Abstract
The food-borne pathogen Listeria monocytogenes encodes two chitinases, ChiA and ChiB, which allow the bacterium to hydrolyze chitin, the second most abundant polysaccharide in nature. Intriguingly, despite the absence of chitin in human and mammalian hosts, both of the chitinases have been deemed important for infection, through a mechanism that, at least in the case of ChiA, involves modulation of host immune responses. In this study, we show that the expression of the two chitinases is subject to regulation by the listerial agr system, a homologue of the agr quorum-sensing system of Staphylococcus aureus, that has so far been implicated in virulence and biofilm formation. We demonstrate that in addition to these roles, the listerial agr system is required for efficient chitin hydrolysis, as deletion of agrD, encoding the putative precursor of the agr autoinducer, dramatically decreased chitinolytic activity on agar plates. Agr was specifically induced in response to chitin addition in stationary phase and agrD was found to regulate the amount of chiA, but not chiB, transcripts. Although the transcript levels of chiB did not depend on agrD, the extracellular protein levels of both chitinases were reduced in the ΔagrD mutant. The regulatory effect of agr on chiA is potentially mediated through the small RNA LhrA, which we show here to be negatively regulated by agr. LhrA is in turn known to repress chiA translation by binding to the chiA transcript and interfering with ribosome recruitment. Our results highlight a previously unrecognized role of the agr system and suggest that autoinducer-based regulation of chitinolytic systems may be more commonplace than previously thought.
Introduction
Listeria monocytogenes is a Gram-positive food-borne pathogen, and the causative agent of human listeriosis, a disease of varying severity that can prove fatal for immunosusceptible patient groups, such as pregnant women and the elderly.
L. monocytogenes is often isolated from marine environments, as well as from soil, where it lives as a saprophyte [1]–[3]. In these habitats, chitin, a polymer of N-acetylglucosamine (GlcNAc) and the second most abundant carbohydrate in nature, can constitute an important source of carbon and nitrogen [4]–[8]. Many bacteria autochthonous to chitin-rich environments have developed simple to complex chitinolytic systems, which allow them to degrade chitin and use it as a nutrient source [4], [8], [9]. Most importantly, chitin is broken down by chitinases, which are often assisted by lytic polysaccharide monooxygenases (LPMOs) that facilitate chitinase accessibility to the complex crystalline chitin structure [10], [11]. Interestingly, many human and animal pathogens are chitinolytic, and evidence has emerged that chitinases and LMPOs have an additional key role in these pathogens as virulence factors, which promote infection [12].
L. monocytogenes possesses a chitinolytic system that comprises two chitinases (ChiA and ChiB) and a putative LPMO (Lmo2467) [13], [14]. Both of the chitinases, but not Lmo2467, have been found to be important for colloidal chitin hydrolysis on agar plates [13]. ChiB contributes the most to the hydrolysis of this substrate and is the chitinase most induced during growth in soil [13], [15]. In contrast, ChiA has been identified as an important virulence factor associated with enhanced pathogenicity [16], [17]. Specifically, it was recently shown that ChiA helps L. monocytogenes suppress iNOS expression and thereby increases the rate of survival in the host [17].
During growth in rich laboratory media, the two chitinases are expressed only at background levels, and it is known that nutrient-poor conditions as well as the presence of an inducer are required for their full induction [18]. As is common with carbon utilization systems [19], stringent regulatory controls are in place to ensure chitinase expression only occurs under desired conditions. These include transcriptional dependence on the major global regulators σB and PrfA, as well as negative regulation by the small RNA LhrA, which we have previously shown to be negatively controlling translation of chiA, by binding to its mRNA and preventing ribosome recruitment [18], [20].
Another important regulatory machinery in L. monocytogenes is the accessory gene regulator (agr) system, which has orthologs in several Gram-positive bacteria, most notably Staphylococcus aureus [21]–[23]. Based on what is known about the function of the agr orthologs in the other bacteria, the listerial agr system has been proposed to be a quorum-sensing system composed of four main components (agrBDCA), co-transcribed in an operon [24]–[26]. According to this model, agrD encodes a propeptide, which is processed into the mature autoinducing peptide (AIP) and exported outside the cell by the transmembrane protein AgrB. When the extracellular concentration of the AIP reaches a certain threshold the system is activated through the two-component sensing system AgrC-AgrA and exerts regulatory effects on target substrates, as well as induces its own production (positive autoregulation).
The agr system of L. monocytogenes has been found to be important for key functions, such as biofilm formation and virulence [24]–[29]. However, microarray analyses suggested that it might play a far broader role in the bacterium, as they revealed a rather large agr regulon [26], [29]. Still, little is known about other roles of agr, or about its regulatory mechanism.
In this paper, we present a newly-identified role for the agr system in the regulation of the chitinolytic activity of L. monocytogenes. Additionally, we provide evidence that the levels of the sRNA LhrA are negatively regulated by agr and postulate that LhrA could be an effector of the system, mediating the regulatory effect of agr on chiA. Finally, we demonstrate that agr itself responds to the addition of chitin in the extracellular medium.
Materials and Methods
Bacterial strains and standard growth conditions
The ΔagrD mutant and its parental wild-type EGD-e strain were kindly provided by Colin Hill [26]. The chitinase deletion mutants and their parental EGD-e wild-type strain were obtained from Raghupathi et al. [30], and Dr. W. Goebel (Biozentrum, University of Würzburg, Germany), respectively. EGD-e strains from different laboratories may vary genetically. Therefore, mutants were always compared to their parental wild types, although when compared no differences were observed between the two wild-type parental strains.
Bacteria were routinely grown in brain heart infusion (BHI, Oxoid) aerobically at 37 °C, unless stated otherwise.
Colloidal chitin preparation
5 g of chitin from shrimp shells (C9213, Sigma-Aldrich) were treated overnight with 50 mL 36–38% HCl. After treatment, the pH was adjusted to 8 by addition of NaOH. Subsequently, the mixture was centrifuged at 8228 × g for 5 min, and the chitin pellet was separated and washed seven times in ultrapure water.
Chitinase assay
Chitin hydrolysis was assayed on LB (Oxoid) agar plates containing 6 mg/mL acid-hydrolyzed colloidal chitin. Specifically, 10 µL of overnight cultures were spotted on the plates and chitinase activity was assayed by measuring the diameters of the produced clearing zones after 4 days of incubation under aerobic conditions at 30 °C.
Sample collection for total RNA extraction and beta-galactosidase assay
For sample collection, the cells were grown aerobically in LB at 30 °C and 190 rpm overnight. The next day, the cultures were diluted in LB supplemented with 0.05% glucose to an OD600 of 0.05, and thereafter grown at 30 °C and 190 rpm.
