Abstract
Dengue occurs throughout the year in Hanoi, Vietnam, despite winter low temperatures <10°C. During July 2010 to March 2012, we surveyed monthly for Aedes larvae and pupae in 120 houses in 8 Hanoi districts. Aedes albopictus preferred discarded containers in summer and pupal density drastically decreased in winter. Aedes aegypti preferred concrete tanks and this preference increased in winter. Even in winter, the lowest water temperature found in concrete tanks was >14°C, exceeding the developmental zero point of Ae. aegypti. Although jars, drums and concrete tanks were the dominant containers previously (1994–97) in Hanoi, currently the percentage of residences with concrete tanks was still high while jars and drums were quite low. Our study showed that concrete tanks with broken lids allowing mosquitoes access were important winter refuge for Ae. aegypti. We also indicate a concern about concrete tanks serving as foci for Ae. aegypti to expand their distribution in cooler regions.
Introduction
Dengue fever and dengue haemorrhagic fever are very important viral diseases, with an estimated 50 million infections every year and around 3.6 billion people living in areas at risk [1]. Dengue virus is transmitted by Aedes aegypti (L.) and in Southeast Asia to a lesser extent also by Aedes albopictus (Skuse) (Diptera: Culicidae) [2]. In Hanoi northern Vietnam, there were two major outbreaks in 1998 and 2009 and dengue incidence has been increasing yearly since 1999 [3]. Transmission peaks in late summer; however, many dengue cases occur in Hanoi every winter [4], [5].
Although Hanoi has a sub-tropical climate, winters are cool, with minimum temperatures around 10°C [6]. Both Ae. aegypti and Ae. albopictus are found in Hanoi [3]. In Hanoi, these species may experience seasonal constraints in activity due to cold temperature, as the developmental zero point of Ae. aegypti is 13.3°C [7] and that of Ae. albopictus is 12.0°C [8].
Mosquitoes in temperate zones have efficient overwintering mechanisms and hibernation in the egg stage is practiced by most Aedes species [9]. In short day-length conditions, Ae. albopictus collected from East Asia (Shanghai, Beijing, Japan and Korea) enters into diapause [10]. Though Ae. albopictus from Southeast Asia (Taipei, Hong Kong, Thailand, and Malaysia) does not enter into diapause in short day conditions [10], little is known about overwintering of Ae. albopictus in Vietnam.
Within the urban environment in many developing countries, densely packed housing and inadequate drinking-water supplies, poor garbage collection services, and surface-water drainage systems combine to create favorable habitats for the proliferation of insect vectors and reservoirs of communicable diseases [11]. In Hanoi, urbanization has been proceeding rapidly and infrastructure has been developing. During a 1994–1997 survey in Hanoi, the major habitat for Aedes immatures were ceramic jars, drums, and concrete tanks [12]. However, in the 15 years since this survey, the situation may have changed, and urbanization may change Aedes habitat and behavior.
Overall in southeast China, the winter minimum and maximum temperatures have been increasing as has diurnal temperature range. Where rapid urbanization has occurred, land-use changes from urbanization have created urban heat islands [13]. Vietnam has also experienced dramatic economic growth since the Doi Moi Policy started in 1986, also contributing to rapid urbanization in Hanoi. In Hanoi, warming via urbanization may result in greater mosquito survival during winter. However, little is known about winter activity of dengue vectors, as there is little transmission then. Our purpose was to investigate the seasonal activity of Ae. aegypti and Ae.albopictus in Hanoi, especially in winter.
Materials and Methods
Study Area
The study area included eight districts (Hoan Kiem, Dong Da, Hai Ba Trung, Hoang Mai, Thanh Xuan, Thanh Tri, Tu Liem, and Ha Dong) in Hanoi City, Vietnam. These districts had many dengue cases in 2008 and 2009 [4], [5]. We selected 15 houses randomly in each district, a total of 120 houses monthly.
