Abstract
Background
Alcohol-related steatohepatitis is associated with increased oxidative stress, DNA damage, lipotoxicity, and insulin resistance in liver.
Hypothesis
Since inflammation and oxidative stress can promote insulin resistance, effective treatment with anti-oxidants, e.g. N-acetylcysteine (NAC), may restore ethanol-impaired insulin signaling in the liver.
Methods
Adult male Sprague-Dawley rats were fed for 130 days with liquid diets containing 0% or 37% ethanol by caloric content, and simultaneously treated with vehicle or NAC. Chow-fed controls were studied in parallel. Liver tissues were used for histopathology, cytokine activation, and insulin/IGF-1 signaling assays.
Results
We observed significant positive trends of increasing severity of steatohepatitis (P=0.016) with accumulation of neutral lipid (P=0.0002) and triglycerides (P=0.0004) from chow to control, to the ethanol diet, irrespective of NAC treatment. In ethanol-fed rats, NAC reduced inflammation, converted the steatosis from a predominantly micro-vesicular to a mainly macro-vesicular histological pattern, reduced pro-inflammatory cytokine gene expression, ceramide load, and acid sphingomyelinase activity, and increased expression of IGF-1 receptor and IGF-2 in liver. However, NAC did not abrogate ethanol-mediated impairments in signaling through insulin/IGF-1 receptors, IRS-1, Akt, GSK-3β, or p70S6K, nor did it significantly reduce pro-ceramide or GM3 ganglioside gene expression in liver.
Conclusions
Anti-oxidant treatments reduce the severity of chronic alcohol-related steatohepatitis, possibly due to decreased expression of inflammatory mediators and ceramide accumulation, but they do not restore insulin/IGF-1 signaling in liver, most likely due to persistent elevation of GM3 synthase expression. Effective treatment of alcohol-related steatohepatitis most likely requires dual targeting of oxidative stress and insulin/IGF resistance.
Keywords: Hepatic steatosis, steatohepatitis, total enteral nutrition, ethanol, insulin resistance, anti-oxidants, ceramides, lipotoxicity, oxidative stress, GM3 ganglioside
INTRODUCTION
Acute alcohol exposure causes simple hepatic steatosis, but with continued alcohol abuse, this relatively benign state of lipid (mainly triglyceride) accumulation can progress through stages of steatohepatitis, followed by fibrosis, cirrhosis, and finally liver failure (de Alwis and Day, 2008). In addition, chronic alcohol abuse can lead to hepatocellular carcinoma (HCC). Progression of alcohol-related liver disease (ALD) is partly mediated by impaired lipid metabolism and homeostasis, which lead to lipotoxicity, oxidative stress, pro-inflammatory cytokine activation, and insulin resistance (Carter-Kent et al., 2008; Farrell and Larter, 2006). Lipotoxic states themselves activate pro-apoptotic, anti-growth, and anti-survival pathways, and they compromise reparative and regenerative mechanisms in the liver (Day, 2006; Day and James, 1998).
Growing evidence suggests that ceramides are key mediators of lipotoxicity with attendant increased inflammation, oxidative stress, and insulin resistance in both ALD and non-alcoholic fatty liver disease (Chavez et al., 2003; Fernandez-Checa et al., 2005; Holland et al., 2007; Pickersgill et al., 2007; Schutze et al., 1992). Ceramides constitute a family of lipids generated from fatty acid and sphingosine (Liu et al., 1997; Reynolds et al., 2004; Summers, 2006; Tilg and Hotamisligil, 2006). Ceramides are distributed in cell membranes, and in addition to their structural functions, they regulate signaling pathways that mediate growth, proliferation, motility, adhesion, differentiation, senescence, and apoptosis. Ceramides are generated biosynthetically through the actions of ceramide synthase and serine palmitoyltransferase (Hannun et al., 2001; Hauck et al., 2000; Laviad et al., 2008; Mizutani et al., 2005; Shah et al., 2008), or they can be formed catabolically by neutral or acidic sphingomyelinase activity (Holland et al., 2007; Reynolds et al., 2004; Shah et al., 2008). Sphingomyelinases are activated by pro-inflammatory cytokines, e.g. TNF-α (Carter-Kent et al., 2008; Summers, 2006), and pro-apoptotic stimuli including ionizing radiation, Fas, and trophic factor withdrawal (Hannun and Obeid, 2002; Liu et al., 1997; Marchesini and Hannun, 2004; Reynolds et al., 2004). Ceramides impair cellular functions and cause apoptosis by: 1) modulating the phosphorylation states of various proteins, including those that regulate insulin signaling (Silveira et al., 2008); 2) activating enzymes such as interleukin-1β converting enzyme (ICE)-like proteases, which promote apoptosis (Smyth et al., 1996); and 3) inhibiting Akt phosphorylation and kinase activity (Chalfant et al., 1999; Summers et al., 1998; Zhou et al., 1998) through activation of protein phosphatase 2A (Chalfant et al., 1999; Dobrowsky et al., 1993).
Free fatty acid accumulation in hepatocytes stimulates lipogenesis, and factors related to increased stress promote lipotoxicity, which leads to oxidative stress and cell death (Marra et al., 2008). Oxidative stress itself plays a key role in the progression of simple steatosis to steatohepatitis, and the eventual activation pro-fibrogenic cascades (Albano, 2008; Dowman et al., 2009). Susceptibility to oxidative and radical injury rests on maintaining a balance between pro-oxidant and anti-oxidant factors. Glutathione (GSH) is a radical scavenger and key attenuator of oxidative stress in liver, and hepatic glutathione levels are reduced in both ALD and non-alcoholic fatty liver disease (Das et al., 2008; Videla et al., 2004). N-acetylcysteine (NAC), one of the anti-oxidants used to manage acute liver failure caused by acetaminophen poisoning (Marzullo, 2005), acts by donating cysteine residues for GSH synthesis (Aruoma et al., 1989; Atkuri et al., 2007). Therefore, NAC therapy may reduce liver injury and progression from simple steatosis to steatohepatitis in ALD. Reduced inflammation and severity of steatohepatitis effectuated by NAC or other anti-inflammatory agents could lead to decreased generation of toxic lipids, including ceramides, and consequently improve hepatic insulin sensitivity. The present study utilizes a rat model of chronic ethanol feeding to evaluate the potential use of NAC for reducing the severity of alcohol-related steatohepatitis, lipotoxicity, pro-inflammatory cytokine activation, and insulin resistance in the liver.
MATERIALS AND METHODS
Materials
The bicinchoninic acid (BCA) kit to measure protein concentration was purchased from Pierce Chemical Co (Rockford, IL). Histochoice fixative was purchased from Amresco, Inc (Solon, OH). Nile Red fluorescent dye, the Amplex Red Cholesterol Assay Kit, Amplex UltraRed soluble flourophore, and the Akt Pathway Total and Phospho 7-Plex panels were purchased from Invitrogen (Carlsbad, CA). Triglyceride Reagent Kit was purchased from Analox Instruments Ltd (London, UK). The rat cytokine Milliplex xMAP ELISA kit was purchased from Millipore (Billerica, MA). Maxisorp 96-well plates used for enzyme-linked immunosorbant assays (ELISAs) were from Nunc (Thermo Fisher Scientific; Rochester, NY). Superblock-TBS, horseradish peroxidase conjugated antibodies, and SuperSignal Enhanced Chemiluminescence Reagent were from Pierce Chemical Co (Rockford, IL). Monoclonal anti-ceramide and synthetic oligonucleotides used in quantitative polymerase chain reaction (qPCR) assays were purchased from Sigma-Aldrich Co (St. Louis, MO). All other antibodies and immunodetection reagents were purchased from Abcam (Cambridge, MA), Upstate (Billerica, MA), Vector Laboratories (Burlingame, CA), Invitrogen (Carlsbad, CA) or Chemicon (Temecula, CA). Fine chemicals were purchased from CalBiochem (Carlsbad, CA), or Sigma-Aldrich (St Louis, Mo). QIAzol Lysis Reagent for RNA extraction and QuantiTect SYBR Green PCR Mix were obtained from Qiagen, Inc (Valencia, CA). The AMV 1st Strand cDNA Synthesis Kit was purchased from Roche Applied Science (Indianapolis, IN).
Experimental Model
We utilized the Tsukamoto and French intra-gastric feeding model of total enteral nutrition (TEN) to administer the control or ethanol-containing liquid diets (Baumgardner et al., 2008; Ronis et al., 2005; Ronis et al., 2010). In this model, the entire range of pathological changes, including steatosis, steatohepatitis, and fibrosis, can be produced in rodents (Nanji and French, 2003). In brief, adult male Sprague Dawley rats were fitted with intra-gastric cannulae to infuse isocaloric TEN liquid diets (187 kcal/kg/day) containing 0% (N=18) or 37% (N=14) ethanol (caloric content) (Table 1). Ethanol was substituted for carbohydrates (12 g/kg/day). In subsets of control (N=9) and ethanol (N=7) fed rats, NAC (1.2 g/kg/day) was added to the diets. Chow-fed controls (ad libitum) were studied in parallel (N=7). Diets were adjusted weekly to maintain similar weights among the experimental groups, and after 130 days of feeding, rats were sacrificed and livers were harvested for study (Ronis et al., 2010). Liver tissue was immersion fixed in Histochoice and embedded in paraffin for histopathological studies. Fresh snap frozen tissue was used for biochemical and molecular analyses. Our experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. The protocol was compliant with NIH guidelines provided in the Guide for the Care and Use of Laboratory Animals.
Table 1.
Composition of diets
| Diet | Abbreviation* | Composition |
|---|---|---|
| 1 | Chow | Chow ad libitum |
| 2 | Control TEN | Diet containing 41% carbohydrates and 43% fat |
| 3 | Control TEN +NAC | Diet containing 41% carbohydrates, 43% fat and NAC (1.2 g/kg/day) |
| 4 | EtOH TEN | Diet containing 36% carbohydrates from ethanol and 43% fat |
| 5 | EtOH TEN+NAC | Diet containing 36% carbohydrates from ethanol, 43% fat and NAC (1.2 g/kg/day) |
TEN= total enteral nutrition; NAC= N-acetylcysteine; EtOH=ethanol
Histopathology
Histological sections (5 µm thick) were stained with Hematoxylin and Eosin (H&E) and scored under code by a pathologist (SMD) using a semi-quantitative scale (1–4) to assess severity and extent of macro- and micro-steatosis, inflammation, and necrosis (including apoptotic bodies) (Table 2). The scoring system was graded as follows: 1=no significant abnormalities, i.e. expected chord-like architecture, minimal or absent inflammation, necrosis, apoptosis or anisocoria; 2=mild pathological changes, i.e. micro- and macrosteatosis affecting less than 25% hepatocytes, slight derangement of the chord-like architecture, rare scattered foci of inflammation near portal areas; and absent necrosis/apoptosis; 3=moderate steatohepatitis with 25–50% micro- or macrosteatosis, moderate disruption of the chord-like architecture, inflammation extending into lobules, and conspicuous foci of apoptosis; and 4=severe steatohepatitis with micro- and macro-steatosis involving greater than 50% of parenchyma, marked architectural distortion, and severe inflammation with evidence of apoptotic bodies and foci of necrosis.