For estimation of agrA and chitinase gene transcription, the mutant and/or wild-type strains were grown until late-exponential phase (OD600 0.7). At that point the cells were divided into two flasks, in one of which the cells were induced by the addition of colloidal chitin (see above) to a final concentration of 3.3 g/L. Samples for RNA were collected from both flasks (with and without chitin) 15 min and 2 h after induction, corresponding to late exponential and stationary (OD600 approximately 0.9) phase, respectively.
For estimation of lhrA transcription, the wild type and mutant samples were collected at mid-exponential and stationary phase (OD600 approximately 0.35 and 0.9). The same method was applied for the collection of samples for the beta-galactosidase assay, with the exception that the medium additionally contained kanamycin at a concentration of 50 µg/mL.
Total RNA extraction
The cells were lysed with the aid of a FastPrep-24 Instrument (MP Biomedicals) for two rounds of 40 s at speed setting 6.0. Total RNA was extracted with the RNeasy mini kit (Qiagen), according to the manufacturer's directions. RNA concentration was measured in a Nanodrop 1000 spectrophotometer and the integrity of RNA was confirmed by visualizing the RNA samples on a 1% agarose gel.
Northern blot analysis
For the northern blot analysis of agrA transcripts, 5 µg of RNA samples were denatured and separated on a 1% agarose gel containing 10 mg/mL ethidium bromide. After visualization under UV light (loading control), the RNA from the gel was transferred to a Hybond-N+ nylon membrane (GE Healthcare Life Sciences) by overnight capillary blotting. The radioactive probes were generated by PCR amplification and were subsequently 32P-radioactively-labeled using the Ready-to-Go DNA labeling Beads (GE Healthcare Life Sciences). Primers AgrA-F: CGAATGCCTACACATCAAGG and AgrA-R: CTTCACCACACCTTTTGTCG were used for the amplification of the agrA probe, while the primers for the chiA and chiB probes were the same as described in [18]. RNA hybridization was performed overnight at 65 °C in 0.5 M sodium phosphate buffer pH 7.2 containing 7% SDS. After washing in 20 mM sodium phosphate buffer pH 7.2 containing 1% SDS, the radioactive signal was detected with the aid of a Cyclone Plus Phosphor Imager (PerkinElmer) and analyzed with the accompanying OptiQuant software. Differences between the amounts of transcripts were considered relevant only if they exceeded 2-fold.
The northern blot analysis of lhrA was performed as described in [20].
Beta-galactosidase assay
Previously constructed [20] fusions of the pTCV-lac vector with lhrA promoter fragments were introduced into the ΔagrD mutant and its parental EGD-e strain by means of electroporation [31]. The beta-galactosidase assay was performed as described in [32].
Protein precipitation of bacterial supernatants
For sample collection of GlcNAc-induced cells, the cells were initially grown aerobically in LB at 30 °C and 190 rpm overnight. After the overnight incubation half of the cells were induced by dilution in LB (final OD600 of 0.05) supplemented with 1 g/L GlcNAc (Sigma-Aldrich). After overnight growth at 30 °C and 190 rpm, the OD600 of the uninduced cells was recorded. 9 mL of induced cells were spun down at 4629 × g at 0°C for 7 min. The supernatants were transferred to new tubes and the proteins were precipitated, by addition of 0.02% sodium deoxycholate (Sigma-Aldrich) and incubation for 30 min at 4°C, followed by addition of 10% trichloroacetic acid (Sigma-Aldrich) and overnight incubation at 4°C. The precipitated proteins were separated from the supernatant by centrifugation at 15000 × g for 15 min. Subsequently, the protein pellets were washed twice in 1.5 mL and 500 µL of ice cold 99% ethanol. Pellets were completely dried in a vacuum centrifuge and resuspended in 50 mM TrisHCl pH 8.0. The volumes of TrisHCl used for resuspension were calculated so that the samples would be normalized based on the recorded OD600 (50 µL of TrisHCl pH 8.0 per OD600 1). 10 µL of the samples were used for the western blot analysis.
For sample collection of chitin-induced cells, the cells were initially grown aerobically in LB at 30 °C and 190 rpm overnight. The next day, the cultures were diluted in LB supplemented with 0.05% glucose to an OD600 of 0.05, and thereafter grown at 30 °C and 190 rpm. Upon reaching an OD600 of approximately 0.7, part of the cells were induced by transfer of 10 mL of culture to flasks containing 0.1 g of colloidal chitin (to reach a final concentration of 10 g/L). After overnight growth, the flasks were left to stand for 30 min, and the unhydrolyzed chitin fraction was separated from the culture by gravity. 9 mL of the culture were analyzed as described above for the GlcNAc-induced samples, with the exception that 5 µL and 1.25 µL instead of 10 µL were analyzed in the western blots of Fig. 5A and 5B, respectively. In parallel, the chitin fraction separated by gravity was washed twice in 1 mL 0.9% saline solution to remove bacterial cells. Subsequently, the chitin particles were separated by centrifugation at 16000 × g for 5 min. The pellet was resuspended in 20 µL of SDS-PAGE sample buffer, and boiled for 10 min at 99°C. 10 µL of the samples were used for western blot analysis.
Figure 5.
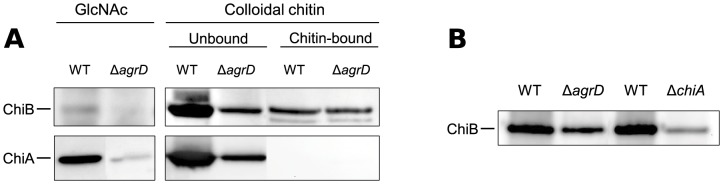
A. Western blot analysis of culture supernatants of wild type EGD-e and the ΔagrD mutant.The bacteria were grown overnight at 30 °C in LB+0.05% glucose, supplemented with either colloidal chitin or GlcNAc. In the case of colloidal chitin, two fractions are presented, representing the proteins remaining free in the supernatant (unbound), and those that remained bound to the chitin (chitin-bound). It should be noted that the loading of the two fractions was unequal. For comparison, the loaded amounts were such that the samples of the chitin-bound fraction represent a five times larger fraction of the total supernatant than the “unbound” samples. The results depicted here were reproduced in three independent experiments, except for the analysis of the chitin-bound proteins, which was confirmed in a biological duplicate. B. Western blot analysis of culture supernatants of mutants lacking chiA and agrD , with their respective wild-type parental strains. Samples were collected after overnight growth at 30 °C in LB+0.05% glucose, supplemented with colloidal chitin. Only the proteins remaining free in the supernatant and not bound to chitin are presented. The results were reproduced in a biological triplicate, collected and analyzed during the course of two independent experiments.
