Abstract
Objective
This study was designed to investigate the impact of body mass index (BMI) on short- and long-term outcomes after initial revascularization with coronary artery bypass graft (CABG) surgery.
Methods
4916 Chinese who consecutively underwent isolated, primary CABG at the Cardiovascular Institute of Fuwai Hospital from January 1, 1999 to December 31, 2005 were included in this study. They were classified based on BMI as follows: underweight: <18.5 kg/m2, normal weight: 18.5 to 23.9 kg/m2, overweight: 24 to 27.9 kg/m2, obesity: 28 to 32 kg/m2, and severe obesity: >32 kg/m2. Short (in-hospital) and long-term (5-years) major post-operative complications and mortalities were compared among various BMI groups after initial revascularization.
Results
Multiple regression analysis of five years follow-up of clinical end points indicated that various BMI groups were not associated with significant differences in 5 years mortality and MACCE, however, old age, smoking, hypertension, myocardial infarction and heart failure were the risk factor for the mortality.
Conclusions
In this large-scale study with long term follow-up after primary CABG in an exclusively ethnic Chinese population, we found that different BMI groups were not significantly associated with 5-years mortality and MACCE, however, old age, smoking, hypertension, myocardial infarction and heart failure were the risk factors of post-operative mortality, and old age, hypertension and heart failure increased the rate of MACCE.
Introduction
Obesity is a common and growing health problem; almost one third of American adults are obese [1]. Annual health care costs attributable to obesity have been estimated to be approximately $68 billion, with an additional $30 billion being spent on weight-reduction programs and special diet [1]. The relationship of obesity to long-term survival is complex and most studies have found a J- or U-shaped curve with increasing mortalities in the underweight or very obese [2], [3]. However, when the data have been adjusted for smoking and concurrent illness, the relationship has been more linear and the risk of death rises as body mass index (BMI) increases [4]. BMI is a validated measure of adiposity [5] and has been consistently used to analyze of obesity and mortality. Obese adults are at an increased risk of cardiovascular mortality [6]. These studies have been limited by failing to account for important confounding factors, such as smoking and comorbidity. Furthermore, no long-term data are available on the impact of BMI on survival in a large series of patients underwent coronary artery bypass graft (CABG) surgeries.
Studies have identified factors that contribute to balance in preventing preoperative adverse events, and potentially improving quality of life [7]. Body fat (commonly described by BMI) in CABG patients is an independent risk factor for blood loss: very low BMI (<18.5 kg/m2) has been identified as increasing blood loss [8]–[10].
This study retrospectively evaluated the effects of BMI on short and long term outcomes in Chinese patients who underwent isolated, primary CABG [11]–[13].
Methods
Study Population
This was a retrospective, observational study of consecutive patients who underwent isolated primary CABG at the Fuwai Hospital. The study protocol was approved by the the Ethics Committee of Fuwai Hospital, and the written informed consent was waived. 4916 patients who consecutively underwent isolated, primary CABG from January 1, 1999 to December 30, 2005 at Fuwai Hospital in Beijing, China were included in this study. BMI was defined as weight, in kilogram, divided by height in meter squared. As the current World Health Organization's (WHO) definition of adult overweight (BMI>25 kg/m2) and obesity (BMI>30 kg/m2) may not be applicable to the Chinese population, thus it had been suggested that the definition of using BMI for overweight or obesity for the Chinese population is different from that for the North American or European populations because obesity-associated metabolism is lower in Chinese individuals than in North American or European populations [14]. Therefore, we adopted the Chinese BMI definition proposed by the Working Group on Obesity in China (WGOC) [15] and described in the Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults [16]to define overweight or obesity, as follows: BMI<18.5 kg/m2 (underweight), 18.5≤BMI<24 kg/m2 (normal weight), 24≤BMI<28 kg/m2 (overweight), and BMI≥28 kg/m2 (obese). To investigate the severe obese in this study, we modified that defined as underweight (<18.5 kg/m2), normal weight (BMI 18.5 to 23.9 kg/m2), overweight (BMI 24 to 27.9 kg/m2), obesity (BMI 28 to 32 kg/m2), and severe obesity (BMI>32 kg/m2).
Isolated primary CABG was defined as coronary artery bypass graft surgery alone for the first time with or without cardiopulmonary bypass. Patients who underwent combined cardiac surgical procedures were excluded. Data were collected from hospital medical records.
Outcome events definition
Renal failure was defined as a need for dialysis to treat prolonged oliguria or anuria [17]; stroke as central neurological deficit persisting more than 72 h; coma as being unresponsive for more than 24 h; encephalopathy as reversible neurological deficit (recovery within 72 h of onset); low cardiac output syndrome (LCOS) as cardiac index lower than 2.0 l/min per m2 and left ventricular assist (LVAD), intra-aortic balloon pump (IABP) and inotropic support after surgery. Outcomes were recorded from follow-up included death and major adverse cardiac and cerebrovascular events (MACCE). MACCE was defined as permanent or transient stroke, coma, perioperative myocardial infarction (MI), heart block, and cardiac arrest [18]–[20].