Table 2.
Liver histopathology scoring system
| Grade | Classification | Features |
|---|---|---|
| 1 | Normal | Normal liver chords, absent or minimal steatosis, inflammation, necrosis or apoptosis. |
| 2 | Mild | Slight derangement of the chord-like architecture, micro- or macrosteatosis affecting < than 25% hepatocytes, rare scattered foci of inflammation near portal areas and absent necrosis/apoptosis. |
| 3 | Moderate | Moderate disruption of the chord-like architecture, 25–50% hepatocytes with micro- or macrosteatosis, inflammation extending into lobules, and conspicuous foci of apoptosis. |
| 4 | Severe | Marked architectural distortion, micro- and/or macrosteatosis involving greater than 50% of parenchyma, severe inflammation with evidence of apoptotic bodies and foci of necrosis. |
This semi-quantitative grading scheme was used to evaluate liver histopathology in Sprague-Dawley adult male rats that were subjected to chronic feeding with control or ethanol-containing transenteral nutrition (TEN) diets and concomitant treatment with vehicle or NAC. Chow fed controls were simultaneously studied. The liver histopathology was reviewed under code by a pathologist.
Lipid studies
Lipids were extracted from fresh frozen liver with 2:1 chloroform-methanol (Lyn-Cook et al., 2009). Lipid content was measured using the Nile red fluorescence-based microplate assay as described (Lyn-Cook et al., 2009). Cholesterol was measured with the Cholesterol Amplex Red kit, and triglycerides were measured using the Analox Instruments GM7 Analyzer. Results were normalized to sample weight. Ceramide immunoreactivity was measured in lipid extracts using a direct-binding ELISA (Brade et al., 2000). In brief, duplicate 50 µl samples were dried onto the bottom surfaces of 96-well Polysorp plates. Samples were blocked for 1 hour at room temperature with Superblock-TBS. The samples were incubated with mouse monoclonal anti-ceramide (2.0 µg/ml) overnight at 4°C with gentle platform agitation. Immunoreactivity was detected with horseradish peroxidase-conjugated anti-mouse IgG (1:10000) and enhanced chemiluminescence substrate. Luminescence was quantified in a Topcount machine. Between steps, the wells were washed 3 times with PBS (Brade et al., 2000). C6 synthetic ceramide (500 ng) served as a positive control. Negative controls included reactions in which the primary or secondary antibody was omitted.
Multiplex ELISA
We used bead-based multiplex ELISAs to examine the integrity of insulin and IGF-1 signaling networks, or measure immunoreactivity to selected pro-inflammatory cytokines and chemokines in liver tissue. With regard to insulin/IGF-1 signaling, we measured immunoreactivity to the insulin receptor (IR), IGF-1 receptor (IGF-1R), IRS-1, Akt, glycogen synthase kinase 3β (GSK-3β), p70S6 kinase (p70S6K), and proline-rich Akt substrate 40 (PRAS40), and pYpY1162/1163-IR, pYpY1135/1136-IGF-1R, pS312-IRS-1, pS473-Akt, pS9-GSK3β, pTpS421/424-p70S6K, and pT246-PRAS40 according to the manufacturer's protocol. Regarding pro-inflammatory cytokine and chemokine activation, we measured immunoreactivity to interleukin (IL) 1β, IL-2, IL-4, IL-6, TNF-α, GM-CSF, GRO/KC, and VEGF. Liver tissues were homogenized in buffer containing 50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 2 mM EGTA, 10 mM EDTA, 100 mM NaF, 1 mM Na4P2O7, 2 mM Na3VO4, and protease and phosphatase inhibitors (Lyn-Cook et al., 2009). Samples containing 200 µg protein were incubated with the beads, and captured antigens were detected with biotinylated secondary antibody and phycoerythrin-conjugated Streptavidin. Plates were read in a Bio-Plex 200 system (Bio-Rad, Hercules, CA). Data are expressed as fluorescence light units corrected for protein concentration.
Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR) Assays
Liver tissue was homogenized in QIAzol reagent. RNA isolated using the EZ1 RNA Universal Tissue Kit and the BIO Robot EZ1 (Qiagen Inc., Valencia, CA), was reverse transcribed with random oligodeoxynucleotide primers and the AMV First Strand cDNA synthesis kit. The cDNAs were used to measure gene expression by qPCR with gene-specific primer pairs (Supplementary Table 1). Primers were designed using MacVector 10 software (MacVector, Inc., Cary, NC) and target specificity was verified using NCBI-BLAST (Basic Local Alignment Search Tool). The Master ep realplex instrument and software (Eppendorf AG, Hamburg, Germany) were used to detect amplified signals from triplicate reactions. Relative mRNA abundance was calculated from the ng ratios of specific mRNA to 18S measured in the same samples (Moroz et al., 2008). Template-free reactions served as negative controls.
Statistical Analysis
Data depicted in box plots reflect group median, 95% confidence interval limits and range (whiskers), while tabulated data reflect means ± SEMs for each group. Intergroup comparisons were made using one-way analysis of variance (ANOVA) with Tukey post-hoc tests. In addition, we performed linear trend analysis to gauge the impact of ethanol relative to chow and control TEN with regard to severity of steatosis, lipid/triglyceride accumulation, and ceramide immunoreactivity in liver. Data were analyzed using GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA). Significant P-values (<0.05) are shown within the panels or tables.
RESULTS
General characteristics of the model (Ronis et al., 2010)
Previous studies using samples from this model demonstrated significantly increased serum levels of alanine aminotransferase (ALT) activity, hepatic apoptosis indices, and endonuclease G immunoreactivity in ethanol-exposed relative to chow and TEN-fed controls (Supplementary Table 2). Treatment with NAC significantly reduced serum ALT and hepatic apoptosis and endonuclease G staining in ethanol-fed rats (Supplementary Table 2). Chronic ethanol exposure significantly reduced hepatic glutathione and increased bovine serum albumin adducted with malondialdehyde and lipid peroxide, while concomitant treatment with NAC inhibited these effects of alcohol on oxidative stress mediators. The levels of thiobarbituric-reactive products in liver were unaffected by ethanol exposure or NAC treatment (Supplementary Table 3). Chronic ethanol exposure increased the expression of pro-fibrogenesis genes (mRNAs), but did not significantly alter hepatic hydroxyproline content. Quantitative RT-PCR analysis detected significantly elevated levels of α-1collagen Types I and III, and matrix metalloproteinase (MMP) 3, MMP-9, and MMP-13, but not TIMP-1 in ethanol-exposed relative to control livers (Supplementary Table 4). Concomitant NAC treatment significantly reduced hepatic expression of MMP-9 in ethanol-exposed livers, but had no significant effect on the expression of the other pro-collagen genes examined (Supplementary Table 4).
Effects of ethanol and NAC on steatohepatitis
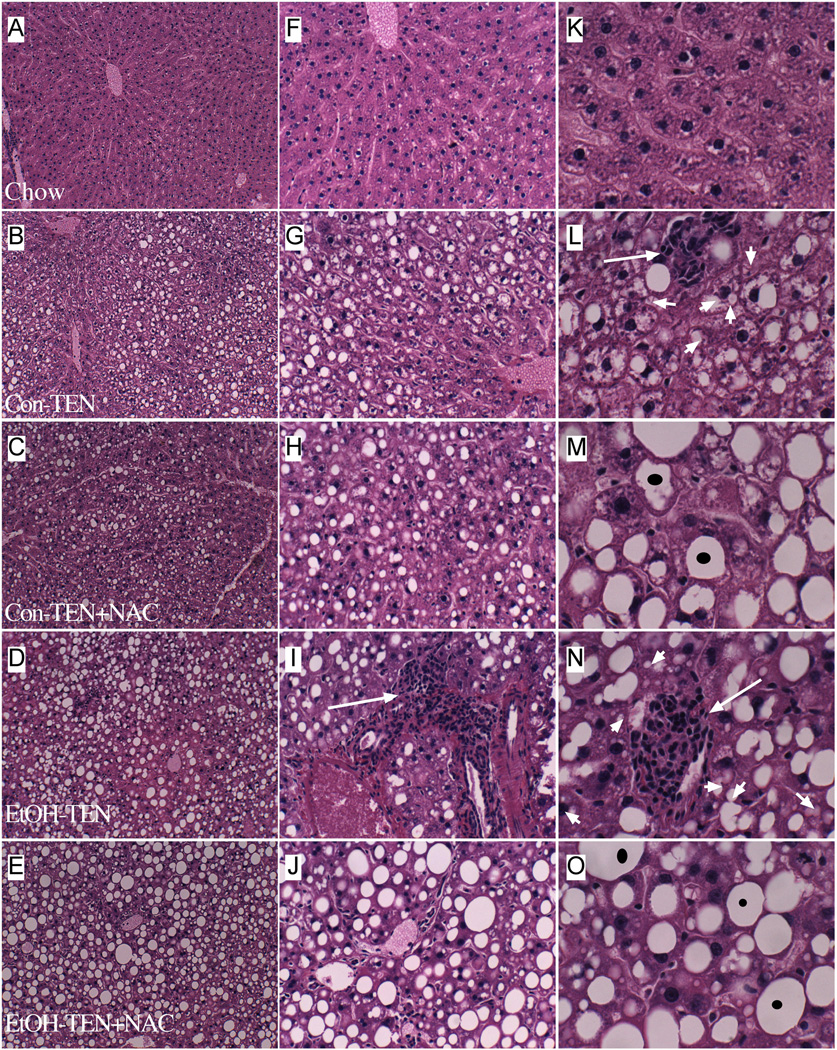
H&E stained sections of chow-fed livers demonstrated the expected chord-like architecture with minimal or absent portal lympho-mononuclear inflammatory cell infiltrates, and scattered hepatocytes with macrosteatosis (Figures 1A, 1F, 1K). Control-TEN livers exhibited micro-vesicular steatosis that was predominantly distributed in Zone 2, but extended close to Zones 1 and 3. In addition, control-TEN livers contained small foci of lobular lympho-mononuclear cell inflammation and scattered apoptotic bodies, and the regular chord-like architecture was mildly disrupted (Figures 1B, 1G, 1L). Treatment of control-TEN rats with NAC converted the predominantly micro-steatosis to macrosteatosis, and minimized the inflammation. However, the presence of scattered apoptotic bodies and mildly disrupted hepatic chord architecture persisted despite NAC treatment (Figures 1C, 1H, 1M).