Western blot analysis
The samples were separated in 4–12% NuPAGE Bis-Tris gels (Invitrogen) under reducing conditions, and then transferred to a polyvinylidene difluoride (PVDF) membrane by use of the iBlot dry blotting system (Invitrogen). Western blot analysis was carried out with the use of the Western Breeze Chemiluminescent Kit-Anti-Rabbit (Invitrogen), according to the manufacturer's instructions. The anti-ChiA and anti-ChiB antibodies used for the analysis were raised in New Zealand white rabbits by Covalab. Purified N-terminally-HisTagged ChiA and ChiB proteins were used for immunization, respectively. Polyclonal serum was obtained after 53 days. Specificity was confirmed by comparison of blots of culture supernatants of wild-type EGD-e to blots of culture supernatants of ΔchiA and ΔchiB mutant strains (Fig. S1).
Image processing
For presentation purposes, contrast and coloring were adjusted with Pixelmator.
Results
A ΔagrD mutant is impaired in chitin hydrolysis
In L. monocytogenes agrD is predicted to encode the premature form of the autoinducing peptide of the agr system [26], [33]. In order to investigate the role of agr in chitin hydrolysis, we tested a mutant lacking agrD for chitinolytic activity on LB-chitin agar plates at 30 °C. We found the chitinolytic activity of the mutant to be clearly reduced when compared to that of the wild-type EGD-e (Fig. 1). This observation was supported by experiments in liquid cultures, where after overnight growth in LB supplemented with 0.05% glucose and 3.3 g/L colloidal chitin, the amount of unhydrolyzed chitin remaining in the supernatant was markedly larger in the case of the mutant (results not shown). No growth differences that could account for the observed phenotype were recorded between the two strains in LB medium supplemented with 0.05% glucose or on BHI and LB-chitin agar plates at 30 °C (data not shown). Similarly, the numbers of colony-forming units were comparable at selected points during growth in LB medium supplemented with 0.05% glucose and 3.3 g/L colloidal chitin.
Figure 1. Chitinase activity of the wild-type EGD-e and its isogenic mutant lacking agrD.
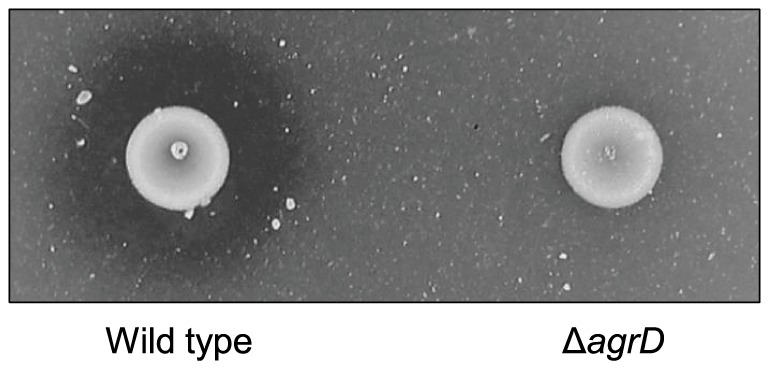
Bacterial cultures were spotted on LB-colloidal chitin agar plates and incubated at 30 °C for 3 days. The results presented here are representative of four independent experiments.
Deletion of agrD results in decreased transcripts of chiA but not of chiB
The impaired chitinolytic activity exhibited by the agr mutant could be the result of decreased expression of the chitinase genes chiA and/or chiB. To examine if agrD influences transcription of chiA and chiB, we compared the amounts of chiA and chiB mRNA produced in the wild-type and mutant strains. Transcripts were quantified 15 minutes and 2 hours following chitin addition to exponentially-growing cultures, at which time points the cells had reached late-exponential and stationary growth, respectively (Fig. 2).
Figure 2. Northern blot analysis of chiA and chiB mRNA in the wild-type EGD-e and the ΔagrD mutant.
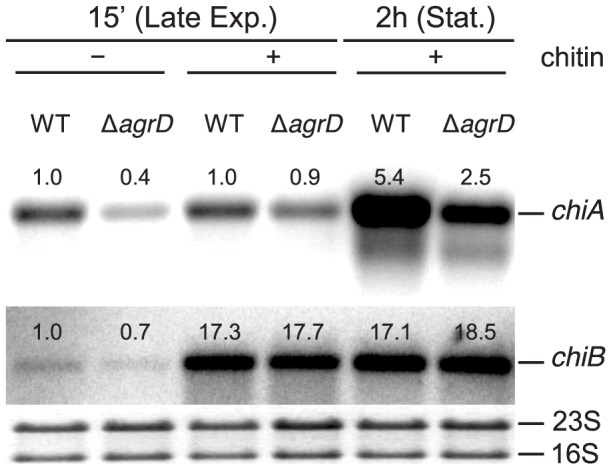
Samples were taken 15-exponential and early stationary phase of growth, respectively. At the 15 min time point, RNA was also extracted from cultures grown without chitin and used as a reference. The numbers above the bands correspond to the relative fold change in relation to lane 1, i.e. to the transcript levels of wild-type bacteria in late exponential phase in medium without chitin. The loading control can be seen below each band. The results presented here are representative of a biological triplicate, collected and analyzed during the course of two independent experiments.
In the absence of chitin, only low levels of chiA and chiB transcripts were observed in the late exponential phase (Fig. 2). In stationary phase, both transcripts are undetectable by northern blot [18] and were, therefore, not investigated. 15 minutes after chitin addition, chiB transcripts were induced to similarly high levels in the wild type and the ΔagrD mutant and remained as such until at least 2 h after induction. In contrast, chiA transcripts were only induced 2 h after chitin addition, when the cells had reached stationary phase, at which point the amount of chiA mRNA was approximately two-fold lower in the mutant lacking agrD compared to the wild type.
Therefore, it appears that agrD exerts a positive effect on chiA, but not chiB, expression at the transcript level.
Deletion of agrD results in increased amounts of LhrA, a repressor of chiA
The product of the small RNA LhrA negatively regulates chitinase A, by binding to the chiA transcript and interfering with ribosome recruitment [20]. We hypothesized that the regulation of ChiA by agr might not be direct, but mediated through LhrA instead. To investigate that, we compared the expression of lhrA between the wild-type strain and the mutant lacking agrD, in mid-exponentially growing cells, as well as in cells that had reached stationary phase.
