In agreement with this, Wang et al13 reported that lumen formation in the zebrafish intersegmental vessels proceeds via a cord hollowing mechanism driven, in part, by the formation of intracellular vacuoles. The molecular regulators of vascular lumen formation modulate a variety of cellular processes, including cell-cell adhesion, extracellular matrix degradation, polarity, and motility.14 Although this list continues to grow, our understanding of the molecular complexity of endothelial lumen formation remains in its infancy.
Human interferon-induced transmembrane protein 1 (IFITM1), which was originally identified as a downstream target of interferon stimulation,15 belongs to the IFITM gene family, along with IFITM2, IFITM3, IFITM10, and bone-specific IFITM5/BRIL.16,17 IFITM1 exhibits a diverse range of cellular functions that are cell-type and context dependent, including proliferation, adhesion, and cellular resistance to viral infection.18,19 The 7 Ifitm/fragilis genes in mice are homologs, but not orthologs, of the human genes. Only a single ancestral gene was passed to each species, where it then duplicated independently within each lineage. Thus, the gene families may have acquired completely independent functions.20
Over twenty years ago, a study reported that blood vessel ECs in several adult organs express human IFITM1.21 Moreover, IFITM1 expression is induced in cultured ECs in response to interferon22 and IFITM1 may be a pan-endothelial marker.23 Surprisingly, however, the function of IFITM1 in ECs has not been investigated. Therefore, we asked whether IFITM1 regulates the formation of functional EC-lined blood vessels. To determine the cellular mechanisms of IFITM1 function in ECs, we used RNAi and 3D in vitro models of vessel formation. We examined the role of human IFITM1 during vessel formation in vivo using a murine xeno-transplant vascular bed model:24 a strategy to avoid inferring gene function by comparing non-orthologous human and mouse IFITM/fragilis genes.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Supplement.
RESULTS
Endothelial IFITM1 expression is regulated during angiogenesis in vitro
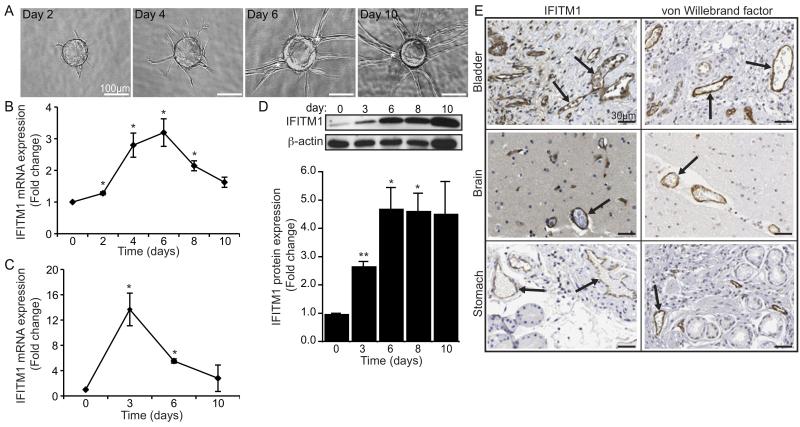
We previously developed an in vitro angiogenesis assay, in which human ECs sprout from the surface of beads embedded in fibrin gels to form microvessels.12 In this model, ECs undergo a series of coordinated morphological changes that recapitulate the critical stages of in vivo angiogenesis, including proliferation, migration, sprouting, branching, and lumen formation (Figure 1A). Using this assay, we performed microarray analyses to examine temporal gene expression changes in ECs actively undergoing tube formation (unpublished data). Notably, we identified IFITM1 as being strongly induced on day 4—correlating with the onset of lumen formation—with expression peaking on day 6 (Figure 1B). This expression pattern was confirmed independently using qRT-PCR (Figure 1C). In addition, western blot analysis revealed a gradual and cumulative increase of IFITM1 protein in ECs over the course of 10 days (Figure 1D). To determine the expression of IFITM1 by ECs in vivo, we examined normal human tissue immunohistochemically stained with an IFITM1 antibody. As shown in Figure 1E, IFITM1 was expressed by blood vessel ECs in the bladder, brain, and stomach and exhibited a staining pattern similar to the EC marker, von Willebrand factor. Thus, endothelial IFITM1 expression correlates with vessel maturation: it is induced by ECs during the maturation stages of angiogenesis in vitro and is stably expressed by quiescent microvascular ECs in vivo.
Figure 1. IFITM1 is regulated by ECs during in vitro angiogenesis and expressed by blood vessels in vivo.