Figure 1.
Effects of chronic ethanol feeding and NAC treatment on steatohepatitis. Adult male Sprague-Dawley rats were fed for 130 days with chow (A, F, K), control TEN (B, G, L), control TEN supplemented with NAC (C, H, M), ethanol TEN (37% by caloric content; D, I, N), or ethanol TEN supplemented with NAC (E, J, O). Paraffin-embedded histological sections (5 µm thick) of liver were stained with H&E and photographed at 40× (A–E), 160× (F–J), and 400× (K–O) magnifications. Note reduced inflammation and shift from microvesicular steatosis to macrovesicular steatosis associated with NAC treatments, but persistence of hepatic chord architecture disarray in the control liquid + ethanol livers despite NAC treatment.
Chronic ethanol feeding caused moderate to severe steatohepatitis that was pan-lobular, i.e. distributed from Zone 1 through Zone 3, and mainly micro-vesicular in nature (Figures 1D, 1I, 1N). Inflammation was prominent, multi-focal, and nodular. Apoptotic bodies were abundant, and the hepatic architecture was markedly disorganized. Co-treatment with NAC reduced the inflammation and converted the predominantly micro-vesicular steatosis to mainly macro-vesicular steatosis (Figures 1E, 1J, 1O). Semi-quantitative grading of the liver histology under code, using the 1 to 4 scale (see Methods) revealed a significant trend of increasing severity of steatohepatitis, with minimal injury observed in chow-fed rats, to mild or moderate disease in the control-TEN group, to severe liver injury in the ethanol-TEN fed rats (P=0.0157; Figure 2A). Overall, there were no discernible effects of the NAC treatments on the mean scores assigned to livers of control- or ethanol-TEN fed rats.
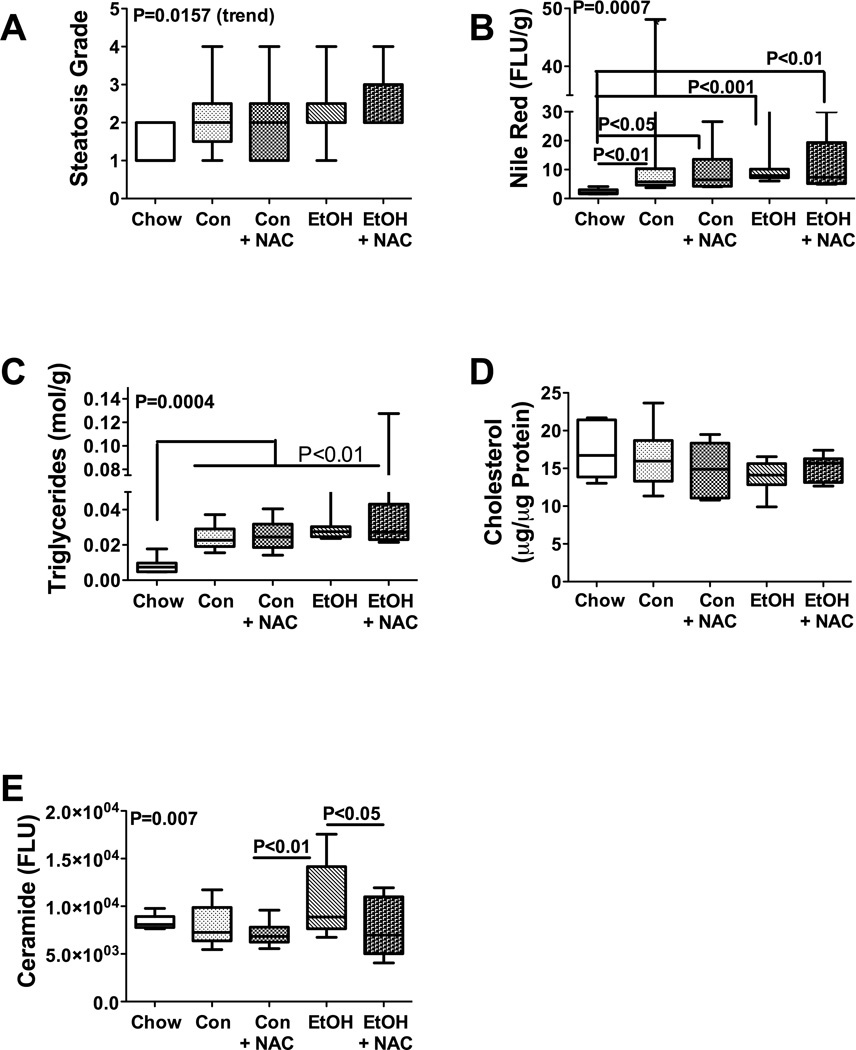
Figure 2.
Lipid and ceramide accumulations in liver following chronic ethanol and NAC treatment. (A) Severity of hepatic steatosis observed in H&E stained histological sections was graded blind on a scale from 1 to 4. (B–E) Lipid extracted from fresh frozen liver tissue was analyzed for (B) neutral lipid content using the Nile Red assay, (C) triglycerides, (D) cholesterol, or (E) ceramide immunoreactivity (see Material and Methods), and values were normalized to tissue sample weight or protein content. (F) CYP2E1 expression was measured by qRT-PCR analysis. Box plots depict medians (horizontal bars), 95% confidence interval limits, and range (whiskers). Inter-group comparisons were made using one-way ANOVA with the Tukey multiple comparison and linear trend post hoc tests. Significant P-values are indicated within the panels. Significant post-hoc linear trend analysis results are shown in the upper left quadrant of each panel.
Hepatic steatosis was further characterized by measuring neutral lipid, triglyceride, cholesterol, and ceramide content. The Nile Red assay demonstrated generally higher mean levels of neutral lipids in livers of TEN-fed relative to chow-fed rats. A borderline significant trend was observed indicating increased hepatic neutral lipid accumulation with respect to diet and ethanol feeding, i.e. from chow, to control-TEN, and then ethanol-TEN feeding (P=0.05; R2=0.097). In addition, chronic ethanol feeding, with or without NAC treatment, significantly increased hepatic neutral lipid content relative to chow-fed but not TEN-fed controls (Figure 2B). With regard to triglycerides, a significant positive trend for increasing hepatic triglyceride content with respect to treatment group (Chow < Control TEN < Ethanol TEN) was demonstrated (R2=0.29; P=0.0004). The mean hepatic triglyceride content was significantly higher in the control-TEN and ethanol-TEN relative to chow fed rats, irrespective of NAC treatment (all P<0.01; Figure 2C). In contrast, mean hepatic cholesterol levels were similar in all 5 groups (Figure 2D). This result differs from findings in other studies in which chronic ethanol feeding significantly increased hepatic cholesterol levels (Wang et al., 2010). However, our result could be due to the use of TEN rather than a standard Lieber-De Carli diet. Finally, although no significant trend relating diet or treatment to hepatic ceramide content was observed, ANOVA tests demonstrated significant inter-group differences (P=0.007) with the highest level in the ethanol-TEN group, and significant differences in the mean levels between ethanol-TEN and ethanol-TEN+NAC (P<0.05; Figure 2E). This indicates that ethanol-induced increases in hepatic ceramide can be reduced by NAC treatment.
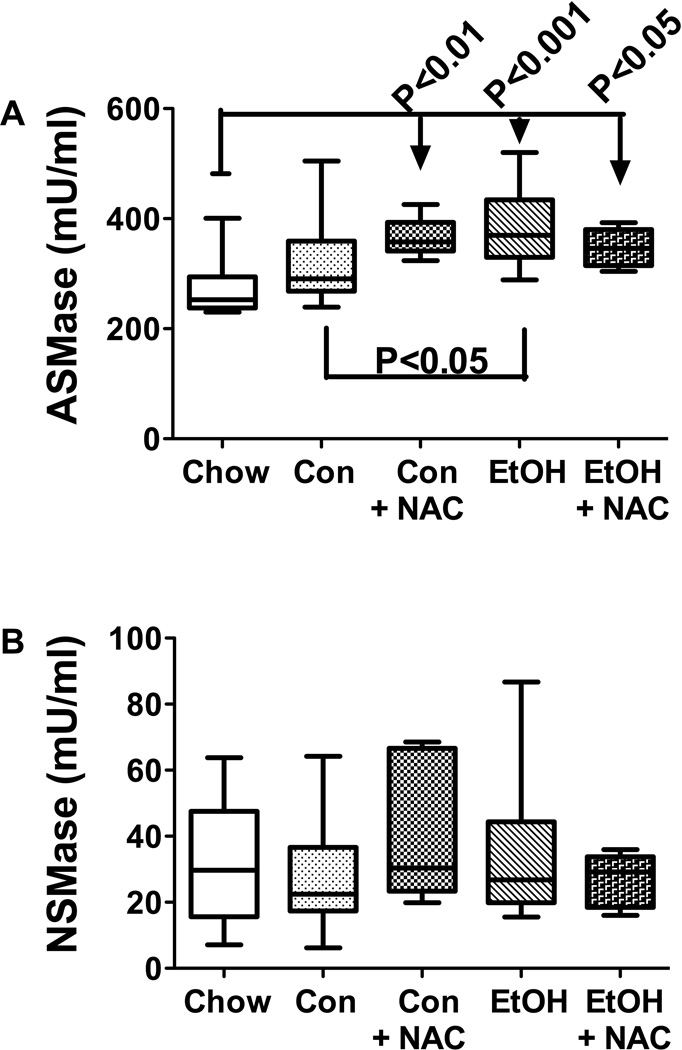
To determine if increased hepatic ceramide was mediated by increased pro-ceramide gene expression or sphingomyelinase enzymatic activity, we measured mRNA levels of ceramide synthases 1, 2, and 4 (CERS), ceramidase 2 and 3 (CERD), serine palmitoyl transferase 1 and 2 (SPTLC), and sphingomyelinase 1 and 3 (SMPD), UDP-glucose ceramide glucosyltransferae (UGCG), and GM3 synthase by qRT-PCR analysis (Table 3), and acid (ASMase) and neutral (NSMase) sphingomyelinase activities using a commercial assay (Invitrogen, Carlsbad, CA) (Figure 3). The genes selected for analysis were based on previous studies demonstrating which mRNA transcripts were modulated with increasing steatohepatitis in animal models (de la Monte et al., 2009; Lyn-Cook et al., 2009; Shah et al., 2008). In the present study, similar mean levels of CERS2, CERD2, SPTLC2, and SMPD1 were measured in all experimental groups (Table 3). In contrast, CERS1 expression was significantly elevated in the control-TEN+NAC and ethanol-TEN+NAC groups relative to the control-TEN and chow-fed groups. CERS4 gene expression was highest in the ethanol-TEN+NAC group, and significantly elevated relative to all control groups. CERD3 expression was significantly higher in livers of ethanol-TEN±NAC rats relative to control-TEN fed rats. In addition, NAC treatment of control-TEN fed rats also significantly elevated hepatic CERD3 expression. The most striking effect of chronic ethanol feeding was to increase hepatic GM3 expression relative to all control group. Although NAC abated this effect of ethanol, hepatic GM3 expression was still significantly elevated relative to all control groups, including chow (Table 3). On the other hand, ASMase activity was significantly elevated in livers from control-TEN+NAC, ethanol-TEN, and ethanol-TEN+NAC relative to chow fed rats (Figure 3A). Moreover, ethanol-TEN feeding significantly increased ASMase activity relative control-TEN, while NAC treatment abolished that difference. The mean levels of NSMase activity were similar in the five study groups (Figure 3B). These findings suggest that NAC treatment may reduce hepatic ceramide accumulation in ALD through inhibition of ASMase activity.