Entomological Survey
All indoor and outdoor containers were surveyed to confirm the presence of Aedes immatures. Pupae were brought to the laboratory and reared to adults for species identification. Buckets, drums, jars, concrete tanks, toilet concrete tanks, and wells were sampled by sweeping them five times with a small net. Bonsai, trees grown in containers, plant saucers, and aquarium without fish were included in others. The number of pupae was estimated from mean interval percentage recovery [14], and small containers were emptied to collect pupae. All water-holding containers, both indoors and outdoors, were inspected only after obtaining the necessary permission from the occupants.
Temperature Measurement
Our preliminary survey showed that residents seldom use ceramic jars and drums for storing water and concrete tanks are preferred. January is the coldest month in Hanoi [6]. We measured water temperatures in seven concrete tanks and one metal tank by data loggers (HOBO Pendant Temperature/Light Data Logger 8K-UA-002-08, Onset, MA, USA) in January 2011 and January 2012 (Fig. 1). The concrete tanks in Hoang Mai in 2012 and Tu Liem in 2011 and 2012 were outdoors and set in the ground. The other concrete tanks were in basements (Dong Da, Hai Ba Trung, Hoang Mai in 2011, Thanh Xuan, Thanh Tri, and Ha Dong). A metal tank in Hoan Kiem was under the roof of a house in 2011 and on the roof of another in 2012. We also measured room temperature in health centers of Hoang Mai in January 2011 and 2012 and Ha Dong in January 2011 using the same data logger. The room temperature of Ha Dong in January 2012 was measured by a thermo recorder (TR-72U, T&D Corporation, Matsumoto City, Japan).
Figure 1. Typical water tanks in Hanoi.

A) Roof-top metal tank. B) Underground concrete tank. C) Above-ground concrete tank.
We also obtained the outside air temperature data measured in Dong Da district from January 2010 to October 2012 [15].
Statistical Analysis
The lowest temperature and January mean temperature data in 2011 and 2012 were subjected to analysis of variance (ANOVA) to compare the values of different medium and location. When the F-test was significant (p<0.05), the treatment means were compared using Tukey’s significantly different test. To examine which type of container was used by mosquitoes in summer and winter, habitat preference was analyzed by G-test. Similar types of containers were grouped if expected frequencies were <3. Multiple correspondence analysis was conducted to analyze the relationship between species (Ae. aegypti and Ae. albopictus), season (summer and winter) and container type. Mutiple correspondence analysis is a graphic technique that shows each category as a point in a type of scatter plot. The positions of the category-points on the graph mean similarity or association between categories. All data were analyzed using R (version 2.6.2) software.
Results
The lowest Hanoi outside air temperature in January was 9.0°C in 2011 and 8.0°C in 2012 [15]. The mean outside air temperatures were lower than room temperatures, metal tank, and the concrete tank temperatures (Table 1). The mean concrete tank water temperature was higher than other temperatures in 2011 and 2012. The difference was not significant between the mean of outside air temperature and metal tank water temperature. However, outside air temperature and room temperature were significantly different. The mean outside air temperature was lowest in January 2011 and 2012, though it was not significantly different from means of the room temperature and metal tank water temperature (Table 2). Concrete tanks were warmer in January 2011 and 2012 compared to outside and room temperatures.
Table 1. The lowest temperature in 2011 and 2012.
| Medium | Place | n | Temperature (°C) (mean±SD)5) |
| Air | Outside1) | 2 | 8.5±0.7 a |
| Room2) | 4 | 13.4±1.0 b | |
| Water | Metal tank3) | 2 | 11.3±1.2 ab |
| Concrete tank4) | 14 | 18.2±1.9 c |
National Hydro-Meteorological Service (2012).
Room temperature in public health centers of Hoang Mai and Ha Dong districts.
Metal tank set in Hoan Kiem district.
Concrete tanks set in seven districts (Dong Da, Hai Ba Trung, Hoang Mai, Thanh Xuan, Thanh Tri, Tu Liem, and Ha Dong).