Statistical analysis
SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Patient demographics, perioperative and follow up outcomes were analyzed. Demographic data are summarized in Tables 1 and 2. Continuous parameters are described by mean and standard deviations, and frequencies describe categorical variable. Comparisons of numerical variables between two groups were performed with Student's test or Mann–Whitney U test if not meeting the normal distribution. ANOVA was used to compare numerical variables between more than two groups. Comparisons of categorical variables were performed with Chi-square tests. Correlations between variables were measured using Spearman rank correlation coefficient. Cumulative incidence of mortalities and MACCE of each BMI groups at 5 year were estimated by Kaplan-Meier method and compared by log-rank test. Multiple Cox regression model and multiple logistic regression model were used to analysis follow-up mortalities and MACCE separately. Covariates included in multiple regression models were baseline variables that showed significant difference among 5 BMI groups. A two-tailed P value <0.05 was considered as statistically significance.
Table 1. Baseline Characteristics of the Patients in All Five BMI Groups.
| Variable | BMI(kg/m2) | ||||||
| <18.5 | 18.5–23.9 | 24–27.9 | 28–32 | >32 | Total | P-value | |
| (n = 31) | (n = 1387) | (n = 2496) | (n = 849) | (n = 153) | (n = 4916) | ||
| Age(yrs) | 67.1±6.2 | 61.8±8.4 | 59.6±8.5 | 58.8±8.7 | 59.2±8.6 | 60.1±8.6 | <.0001 |
| BMI(kg/m2) | 17.4±1.0 | 22.3±1.3 | 25.9±1.1 | 29.4±1.1 | 50.5±49.8 | 26.2±10.1 | <.0001 |
| Men | 20(64.5%) | 1110(80.0%) | 2133(85.5%) | 729(85.9%) | 123(80.4%) | 4115(83.7%) | <.0001 |
| Smoking | 10(32.3%) | 672(48.5%) | 1348(54.0%) | 496(58.4%) | 86(56.2%) | 2612(53.1%) | <.0001 |
| Family History | 1(3.2%) | 107(7.7%) | 206(8.3%) | 63(7.4%) | 6(3.9%) | 383(7.8%) | 0.2869 |
| Hypertension | 15(48.4%) | 784(56.5%) | 1535(61.5%) | 571(67.3%) | 107(70.0%) | 3012(61.2%) | <.0001 |
| Hyperlipidemia | 8(25.8%) | 480(34.6%) | 936(37.5%) | 345(40.6%) | 69(45.1%) | 1838(37.4%) | 0.0070 |
| Diabetes Mellitus | 7(22.6%) | 388(28.0%) | 633(25.4%) | 205(24.2%) | 36(23.5%) | 1269(25.8%) | 0.2427 |
| History of Renal Failure | 0 | 17(1.2%) | 16(0.6%) | 3(0.4%) | 0 | 36(0.7%) | 0.0929 |
| Creatinine (µmol/L) | 193.0±328.9 | 103.5±101.5 | 109.1±111.0 | 122.2±161.9 | 104.8±154.8 | 110.0±130.0 | 0.1180 |
| Cerebrovascular Events | 5(16.1%) | 94(6.8%) | 163(6.5%) | 45(5. 3%) | 8(5. 2%) | 315(6. 4%) | 0.1183 |
| Peripheral Artery Disease | 6(19. 4%) | 152(11.0%) | 212(8. 5%) | 58(6.8%) | 18(11.8%) | 446(9. 1%) | 0.0016 |
| Thrombolytic Therapy | 3(9.7%) | 133(9.6%) | 248(10.0%) | 56(6.6%) | 8(5.2%) | 488(9.1%) | 0.0196 |
| Unstable Angina Pectoris | 1(3.2%) | 152(11.0%) | 234(9.4%) | 87(10.3%) | 24(15.7%) | 498(10.1%) | 0.1186 |
| Myocardial Infarction | 11(35.5%) | 682(49.2%) | 1229(49.2%) | 397(46.8%) | 73(47.7%) | 2392(48.7%) | 0.4177 |
| Diseased Coronary Artery | 2.9±0.2 | 2.8±0.5 | 2.8±0.5 | 2.8±0.5 | 2.8±0.5 | 2.8±0.5 | 0.4558 |
| Left Main Disease | 8(25.8%) | 504(36.3%) | 700(28.0%) | 230(27.1%) | 35(22.9%) | 1477(30.0%) | <.0001 |
| Heart Failure | 1(3.2%) | 37(2.7%) | 45(1.8%) | 9(1.1%) | 6(4.0%) | 98(2.0%) | 0.0311 |
| Atrial Fibrillation | 0 | 41(3.0%) | 38(1.5%) | 17(2.0%) | 5(3.3%) | 101(2.1%) | 0.0278 |
| LVEF (%) | 59.4±9.7 | 60.0±10.1 | 59.7±9.4 | 59.2±9.1 | 58.2±8.9 | 59.5±9.5 | 0.2901 |
| Intravenous Nitrate | 0.06±0.2 | 0.06±0.2 | 0.05±0.2 | 0.06±0.2 | 0.09±0.3 | 0.06±0.2 | 0.1968 |
BMI: body mass index; LVEF: left ventricular ejection fraction. LVEF: left ventricular ejection fraction; ANOVA test was used to analyze continuous variables, χ2 test for categorical variables. P<0.05 was accepted as statistically significant.