Table 3.
Effects of chronic ethanol feeding and treatment with N-acetylcysteine on pro-sphingolipid gene expression in liver
| GENE | Chow | C-TEN | C-TEN−NAC | Et-TEN | Et-TEN+NAC | F-Ratio | P-Value |
|---|---|---|---|---|---|---|---|
| CERS1 | 0.02521a ±0.00196 |
0.02434b ±0.00135 |
0.03277b* ±0.00217 |
0.02970 ±0.0022 |
0.03398a-***
b** ±0.00199 |
18.85 | 0.0008 |
| CERS2 | 0.2929 ± 0.069 |
0.6555 ± 0.1658 |
0.2738 ± 0.1064 |
0.4751 ± 0.1121 |
0.5806 ± 0.1323 |
7.297 | 0.12 |
| CERS4 | 0.02588a ±0.002796 |
0.02517a ±0.0009467 |
0.02825b ±0.002721 |
0.03106 ±0.001883 |
0.04335a***; b* ±0.003032 |
27.00 | <0.0001 |
| CERD2 | 0.0006725 ± 0.000228 |
0.0009465 ± 0.0002361 |
0.0004346 ± 0.0001164 |
0.001056 ± 0.00025 |
0.001797 ± 0.001044 |
4.137 | |
| CERD3 | 0.2567a ±0.0234 |
0.2740b ±0.0249 |
0.5914a-**; b** ±0.0909 |
0.5428a-***; b*** ±0.0482 |
0.4960a-**; b*** ±0.0328 |
38.30 | <0.0001 |
| SMPD1 | 0.1037 ± 0.0215 |
0.1749 ± 0.0367 |
0.1015 ± 0.0331 |
0.1404 ± 0.0237 |
0.1345 ± 0.0347 |
3.198 | |
| SMPD3 | 0.05598a ± 005982 |
0.09191a-* ± 0.00748 |
0.1036a-** ± 0.0105 |
0.09724a-* ± 0.00846 |
0.08731 ± 0.00896 |
4.04 | 0.0049 |
| SPTLC1 | 0.0000187a ±0.0000059 |
0.00000847 ± 0.0000022 |
0.0000095 ± 0.0000010 |
0.0000043a-* ± 0.0000006 |
0.0000073 ± 0.0000021 |
8.354 | 0.079 |
| SPTLC2 | 0.001612 ± 0.000984 |
0.001213 ± 0.000299 |
0.001224 ± 0.000418 |
0.00172 ± 0.000561 |
0.001217 ± 0.000395 |
1.271 | |
| UGCG | 1.484 ± 0.3261 |
0.7152 ± 0.0666 |
0.9944 ± 0.1719 |
0.9889 ± 0.2521 |
0.8111 ±0.0894 |
6.097 | |
| GM3SYN | 1.185a ± 0.136 |
1.212a ±0.083 |
1.396b ±0.094 |
3.436a***
b*** ±0.207 |
2.327a***br/>±0.169 | 55.92 | <0.0001 |
Effects of chronic ethanol exposure and NAC treatment on pro-ceramide gene expression in liver. RNA extracted from liver was reverse transcribed and the cDNAs were used to measure ceramide synthases (CERS), N-acylsphingosine amidohydrolase (ceramidase; CERD2-neutral; CERD3-alkaline), serine palmitoyltransferases (SPTLC), sphingomyelin phosphodiesterases (SMPD), UDP-glucose ceramide glucosyltransferase (UGCG), and GM3SYN= GM3 synthase expression by qPCR using gene-specific primers (Supplementary Table 1).
Abbreviations: C=Control; Et=Ethanol; TEN=Transenteral nutrition; NAC=N-acetylcysteine. Gene expression levels were normalized to 18S rRNA measured in the same samples. Results depict mean ± S.E.M. of mRNA /18S rRNA (×10−6) calculated for each group. Inter group statistical comparisons were made by one-way ANOVA with the Kurskall-Wallis post hoc test.
Superscripted letters indicate inter-group comparisons that are statistically significant;
<0.05;
P<0.01;
P<0.001 relative group with superscripted letter but no asterisk
Figure 3.
Role of sphingomyelinase activity as a mediator of increased hepatic ceramide levels following chronic ethanol feeding, and therapeutic effects of NAC. (A) Acid (ASMase) and (B) neutral (NSMase) sphingomyelinase activities were measured using a standard fluorometric assay and results were normalized to protein concentration. Inter-group comparisons were made using one-way ANOVA with the Tukey multiple comparison tests. Significant P-values are indicated within the panels.
Effects of ethanol and NAC treatments on cytokine levels in liver
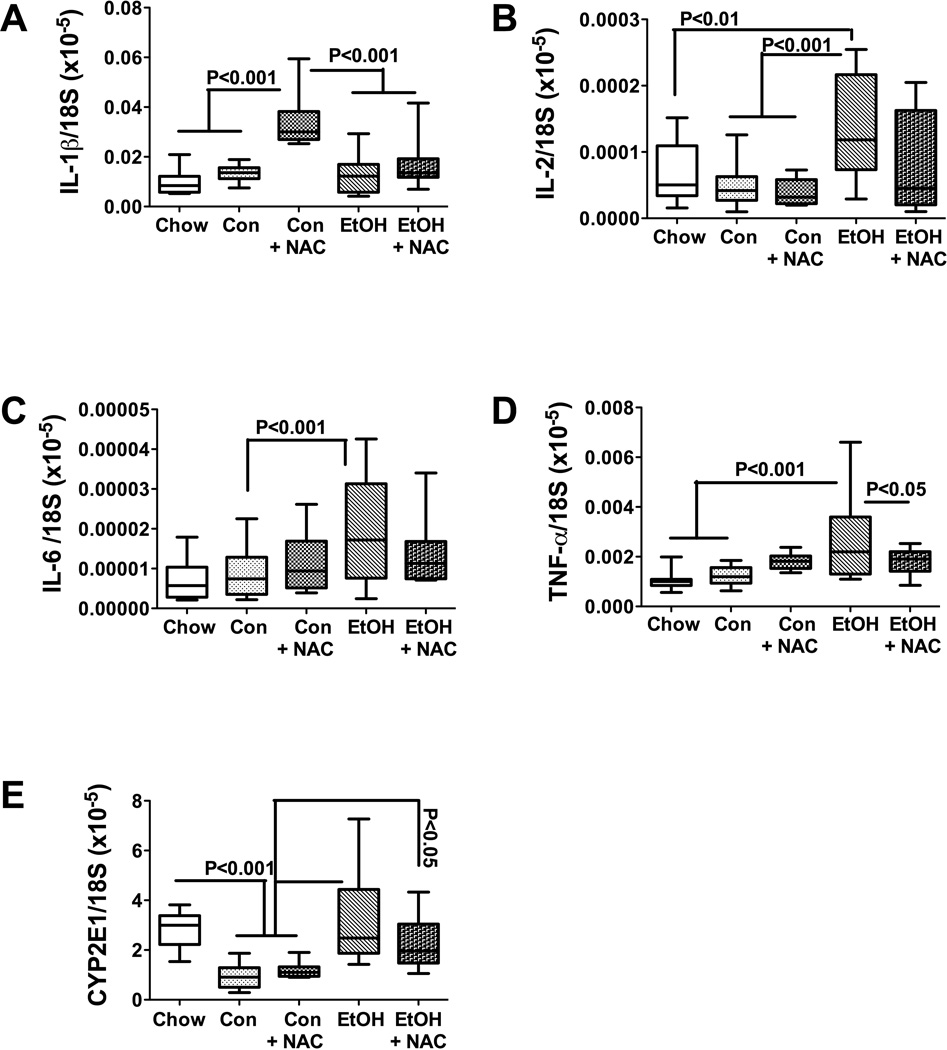
Activation of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, is a common feature of steatohepatitis (Carter-Kent et al., 2008; Larter and Farrell, 2006; Tilg and Hotamisligil, 2006) (McClain et al., 1999; McClain and Cohen, 1989). In addition, ALD is associated with increased levels of GRO/KC (Maltby et al., 1996; Ohki et al., 1998; Shiratori et al., 1993), which is a small CXC cytokine with mitogenic properties that mediates inflammation and angiogenesis (Haskill et al., 1990). We measured hepatic levels of these cytokines, plus IL-2, IL-4, GM-CSF, and VEGF by multiplex ELISA to determine the degree to which chronic ethanol feeding stimulates pro-inflammatory states and angiogenesis, and whether NAC treatment reduces cytokine activation. The studies demonstrated similar mean levels of TNF-α, IL-4, IL-6, and VEGF among the five study groups (Table 4; Figure 4). IL-1β expression was significantly higher in livers of control-TEN (P=0.036) but not in ethanol-TEN relative to chow fed rats (Figure 4A). IL-2 expression was significantly higher in livers of ethanol-TEN ± NAC treated compared with chow-fed rats (P<0.05). Although the expression levels of GM-CSF and GRO/KC were generally higher in livers of control- and ethanol-TEN fed relative to chow-fed rats, significant differences were only observed between the chow and control-TEN fed groups (Figures 4F-P<0.001; and 4G-P<0.01). In aggregate, we observed significantly increased IL-2 immunoreactivity in ethanol-TEN fed rats relative to controls, but minimal or no significant modulation of pro-inflammatory cytokine expression mediated by the NAC treatments.