(A) Representative images showing morphologic progression of developing EC sprouts during in vitro angiogenesis for 10 days. Asterisks indicate vessel lumens. (B-C) ECs were harvested from angiogenesis assays at the indicated times and IFITM1 mRNA expression was analyzed by microarray (B) or qRT-PCR (C). Data are represented as fold change over day 0±SEM (n=3). *P<0.05. (D) ECs were harvested from angiogenesis assays at the indicated times and IFITM1 protein was examined by western blot. Blots were probed for β-actin as a loading control. Densitometry values normalized to β-actin are expressed as fold change over day 0±SEM (n=3). *P<0.05, **P<0.005. (E) Human tissue sections were immunohistochemically stained for IFITM1 (left panels) or von Willebrand factor (right panels) and counterstained with hematoxylin. Arrows point to blood vessels. Images are from The Human Protein Atlas, with permission.
IFITM1 regulates EC lumen formation during angiogenesis in vitro
To elucidate the function of IFITM1 in ECs, we used RNAi to knock down expression and then examined the ability of the cells to undergo morphogenetic events during angiogenesis. Human IFITM1 exhibits approximately 70-80% mRNA sequence identity with two of its family members, IFITM2 and IFITM3 (Figure S-IA). Thus, we confirmed the specificity of the siRNA for IFITM1 transcripts. As shown in Figure S-IB, transfection of ECs with IFITM1 siRNA had no significant effect on expression of either IFITM2 or IFITM3 mRNA. In contrast, IFITM1 mRNA was significantly reduced (>95%), as was protein expression, as confirmed by western blot (Figure 2A-B). The residual protein band observed following IFITM1 siRNA treatment was not IFITM1 protein, but rather IFITM3 protein, which was also detected by the IFITM1 antibody, as demonstrated by the addition of IFITM3 siRNA (Figure 2B, compare lanes 2 and 3). Knockdown of IFITM1 was persistent, with inhibition still greater than 80% at 7 days post-transfection (Figure S-IC).
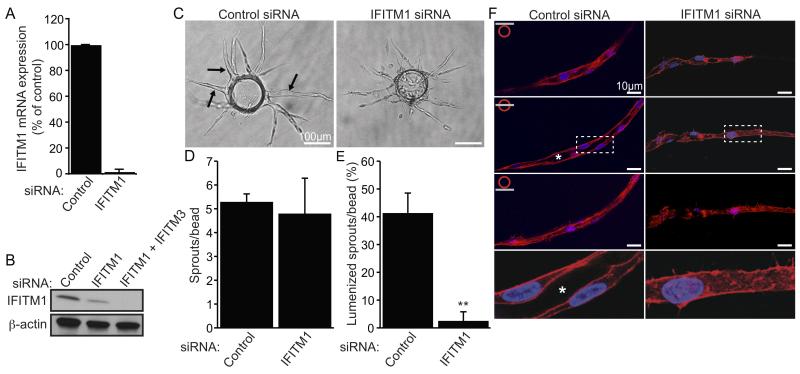
Figure 2. IFITM1 is required for EC lumen formation during angiogenesis in vitro.
(A) ECs were transfected with control or IFITM1 siRNAs and knockdown of IFITM1 mRNA was assessed by qRT-PCR 24 hours later. Data are represented as percent of control expression (set to 100)±SEM (n=3). (B) ECs were transfected with indicated siRNAs and knockdown of IFITM1 protein was examined by western blot 72 hours later. Residual signal detected following IFITM1 siRNA treatment was IFITM3 protein (compare lanes 2 and 3). Blots were probed for β-actin as a loading control. (C) ECs transfected with control or IFITM1 siRNAs were seeded into angiogenesis assays for 6 days and then photographed. Arrows indicate vessels containing a continuous intercellular lumen. (D-E) Assays described in C were analyzed for the number of sprouts (D) and percentage of lumenized sprouts (E). Values are mean±SEM (n=3). **P<0.005. (F) Assays described in C were stained for F-actin (phalloidin, red) and nuclei (DAPI, blue) and visualized using confocal fluorescence microscopy. Images of stained vessels were captured at three focal planes (top, center, bottom). Enlarged images of center cross sections (dotted white boxes) are shown in the bottom panels. White asterisks indicate vessel lumens.
When we tested the effect of IFITM1 knockdown in ECs during angiogenesis in vitro, we found that while there was no significant effect on sprouting, EC lumen formation was almost completely inhibited (Figure 2C-E). Similar results were obtained using an independent IFITM1 siRNA (Figure S-IIA-E). Next, to better visualize EC lumens within fibrin matrices, control and IFITM1 siRNA-treated cultures were stained for F-actin and examined using confocal microscopy. Control vessels contained a continuous lumen surrounded by a single layer of ECs along the length of the vessel (Figure 2F, left panels). In contrast, IFITM1 knockdown vessels lacked a lumen and instead were organized into cords of ECs (Figure 2F, right panels). Collectively, these results demonstrate that IFITM1 expression is required for EC lumen formation during angiogenesis in vitro.