Different letters following means in a row indicate significant difference at p<0.05 by Tukey’s test.
Table 2. January average temperature in 2011 and 2012.
| Medium | Place | n | Temperature (°C) (mean±SD)5) |
| Air | Outside1) | 2 | 13.7±1.3 a |
| Room2) | 4 | 16.2±1.2 a | |
| Water | Metal tank3) | 2 | 17.3±1.6 ab |
| Concrete tank4) | 14 | 20.0±1.7b |
National Hydro-Meteorological Service (2012).
Room temperature of Public health centers in Hoang Mai and Ha Dong.
Metal tank set in Hoan Kiem.
Concrete tanks set in seven districts (Dong Da, Hai Ba Trung, Hoang Mai, Thanh Xuan, Thanh Tri, Tu Liem, and Ha Dong).
Different letters following means in a row indicate significant difference at p<0.05 by Tukey’s test.
Peaks of Ae. aegypti pupation were seen in October and December, 2010, and in May and October, 2011 (Fig. 2). Pupation of Ae. albopictus peaked only twice October 2010 and May 2011. Compared to Ae. albopictus, the density of Ae. aegypti was higher during winter.
Figure 2. Seasonal occurrence of Ae. aegypti and Ae. albopictus in Hanoi from July 2010 to March 2012.
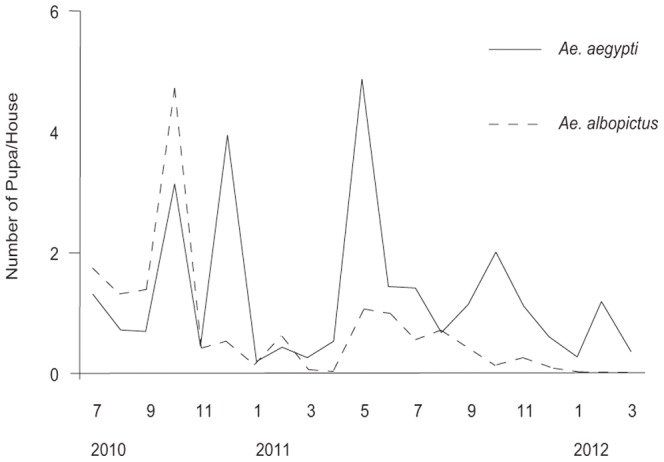
Error bars indicate standard error.
Concrete tanks, flower vases, and discarded containers were abundant both in summer and winter (Table 3). Discarded containers were most abundant (31.4% of all habitat types) in winter, and 368 discarded bottles were examined in one house in March 2011. Aedes aegypti preferred concrete tanks through the year. Forty-five percent of all Ae. aegypti were collected from concrete tanks in summer, and 71% of all Ae. aegypti were from concrete tanks in winter. For Ae. aegypti, the frequency of preferred containers was significantly different between summer and winter (G = 292.68 d.f. = 5, P<0.001). Habitat preference was significantly different between Ae. aegypti and Ae. albopictus in summer (G = 654.63, d.f. = 5, P<0.001) and in winter (G = 81.84, d.f. = 5, P<0.001). Although most Ae. albopictus were collected from discarded containers, the frequency of preferred containers in summer was not concentrated on a particular container, as was the case for concrete tanks in Ae. aegypti. However, in the winter, most Ae. albopictus were collected from concrete tanks (41%) with 38% in other containers. There was a significant difference in habitat preference between summer and winter for Ae. albopictus (G = 124.37, d.f. = 5, P<0.001).