Table 4.
Influence of chronic ethanol feeding and NAC treatment on pro-inflammatory cytokine levels in liver tissue
| Protein | Chow | C-TEN | C-TEN+NAC | Et-TEN | Et-TEN+NAC | F-Ratio | P-Value |
|---|---|---|---|---|---|---|---|
| IL-1β | 31.63 ± 4.321 |
85.68 ± 21.36 |
56.47 ± 6.103 |
67.16 ± 16.68 |
52.02 ± 4.738 |
10.31 | 0.036 |
| IL-2 | 392.6 ± 45.03 |
929.5 ± 237 |
521.1 ± 80.05 |
976.5 ± 280.6 |
692.6 ± 48.36 |
10.77 | 0.029 |
| IL-4 | 17.97 ± 2.545 |
40.19 ± 11.3 |
25.59 ± 5.391 |
36.16 ± 17.56 |
25.97 ± 3.068 |
4.25 | |
| IL-6 | 229.8 ± 27.34 |
548.7 ± 176.3 |
355.4 ± 35.12 |
420.7 ± 110.2 |
434.6 ± 44.05 |
7.31 | 0.12 |
| GM-CSF | 36.07a ± 7.817 |
195.1a-*** ± 36.58 |
82.28 ± 12.83 |
142.1 ± 43.76 |
83.83 ± 10.96 |
18.05 | 0.001 |
| GRO/KC | 98.33a ± 7.55 |
199.9a-** ± 34.37 |
142.4 ± 14.39 |
180.7 ± 43.24 |
123.5 ± 5.058 |
12.90 | 0.012 |
| TNF-α | 11.34 ± 1.898 |
14.08 ± 3.828 |
15.03 ± 2.336 |
15.82 ± 4.465 |
13.74 ± 1.865 |
3.45 | |
| VEGF | 11.3 ± 1.889 |
21.5 ± 5.301 |
14.59 ± 2.562 |
27.81 ± 8.942 |
14.02 ± 0.8307 |
2.23 |
Effects of chronic ethanol and NAC treatment on pro-inflammatory cytokine and vascular endothelial growth factor (VEGF) expression in liver. Liver protein homogenates were used to measure immunoreactivity to IL-1β, IL-2, IL-4, IL-6, TNF-α, GM-CSF, GRO/KC, and VEGF by multiplex ELISA. Immunoreactivity was normalized to protein concentration and data reflect mean ± S.E.M. of fluorescence light units (arbitrary). Inter-group comparisons were made using one-way ANOVA. F-Ratios and significant P-values are indicated in the far right columns. Significant inter-group differences were detected with the post-hoc Kruskal-Wallis multiple comparison tests.
Superscript “a” indicates the comparison groups and
P<0.05;
P<0.01;
P<0.001.
Figure 4.
Effects of chronic ethanol and NAC treatment on pro-inflammatory cytokine and CYP2E1 gene expression in liver. RNA extracted from liver was reverse transcribed and the cDNAs were used to measure to (A) IL-1β, (B) IL-2, (C) IL-6, (D) TNF-α, and (E) CYP2E1 by qPCR using gene-specific primers (Supplementary Table 1). Inter-group comparisons were made using one-way ANOVA with the Tukey multiple comparison tests. Significant P-values are indicated within the panels.
Effects of ethanol and NAC on upstream insulin and IGF signaling mechanisms in liver
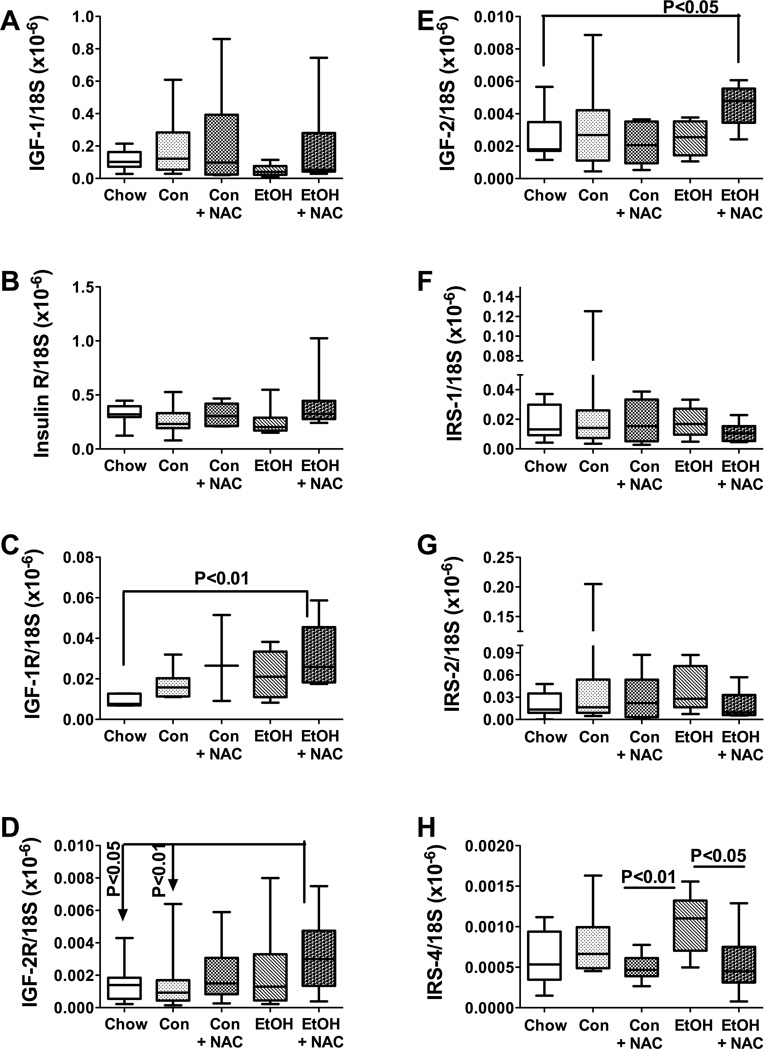
Previous studies demonstrated that steatohepatitis produced by high fat diet or chronic ethanol feeding impairs insulin and IGF signaling in the liver (de la Monte et al., 2008; Svegliati-Baroni et al., 2006; Yeon et al., 2003). We re-investigated this concept and also interrogated the potential therapeutic effects of NAC on ethanol-mediated inhibition of insulin/IGF signaling by measuring mRNA levels of insulin, IGF-1, and IGF-2 polypeptides, their corresponding receptors, and IRS-1, IRS-2, and IRS-4 by qRT-PCR analysis (Figure 5), and IR, pYpY1162/1163-IR, IGF-1R, pYpY1135/1136-IGF-1R, IRS-1, pS312-IRS-1, Akt, pS473-Akt, GSK-3β, pS9-GSK3β, p70S6K, pTpS421/424-p70S6K, PRAS40, and pT246-PRAS40 immunoreactivities by multiplex ELISA (Figures 6 and 7). The qRT-PCR analyses demonstrated similar mean levels of insulin, IGF-1, IR, IRS-1, and IRS-2 expression in all groups. Hepatic IGF-2 polypeptide (P<0.05; Figure 5E) and IGF-1R (P<0.01;Figure 5C) mRNA levels were significantly higher in ethanol-TEN+NAC than in chow-fed rats. In addition, IGF-2R expression was significantly higher in livers of ethanol-TEN+NAC compared with chow and control-TEN fed rats (Figure 5D). Finally, with regard to IRS-4, the mean hepatic mRNA levels were similar for chow, control- and ethanol-TEN fed rats, but the levels were significantly reduced by the NAC treatments relative to the corresponding control- and ethanol-TEN groups (Figure 5H).
Figure 5.
Effects of chronic ethanol and NAC treatment on insulin/IGF signaling network genes. RNA extracted from liver was reverse transcribed to measure (A) IGF-1, (B) insulin receptor, (C) IGF-1 receptor, (D) IGF-2 receptor, (E) IGF-2, (F) IRS-1, (G) IRS-2, and (H) IRS-4 mRNA expression by PCR. Inter-group comparisons were made using one-way ANOVA with the Tukey multiple comparison tests. Significant P-values are indicated within the panels.
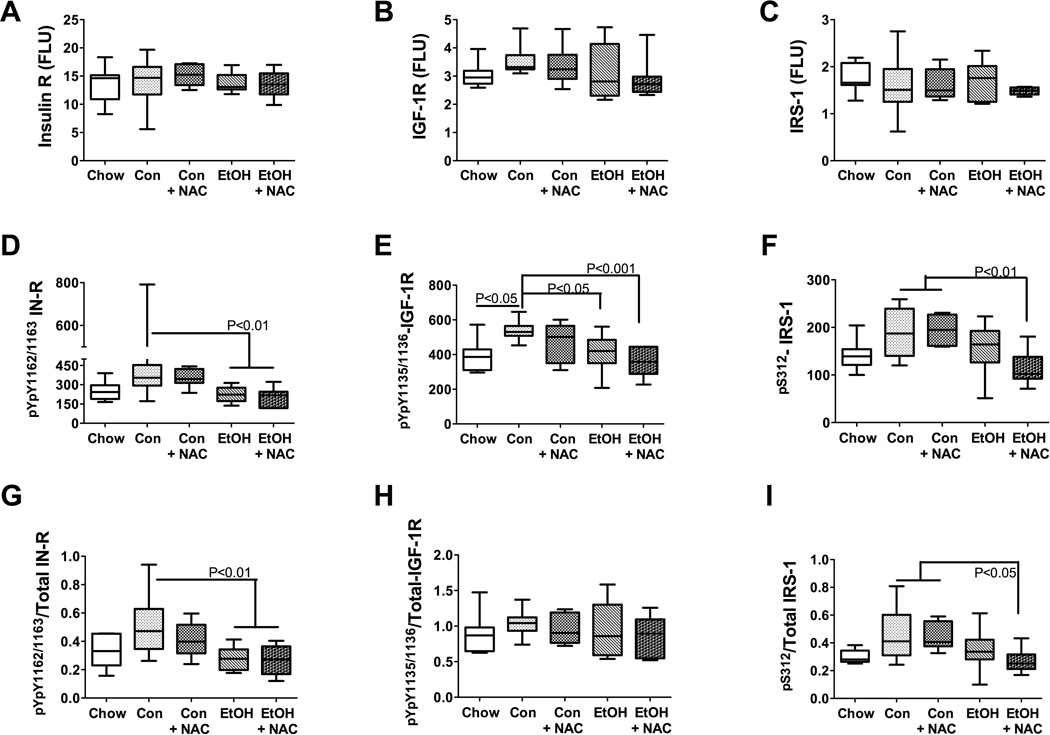
Figure 6.