Table 2. Post-operative Characteristics of All Five BMI Groups.
| Variable | BMI(kg/m2) | ||||||
| <18.5 | 18.5–23.9 | 24–27.9 | 28–32 | >32 | Total | P-value | |
| (n = 31) | (n = 1387) | (n = 2496) | (n = 849) | (n = 153) | (n = 4916) | ||
| Postoperative Complications | 0 | 4(0.3%) | 14(0.6%) | 2(0.2%) | 0 | 20(0.4%) | 0.5076 |
| Epinephrine | 7(22.6%) | 181(13.1%) | 246(9.9%) | 109(12.8%) | 14(9.2%) | 557(11.3%) | 0.0030* |
| Stroke | 0 | 4(0.3%) | 9(0.4%) | 4(0.5%) | 2(1.3%) | 19(0.4%) | 0.4010 |
| Duration of ICU Stay (h) | 69.4±74.7 | 63.4±61.3 | 59.9±57.8 | 60.3±53.6 | 65.5±60.2 | 61.2±58.3 | 0.3025 |
| Ventilation Time (h) | 14.8±7.6 | 17.6±32.2 | 16.1±21.4 | 16.4±17.6 | 18.6±28.4 | 16.7±24.6 | 0.3841 |
| Atrial Fibrillation | 3(9.7%) | 133(9.6%) | 215(8.6%) | 56(6.6%) | 14(9.2%) | 421(8.6%) | 0.1858 |
| Renal Failure | 0 | 6(0.4%) | 6 (0.2%) | 2(0.2%) | 0 | 14(0.3%) | 0.7655 |
| Coma | 0 | 7(0.5%) | 9(0.4%) | 3(0.4%) | 0 | 19(0.4%) | 0.8639 |
| Mortality | 2(6.5%) | 62(%4.5) | 102(4.1%) | 37(4.4%) | 7(4.6%) | 210(4.3%) | 0.9446 |
| Myocardial Infarction | 0 | 19(1.4%) | 37(1.5%) | 14(1.7%) | 3(2.0%) | 73(1.5%) | 0.9119 |
| Repeated Revascularization | 3(9.7%) | 48(3.5%) | 110(4.4%) | 44(5.2%) | 4(2.6%) | 209(4.3%) | 0.1191 |
| Stroke | 6(19.4%) | 172(12.4%) | 285(11.4%) | 102(12.0%) | 22(14.4%) | 587(11.9%) | 0.4906 |
| MACCE | 11(35.5%) | 287(20.7%) | 498(20.0%) | 183(21.6%) | 32(20.9%) | 1011(20.6%) | 0.2549 |
MACCE: major adverse cardiac and cerebrovascular events. BMI: body mass index; ICU: intensive care unit; Student's t test was used to analyze continuous variables, χ2 test for categorical variables. P values: comparison between overweight and control groups; * statistical differences between BMI<18.5 group and the rest of the BMI groups. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 shows patient demographic data. Underweight group tended to be older (67.1±6.2) than patients in other groups. A higher number of underweight patients presented as emergency cases, whereas more elective procedures were performed in the obese group. Hyperlipidemia was more prevalent in all the obese groups (45.1% vs. 25.8%, 34.6%, 37.5%, 40.6%, 37.4% respectively, p<0.01), while peripheral and cerebrovascular disease prevailed in the lean cohort (peripheral artery disease: 19. 4% vs. 11.0%, 8. 5%, 6.8%, 11.8%, 9.1%; cerebrovascular events:16.1% vs. 6.8%, 6.5%, 5. 3%, 5. 2%, 6.4%. p<0.05). Renal function was similar across the population.
Perioperative outcomes
Table 2 shows the results of the five groups. Among all 5 groups, there are no differences in the in-hospital postoperative complications (P = 0.051). Patients in underweight group administered more epinephrine compared with other groups (22.6% vs. 13.1%, 9.9%, 12.8%, 9.2%, 11.3% respectively. P<0.05). There was no difference in in-hospital mortality. There were no differences in stroke, length of ICU stay, ventilation time, atrial fibrillation, renal failure, composite cerebral complication, and coma (p>0.05).