Based on our results, deletion of agrD resulted in an approximately 2.5-fold increase in the levels of LhrA (Fig. 3), pointing to agr being a negative regulator of the small RNA. This supports our hypothesis that the decreased levels of chiA mRNA observed for the ΔagrD mutant were produced by the excessive LhrA amounts in the mutant background. Indeed, a decrease in chiA transcripts would be expected in the case of increased expression of LhrA, as the excessive LhrA would likely destabilize chiA transcripts by pairing with them and thereby promoting their degradation. Such a mechanism has been shown previously for another LhrA substrate [34]. It remains to be investigated whether the expected decrease in chiA transcripts in this case is enough to produce the chiA levels observed for the ΔagrD mutant, or whether additional regulatory interactions are involved.
Figure 3. Northern blot analysis of lhrA transcripts in the wild-type EGD-e and the ΔagrD mutant.
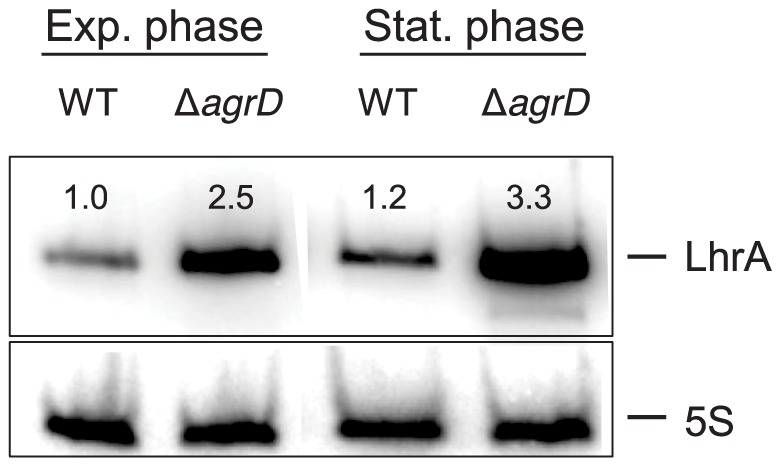
The two strains were grown at 30 °C in LB+0,05% glucose to mid-exponential and stationary phase. The numbers above the bands correspond to the relative fold change in relation to lane 1, i.e. to the transcript levels of wild-type bacteria in mid-exponential phase. The results are normalized to the 5S loading control, shown below each band and are representative of a biological triplicate, collected and analyzed during the course of two independent experiments.
Investigation of lhrA transcription in the agrD mutant
To investigate whether LhrA regulation via agr occurs directly at the transcriptional level or rather post-transcriptionally, we compared lhrA transcription between the wild type and the ΔagrD mutant. To this end, we introduced two plasmids into the wild-type and mutant strains, carrying either the entire lhrA promoter ranging from position −157 to +71 relative to the transcriptional start site (LhrAp-157), or a truncated version of the promoter ranging from position −61 to +71 (LhrAp-61), fused to a promoter-less lacZ gene (for details see [20]).
lhrA transcription was quantified through measurements of specific beta-galactosidase activity in mid-exponential and stationary phase (Fig. 4). No significant differences were recorded between the full and truncated versions of the promoter for either strain, suggesting that there was no binding site for an agr-dependent transcriptional regulator between positions −157 and −61.
Figure 4. Effect of agrD deletion on transcription of lhrA.
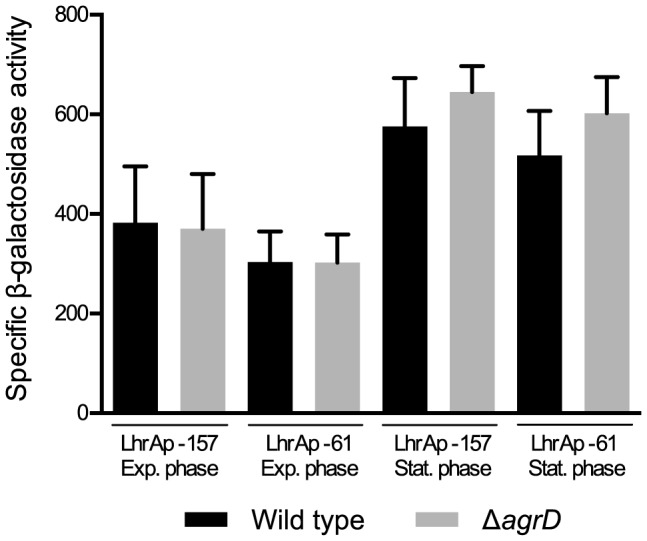
The wild type and agrD mutant containing transcriptional lhrA-lacZ fusions were grown at 30 °C in LB+0.05% glucose supplemented with kanamycin. Samples were collected in mid-exponential and stationary phase and β-galactosidase activity was measured. The results presented here are means of three independent experiments performed in duplicates.
Upon comparison of lhrA transcription between the mutant and the wild-type strains, a small reproducible increase in transcription in the stationary phase was recorded for the mutant, but was not found to be statistically significant. We therefore propose that the regulation of LhrA by agr is exerted mainly at the post-transcriptional level.
The extracellular levels of both ChiA and ChiB are reduced in the ΔagrD mutant
The deletion of agrD gave rise to an almost complete abolishment of chitinolytic activity on chitin agar plates (Fig. 1). However, comparison of the transcripts of chiA and chiB between the wild type and the mutant lacking agrD showed only limited effects of agrD on the levels of chiA (Fig. 2). Therefore, we hypothesized that agrD may have an additional impact on chitinase production at the post-transcriptional level. To confirm this, we carried out western blot analysis to compare the extracellular levels of secreted ChiA and ChiB after overnight incubation.
However, this type of analysis was complicated by the fact that it necessitated the addition of chitin as an inducer of chitinase expression, which, when added, bound part of the secreted chitinases (chitin-bound fraction of Fig. 5A). As this may influence the comparisons, we included both the bound and unbound chitinase fractions in the analysis (Fig. 5A). Although more unhydrolyzed chitin remained in the mutant cultures after the overnight incubation, we found the wild-type and mutant cultures to contain comparable amounts of chitin-bound ChiB, thus suggesting that chitin-binding should not be an important factor in this experimental setting. In support of that, we also found the amount of chitin-bound ChiB to comprise only a small fraction of the total amount of secreted ChiB. Binding of ChiA to chitin should not affect the results either, as we could practically detect no ChiA bound to chitin (Fig. 5A). A potential explanation for this is that ChiA lacks a chitin-binding domain, and thereby presumably has lower binding affinity towards colloidal chitin.
After the overnight incubation, both ChiA and ChiB could be detected unbound in the supernatant fractions in high amounts, but their levels were lower in the ΔagrD mutant compared to the wild-type strain (Fig. 5A). This suggests that agrD ultimately affects production and secretion of both chitinases. This should be the result of post-transcriptional regulation, at least in the case of ChiB, where no transcriptional difference was observed (Fig. 2).