Effects of chronic ethanol and NAC treatment on upstream mediators of insulin/IGF signaling. Liver protein homogenates were used to measure immunoreactivity corresponding to (A) insulin receptor (R), (B) IGF-1R, (C) IRS-1, (D) pYpY1162/1163-IR, (E) pYpY1135/1136-IGF-1R, (F) pS312-IRS-1 with a bead-based Multiplex ELISA platform (see Methods and Methods). (G–I) In addition, the phosphorylated/total protein ratios for were calculated to assess relative levels of phosphorylation of each protein. Data were analyzed statistically using one-way ANOVA with the Tukey multiple comparison tests. Significant P-values are indicated within the panels.
Figure 7.
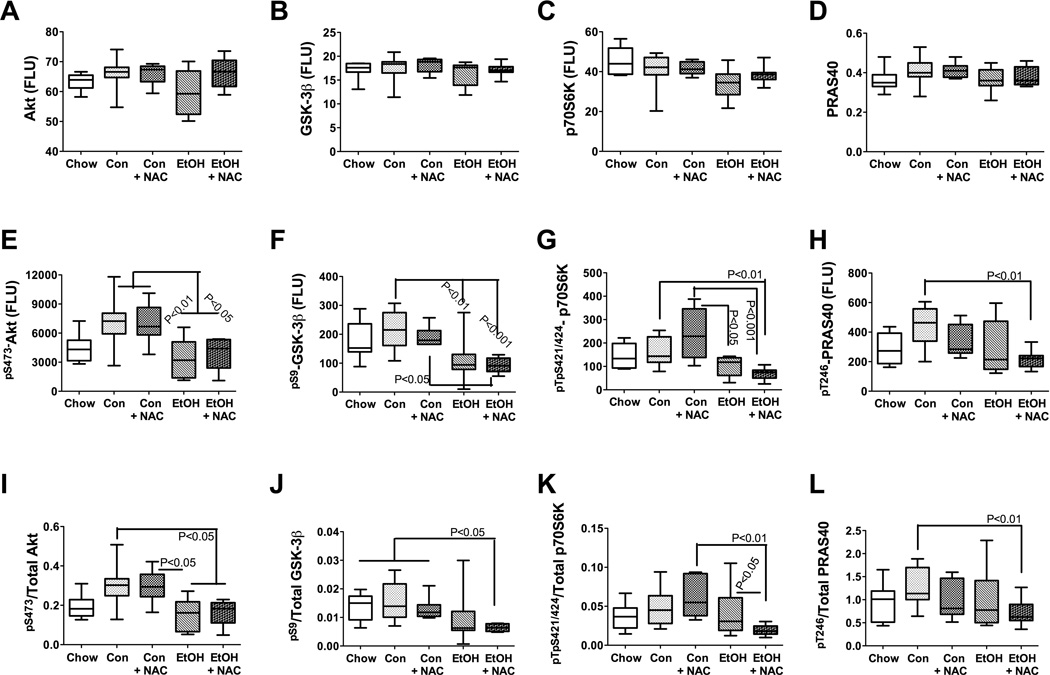
Effects of chronic ethanol, and NAC treatment on the downstream mediators of insulin/IGF signaling. Liver protein homogenates were used in bead-based Multiplex ELISAs to measure (A) Akt, (B) GSK-3β, (C) p70S6K, (D) PRAS40, (E) pS473-Akt, (F) pS9-GSK3β, (G) pTpS421/424-p70S6K and (H) pT246-PRAS40 immunoreactivities. (I–L) In addition, the phosphorylated/total protein ratios for were calculated to assess relative levels of phosphorylation of each protein. Data were analyzed statistically using one-way ANOVA with the Tukey multiple comparison tests. Significant P-values are indicated within the panels.
Analysis of the upstream signaling networks using multiplex ELISAs revealed similar mean levels of IR, IGF-1R, and IRS-1 in all groups (Figures 6A–6C). The mean hepatic levels of pYpY1162/1163-IR (Figure 6D) and the pYpY1162/1163-IR/Total IR ratio (Figure 6G) were significantly reduced in ethanol-TEN ± NAC relative to control-TEN fed rats (both P<0.01). The mean hepatic levels of pYpY1135/1136-IGF-1R were significantly lower in ethanol-TEN (P<0.05) and ethanol-TEN+NAC (P<0.001) relative to control-TEN fed rats (Figure 6E). Finally, the mean hepatic pS312-IRS-1 and the pS312-IRS-1/total IRS-1 ratio were significantly reduced in ethanol-TEN+NAC relative to control-TEN ± NAC fed rats (Figures 6F and 6I). Therefore, chronic ethanol feeding suppressed hepatic signaling through IR, IGF-1R, and IRS-1, with no demonstrated protective effects of the NAC treatments.
Downstream signaling through insulin/IGF-1 in liver, showed similar mean levels of Akt, GSK-3β, p70S6K, and PRAS40 in all five groups (Figures 7A–7D). Hepatic pS473-pAkt and the pS473-pAkt /total Akt ratio were significantly reduced by chronic ethanol-TEN relative to control-TEN feeding, and NAC treatment had no significant effect concerning the responses to ethanol (Figures 7E and 7I). The mean hepatic levels of pS9-GSK-3β were significantly reduced in the ethanol-TEN relative to control-TEN fed rats (Figure 7F), and the mean pS9-GSK-3β/GSK-3β ratio was significantly lower in ethanol-TEN+NAC fed relative to all control groups (P<0.05; Figure 7J). Signaling through p70S6K was also impaired by ethanol as demonstrated by the significantly lower mean levels pTpS421/424-p70S6K in ethanol-TEN relative to control-TEN, particularly with respect to the control-TEN+NAC treated group (Figure 7G). The mean ratio of pTpS421/424-p70S6K/total p70S6K was significantly lower in the ethanol-TEN+NAC relative to the control-TEN+NAC (P<0.01), and ethanol-TEN (P<0.05) fed groups (Figure 7K). Finally, the mean hepatic levels of pT246-PRAS40 and the pT246-PRAS40/total PRAS40 ratio were significantly reduced in livers of ethanol-TEN+NAC treated relative to control- TEN fed rats (both P<0.01; Figures 7H and 7L).
DISCUSSION
Total enteral nutrition (TEN) diets caused hepatic steatosis with mild inflammation, whereas superimposed chronic ethanol feeding strikingly increased the severity of steatohepatitis. Biochemical studies confirmed that the TEN diets increased hepatic lipid content (mainly triglycerides) relative to chow feeding, and that the addition of ethanol to the diets caused only modest further increases total hepatic lipid over TEN alone. Therefore, factors other than simply lipid load must play critical roles in mediating severity of steatohepatitis. One consideration is that ethanol may alter the composition of lipids accumulated in hepatocytes, and perhaps render them cytotoxic. It is noteworthy that the lipid droplets found in ethanol-TEN fed rat livers were mainly micro-vesicular and associated with prominent inflammation, apoptosis, necrosis, and disarray of the hepatic cord architecture, while in control-TEN fed rats, the lipid droplets were mixed micro- and macrovesicular, and the inflammation and cell injury were far less severe. Given the similar overall mean triglyceride contents in livers of control-TEN and ethanol-TEN fed rats, other factors must account for the striking differences in the levels of inflammation and injury. Corresponding with earlier findings (Lyn-Cook et al., 2009), it appears that the micro-vesicular pattern of steatosis may mark a pathological state, whereas the macro-vesicular pattern of steatosis may be more benign. In this regard, it is noteworthy that the major effects of NAC were to reduce inflammation and shift hepatic steatosis from a micro-vesicular to mainly macro-vesicular pattern with accompanying reductions in apoptosis, necrosis, and architectural disarray in both groups, yet total hepatic lipid content was not significantly reduced by NAC.
Oxidative stress contributes to progression of simple steatosis to steatohepatitis (Albano, 2008; Dowman et al., 2009), while radical scavengers attenuate oxidative stress. Since hepatic glutathione levels in ALD and non-alcoholic liver disease are reduced (Das et al., 2008; Videla et al., 2004), we investigated the therapeutic effects of NAC, an anti-oxidant that promotes glutathione synthesis (Aruoma et al., 1989; Atkuri et al., 2007), on the severity of alcohol-induced steatohepatitis. In the present study, NAC reduced inflammation, but not lipid content (Nile Red and triglyceride assays) in liver. However, NAC treatment shifted the sizes of intra-hepatic lipid droplets, converting them from microvesicular to mainly macrovesicular in nature. These findings correspond with results obtained in an earlier study showing that with progression of non-alcoholic steatohepatitis, the histopathology shifted from predominantly macro-vesicular to micro-vesicular steatosis, and was associated with increased inflammation, apoptosis, disorganization of the hepatic lobular architecture, and increased ceramide levels in liver (Lyn-Cook et al., 2009). However, contrary to findings in other models (Cai et al., 2005; McClain et al., 1999; Ronis et al., 2008; Wang et al., 2009), we did not observed a significant role for NAC inhibition of pro-inflammatory cytokines in relation to the reduced severity of alcohol-related steatohepatitis. Moreover, we did not detect significant ethanol-associated increases in pro-inflammatory cytokines in liver. Although the latter observation is not in agreement with previous reports (Thakur et al., 2006a; Thakur et al., 2006b), the discrepancies could be explained by the fact that in those studies, cytokine activation was measured in isolated Kupffer cells, whereas our assays were performed with liver homogenates. The use of tissue homogenates probably masked any differences present in Kupffer cells, which represent a relatively small percentage of the liver cell population.