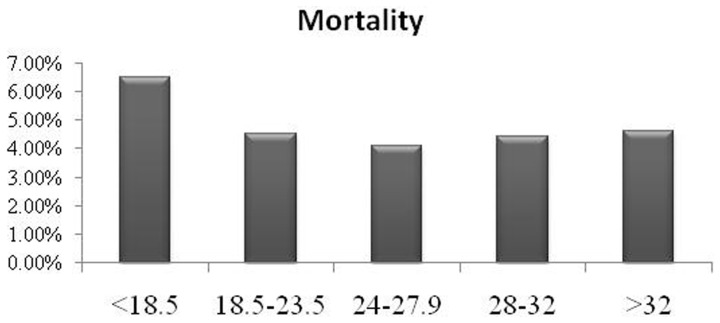
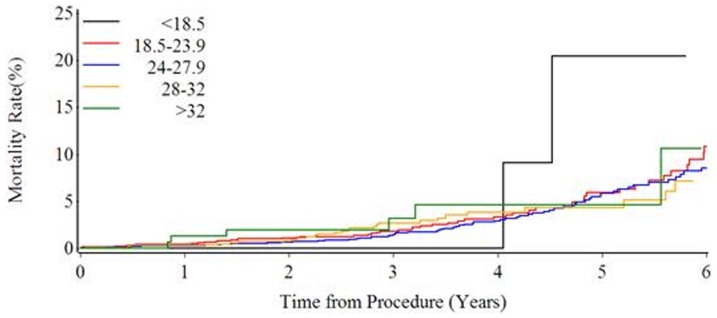
The median follow up duration was 59.3 months in this population. There were no significant differences in myocardial infarction, stroke and revascularization rate (p>0.05). There were also no significant differences in mortality (p>0.05) and MACCE (p>0.05). However, the mortality and MACCE tend to be higher in underweight group than that in other groups, though no significant difference was found (mortality: 6.5% VS. 4.5%, 4.1%, 4.4%, 4.6%, 4.3% respectively; MACCE: 35.5% vs. 20.7%, 20.0%, 21.6%, 20.9%, 20.6% respectively P>0.05; Figure 1–2). Figure 3 shows the Kaplan-Meier curves demonstrated the mortality, and there was no significant difference among 5 groups (Log-rank test P = 0.7909).
Figure 1. Illustrating the mortality rates at the end of 5-year follow-up in all five body mass index (BMI) groups.
Figure 2. Illustrating the major adverse cardiac and cerebrovascular events (MACCE) rates at the end of 5-year follow-up in all five body mass index (BMI) groups.
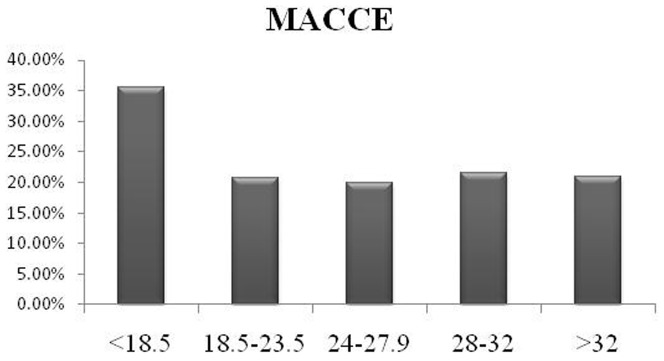
Figure 3. Kaplan-Meier curves of the 5-year mortality in all five body mass index (BMI) groups.
The long-term follow-up mortality: Multivariable Cox regression model was used to adjust the confounders and analysis the association between BMI groups and mortality. Covariates in this model included age, BMI, gender, smoking, hypertension, hyperlipidemia, peripheral artery disease, MI, thrombolytic therapy, left main coronary artery disease, heart failure. As demonstrated in Table 3, old age, smoking, hypertension, MI and heart failure are associated with increased long-term follow-up mortalities [Hazard Ratio (HR)(95% CI):1.053(1.035–1.072), 1.464(1.082–1.980), 1.751(1.292–2.372),1.394(1.034–1.880), 2.817(1.544–5.140).p<0.05]. There were no significant differences found in all 5 BMI groups (p>0.05).