In order to confirm the results with a soluble chitinase inducer we repeated the experiment with GlcNAC, which, in contrast to chitin, induces expression of only chiA and not chiB [18]. In support of the previous experiments, we found the amount of ChiA in the supernatant to be clearly reduced in the mutant lacking agrD (Fig. 5A). As chiB is not induced by GlcNAc, the overall levels of this chitinase in the supernatant were very low, thus hindering the comparison between the wild type and mutant. Nevertheless, there appeared to be a small decrease in the amount of secreted ChiB in the absence of agrD.
Taken together, our results show that deletion of agrD ultimately decreases production of both ChiA and ChiB.
Deletion of chiA affects the amount of secreted ChiB
We have previously shown that although ChiB appears to be the main chitinase involved in colloidal chitin hydrolysis, a ΔchiA mutant strain is also severely impaired in chitin hydrolysis on chitin agar plates [13]. The level of difference appears to be disproportionate to the expected contribution of the deletion of chiA alone. Similarly, in our present study, deletion of agrD greatly affected the extracellular levels of ChiB, although no transcriptional effect was recorded. These two observations raised the question whether the reduced levels of ChiB observed for the agr mutant could be the result of the decreased production of ChiA caused by the agr deletion, rather than a direct result of the deletion itself. To investigate this possibility, we tested a ΔchiA mutant strain for impaired production of ChiB.
ChiB production was compared with the aid of western blotting, carried out as described above; however, only the free, non-chitin-bound protein fraction was analyzed. As can be seen in Fig. 5B, the levels of extracellular ChiB were greatly decreased in the ΔchiA mutant, confirming our assumption that chiA influences production of ChiB. No such effect was observed on the ChiA levels when the wild-type was compared to a ΔchiB mutant (Figure S1A). These observations suggest that, indeed, the reduced levels of ChiB observed for the ΔagrD mutant may be related to the decreased ChiA production in the mutant. However, this should be further confirmed, as our results do not disprove the existence of chiA-independent but agr-dependent post-transcriptional regulation. In addition, the reduced levels of ChiB in the two mutants should be confirmed in an assay using a soluble inducer that does not bind ChiB, instead of colloidal chitin.
Agr is specifically induced by chitin in stationary phase
The observation that the agr system participates in the regulation of the chitinases prompted us to investigate how agr itself responds to chitin induction. To examine that, we looked for changes in the agr transcripts following chitin addition, with the aid of northern blot analysis (Fig. 6).
Figure 6. Northern blot analysis of agrA mRNA in the wild-type EGD-e strain in response to chitin addition.
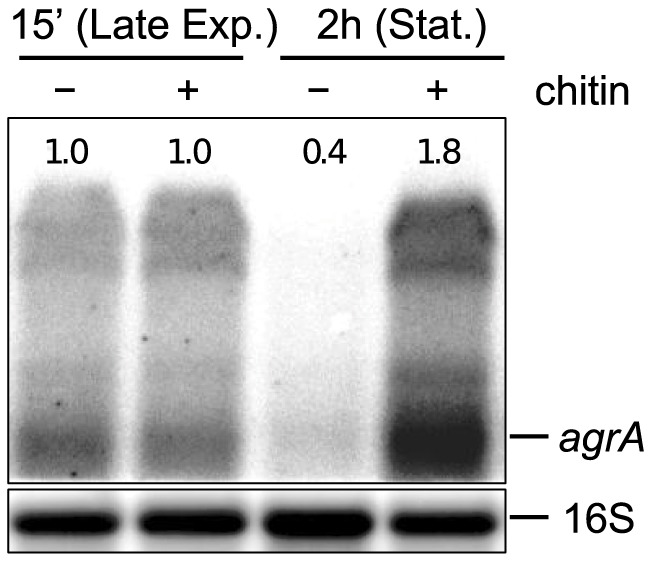
Samples were taken 15-exponential and early stationary phase of growth, respectively. The numbers above the bands correspond to the relative fold change in relation to lane 1, i.e. to the agrA transcript level 15 min after induction in medium without chitin. The loading control, probed for 16S RNA, can be seen below each band. The results presented here were reproduced in three independent experiments.
As a measure of the operon transcription, we quantified the transcripts of agrA 15 min and 2 h after chitin addition, which correspond to late exponential and stationary phase, respectively. Based on previous studies [24], [25], [35], agrA transcripts exist both as part of the whole operon in one long transcript, as well as in smaller fractions, likely resulting from degradation and further processing of the long transcript. In agreement with that, upon probing with the agrA-specific probe we identified two bands, likely corresponding to the mRNA of the whole operon, and the individual agrA transcripts (Fig. 6)
From the northern blot analysis it can be seen that in the absence of chitin agr transcription decreased in stationary phase compared to late exponential. A similar decrease in agr transcription in stationary phase has been reported previously for Clostridium botulinum agrBD1 and agrBD2 [36], and was also recorded for L. monocytogenes in luminescence reporter experiments for cells growing in LB at 37 °C [37]. Nevertheless, it was not noted in other studies investigating agr transcription in BHI at 37 °C [24], [25]. The reasons for this discrepancy are not clear, but may be related to differences in the conditions and the time-points of the measurements.
15 min after chitin addition, in late exponential phase, the levels of agrA appeared to be unchanged compared to the uninduced state. However, a clear upregulation in the presence of chitin was observed 2 h after induction, when the cells had reached stationary phase. A similar induction in stationary phase was observed when beta-chitin, instead of colloidal chitin, was used for the induction (results not shown). These results suggest that agr responds, directly or indirectly, to the presence of chitin in stationary phase, which strengthens the notion that agr is a regulatory component controlling the chitinolytic system of L. monocytogenes.
Discussion
Numerous bacterial organisms are chitinolytic and produce chitinases that aid them in nutrient acquisition, as well as in virulence [4], [8], [12], [16], [38]. However, chitinase production is not constant throughout bacterial growth. Rather, it occurs mostly within a narrow window of nutrient limitation under the presence of an inducer, and is considered to be subject to stringent regulatory controls [5], [18], [39], [40].
In L. monocytogenes the regulatory mechanisms governing chitinase regulation are proving to be extremely complex, and include the central regulators PrfA and σB, as well as the sRNA LhrA [18], [20]. In this study, we identified the agr system of L. monocytogenes as being additionally involved in the regulation of the chitinolytic system. Specifically, we found that deletion of agrD, the presumed precursor of the agr autoinducing peptide [26], [33], dramatically decreased the chitinolytic activity of the bacterium by interfering with the production and secretion of both listerial chitinases.