This study demonstrated that an important consequence of the TEN diets was to increase hepatic ceramide levels. Moreover, the increased levels of ceramide in TEN fed rats, with our without superimposed ethanol and/or NAC treatments, were likely mediated by the combined effects of increased acid sphingomyelinase activity, and increased expression of ceramide synthase 1 and ceramide synthase 4 (ethanol-TEN). In a recent study, increased sphingomyelinase activity was associated with hepatic steatosis caused by chronic high fat feeding (Chocian et al., 2010). Mechanistically, sphingomyelinase activity can be induced by activation of hepatic stellate cells (Moles et al., 2010), and sphingomyelinases are activated by oxidative stress, reactive oxygen species, and reactive nitrogen species, and inhibited by glutathione and other anti-oxidants (Ichi et al., 2009; Levy et al., 2006; Navas et al., 2002; Won and Singh, 2006). Therefore, increased oxidative stress was probably an important factor contributing to the increased levels of acid sphingomyelinase activity, and the attendant increased ceramide levels in livers of ethanol fed rats.
As ceramides can cause cytotoxicity, inflammation, apoptosis, and insulin resistance (Hannun and Obeid, 2008; Holland and Summers, 2008; Morales et al., 2007; Summers, 2006), the NAC-associated improvements in alcohol-induced steatohepatitis could have been mediated by reduced oxidative stress-stimulated sphingomyelinase activity, and consequential reductions in ceramide accumulation. Mechanistically, anti-oxidant treatments may help stabilize membranes of large lipid globules, preventing their rupture and release of cytotoxic lipids, including ceramides. In addition, recent preliminary studies using a precision-cut slice culture model revealed that chemical inhibition of ceramide synthases significantly reduces oxidative stress, lipotoxicity, mitochondrial dysfunction, and insulin resistance in ethanol exposed livers (Setshedi et al., 2010). Therefore, inhibition of ceramide accumulation by reducing sphingomyelin degradation or ceramide synthesis could be therapeutically effective for chronic alcoholic liver disease, although inhibition of ceramide synthesis may be more efficacious in restoring hepatic insulin responsiveness.
Gangliosides are glycosphingolipids that contain sialic acid, and are expressed on mammalian plasma membranes. Gangliosides, like ceramides, are important signaling molecules that can promote insulin resistance (Holland et al., 2007; Yamashita et al., 2003), particularly in response to inflammatory mediators such as TNF-α (Tagami et al., 2002). Insulin-resistance induced by TNF-α was shown to be associated with increased GM3 and reduced insulin receptor accumulation in detergent-resistant membrane microdomains, together with uncoupling of insulin receptor-IRS-1 signaling (Kabayama et al., 2005; Kabayama et al., 2007), Correspondingly, chemical inhibition of GM3 restores insulin responsiveness in leptin-deficient, insulin-resistant states (van Eijk et al., 2009). Our studies demonstrated striking and significantly increased expression of GM3 synthase in ethanol-fed rats, with only modest reductions in GM3 synthase gene expression in livers of the NAC-treated rats. Therefore, ethanol-induced hepatic insulin resistance was likely mediated by the combined effects of increased ceramide and GM3 ganglioside levels in liver. The limited reductions in hepatic ceramide levels and GM3 synthase expression effectuated by NAC treatment correspond with the modest rescue effects of NAC on the ethanol-induced hepatic insulin resistance.
Steatohepatitis produced by ethanol or chronic high fat feeding leads to impaired insulin and IGF signaling in liver (de la Monte et al., 2008; Svegliati-Baroni et al., 2006; Yeon et al., 2003). Included among the factors contributing to this phenotype are reduced hepatic expression of insulin/IGF polypeptides, their receptors, and IRS genes (de la Monte et al., 2008), and reduced phosphorylation of receptor tyrosine kinases and IRS proteins (de la Monte et al., 2008; Yeon et al., 2003). In addition, chronic ethanol exposure causes insulin resistance with inhibition of signaling downstream through growth and survival pathways (He et al., 2006; Mohr et al., 1998; Pang et al., 2009; Ronis et al., 2008; Sasaki and Wands, 1994). Correspondingly, in the present study, the main effects of chronic ethanol feeding were to inhibit phosphorylation and signaling through the insulin receptor, IGF-1 receptor, and IRS-1, as well as through Akt and P76S6K, while increasing activation of GSK-3β.
Reduced levels of pS473-pAkt indicate constitutive inhibition of growth and survival signaling in liver (Nawano et al., 1999), while the reduced levels of pS9-GSK-3β, reflect constitutively increased GSK-3β activity, which also inhibits cell survival (Cross et al., 1995). The findings of reduced Akt and increased GSK-3β activities in ethanol-exposed livers are consistent with previous reports (de la Monte et al., 2008; Yeon et al., 2003). In addition to the impairments in survival and carbohydrate metabolism, we demonstrated significant ethanol inhibition of signaling downstream of PI3 kinase-Akt through p70S6K, and mTOR-dependent PRAS40 pathways, indicating that chronic ethanol feeding inhibits protein synthesis (Berven and Crouch, 2000; Nascimento and Ouwens, 2009). Ethanol inhibition of p70S6K phosphorylation could have been mediated by inhibition of Akt, mTOR, or both, as the multiplex-ELISA detected pTpS421/424-p70S6K; the T421 and S424 residues on p70S6K are phosphorylated by Akt and mTOR (Guillen et al., 2006). Since none of the inhibitory effects of ethanol on insulin/IGF/Akt signaling were abrogated by NAC treatment, anti-oxidant therapy alone would probably not be effective in restoring hepatic function or reducing progression of alcohol-related steatohepatitis.
In conclusion, this study demonstrates that NAC treatment has a role in reducing the severity of alcohol-induced steatohepatitis, but the therapeutic benefits are limited due to persistence of insulin/IGF signaling impairments in liver. In addition to decreasing inflammation, NAC reduced ceramide levels in liver. This effect was mediated by reductions in sphingomyelinase activity rather than changes in gene expression. We hypothesize that the NAC-mediated reductions in oxidative stress (Ronis et al., 2005; Samuhasaneeto et al., 2007) led to improved membrane stabilization and attendant reductions in lipid breakdown with release of toxic sphingolipids, including ceramides. We propose that the histological correlate of this phenomenon is the NAC-associated shift in the pattern of steatosis from micro-vesicular to macro-vesicular. The findings herein reinforce our hypothesis that ethanol-induced steatohepatitis has dual independent but interactive pathogenic mechanisms: oxidative stress/inflammation and insulin/IGF resistance. While NAC and other anti-oxidant and radical scavenger treatments may aid in reducing the severity of oxidative stress/inflammation, additional therapeutic agents such as insulin sensitizers are probably needed to arrest the insulin/IGF-1 resistance arm of this disease, as previously suggested (de Oliveira et al., 2008; Pang et al., 2009).
Supplementary Material
Acknowledgments
Supported by AA-11431, AA-12908, K24-AA-16126 from the National Institutes of Health and the Gastroenterology Foundation of South Africa
REFERENCES
- Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med. 2008;29(1–2):9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Atkuri KR, Mantovani JJ, Herzenberg LA. N-Acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007;7(4):355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJ. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G27–G38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- Berven LA, Crouch MF. Cellular function of p70S6K: a role in regulating cell motility. Immunol Cell Biol. 2000;78(4):447–451. doi: 10.1046/j.1440-1711.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- Brade L, Vielhaber G, Heinz E, Brade H. In vitro characterization of anti-glucosylceramide rabbit antisera. Glycobiology. 2000;10(6):629–636. doi: 10.1093/glycob/10.6.629. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11(2):183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008;103(4):1036–1042. doi: 10.1111/j.1572-0241.2007.01709.x. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274(29):20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278(12):10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- Chocian G, Chabowski A, Zendzian-Piotrowska M, Harasim E, Lukaszuk B, Gorski J. High fat diet induces ceramide and sphingomyelin formation in rat's liver nuclei. Mol Cell Biochem. 2010 doi: 10.1007/s11010-010-0409-6. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Das KS, Balakrishnan V, Mukherjee S, Vasudevan DM. Evaluation of blood oxidative stress-related parameters in alcoholic liver disease and non-alcoholic fatty liver disease. Scand J Clin Lab Invest. 2008;68(4):323–334. doi: 10.1080/00365510701673383. [DOI] [PubMed] [Google Scholar]
- Day CP. From fat to inflammation. Gastroenterology. 2006;130(1):207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48(Suppl 1):S104–S112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Lawton M, Longato L. Nitrosamine exposure exacerbates high fat diet-mediated type 2 diabetes mellitus, non-alcoholic steatohepatitis, and neurodegeneration with cognitive impairment. Mol Neurodegener. 2009;4:54. doi: 10.1186/1750-1326-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Yeon JE, Tong M, Longato L, Chaudhry R, Pang MY, Duan K, Wands JR. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol. 2008;23(8 Pt 2):e477–e486. doi: 10.1111/j.1440-1746.2008.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira CP, Stefano JT, de Siqueira ER, Silva LS, de Campos Mazo DF, Lima VM, Furuya CK, Mello ES, Souza FG, Rabello F, Santos TE, Nogueira MA, Caldwell SH, Alves VA, Carrilho FJ. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008;38(2):159–165. doi: 10.1111/j.1872-034X.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268(21):15523–15530. [PubMed] [Google Scholar]
- Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2009 doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Colell A, Mari M, Garcia-Ruiz C. Ceramide, tumor necrosis factor and alcohol-induced liver disease. Alcohol Clin Exp Res. 2005;29(11 Suppl):151S–157S. [PubMed] [Google Scholar]
- Guillen C, Navarro P, Robledo M, Valverde AM, Benito M. Differential mitogenic signaling in insulin receptor-deficient fetal pancreatic beta-cells. Endocrinology. 2006;147(4):1959–1968. doi: 10.1210/en.2005-0831. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40(16):4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277(29):25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Haskill S, Peace A, Morris J, Sporn SA, Anisowicz A, Lee SW, Smith T, Martin G, Ralph P, Sager R. Identification of three related human GRO genes encoding cytokine functions. Proc Natl Acad Sci U S A. 1990;87(19):7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck CR, Grassme H, Bock J, Jendrossek V, Ferlinz K, Meyer TF, Gulbins E. Acid sphingomyelinase is involved in CEACAM receptor-mediated phagocytosis of Neisseria gonorrhoeae. FEBS Lett. 2000;478(3):260–266. doi: 10.1016/s0014-5793(00)01851-2. [DOI] [PubMed] [Google Scholar]