To confirm these initial findings, we examined the effects of IFITM1 knockdown on EC lumenogenesis using a second in vitro assay. In this system, ECs are suspended as single cells within a 3D collagen matrix and stimulated to undergo vacuole and lumen formation.11 As shown in Figure 3A-B, ECs transfected with control siRNA formed large multicellular lumens within the collagen matrix. In contrast, knockdown of IFITM1 permitted only the formation of intracellular vacuoles and rudimentary intercellular lumens (Figure 3A-B). IFITM1 knockdown using a second IFITM1 siRNA produced similar results (Figure S-IIF-G). Thus, IFITM1 is required for EC lumen formation within collagen matrices, consistent with our observations during angiogenesis within fibrin matrices (Figure 2).
Figure 3. Retroviral delivery of IFITM1 rescues the IFITM1-knockdown phenotype.
(A-B) ECs transfected with control or IFITM1 siRNAs were seeded into lumenogenesis assays and allowed to undergo morphogenesis for 48 hours. (A) Images from a representative experiment are shown. Arrows point to large multicellular lumens in control cultures. Arrowheads indicate small intercellular lumens in IFITM1-knockdown cultures. (B) Assays were quantified for the mean lumenal area±SEM (n=3). *P<0.05. (C) ECs were transduced with the indicated retroviruses and ectopically expressed FLAG-IFITM1 protein was examined by western blot 72 hours later using a FLAG antibody. Blots were probed for β-actin as a loading control. (D-E) ECs were transduced with retroviruses encoding GFP or FLAG-IFITM1, transfected with siRNAs as indicated, and seeded into lumenogenesis assays for 48 hours. (D) Images from a representative experiment are shown. Arrows point to large multicellular lumens. Arrowheads indicate small intercellular lumens. (E) Assays were quantified for mean lumenal area±SEM. **P<0.005.
Finally, we performed rescue experiments to determine whether re-expressing IFITM1 in IFITM1-knockdown ECs could restore lumen formation. We designed an IFITM1 siRNA (IFITM1 #2) targeting the 3′ untranslated region of IFITM1 mRNA and generated a retrovirus encoding an N-terminally FLAG-tagged IFITM1 protein lacking the 3′ siRNA-targeted sequence. Transfection of ECs with IFITM1 #2 siRNA knocked down both IFITM1 mRNA and protein (Figure S-IIA-B) and as mentioned above, impaired EC lumen formation in vitro (Figure S-IIC-G). Expression of the FLAG-IFITM1 protein in transduced ECs was confirmed by western blot using a FLAG-specific antibody (Figure 3C). As shown in Figure 3D-E, the FLAG-IFITM1 retrovirus significantly rescued lumen formation in IFITM1 #2 siRNA-treated ECs, compared to the control GFP retrovirus. Specifically, large multicellular lumens were observed in the presence of the FLAG-IFITM1 retrovirus that morphologically resembled control cultures (Figure 3C, compare left and right panels). Thus, stable re-expression of IFITM1 protein restores lumen formation in IFITM1-knockdown ECs. We conclude from this series of experiments that IFITM1 regulates EC lumenogenesis in both fibrin and collagen matrices in vitro.