A moderate effect of the deletion was already seen on the transcript levels of chiA, but not of chiB. The observed reduction in the transcript levels of chiA is in agreement with a previous study by Riedel and colleagues [26], who recorded a similar effect in a microarray setting, albeit under different conditions. However, this effect was not seen in a microarray study by Garmyn and colleagues [29], who studied a mutant lacking agrA. This discrepancy may be a result of the conditions used for the microarray assay, which involved rich medium, 37 °C and no chitinase inducer, i.e. conditions under which chitinase transcription is normally very low [18]. No transcriptional effect was described for chiB in either of the two microarray studies, which is in accordance with our results.
Despite the lack of an effect at the transcript level, we found the extracellular levels of ChiB to also be reduced in the ΔagrD mutant, suggesting a post-transcriptional effect. This may be related to the altered expression of chiA in the mutant, as the deletion of chiA appears to cause a decrease in the production of ChiB. The exact nature of the post-transcriptional effect on chiB remains unknown, but mechanisms such as modulation of translation, protein stability and/or secretion could be involved.
Interestingly, we also found agr itself to be induced upon chitin addition in stationary phase, in a manner similar to that seen for chiA.
In S. aureus, agr-based regulation is mainly mediated through the sRNA RNAIII that acts as an effector for the system [21]. However, in Listeria no sRNA has been identified in connection with the agr system so far. The recent recognition of the sRNA LhrA as a negative regulator of chiA [20] prompted us to investigate whether it could be an intermediate component, mediating, at least partially, the response between agr and chiA. In support of this hypothesis, we found agrD to be a negative regulator of LhrA. This correlates with the specific decrease in the transcripts of chiA, but not chiB, that we saw in the case of ΔagrD mutant, given that LhrA has been found to exert a negative effect on the levels of the chiA mRNA [20]. Interestingly, in agreement with our results, Garmyn et al. [29] found agrA to repress transcription of lmo2257, which is an open reading frame overlapping with the lhrA gene.
Although this finding implicates LhrA in the agr response, LhrA does not appear to be an effector of agr similar to the staphylococcal RNAIII, as, in contrast to RNAIII [21], we found that LhrA is mainly regulated by agrD at the post-transcriptional level. The mechanism of regulation could involve decreased LhrA stability through the action of agr.
The regulatory effect of agr on LhrA implies that at least part of the regulation exerted by agr on chiA is through LhrA. However, due to the large number of genes that are under agr regulation in L. monocytogenes, the regulation of the chitinases via agr is likely mediated at different levels, and extends further than LhrA. For example, PrfA, which is itself a regulator of the chitinases, is deregulated in the ΔagrD mutant background [18], [26].
The implication of the agr system in chitin response is of great interest, as so far relatively little is known about the targets and role of agr in Listeria. Although not directly proven in Listeria, the system has been baptized as ”quorum-sensing” [26], given that agr is a recognized quorum-sensing (QS) system in other bacteria [23]. However, the discovery of environmental cues to which agr responds, such as temperature, has led other authors to propose that agr should not be viewed as a QS system in the strict sense, but should be considered to have pleiotropic effects of relevance to the environmental lifestyle of the bacterium [29], [41]. Our discovery that agr regulates chitinolytic activity further supports this view.
The importance of QS-like systems in non-strictly population-dependent responses has been underscored before, and has led to the formulation of different terminologies and theories regarding the role and evolution of such systems in bacteria [42], [43]. Overall, autoinducing molecules have been proposed to be secreted as proxies that estimate the efficiency of producing extracellular diffusible effectors, by taking into account parameters such as cell density, diffusion limitations and spatial distribution [43]–[45]. According to these models, QS systems then guide the production of enzymes only when autoinducer recovery, as a measure of enzyme recovery, exceeds a certain threshold. Enzymes regulated in this way have been suggested to include bacterial chitinases [46], and, therefore, similar arrangements might also be relevant for Listeria. In soil habitats where Listeria is autochthonous [3], competition for the scarce resources may pressure for the production of energy-costly systems, such as the chitinolytic system, only at opportune moments [6], [46], [47]. Using an autoinducer for the sensing, instead of the chitinase macromolecule, could offer the advantage of being much more economical in production, while only needing to be produced in low numbers, as it can provide a fast response due to the positive autoregulatory feedback [45].
In support of these views, links between QS systems and chitin utilization have been reported in other bacteria as well. In Pseudomonas aeruginosa, the chitinase ChiC and the chitin-binding protein CbpD have been identified as part of the QS regulon [48], [49]. In Chromobacterium violaceum the production of a set of chitinases is positively controlled by the QS endogenous N-hexanoyl-L-homoserine lactone (HHL) signal [50]. In contrast, negative regulation of chitinase activity by the QS system has been reported for Vibrio harveyi [51]. Finally, there is also evidence for QS-based control of chitinase activity in Serratia proteamaculans [52], as well as in Serratia marsescens [53], a model organism for the study of chitinolytic systems. Nevertheless, to our knowledge, regulation of chitinase production by QS systems had only been reported in Gram-negative bacteria so far, whose QS systems vary significantly and structurally from the Gram-positive ones. The discovery that the Gram-positive listerial agr system is also involved in chitin degradation suggests that this mechanism may be more intrinsic and widespread than previously recognized. In this respect, it would be of interest to examine whether homologous agr systems of other pathogenic chitinolytic bacteria, such as clostridia and Enterococcus faecalis [22], carry out similar regulatory functions in the respective organisms.
Although agr may appear as an ideal autoinducer-based sensing system for chitinase induction, our results show that it may only be partly responsible for the induction of the chitinolytic system of L. monocytogenes. Indeed, although the production of the two chitinases was decreased in the agr mutant, both chitinases were still induced and expressed in considerable amounts in the mutant background. Therefore, other factors that promote chitinase induction in Listeria do exist. This is not surprising, given the complexity of the chitinase regulatory system, as well as the fact that chitinase induction is subject to more than one parameter, each of which is likely represented by a different input. Namely, a number of conditions, including chitin availability, as well as the absence of easily fermentable sugars, should all be simultaneously met in order for the chitinases to be fully induced [5], [18] One such condition, monitored by agr, could be the retributability/efficiency of macromolecule production.
An open question is whether agr is additionally involved in the regulation of the listerial chitinolytic system during infection. The fact that agr specifically targets chiA opens up this possibility, as chiA has been shown to be a virulence factor that downregulates iNOS expression in the host [17]. Since the regulatory pathway behind the induction of chiA during infection remains elusive, it would be of interest to examine whether it involves agr, especially as agr itself has previously been found to be important for pathogenicity [26].