Table 3. Long Term Mortality.
| Variables | β | std | P-value | HR | HR 95% CI | |
| BMI <18.5 vs. 18.5–23.9 | 0.23779 | 0.72457 | 0.7428 | 1.268 | 0.307 | 5.248 |
| BMI 24–27.9 vs. 18.5–23.9 | −0.03860 | 0.16625 | 0.8164 | 0.962 | 0.695 | 1.333 |
| BMI 28–32 vs. 18.5–23.9 | −0.07927 | 0.21944 | 0.7179 | 0.924 | 0.601 | 1.420 |
| BMI>32 vs. 18.5–23.9 | 0.34369 | 0.40349 | 0.3943 | 1.410 | 0.639 | 3.110 |
| Age | 0.05208 | 0.00904 | <.0001 | 1.053 | 1.035 | 1.072 |
| Gender: Male vs. Female | 0.35880 | 0.24604 | 0.1448 | 1.432 | 0.884 | 2.319 |
| Smoking | 0.38097 | 0.15420 | 0.0135 | 1.464 | 1.082 | 1.980 |
| Hypertension | 0.55994 | 0.15509 | 0.0003 | 1.751 | 1.292 | 2.372 |
| Hyperlipidemia | −0.13044 | 0.15397 | 0.3969 | 0.878 | 0.649 | 1.187 |
| Peripheral Artery Disease | 0.39404 | 0.28771 | 0.1708 | 1.483 | 0.844 | 2.606 |
| Thrombolytic Therapy | −0.01460 | 0.22105 | 0.9473 | 0.986 | 0.639 | 1.520 |
| Myocardial Infarction | 0.33206 | 0.15257 | 0.0295 | 1.394 | 1.034 | 1.880 |
| Heart Failure | 1.03561 | 0.30684 | 0.0007 | 2.817 | 1.544 | 5.140 |
| Left Main Disease | 0.10601 | 0.14930 | 0.4777 | 1.112 | 0.830 | 1.490 |
BMI: body mass index; Multivariable Cox regression model was used to analyze categorical variables. β: std: standard deviation; HR: hazard ratio; CI: confidence interval. P values: comparison between the other and normal weight group; P<0.05 was considered as statistically significant.
The 5 years follow-up MACCE: Multivariable logistic regression model was used to adjust the confounding factors and analysis the association between BMI groups and MACCE. Table 4 showed old age, hypertension and heart failure are associated with increased the rate of MACCE [HR (95% CI): 1.026(1.017–1.035), 1.254(1.080–1.455), 1.583(1.014–2.472). p<0.05], while there were no significant associations between BMI groups and MACCE (p>0.05).
Table 4. Major Adverse Cardiac and Cerebrovascular Events (MACCE) at the End of Five Years.
| Variables | β | std | P-value | OR | OR 95% CI | |
| BMI<18.5 vs. 18.5–23.9 | 0.5252 | 0.3076 | 0.0878 | 1.997 | 0.939 | 4.248 |
| BMI 24–27.9 vs. 18.5–23.9 | −0.1738 | 0.0974 | 0.0744 | 0.993 | 0.841 | 1.172 |
| BMI 28–32 vs. 18.5–23.9 | −0.0677 | 0.1111 | 0.5420 | 1.104 | 0.891 | 1.367 |
| BMI>32 vs. 18.5–23.9 | −0.1173 | 0.1790 | 0.5124 | 1.050 | 0.694 | 1.591 |
| Age | 0.0257 | 0.00445 | <.0001 | 1.026 | 1.017 | 1.035 |
| Gender: Male vs. Female | 0.0165 | 0.0525 | 0.7537 | 1.034 | 0.841 | 1.270 |
| Smoking | 0.0700 | 0.0388 | 0.0715 | 1.150 | 0.988 | 1.339 |
| Hypertension | 0.1130 | 0.0380 | 0.0030 | 1.254 | 1.080 | 1.455 |
| Hyperlipidemia | −0.0150 | 0.0371 | 0.6855 | 0.970 | 0.839 | 1.122 |
| Peripheral Artery Disease | −0.0965 | 0.0638 | 0.1302 | 0.824 | 0.642 | 1.059 |
| Thrombolytic Therapy | 0.0389 | 0.0634 | 0.5395 | 1.081 | 0.843 | 1.386 |
| Myocardial Infarction | 0.0391 | 0.0375 | 0.2971 | 1.081 | 0.934 | 1.252 |
| Heart Failure | 0.2297 | 0.1137 | 0.0434 | 1.583 | 1.014 | 2.472 |
| Left Main Disease | 0.00983 | 0.0389 | 0.8003 | 1.020 | 0.876 | 1.188 |
BMI: body mass index; multivariable logistic regression model was used to analyze categorical variables; β: std: standard deviation; HR: hazard ratio; CI: confidence interval. P values: comparison between the other and normal weight group; P<0.05 was accepted as statistically significant.
Discussion
Obesity is a common and increasingly prevalent health issue throughout the world. It has been associated with the development of diabetes mellitus, hypertension, cardiovascular disease and heart failure [21], [22]. The relation between BMI and surgical outcomes is complex [23].Recent studies have described “obesity paradox” phenomena [11], [24]–[29]. Despite the association of obesity with chronic disease that lead to early death, improved survival has been observed in obese patients with heart failure and following CABG surgery [30], [31]. Obesity was found to be a significant factor associated with smaller infarction size following MI. However, most of these reports were in the western populations.