Table 3. Relative percentages of containers (number of containers), Ae. aegypti and Ae. albopictus collected from containers from July to October and from December to March in 2010 and 2011.
| Container type1) | July – October | December – March | ||||
| container | aegypti | albopictus | container | aegypti | albopictus | |
| (n = 2,280) | (n = 1,438) | (n = 1,302) | (n = 2,173) | (n = 992) | (n = 175) | |
| Bucket | 17.4(397) | 27.6(397) | 10.5(137) | 9.5(206) | 5.4 (53) | 0 (0) |
| Drum | 1.0 (22) | 0.5 (7) | 0.3 (3) | 0.6 (14) | 0 (0) | 3.8 (7) |
| Flower vase | 19.2(437) | 2.1 (30) | 3.3 (43) | 21.2(460) | 1.1 (11) | 1.7 (3) |
| Jar | 3.6 (82) | 9.0 (130) | 17.4(227) | 3.3 (72) | 4.0 (40) | 5.7 (10) |
| Concrete tank | 24.4(556) | 44.7(643) | 15.4(200) | 20.8(453) | 70.6(700) | 40.8(71) |
| TCT2) | 0.2 (5) | 0.3 (4) | 0 (0) | 0.3 (6) | 0 (0) | 0 (0) |
| Tire | 0.8 (19) | 0.1 (1) | 2.8 (37) | 0.2 (4) | 0 (0) | 0 (0) |
| Discarded | 18.3(417) | 5.7 (82) | 30.0(390) | 31.4(683) | 2.2 (22) | 9.7 (17) |
| Well | 1.5 (34) | 0.7 (10) | 1.0 (13) | 1.3 (29) | 0.7 (7) | 0 (0) |
| Others3) | 13.6(311) | 9.4(135) | 19.4(252) | 11.3(246) | 16.0(159) | 38.3 (67) |
Values are grouped as follows so that resulting expected frequencies are large enough; Bucket+Drum, Concrete tank+Toilet concrete tank, Tire+Discarded.
Toilet concrete tank.
Others include Bonsai, plant saucer, and aquarium.
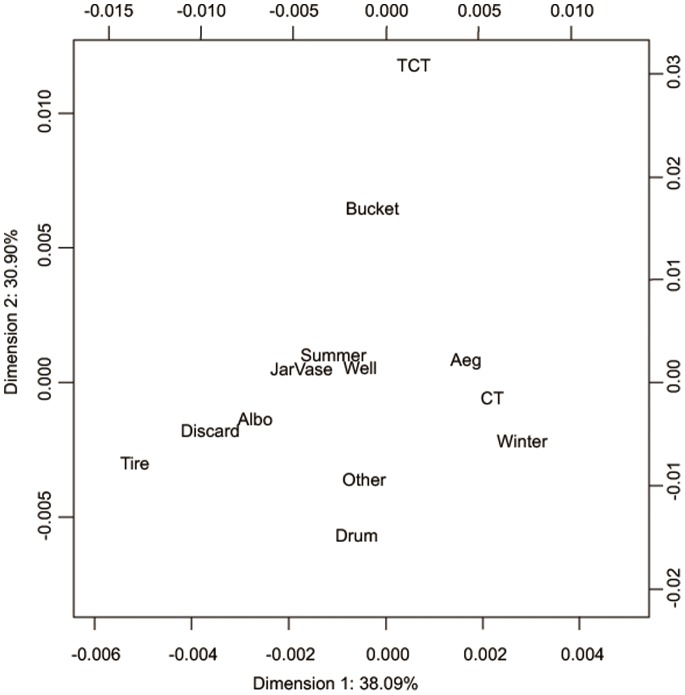
Multiple correspondence analysis enabled 68.99% of total inertia to be explained by two dimensions, with the first accounting for 38.09% and the second 30.90% (Fig. 3). The analysis showed that Ae. albopictus was closely related to discarded containers. Meanwhile, concrete tanks were related to both Ae. aegypti and winter.
Figure 3. Multiple correspondence analysis of mosquito species, season, and container type.
Mosquito species (Ae. aegypti and Ae. albopictus), season (summer and winter), and container type (bucket, concrete tank, discarded container, drum, jar, other containers, tire, toilet concrete tank, and flower vase). Mos. aeg; Ae. aegypti, Mos. albo; Ae. albopictus, C.; Container, CT; concrete tank, Discard; discarded container, Other; other containers, S; Summer, TCT; toilet concrete tank, W; Winter.