Supporting Information
A. Western blot analysis of culture supernatants using an anti-ChiA antibody. The specificity of the anti-ChiA antibody was confirmed by comparison of wild-type and ΔchiA cultures grown at 30 °C in LB+0.05% glucose supplemented with colloidal chitin. Comparison of the wild type to a ΔchiB mutant revealed no substantial differences in the production of ChiA. B. Western blot analysis of culture supernatants using an anti-ChiB antibody. The specificity of the anti-ChiB antibody was confirmed by comparison of wild-type and ΔchiB cultures grown at 30°C in LB+0.05% glucose supplemented with colloidal chitin.
(PDF)
Acknowledgments
We would like to thank Ewa Maria Kuninska and Christel Galschiøt Buerholt for excellent technical assistance, Anita Nielsen for designing the agrA probe for the northern blot analysis, and Rikki Franklin Frederiksen for critical reading of the manuscript.
Funding Statement
This work was supported by grants from The Danish Council for Independent Research - Technology and Production Sciences (09-067081), the Danish AgriFish Agency (3304-FVFP-09-F-013-1), the Danish Dairy foundation and The Danish Council for Independent Research – Natural Sciences (12-1247735, to B.H.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bou-m'handi N, Jacquet C, El Marrakchi A, Martin P (2007) Phenotypic and molecular characterization of Listeria monocytogenes strains isolated from a marine environment in Morocco. Foodborne Pathog Dis 4: 409–417. [DOI] [PubMed] [Google Scholar]
- 2. Locatelli A, Depret G, Jolivet C, Henry S, Dequiedt S, et al. (2013) Nation-wide study of the occurrence of Listeria monocytogenes in French soils using culture-based and molecular detection methods. J Microbiol Methods 93: 242–250. [DOI] [PubMed] [Google Scholar]
- 3. Freitag NE, Port GC, Miner MD (2009) Listeria monocytogenes - from saprophyte to intracellular pathogen. Nat Rev Microbiol 7: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gooday GW (1990) The ecology of chitin degradation. Adv Microb Ecol 11: 387–430. [Google Scholar]
- 5. Keyhani NO, Roseman S (1999) Physiological aspects of chitin catabolism in marine bacteria. Biochim Biophys Acta 1473: 108–122. [DOI] [PubMed] [Google Scholar]
- 6. Aldén L, Demoling F, Bååth E (2001) Rapid method of determining factors limiting bacterial growth in soil. Appl Environ Microbiol 67: 1830–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aluwihare LI, Repeta DJ, Pantoja S, Johnson CG (2005) Two chemically distinct pools of organic nitrogen accumulate in the ocean. Science 308: 1007–1010. [DOI] [PubMed] [Google Scholar]
- 8. Beier S, Bertilsson S (2013) Bacterial chitin degradation-mechanisms and ecophysiological strategies. Front Microbiol 4: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gooday GW (1990) Physiology of microbial degradation of chitin and chitosan. Biodegradation 1: 177–190. [Google Scholar]
- 10. Vaaje-Kolstad G, Westereng B, Horn SJ, Liu Z, Zhai H, et al. (2010) An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330: 219–222. [DOI] [PubMed] [Google Scholar]
- 11. Aachmann FL, Sørlie M, Skjåk-Bræk G, Eijsink VGH, Vaaje-Kolstad G (2012) NMR structure of a lytic polysaccharide monooxygenase provides insight into copper binding, protein dynamics, and substrate interactions. Proc Natl Acad Sci U S A 109: 18779–18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frederiksen RF, Paspaliari DK, Larsen T, Storgaard BG, Larsen MH, et al. (2013) Bacterial chitinases and chitin-binding proteins as virulence factors. Microbiology 159: 833–847. [DOI] [PubMed] [Google Scholar]
- 13. Leisner JJ, Larsen MH, Jørgensen RL, Brøndsted L, Thomsen LE, et al. (2008) Chitin hydrolysis by Listeria spp., including L. monocytogenes . Appl Environ Microbiol 74: 3823–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leisner JJ, Larsen MH, Ingmer H, Petersen BO, Duus JØ, et al. (2009) Cloning and comparison of phylogenetically related chitinases from Listeria monocytogenes EGD and Enterococcus faecalis V583. J Appl Microbiol 107: 2080–2087. [DOI] [PubMed] [Google Scholar]
- 15. Piveteau P, Depret G, Pivato B, Garmyn D, Hartmann A (2011) Changes in gene expression during adaptation of Listeria monocytogenes to the soil environment. PLoS One 6: e24881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaudhuri S, Bruno JC, Alonzo F, Xayarath B, Cianciotto NP, et al. (2010) Contribution of chitinases to Listeria monocytogenes pathogenesis. Appl Environ Microbiol 76: 7302–7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaudhuri S, Gantner BN, Ye RD, Cianciotto NP, Freitag NE (2013) The Listeria monocytogenes ChiA chitinase enhances virulence through suppression of host innate immunity. MBio 4: e00617–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larsen MH, Leisner JJ, Ingmer H (2010) The chitinolytic activity of Listeria monocytogenes EGD is regulated by carbohydrates but also by the virulence regulator PrfA. Appl Environ Microbiol 76: 6470–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Görke B, Stülke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6: 613–624. [DOI] [PubMed] [Google Scholar]
- 20. Nielsen JS, Larsen MH, Lillebæk EMS, Bergholz TM, Christiansen MHG, et al. (2011) A small RNA controls expression of the chitinase ChiA in Listeria monocytogenes . PLoS One 6: e19019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Novick RP, Geisinger E (2008) Quorum sensing in staphylococci. Annu Rev Genet 42: 541–564. [DOI] [PubMed] [Google Scholar]
- 22. Wuster A, Babu MM (2008) Conservation and evolutionary dynamics of the agr cell-to-cell communication system across firmicutes. J Bacteriol 190: 743–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gray B, Hall P, Gresham H (2013) Targeting agr- and agr-like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections. Sensors 13: 5130–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Autret N, Raynaud C, Dubail I, Berche P, Charbit A (2003) Identification of the agr locus of Listeria monocytogenes: Role in bacterial virulence. Infect Immun 71: 4463–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rieu A, Weidmann S, Garmyn D, Piveteau P, Guzzo J (2007) agr system of Listeria monocytogenes EGD-e: role in adherence and differential expression pattern. Appl Environ Microbiol 73: 6125–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riedel CU, Monk IR, Casey PG, Waidmann MS, Gahan CGM, et al. (2009) AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes . Mol Microbiol 71: 1177–1189. [DOI] [PubMed] [Google Scholar]
- 27. Rieu A, Briandet R, Habimana O, Garmyn D, Guzzo J, et al. (2008) Listeria monocytogenes EGD-e biofilms: no mushrooms but a network of knitted chains. Appl Environ Microbiol 74: 4491–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schauer K, Geginat G, Liang C, Goebel W, Dandekar T, et al. (2010) Deciphering the intracellular metabolism of Listeria monocytogenes by mutant screening and modelling. BMC Genomics 11: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garmyn D, Augagneur Y, Gal L, Vivant A-L, Piveteau P (2012) Listeria monocytogenes differential transcriptome analysis reveals temperature-dependent Agr regulation and suggests overlaps with other regulons. PLoS One 7: e43154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghupathi PK, Paulander W, Paspaliari DK, Larsen MH (2013) The chitinases of Listeria monocytogenes contribute to colonisation of Caenorhabditis elegans. Submitted manuscript.