- He XP, Butler L, Liu X, McNamara JO. The tyrosine receptor kinase B ligand, neurotrophin-4, is not required for either epileptogenesis or tyrosine receptor kinase B activation in the kindling model. Neuroscience. 2006;141(1):515–520. doi: 10.1016/j.neuroscience.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65(6 Pt 2):S39–S46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29(4):381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichi I, Kamikawa C, Nakagawa T, Kobayashi K, Kataoka R, Nagata E, Kitamura Y, Nakazaki C, Matsura T, Kojo S. Neutral sphingomyelinase-induced ceramide accumulation by oxidative stress during carbon tetrachloride intoxication. Toxicology. 2009;261(1–2):33–40. doi: 10.1016/j.tox.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Kabayama K, Sato T, Kitamura F, Uemura S, Kang BW, Igarashi Y, Inokuchi J. TNFalpha-induced insulin resistance in adipocytes as a membrane microdomain disorder: involvement of ganglioside GM3. Glycobiology. 2005;15(1):21–29. doi: 10.1093/glycob/cwh135. [DOI] [PubMed] [Google Scholar]
- Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci U S A. 2007;104(34):13678–13683. doi: 10.1073/pnas.0703650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larter CZ, Farrell GC. Insulin resistance, adiponectin, cytokines in NASH: Which is the best target to treat? J Hepatol. 2006;44(2):253–261. doi: 10.1016/j.jhep.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH, Jr, Futerman AH. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem. 2008;283(9):5677–5684. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun. 2006;344(3):900–905. doi: 10.1016/j.bbrc.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Obeid LM, Hannun YA. Sphingomyelinases in cell regulation. Semin Cell Dev Biol. 1997;8(3):311–322. doi: 10.1006/scdb.1997.0153. [DOI] [PubMed] [Google Scholar]
- Lyn-Cook LE, Jr, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, Mark P, Wands JR, Xu H, de la Monte SM. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;16(4):715–729. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby J, Wright S, Bird G, Sheron N. Chemokine levels in human liver homogenates: associations between GRO alpha and histopathological evidence of alcoholic hepatitis. Hepatology. 1996;24(5):1156–1160. doi: 10.1053/jhep.1996.v24.pm0008903391. [DOI] [PubMed] [Google Scholar]
- Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82(1):27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14(2):72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Marzullo L. An update of N-acetylcysteine treatment for acute acetaminophen toxicity in children. Curr Opin Pediatr. 2005;17(2):239–245. doi: 10.1097/01.mop.0000152622.05168.9e. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19(2):205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9(3):349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J. 2005;390(Pt 1):263–271. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr L, Tanaka S, Wands JR. Ethanol inhibits hepatocyte proliferation through the IRS-1 signal transduction pathway in transgenic mice. Gastroenterology. 1998;115:1558–1565. doi: 10.1016/s0016-5085(98)70036-8. [DOI] [PubMed] [Google Scholar]
- Moles A, Tarrats N, Morales A, Dominguez M, Bataller R, Caballeria J, Garcia-Ruiz C, Fernandez-Checa JC, Mari M. Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. Am J Pathol. 2010;177(3):1214–1224. doi: 10.2353/ajpath.2010.091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12(5):923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- Moroz N, Tong M, Longato L, Xu H, de la Monte SM. Limited Alzheimer-type neurodegeneration in experimental obesity and type 2 diabetes mellitus. J Alzheimers Dis. 2008;15(1):29–44. doi: 10.3233/jad-2008-15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA, French SW. Animal models of alcoholic liver disease--focus on the intragastric feeding model. Alcohol Res Health. 2003;27(4):325–330. [PMC free article] [PubMed] [Google Scholar]
- Nascimento EB, Ouwens DM. PRAS40: target or modulator of mTORC1 signalling and insulin action? Arch Physiol Biochem. 2009;115(4):163–175. doi: 10.1080/13813450902988580. [DOI] [PubMed] [Google Scholar]
- Navas P, Fernandez-Ayala DM, Martin SF, Lopez-Lluch G, De Caboa R, Rodriguez-Aguilera JC, Villalba JM. Ceramide-dependent caspase 3 activation is prevented by coenzyme Q from plasma membrane in serum-deprived cells. Free Radic Res. 2002;36(4):369–374. doi: 10.1080/10715760290021207. [DOI] [PubMed] [Google Scholar]
- Nawano M, Ueta K, Oku A, Arakawa K, Saito A, Funaki M, Anai M, Kikuchi M, Oka Y, Asano T. Hyperglycemia impairs the insulin signaling step between PI 3-kinase and Akt/PKB activations in ZDF rat liver. Biochem Biophys Res Commun. 1999;266(1):252–256. doi: 10.1006/bbrc.1999.1797. [DOI] [PubMed] [Google Scholar]
- Ohki E, Kato S, Ohgo H, Mizukami T, Fukuda M, Tamai H, Okamura Y, Matsumoto M, Suzuki H, Yokoyama H, Ishii H. Effect of chronic ethanol feeding on endotoxin-induced hepatic injury: role of adhesion molecules on leukocytes and hepatic sinusoid. Alcohol Clin Exp Res. 1998;22(3 Suppl):129S–132S. doi: 10.1111/acer.1998.22.s3_part1.129s. [DOI] [PubMed] [Google Scholar]
- Pang M, de la Monte SM, Longato L, Tong M, He J, Chaudhry R, Duan K, Ouh J, Wands JR. PPARdelta agonist attenuates alcohol-induced hepatic insulin resistance and improves liver injury and repair. J Hepatol. 2009;50(6):1192–1201. doi: 10.1016/j.jhep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickersgill L, Litherland GJ, Greenberg AS, Walker M, Yeaman SJ. Key role for ceramides in mediating insulin resistance in human muscle cells. J Biol Chem. 2007;282(17):12583–12589. doi: 10.1074/jbc.M611157200. [DOI] [PubMed] [Google Scholar]
- Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206(2):169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Butura A, Korourian S, Shankar K, Simpson P, Badeaux J, Albano E, Ingelman-Sundberg M, Badger TM. Cytokine and chemokine expression associated with steatohepatitis and hepatocyte proliferation in rats fed ethanol via total enteral nutrition. Exp Biol Med (Maywood) 2008;233(3):344–355. doi: 10.3181/0707-RM-203. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Butura A, Sampey BP, Shankar K, Prior RL, Korourian S, Albano E, Ingelman-Sundberg M, Petersen DR, Badger TM. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med. 2005;39(5):619–630. doi: 10.1016/j.freeradbiomed.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronis MJ, Korourian S, Blackburn ML, Badeaux J, Badger TM. The role of ethanol metabolism in development of alcoholic steatohepatitis in the rat. Alcohol. 2010;44(2):157–169. doi: 10.1016/j.alcohol.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuhasaneeto S, Thong-Ngam D, Kulaputana O, Patumraj S, Klaikeaw N. Effects of N-acetylcysteine on oxidative stress in rats with non-alcoholic steatohepatitis. J Med Assoc Thai. 2007;90(4):788–797. [PubMed] [Google Scholar]
- Sasaki Y, Wands JR. Ethanol impairs insulin receptor substrate-1 mediated signal transduction during rat liver regeneration. Biochem Biophys Res Commun. 1994;199(1):403–409. doi: 10.1006/bbrc.1994.1243. [DOI] [PubMed] [Google Scholar]
- Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Setshedi M, Tong M, Feng D, Longato L, Ramirez T, Le R, Wands JR, de la Monte SM. Ceramide inhibitors and PPAR agonists ameliorate alcohol-induced steatohepatitis in an ex vivo liver slice culture model. Hepatology. 2010;52(Supplement 1):121A. [Google Scholar]
- Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem. 2008;283(20):13538–13548. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori Y, Takada H, Hikiba Y, Okano K, Niwa Y, Matsumura M, Komatsu Y, Omata M. Increased release of KC/gro protein, intercrine cytokine family, from hepatocytes of the chronically ethanol fed rats. Biochem Biophys Res Commun. 1993;197(1):319–325. doi: 10.1006/bbrc.1993.2478. [DOI] [PubMed] [Google Scholar]
- Silveira LR, Fiamoncini J, Hirabara SM, Procopio J, Cambiaghi TD, Pinheiro CH, Lopes LR, Curi R. Updating the effects of fatty acids on skeletal muscle. J Cell Physiol. 2008;217(1):1–12. doi: 10.1002/jcp.21514. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Perry DK, Zhang J, Poirier GG, Hannun YA, Obeid LM. prICE: a downstream target for ceramide-induced apoptosis and for the inhibitory action of Bcl-2. Biochem J. 1996;316(Pt 1):25–28. doi: 10.1042/bj3160025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18(9):5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svegliati-Baroni G, Candelaresi C, Saccomanno S, Ferretti G, Bachetti T, Marzioni M, De Minicis S, Nobili L, Salzano R, Omenetti A, Pacetti D, Sigmund S, Benedetti A, Casini A. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol. 2006;169(3):846–860. doi: 10.2353/ajpath.2006.050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami S, Inokuchi JiJ, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, Sakaue S, Igarashi Y. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 2002;277(5):3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006a;290(5):G998–G1007. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006b;79(6):1348–1356. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131(3):934–945. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- van Eijk M, Aten J, Bijl N, Ottenhoff R, van Roomen CP, Dubbelhuis PF, Seeman I, Ghauharali-van der Vlugt K, Overkleeft HS, Arbeeny C, Groen AK, Aerts JM. Reducing glycosphingolipid content in adipose tissue of obese mice restores insulin sensitivity, adipogenesis and reduces inflammation. PLoS One. 2009;4(3):e4723. doi: 10.1371/journal.pone.0004723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quinones L, Varela N, Contreras J, Lazarte R, Csendes A, Rojas J, Maluenda F, Burdiles P, Diaz JC, Smok G, Thielemann L, Poniachik J. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106(3):261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- Wang Y, Seitz HK, Wang XD. Moderate Alcohol Consumption Aggravates High-Fat Diet Induced Steatohepatitis in Rats. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yao T, Song Z. Chronic alcohol consumption disrupted cholesterol homeostasis in rats: down-regulation of low-density lipoprotein receptor and enhancement of cholesterol biosynthesis pathway in the liver. Alcohol Clin Exp Res. 2010;34(3):471–478. doi: 10.1111/j.1530-0277.2009.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JS, Singh I. Sphingolipid signaling and redox regulation. Free Radic Biol Med. 2006;40(11):1875–1888. doi: 10.1016/j.freeradbiomed.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A. 2003;100(6):3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon JE, Califano S, Xu J, Wands JR, De La Monte SM. Potential role of PTEN phosphatase in ethanol-impaired survival signaling in the liver. Hepatology. 2003;38(3):703–714. doi: 10.1053/jhep.2003.50368. [DOI] [PubMed] [Google Scholar]
- Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273(26):16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.