Human IFITM1 is required for blood vessel formation in vivo
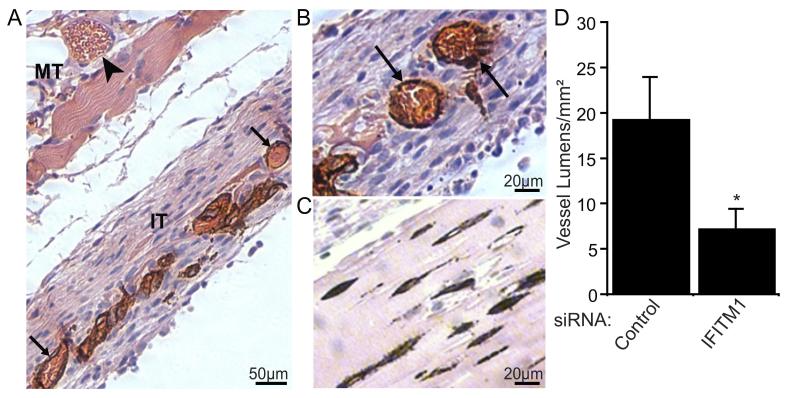
Our knockdown studies demonstrate that IFITM1 is required for endothelial lumen formation in vitro. Therefore, we next wanted to test whether IFITM1 might similarly regulate vessel formation in vivo. There are 7 Ifitm/fragilis genes in mice. However, phylogenetic analyses performed by us and others indicate that IFITM/fragilis genes in human and mouse are not orthologs, but rather they are homologs (Figure S-III).20 The IFITM/fragilis gene family in each species arose from a single common ancestral gene that was subsequently duplicated independently within each lineage. Thus, it is not possible to directly compare individual IFITM/fragilis gene functions between the two species. Therefore, we turned to a xeno-transplant vascular bed model, whereby human ECs suspended in a fibrin gel form microvessels that anastomose with the host vasculature when implanted under the skin of a mouse (Figure 4A).24 As shown in Figure 4B, control siRNA-treated ECs organized into microvessels within the implant tissue and formed functional anastomoses with host vessels, as evidenced by the presence of mouse erythrocytes within the human vessels. In sharp contrast, IFITM1-knockdown ECs failed to form functional vessels (Figure 4C). Instead, only rudimentary vessel-like EC cords lacking a distinct lumen were observed (Figure 4C-D). Notably, these non-perfused vessels were unable to support the robust stromal cell growth observed in control tissues (Figure 4A-C). The failure to form lumenized structures was not due to a defect in cell migration, as control and IFITM1-knockdown cells showed similar migratory rates in 3D collagen gels (control, 0.27±0.04μm/min; IFITM1-knockdown, 0.29±0.05μm/min). These transplant studies are entirely consistent with our in vitro data and demonstrate that IFITM1 expression is also required for human vessels to form lumens in vivo.
Figure 4. Human IFITM1 is required for vessel formation in vivo.
Fibrin tissue constructs containing ECs transfected with siRNAs were implanted subcutaneously into ICR-SCID mice and harvested 8 days later. (A) Control siRNA-treated tissue stained with human CD31 antibody and counterstained with hematoxylin and eosin. Perfused vessels lined by human ECs stained positive for CD31 (brown staining) in the implant tissue (IT, arrows), while host vessels showed no CD31 staining in the mouse tissue (MT, arrowhead). (B-C) Representative histological sections of implant tissue constructs containing control (B) or IFITM1 (C) siRNA-transfected ECs immunohistochemically stained for human CD31 and counterstained with hematoxylin and eosin. Arrows indicate perfused human EC-lined vessels. (D) Human vessel formation within the implant tissues was quantified by counting CD31+ vessel lumens. Data are mean vessel lumens/unit area of implant tissue±SEM (n=3). *P<0.05.
IFITM1 is dispensable for early stages of lumenogenesis
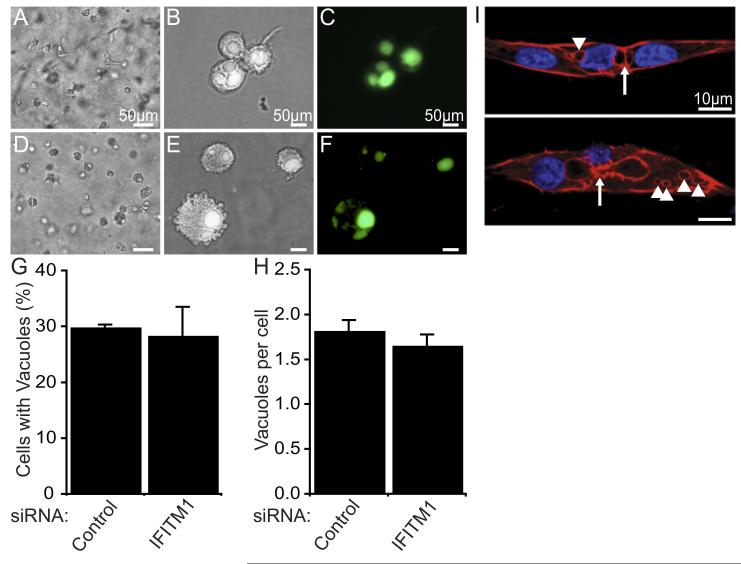
EC lumen formation has been proposed to occur via a step-wise process, beginning with the formation of intracellular pinocytic vacuoles, followed by intracellular vacuole fusion, and ending with intercellular coalescence of neighboring EC vacuoles. Expansion and remodeling then follows to form a continuous vessel lumen.8,9,25 To determine whether IFITM1 is required for pinocytic vacuole formation, we examined the process of lumen formation in collagen matrices in the presence of FITC-dextran.26 Over the course of 4 hours, newly formed pinocytic vacuoles become fluorescently labeled and could be easily visualized (Figure 5A-F). Interestingly, siRNA-mediated suppression of IFITM1 had no effect on the ability of ECs to form intracellular vacuoles (Figure 5G-H). Next, we examined EC vacuole formation during angiogenesis within fibrin matrices by staining for F-actin, which associates with EC vacuole membranes during lumen formation.26 Consistent with our observations in collagen matrices, several distinct intracellular vacuoles were present in ECs in fibrin gels even in the absence of IFITM1 expression (Figure 5I). Moreover, we also found evidence of incomplete intercellular vacuole fusions between neighboring ECs within individual sprouts. Collectively, these studies demonstrate that IFITM1 expression is dispensable for EC intracellular vacuole formation and suggest that IFITM1 regulates a subsequent morphologic event during EC lumen formation.