- 31. Park SF, Stewart GS (1990) High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94: 129–132. [DOI] [PubMed] [Google Scholar]
- 32. Christiansen JK, Larsen MH, Ingmer H, Søgaard-Andersen L, Kallipolitis BH (2004) The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence. J Bacteriol 186: 3355–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garmyn D, Gal L, Lemaître J-P, Hartmann A, Piveteau P (2009) Communication and autoinduction in the species Listeria monocytogenes: A central role for the agr system. Commun Integr Biol 2: 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nielsen JS, Lei LK, Ebersbach T, Olsen AS, Klitgaard JK, et al. (2010) Defining a role for Hfq in Gram-positive bacteria: evidence for Hfq-dependent antisense regulation in Listeria monocytogenes . Nucleic Acids Res 38: 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, et al. (2009) The Listeria transcriptional landscape from saprophytism to virulence. Nature 459: 950–956. [DOI] [PubMed] [Google Scholar]
- 36. Cooksley CM, Davis IJ, Winzer K, Chan WC, Peck MW, et al. (2010) Regulation of neurotoxin production and sporulation by a putative agrBD signaling system in proteolytic Clostridium botulinum . Appl Environ Microbiol 76: 4448–4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waidmann MS (2012) The Listeria monocytogenes agr system: Autoregulation and role in virulence. PhD thesis, University of Ulm. Available: vts.uni-ulm.de/docs/2012/8122/vts_8122_11850.pdf. Accessed 19 December 2013.
- 38. DebRoy S, Dao J, Söderberg M, Rossier O, Cianciotto NP (2006) Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc Natl Acad Sci U S A 103: 19146–19151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suzuki K, Suzuki M, Taiyoji M, Nikaidou N, Watanabe T (1998) Chitin binding protein (CBP21) in the culture supernatant of Serratia marcescens 2170. Biosci Biotechnol Biochem 62: 128–135. [DOI] [PubMed] [Google Scholar]
- 40. Vaaje-Kolstad G, Bøhle LA, Gåseidnes S, Dalhus B, Bjørås M, et al. (2012) Characterization of the chitinolytic machinery of Enterococcus faecalis V583 and high-resolution structure of its oxidative CBM33 enzyme. J Mol Biol 416: 239–254. [DOI] [PubMed] [Google Scholar]
- 41. Garmyn D, Gal L, Briandet R, Guilbaud M, Lemaître J-P, et al. (2011) Evidence of autoinduction heterogeneity via expression of the Agr system of Listeria monocytogenes at the single-cell level. Appl Environ Microbiol 77: 6286–6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Platt TG, Fuqua C (2010) What's in a name? The semantics of quorum sensing. Trends Microbiol 18: 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. West SA, Winzer K, Gardner A, Diggle SP (2012) Quorum sensing and the confusion about diffusion. Trends Microbiol 20: 586–594. [DOI] [PubMed] [Google Scholar]
- 44. Redfield RJ (2002) Is quorum sensing a side effect of diffusion sensing? Trends Microbiol 10: 365–370. [DOI] [PubMed] [Google Scholar]
- 45. Hense BA, Kuttler C, Müller J, Rothballer M, Hartmann A, et al. (2007) Does efficiency sensing unify diffusion and quorum sensing? Nat Rev Microbiol 5: 230–239. [DOI] [PubMed] [Google Scholar]
- 46. DeAngelis KM, Lindow SE, Firestone MK (2008) Bacterial quorum sensing and nitrogen cycling in rhizosphere soil. FEMS Microbiol Ecol 66: 197–207. [DOI] [PubMed] [Google Scholar]
- 47. Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37: 937–944. [Google Scholar]
- 48. Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, et al. (2003) Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22: 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nouwens AS, Beatson SA, Whitchurch CB, Walsh BJ, Schweizer HP, et al. (2003) Proteome analysis of extracellular proteins regulated by the las and rhl quorum sensing systems in Pseudomonas aeruginosa PAO1. Microbiology 149: 1311–1322. [DOI] [PubMed] [Google Scholar]
- 50. Chernin LS, Winson MK, Thompson JM, Haran S, Bycroft BW, et al. (1998) Chitinolytic activity in Chromobacterium violaceum: substrate analysis and regulation by quorum sensing. J Bacteriol 180: 4435–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Defoirdt T, Darshanee Ruwandeepika HA, Karunasagar I, Boon N, Bossier P (2010) Quorum sensing negatively regulates chitinase in Vibrio harveyi . Environ Microbiol Rep 2: 44–49. [DOI] [PubMed] [Google Scholar]
- 52. Christensen AB, Riedel K, Eberl L, Flodgaard LR, Molin S, et al. (2003) Quorum-sensing-directed protein expression in Serratia proteamaculans B5a. Microbiology 149: 471–483. [DOI] [PubMed] [Google Scholar]
- 53. Coulthurst SJ, Williamson NR, Harris AKP, Spring DR, Salmond GPC (2006) Metabolic and regulatory engineering of Serratia marcescens: mimicking phage-mediated horizontal acquisition of antibiotic biosynthesis and quorum-sensing capacities. Microbiology 152: 1899–1911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Western blot analysis of culture supernatants using an anti-ChiA antibody. The specificity of the anti-ChiA antibody was confirmed by comparison of wild-type and ΔchiA cultures grown at 30 °C in LB+0.05% glucose supplemented with colloidal chitin. Comparison of the wild type to a ΔchiB mutant revealed no substantial differences in the production of ChiA. B. Western blot analysis of culture supernatants using an anti-ChiB antibody. The specificity of the anti-ChiB antibody was confirmed by comparison of wild-type and ΔchiB cultures grown at 30°C in LB+0.05% glucose supplemented with colloidal chitin.
(PDF)