This is the first and the largest study to date in examining outcomes after CABG surgery in the Chinese population, stratified according to BMI. In this study, the worse outcomes were seen in patients at the body mass with a BMI<18.5 kg/m2, although this accounted for only 0.63% of the sample size that is consistent with previous reports in western population [2], [3]. Compared with obese patients, underweight patients in this series had a higher rate of epinephrine administration and MACCE that somehow suggesting that underweight weight is a possible risk factor in patients with isolated CABG.A previous report indicated that fat mass is the main energy storage of the body [32], supply energy in underweight patients undergoing CABG may confer survival advantages.
We found no significant differences in in-hospital mortality in all 5 groups. However, although 5-years mortality rate tend to be higher in underweight group than other groups, there was no statistically significant difference seen among all 5 groups. Thus the present study suggested a possible link between the underweight and the mortality, which has been showed in previous studies [33], [34]. Further studies, especially large sample size of the underweight patients are needed to confirm these findings. Because there was no BMI>40 kg/m2 available in our database that may due to racial differences, we are unable to find out the mortality in extreme severe obese patients in Chinese patients.
Traditionally, obesity has been viewed as a risk factor for postoperative mortality in patients undergoing CABG surgery [10]. Several studies have explored this potential association reported with mixed results. Some studies found a higher incidence of mortality in the postoperative and mid-term follow-up after CABG in obese patients [24]–[27], whereas others did not [12], [13], [30], [31], [35]–[41]. There are reports of increased operative mortality in morbidly obese individuals [11], [28]. We did not find a significant association between obesity and mortality either immediately after surgery or at the end of 5-year follow-up even after multivariable Cox regression analysis. However, we did find that old age, smoking, hypertension, MI and heart failure were the major risk factors for long-term mortality, and old age, hypertension and heart failure increased the rate of MACCE in ethnic Chinese population after CABG surgery. The inconsistent results between our research and prior ones might be caused of the following aspects: Firstly, our objects is the Chinese, unlike previous white people, where may exist racial differences. The classification criteria of BMI we used also reflected this difference (we use China's national standard which is different from the WHO criteria), and this is also the second reason why our results cannot be directly used to compare prior ones; secondly, unlike some multi-center study, this study is a single center study. However, these factors associated with 5 years mortalities were consistence with Nalysnyk et al. Meta-analysis reports where found that old age, hypertension, and history of prior heart surgery and MI are associated with increased risk of death after CABG [42].
Several statistical methods were applied in this study to test and adjust for bias between the five groups. Multivariable logistic and Cox regression analyses were used to examine whether normal weight was independently related to defined postoperative outcomes. As a result, the derived five groups showed no difference in baseline characteristics and had identical results as the unmatched analysis.
Studies in cardiovascular surgery focused largely on CABG have found mixed results. Jin, et al, postulated that this was due to different BMI classifications and sample sizes [12]. However, what is intriguing about Jin's study was their finding of reduced mortality in mildly obese patients. Similarly, Romero-Corral, in a meta-analytical review, found that overweight CAD patients had the lowest risk of cardiovascular complications and overall mortality. These data differed from what we found in Chinese patients, which may be due to the difference in race(need provide reference number).
Our finding was different from others that higher incidence of renal insufficiency and diabetes mellitus occurs in obese patients [43], [44]. We found no difference in ICU stay in the postoperative period in obese patients compared with non-obese patients that consistent with other repots [45], [46].
In addition, while it is widely accepted that obesity increases the risk of heart disease, a growing number of recent reports document a significant survival benefit in obese patients once they have been diagnosed. This has been termed the “obesity paradox”. The current study supports this observation by demonstrating lower mortality and lower vasoactive medicine requirement in normal weight and mild to moderate obese patients and raises the question why mild to moderate obese patients have better short term outcomes than long term outcomes.
Study limitations
The current study did not show a significant difference in the mortality among different BMI groups, although a trend to increase in the mortality and a higher vasoactive medicine requirement were seen in the underweight group. It needs further studies on the relationship between BMI and outcomes in patients undergoing CABG in the Asian population.
Conclusion
This large-scale study with long term follow-up after primary CABG in an exclusively ethnic Chinese population found that there were no significant associated with 5-year mortality and MACCE with different BMI groups, however, old age, smoking, hypertension, MI and heart failure were the risk factors of follow-up mortality, and old age, hypertension and heart failure increased the rate of MACCE.
Funding Statement
No current external funding sources for this study.