Figure 5. IFITM1 is dispensable for early stages of lumenogenesis.
(A-H) ECs transfected with control (A-C) or IFITM1 siRNAs (D-F) were seeded into lumenogenesis assays in the presence of soluble FITC-conjugated dextran. After 4 hours, collagen gels were digested to release the cells from the 3D matrix for live imaging on collagen-coated coverslips. Representative bright field images of cells within the 3D matrix prior to digestion of the gels are shown (A and D). Cells on coverslips were photographed under bright field (B and E) and fluorescence (C and F) microscopy. The percentage of cells containing fluorescently-labeled intracellular vacuoles (G) and the number of fluorescently-labeled intracellular vacuoles per cell (H) were quantified and expressed as mean±SEM (n=2). (I) IFITM1-knockdown ECs were seeded into angiogenesis assays and fixed 5 days later. IFITM1 knockdown vessels were visualized by staining for F-actin (phalloidin, red) and nuclei (DAPI, blue) and examined by confocal microscopy. Intracellular vacuoles (arrowheads) and incomplete intercellular vacuole fusions (arrows) are indicated.
IFITM1 regulates junctional stability during EC lumen formation
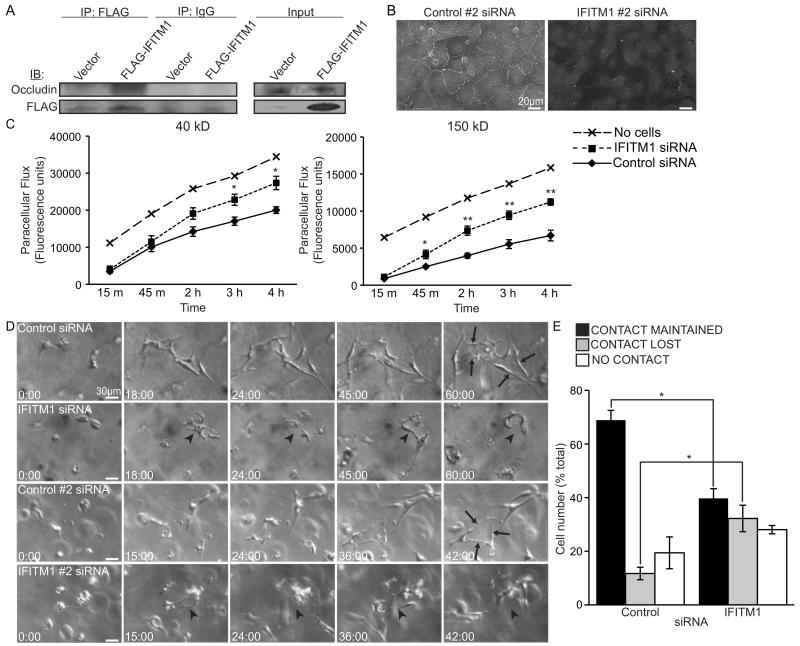
A critical step during EC vessel and lumen maturation is stabilizing EC-EC junctions.27 As IFITM1 was recently shown to associate with the tight junction (TJ) protein occludin in hepatocytes,28 we reasoned that the failure of IFITM1-knockdown ECs to form mature lumens might be explained by their inability to assemble stable TJ adhesion complexes. Therefore, we first confirmed the physical interaction of IFITM1 and occludin proteins in ECs using coimmunoprecipitation (Figure 6A). Next, we examined the effects of IFITM1 knockdown on occludin localization in confluent EC monolayers and found that occludin was almost completely absent from TJs in IFITM1 siRNA-treated ECs (Figure 6B). Knockdown of IFITM1 also caused aberrant cytosolic accumulation of a second TJ protein, claudin-5, providing further evidence for a defect in TJ formation (Figure S-IV). In addition, IFITM1-knockdown EC monolayers exhibited impaired TJ barrier function, as determined by an increase in paracellular permeability (Figure 6C). Lastly, to determine whether endothelial junctional stability was disrupted in IFITM1-knockdown ECs during lumen formation, we used time-lapse video microscopy (Videos S-I-IV). ECs transfected with either of the two control siRNAs established initial intercellular interactions within the 3D collagen matrix that remained relatively stable over time to facilitate the formation, remodeling, and expansion of multicellular lumens (Figure 6D-E and Videos S-I-II). In sharp contrast, ECs transfected with either of the two IFITM1 siRNAs formed unstable, dynamic intercellular interactions (Figure 6D-E and Videos S-III-IV). EC lumens were only formed transiently, cycling through phases of initiation followed by disassembly (Figure 6D-E). Junctional stability was similarly disrupted in IFITM1-knockdown ECs during sprouting angiogenesis in fibrin gels (Figure S-V and data not shown). Collectively, these data demonstrate that IFITM1 is required for the proper localization of occludin and claudin-5 in ECs and suggest that IFITM1 may regulate endothelial TJ stability during lumen formation.