Discussion
Cold temperatures may act as a strong ecological constraint over possible range expansions of Ae. aegypti and Ae. albopictus [16]. Certain populations of Ae. albopictus may extend their summer range considerably north of the 10°C cold month isotherms, but they are not able to survive during cold winter months, which prevents the establishment of permanent populations [9]. Climatic variability and extreme temperatures will have more important effects on reproductive success and population dynamics than winter warming [17].
It may be presumed that Ae. aegypti and Ae. albopictus use different strategies to survive in winter in Hanoi. Aedes aegypti does not enter into diapause [18]. Our results show that Ae. aegypti continued to develop throughout the winter in containers that kept water warm, as the development zero point of Ae. aegypti is 12–13°C [7], [19], [20]. However, it is hard for Ae. aegypti adults to fly and search for a food in winter, because they cannot fly below 17°C [18]. Winter can impose a severe physiological stress on insects, and overwintering sites that are above the ground are generally colder and experience more variable temperature regimes than those underground [21]. Winter severity varies both temporally and spatially and many insects are adapted to take advantage of the milder conditions that can occur [21]. In our study, the average temperature of a room in January was below 17°C in Hanoi, with outside temperatures much lower. As a consequence, the time and space for Ae. aegypti adults to fly and feed might be limited in winter.
Aedes albopictus originating from Taipei, Hong Kong, and Thailand do not enter into diapause [10], which implies that Ae. albopictus from Hanoi may also be a non-diapause race. However, there were fewer Ae. albopictus in winter in Hanoi, compared with Ae. aegypti. Eggs of Ae. albopictus collected from Hanoi delayed hatching under short day length conditions [Tsunoda et al., unpublished data]. These results suggest that most Ae. albopictus enter into diapause and others continue to grow, an apparent bed-hedging strategy [22].
Migration may be an alternative strategy to escape from severe winters. In the Shanghai area of China, diapausing adults of Culex tritaeniorhynchus are blown southward for at least 35 km, possibly as far as 200 km, in autumn [23]. The maximum flight distance of Ae. aegypti is usually 100–200 m [24]–[27], although, in some particular situations, this species can fly distances >400–600 m [28], [29]. The maximum flight distance of Ae. albopictus is longer than that of Ae. aegypti, but at most 100–1,000 m [30], [31]. Therefore, both Ae. aegypti and Ae. albopictus might stay near their natal habitat in winter.
Our results show that Ae. albopictus preferred discarded containers mostly in summer but not in winter. It is likely that water temperature in a bottle and other discarded outside containers fluctuates with outside air temperature. It seems to be difficult for Ae. albopictus to grow in discarded containers in winter, because the average outside temperature in January is close to its developmental zero point, 11–12°C [8], [19].
Although Ae. aegypti is highly endophilic, Ae. albopictus is exophilic in Thailand [32], which suggests for habitat segregation between the species. However, as room air was warmer than outside air in winter, it will be advantageous for Ae. albopictus adults to fly into and take a blood meal in a house. We should point out that the house-entering behavior of Ae. albopictus may be influenced by the presence of Ae. aegypti.
Over the last 15 years, the distribution of containers for Aedes mosquitoes has changed in Hanoi. The entomological survey conducted in 1994–1997 showed that concrete tanks (38.9% in total containers), jars (30.2%), and drums (26.0%) were abundant [12]. However, in our entomological survey, the relative percentages of jars (3.3–3.6%) and drums (0.6–1.0%) were low, though concrete tanks were still high (20.8–24.4%). The reason why residents do not currently use jars and drums is attributed to the increasing popularity of tap water over the last 15 years. Residents usually keep tap water in an underground or above-ground concrete tank. The water is automatically pumped up to a rooftop tank, since tap water pressure is very weak. As a result, people retain existing tanks when they remodel a home or build a new one if a new home is constructed. Since some concrete tanks may be sealed with putty or covered by plastic sheeting and are difficult to detect, our estimate of the number of tanks may be low.