References
- 1. Rosenbaum M, Leibel RL, Hirsch J (1997) Obesity. N Engl J Med 337: 396–407. [DOI] [PubMed] [Google Scholar]
- 2. Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, et al. (1995) Body weight and mortality among women. N Engl J Med 333: 677–685. [DOI] [PubMed] [Google Scholar]
- 3. Harris T, Cook EF, Garrison R, Higgins M, Kannel W, et al. (1988) Body mass index and mortality among nonsmoking older persons. The Framingham Heart Study. JAMA 259: 1520–1524. [PubMed] [Google Scholar]
- 4. Lee IM, Manson JE, Hennekens CH, Paffenbarger RS Jr (1993) Body weight and mortality. A 27-year follow-up of middle-aged men. Jama 270: 2823–2828. [DOI] [PubMed] [Google Scholar]
- 5. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr (1999) Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 341: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 6. Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P (1996) Body weight, cardiovascular risk factors, and coronary mortality. 15-year follow-up of middle-aged men and women in eastern Finland. Circulation 93: 1372–1379. [DOI] [PubMed] [Google Scholar]
- 7. Artham SM, Lavie CJ, Patel HM, Ventura HO (2008) Impact of obesity on the risk of heart failure and its prognosis. J Cardiometab Syndr 3: 155–161. [DOI] [PubMed] [Google Scholar]
- 8. Lavie CJ, Milani RV, Ventura HO (2008) Untangling the heavy cardiovascular burden of obesity. Nat Clin Pract Cardiovasc Med 5: 428–429. [DOI] [PubMed] [Google Scholar]
- 9. Todd Miller M, Lavie CJ, White CJ (2008) Impact of obesity on the pathogenesis and prognosis of coronary heart disease. J Cardiometab Syndr 3: 162–167. [DOI] [PubMed] [Google Scholar]
- 10. Parsonnet V, Dean D, Bernstein AD (1989) A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation 79: I3–12. [PubMed] [Google Scholar]
- 11. Uva MS, Rodrigues V, Monteiro N, Manuel Pedro A, Caria R, et al. (2002) Cardiac surgery and morbid obesity. Rev Port Cardiol 21: 255–264 discussion 267–259. [PubMed] [Google Scholar]
- 12. Jin R, Grunkemeier GL, Furnary AP, Handy JR Jr (2005) Is obesity a risk factor for mortality in coronary artery bypass surgery? Circulation 111: 3359–3365. [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Hammar N, Jakobsson K, Luepker RV, McGovern PG, et al. (2003) Obesity and the risk of early and late mortality after coronary artery bypass graft surgery. Am Heart J 146: 555–560. [DOI] [PubMed] [Google Scholar]
- 14. Razak F, Anand SS, Shannon H, Vuksan V, Davis B, et al. (2007) Defining obesity cut points in a multiethnic population. Circulation 115: 2111–2118. [DOI] [PubMed] [Google Scholar]
- 15. Zhou BF (2002) Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases—report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci 15: 245–252. [PubMed] [Google Scholar]
- 16. Chen C, Lu FC (2004) The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci 17 Suppl: 1–36. [PubMed] [Google Scholar]
- 17. Wang X, Zheng Z, Ao H, Zhang S, Wang Y, et al. (2010) Effects of aprotinin on short-term and long-term outcomes after coronary artery bypass grafting surgery. Ann Thorac Surg 89: 1489–1495. [DOI] [PubMed] [Google Scholar]
- 18. Mangano DT (1990) Perioperative cardiac morbidity. Anesthesiology 72: 153–184. [DOI] [PubMed] [Google Scholar]
- 19. Cao L, Young N, Liu H, Silvestry S, Sun W, et al. (2012) Preoperative aspirin use and outcomes in cardiac surgery patients. Ann Surg 255: 399–404. [DOI] [PubMed] [Google Scholar]
- 20. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, et al. (1990) Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 323: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 21. Kuduvalli M, Grayson AD, Oo AY, Fabri BM, Rashid A (2002) Risk of morbidity and in-hospital mortality in obese patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg 22: 787–793. [DOI] [PubMed] [Google Scholar]
- 22. Fukui T, Takanashi S (2010) Gender differences in clinical and angiographic outcomes after coronary artery bypass surgery. Circ J 74: 2103–2108. [DOI] [PubMed] [Google Scholar]
- 23. Gurm HS, Whitlow PL, Kip KE (2002) The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization. Insights from the bypass angioplasty revascularization investigation (BARI). J Am Coll Cardiol 39: 834–840. [DOI] [PubMed] [Google Scholar]
- 24. Kuduvalli M, Grayson AD, Oo AY, Fabri BM, Rashid A (2003) The effect of obesity on mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg 23: 368–373. [DOI] [PubMed] [Google Scholar]