Figure 6. IFITM1 regulates junctional stability during EC lumen formation.
(A) Whole cell lysates from ECs transfected with vector or FLAG-IFITM1 expression constructs were immunoprecipitated using a FLAG antibody and protein complexes were analyzed by western blot by probing for occludin or FLAG, as indicated. Immunoprecipitation with an IgG antibody was used as a control. IP, immunoprecipitation; IB, immunoblot. (B) ECs were transfected with control #2 or IFITM1 #2 siRNAs, grown to confluence, immunostained for occludin, and visualized using conventional fluorescence microscopy. (C) Paracellular flux of FITC-conjugated 40 kD or 150 kD dextran across confluent monolayers of control or IFITM1 siRNA-transfected ECs was measured by transwell permeability assay. Data are mean fluorescence units±SEM (n=4). *P<0.05, **P<0.005. (D-E) ECs transfected with the indicated siRNAs were seeded into lumenogenesis assays and examined by time-lapse video microscopy. (D) Frame grabs at the indicated times are shown. Control ECs formed stable large multicellular lumens (arrows). IFITM1-knockdown EC lumens were unstable, repeatedly initiating and regressing (arrowheads). (E) Videos were analyzed for the stability of EC-EC contacts by quantifying the number of cells that maintained, lost, or never made contact with a neighboring cell, expressed as a percent of the total number of cells quantified for each condition±SEM (n=3). *P<0.05.
DISCUSSION
In the present work, we define a novel role for IFITM1 in endothelial lumenogenesis during blood vessel formation. Using an siRNA approach to knock down IFITM1 expression, we developed a clear understanding of the cellular mechanisms underlying IFITM1 function in ECs during vascular morphogenesis. While IFITM1 was dispensable for EC sprouting and migration within 3D matrices, it was absolutely required for multicellular lumen formation and subsequent vessel maturation. IFITM1-knockdown ECs proceeded normally through the early stages of lumen formation, which includes the formation of pinocytotic vacuoles and their subsequent intracellular fusion,8,14 while the later phase of intercellular vacuole fusion to yield multicellular lumens invariably failed, apparently due to mislocalization of the TJ proteins occludin and claudin-5.
We believe that our observations using two different in vitro assays reflect the same defect—an inability to stabilize junctions. This was most obvious in collagen gel lumenogenesis assays, in which ECs begin as single cell suspensions in the gel; we observed IFITM1-knockdown ECs coming together, forming temporary contacts, and then separating again before stable intercellular lumens could form. Interestingly, in fibrin gel angiogenesis assays, where cells are in contact from the beginning of the assay, we did not see IFITM1-knockdown ECs migrating away from one another. Rather, we noted that cords of connected ECs migrated (sprouted) away from the bead, but never underwent intercellular lumen formation. While we do not yet have a clear explanation for this difference, it may relate to the local microenvironment, which in the sprouting assay contains fibroblast-derived factors. Indeed, we previously demonstrated that fibroblast-derived matricellular proteins play a critical role in lumen formation, partially by increasing gel stiffness. Thus, it may be that this modified matrix constrains ECs during junctional remodeling, while in the absence of fibroblasts, such as in the collagen gel lumenogenesis assay, ECs more readily migrate away from each other. In support of this hypothesis, we found that in our in vivo model, where fibroblasts are present, cords of IFITM1-knockdown ECs were present, and these cords lacked a lumen.
Further support for the idea that IFITM1 regulates junctional assembly and stability comes from our observation that IFITM1 interacts with the TJ protein occludin in ECs, consistent with a recent report in hepatocytes.28 Importantly, we demonstrated that occludin failed to localize to TJs in the absence of IFITM1 expression. Occludin knockout mice, however, are viable and exhibit normal TJ morphology, with no reported vascular defects.29 These discrepancies may reflect inherent differences between mice and humans30 or might suggest that IFITM1 knockdown has additional effects on endothelial TJ assembly. In agreement with the latter, we also observed aberrant localization of a second TJ protein, claudin-5, in IFITM1-knockdown ECs.
While our data clearly demonstrate dysregulated localization of occludin and claudin-5 in the absence of IFITM1 expression in ECs, the precise molecular mechanisms underlying IFITM1 regulation of TJ assembly and stability remain elusive. One potential mechanism might involve a role for IFITM1 in coordinating the recycling of internalized TJ proteins back to the plasma membrane during TJ remodeling. Indeed, IFITM proteins function in the endosomal pathway to inhibit viral infection18 and internalized occludin and claudin-5 are returned to the plasma membrane from recycling endosomes during TJ remodeling in brain ECs.31 Alternatively, IFITM1 protein may itself be a critical component of the endothelial TJ complex, as IFITM1 has been shown to localize to hepatic TJs.28 Distinguishing between these and other possibilities will be the focus of future studies.
As noted above, our in vivo data using a xeno-transplant vascular bed model24 were consistent with our in vitro observations: in the absence of IFITM1 expression, implanted human ECs failed to form functional, mature, lumenized vessels. This result is further supported by our observation that IFITM1 is expressed by quiescent blood vessel ECs in multiple human tissues, in agreement with previous reports.21,23 Taken together, these data suggest that IFITM1 is required for both the formation and maintenance of blood vessel lumens.
The use of human ECs in these studies proved critical, as our phylogenetic analysis revealed that although the human and mouse IFITM/fragilis genes are homologs, they are not orthologs—a single ancestral gene (predicted to be IFITM5/BRIL) was passed to each lineage where it subsequently underwent independent duplications, a conclusion also drawn by others.19,20,32 Thus, manipulation of mouse fragilis genes could not be used to reliably investigate the function of the human IFITM1 gene. In agreement with this, our studies silencing human IFITM1 in ECs demonstrated that the expression of IFITM2 and IFITM3 was not sufficient to functionally compensate for the loss of IFITM1, whereas mice carrying a deletion of either the entire fragilis locus on chromosome 7 (encompassing five fragilis family members) or only the Ifitm3/fragilis gene had no discernable phenotype, despite a previously defined role in murine embryonic development, suggesting functional redundancy and compensation within the mouse fragilis gene family.19,33
Our studies in vitro and in vivo indicate that IFITM1 regulates EC lumen formation within a diverse range of complex extracellular matrices, suggesting that the function of IFITM1 is not matrix, or by implication, integrin specific. These findings also highlight the potential relevance of IFITM1 function to vessel formation, both within physiologic settings, in which type I collagen dominates the interstitial matrix, and pathologic settings, which are characterized by a provisional matrix rich in vitronectin, fibronectin, and fibrin.34 Our results also implicate IFITM1 in diseases such as diabetes, stroke, and hypoxia/aglycemia, where vessel barrier function is disrupted in association with occludin downregulation.30
In summary, our data define IFITM1 as a novel regulator of EC lumen formation during vascular morphogenesis. We describe a mechanism by which IFITM1 regulates the expansion and maturation of nascent EC lumens into multicellular tubular networks by regulating the formation and/or stability of endothelial TJs. Thus, IFITM1 may prove to be a useful therapeutic target in settings of pathological angiogenesis.
Supplementary Material
SIGNIFICANCE.
The ability to make multicellular tubes is restricted to cells of the epithelial lineage, including ECs, and is a process critical to the formation of a functional vascular network. Our study identifies IFITM1 as a novel regulator of endothelial tube (lumen) formation during angiogenesis. Using a combination of in vitro and in vivo models, we demonstrate that IFITM1 functions to promote maturation of nascent lumens by regulating the assembly and/or stability of intercellular TJ adhesion complexes. These findings suggest that IFITM1 may be a potential therapeutic target for pathological angiogenesis and implicates IFITM1 in diseases associated with disrupted TJs.
ACKNOWLEDGEMENTS
We thank Anna Aledia for assistance with animal surgeries, Dr. Grant MacGregor for help with time-lapse video microscopy, Shohei Takuno for performing phylogenetic analyses, Duc Phan for help with tissue culture, and Dr. Steven George for helpful discussions.