- 25. Kunadian B, Dunning J, Millner RW (2007) Modifiable risk factors remain significant causes of medium term mortality after first time Coronary artery bypass grafting. J Cardiothorac Surg 2: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perrotta S, Nilsson F, Brandrup-Wognsen G, Jeppsson A (2007) Body mass index and outcome after coronary artery bypass surgery. J Cardiovasc Surg (Torino) 48: 239–245. [PubMed] [Google Scholar]
- 27. Prabhakar G, Haan CK, Peterson ED, Coombs LP, Cruzzavala JL, et al. (2002) The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons' database. Ann Thorac Surg 74: 1125–1130 discussion 1130–1121. [DOI] [PubMed] [Google Scholar]
- 28. Baslaim G, Bashore J, Alhoroub K (2008) Impact of obesity on early outcomes after cardiac surgery: experience in a Saudi Arabian center. Ann Thorac Cardiovasc Surg 14: 369–375. [PubMed] [Google Scholar]
- 29. Birkmeyer NJ, Charlesworth DC, Hernandez F, Leavitt BJ, Marrin CA, et al. (1998) Obesity and risk of adverse outcomes associated with coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation 97: 1689–1694. [DOI] [PubMed] [Google Scholar]
- 30. Cruse PJ, Foord R (1973) A five-year prospective study of 23,649 surgical wounds. Arch Surg 107: 206–210. [DOI] [PubMed] [Google Scholar]
- 31. Fasol R, Schindler M, Schumacher B, Schlaudraff K, Hannes W, et al. (1992) The influence of obesity on perioperative morbidity: retrospective study of 502 aortocoronary bypass operations. Thorac Cardiovasc Surg 40: 126–129. [DOI] [PubMed] [Google Scholar]
- 32. Bouillanne O, Dupont-Belmont C, Hay P, Hamon-Vilcot B, Cynober L, et al. (2009) Fat mass protects hospitalized elderly persons against morbidity and mortality. Am J Clin Nutr 90: 505–510. [DOI] [PubMed] [Google Scholar]
- 33. Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, et al. (2009) The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann Thorac Surg 88: S2–22. [DOI] [PubMed] [Google Scholar]
- 34. Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, et al. (2008) Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity (Silver Spring) 16: 442–450. [DOI] [PubMed] [Google Scholar]
- 35. Hamman BL, Filardo G, Hamilton C, Grayburn PA (2006) Effect of body mass index on risk of long-term mortality following coronary artery bypass grafting. Am J Cardiol 98: 734–738. [DOI] [PubMed] [Google Scholar]
- 36. Lindhout AH, Wouters CW, Noyez L (2004) Influence of obesity on in-hospital and early mortality and morbidity after myocardial revascularization. Eur J Cardiothorac Surg 26: 535–541. [DOI] [PubMed] [Google Scholar]
- 37. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, et al. (2005) Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Jama 293: 2126–2130. [DOI] [PubMed] [Google Scholar]
- 38. Orhan G, Bicer Y, Aka SA, Sargin M, Simsek S, et al. (2004) Coronary artery bypass graft operations can be performed safely in obese patients. Eur J Cardiothorac Surg 25: 212–217. [DOI] [PubMed] [Google Scholar]
- 39. Syrakas CA, Neumaier-Prauser P, Angelis I, Kiask T, Kemkes BM, et al. (2007) Is extreme obesity a risk factor for increased in-hospital mortality and postoperative morbidity after cardiac surgery? Results of 2251 obese patients with BMI of 30 to 50. Thorac Cardiovasc Surg 55: 491–493. [DOI] [PubMed] [Google Scholar]
- 40. Vassiliades TA Jr, Nielsen JL, Lonquist JL (2003) Effects of obesity on outcomes in endoscopically assisted coronary artery bypass operations. Heart Surg Forum 6: 99–101. [DOI] [PubMed] [Google Scholar]
- 41. Lavie CJ, Milani RV (2003) Obesity and cardiovascular disease: the hippocrates paradox? J Am Coll Cardiol 42: 677–679. [DOI] [PubMed] [Google Scholar]
- 42. Nalysnyk L, Fahrbach K, Reynolds MW, Zhao SZ, Ross S (2003) Adverse events in coronary artery bypass graft (CABG) trials: a systematic review and analysis. Heart 89: 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pan W, Hindler K, Lee VV, Vaughn WK, Collard CD (2006) Obesity in diabetic patients undergoing coronary artery bypass graft surgery is associated with increased postoperative morbidity. Anesthesiology 104: 441–447. [DOI] [PubMed] [Google Scholar]
- 44. Prasad US, Walker WS, Sang CT, Campanella C, Cameron EW (1991) Influence of obesity on the early and long term results of surgery for coronary artery disease. Eur J Cardiothorac Surg 5: 67–72 discussion 72–63. [DOI] [PubMed] [Google Scholar]
- 45. Engel AM, McDonough S, Smith JM (2009) Does an obese body mass index affect hospital outcomes after coronary artery bypass graft surgery? Ann Thorac Surg 88: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 46. Prapas SN, Panagiotopoulos IA, Hamed Abdelsalam A, Kotsis VN, Protogeros DA, et al. (2007) Predictors of prolonged mechanical ventilation following aorta no-touch off-pump coronary artery bypass surgery. Eur J Cardiothorac Surg 32: 488–492. [DOI] [PubMed] [Google Scholar]