SOURCES OF FUNDING
This work was supported by NIH grants RO1 HL60067 and HL086959. C.C.W.H. receives support from the Chao Family Comprehensive Cancer Center through NCI/NIH Grant P30 CA62203. M.E.Z. receives support from NCI/NIH Grant T32CA009054. The content is solely the responsibility of the authors and doesn’t necessarily represent the official views of the NCI/NIH. K.M.W-R. receives support from an AHA pre-doctoral award. A.H.F. receives support from IGERT Program sponsored by NSF (DGE-0549479) and California Institute for Regenerative Medicine (TG2-01152)
ABBREVIATIONS
- EC
endothelial cell
- IFITM1
interferon-induced transmembrane protein 1
- TJ
tight junction
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 2.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strilic B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara N, Lammert E. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17:505–515. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 6.Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Rigamonti D, Badr A, Zhang J. Ccm1 regulates microvascular morphogenesis during angiogenesis. J Vasc Res. 2010;48:130–140. doi: 10.1159/000316851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 9.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 10.Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF, Iruela-Arispe ML. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol. 2008;443:83–101. doi: 10.1016/S0076-6879(08)02005-3. [DOI] [PubMed] [Google Scholar]
- 12.Nakatsu MN, Hughes CC. An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods Enzymol. 2008;443:65–82. doi: 10.1016/S0076-6879(08)02004-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Kaiser MS, Larson JD, Nasevicius A, Clark KJ, Wadman SA, Roberg-Perez SE, Ekker SC, Hackett PB, McGrail M, Essner JJ. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development. 2010;137:3119–3128. doi: 10.1242/dev.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacharidou A, Stratman AN, Davis GE. Molecular mechanisms controlling vascular lumen formation in three-dimensional extracellular matrices. Cells Tissues Organs. 2011;195:122–143. doi: 10.1159/000331410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman RL, Manly SP, McMahon M, Kerr IM, Stark GR. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984;38:745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- 16.Lewin AR, Reid LE, McMahon M, Stark GR, Kerr IM. Molecular analysis of a human interferon-inducible gene family. Eur J Biochem. 1991;199:417–423. doi: 10.1111/j.1432-1033.1991.tb16139.x. [DOI] [PubMed] [Google Scholar]
- 17.Moffatt P, Gaumond MH, Salois P, Sellin K, Bessette MC, Godin E, de Oliveira PT, Atkins GJ, Nanci A, Thomas G. Bril: a novel bone-specific modulator of mineralization. J Bone Miner Res. 2008;23:1497–1508. doi: 10.1359/jbmr.080412. [DOI] [PubMed] [Google Scholar]
- 18.Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol. 2012;13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegrist F, Ebeling M, Certa U. The small interferon-induced transmembrane genes and proteins. J Interferon Cytokine Res. 2011;31:183–197. doi: 10.1089/jir.2010.0112. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Liu J, Li M, Yang H, Zhang C. Evolutionary dynamics of the interferon-induced transmembrane gene family in vertebrates. PLoS One. 2012;7:e49265. doi: 10.1371/journal.pone.0049265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pumarola-Sune T, Graus F, Chen YX, Cordon-Cardo C, Evans RL. A monoclonal antibody that induces T cell aggregation reacts with vascular endothelial cells and placental trophoblasts. J Immunol. 1986;137:826–829. [PubMed] [Google Scholar]
- 22.Jaffe EA, Armellino D, Lam G, Cordon-Cardo C, Murray HW, Evans RL. IFN-gamma and IFN-alpha induce the expression and synthesis of Leu 13 antigen by cultured human endothelial cells. J Immunol. 1989;143:3961–3966. [PubMed] [Google Scholar]
- 23.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Aledia AS, Popson SA, Him L, Hughes CC, George SC. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A. 2010;16:585–594. doi: 10.1089/ten.tea.2009.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 27.Lampugnani MG, Dejana E. Adherens junctions in endothelial cells regulate vessel maintenance and angiogenesis. Thromb Res. 2007;120(Suppl 2):S1–6. doi: 10.1016/S0049-3848(07)70124-X. [DOI] [PubMed] [Google Scholar]
- 28.Wilkins C, Woodward J, Lau DT, Barnes A, Joyce M, McFarlane N, Tyrrell DL, Gale M., Jr. IFITM1 is a tight junction protein that inhibits hepatitis C virus entry. Hepatology. 2012;57:461–469. doi: 10.1002/hep.26066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem. 2009;284:19053–19066. doi: 10.1074/jbc.M109.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hickford DE, Frankenberg SR, Shaw G, Renfree MB. Evolution of vertebrate interferon inducible transmembrane proteins. BMC Genomics. 2012;13:155. doi: 10.1186/1471-2164-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lange UC, Adams DJ, Lee C, Barton S, Schneider R, Bradley A, Surani MA. Normal germ line establishment in mice carrying a deletion of the Ifitm/Fragilis gene family cluster. Mol Cell Biol. 2008;28:4688–4696. doi: 10.1128/MCB.00272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen. 2004;12:134–147. doi: 10.1111/j.1067-1927.2004.012208.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.