Our study showed that concrete tanks are important habitat for overwinter-survival of Ae. aegypti in Hanoi. Here, as well as potentially in other countries, if such domestic water systems are encouraged, these concrete tanks could serve as foci for range expansion of Ae. aegypti in cooler regions.
Acknowledgments
We appreciate for the assistance of local guides, District health center staff, and staff of the Hanoi Preventive Medicine Center. We also thank for residents for permitting installation of HOBO units to measure water temperature in concrete tanks.
Funding Statement
This work was funded by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) granted by Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Beatty M, Beutels P, Meltzer M, Shepard D, Hombach J (2010) Health economics of Dengue: A systematic literature review and expert panel's assessment. American Journal of Tropical Medicine and Hygiene 84: 473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodhain F, Rosen L (1997) Mosquito vectors and dengue virus-vector relationships. In: Gubler D, Kuno G, editors. Dengue and Dengue Haemorrhagic Fever. Wallingford: CAB International. 45–60.
- 3. Cuong HQ, Hien NT, Duong TN, Phong TV, Cam NN, et al. (2011) Quantifying the emergence of dengue in Hanoi, Vietnam: 1998–2009. PLoS Negl Trop Dis 5: e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health (2009) Analysis of communicable disease data in Vietnam 2008. Hanoi, Vietnam: National Institute of Hygiene and Epidemiology.
- 5.Ministry of Health (2010) Analysis of communicable disease data in Vietnam 2009. Hanoi, Vietnam: National Institute of Hygiene and Epidemiology.
- 6.Weatherbase (2012) Hanoi, Vietnam Travel Weather Averages (Weatherbase). Canty and Associates LLC.
- 7. Bar-Zeev M (1958) The effect of temperature on the growth rate and survival of the immature stages of Aedes aegypti (L.). Bulletin of Entomological Research 49: 157–163. [Google Scholar]
- 8.Hawley W (1988) The biology of Aedes albopictus. Journal of American Mosquito Control Association Supplement 1: 1–40. [PubMed]
- 9.Becker N, Petric D, Zgomba M, Boase C, Madon M, et al.. (2010) Mosquitoes and Their Control. Heidelberg: Springer. 577 p. [Google Scholar]
- 10. Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB Jr (1987) Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science 236: 1114–1116. [DOI] [PubMed] [Google Scholar]
- 11. Knudsen AB, Slooff R (1992) Vector-borne disease problems in rapid urbanization: new approaches to vector control. Bull World Health Organ 70: 1–6. [PMC free article] [PubMed] [Google Scholar]
- 12. Phong TV, Nam VS (1999) Key breeding sites of dengue vectors in Hanoi, Vietnam, 1994–1997. Dengue Bull 23: 67–72. [Google Scholar]
- 13. Zhou L, Dickinson RE, Tian Y, Fang J, Li Q, et al. (2004) Evidence for a significant urbanization effect on climate in China. Proc Natl Acad Sci U S A 101: 9540–9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knox TB, Yen NT, Nam VS, Gatton ML, Kay BH, et al. (2007) Critical evaluation of quantitative sampling methods for Aedes aegypti (Diptera: Culicidae) immatures in water storage containers in Vietnam. J Med Entomol 44: 192–204. [DOI] [PubMed] [Google Scholar]
- 15.National Hydro-Meteorological Service (2012) Meterological Data (2010–2012). Hanoi: the National Centre for Hydro-Meterological Forcasting.
- 16. Hanson SM, Craig GB Jr (1995) Aedes albopictus (Diptera: Culicidae) eggs: field survivorship during northern Indiana winters. J Med Entomol 32: 599–604. [DOI] [PubMed] [Google Scholar]
- 17. Bale JS, Hayward SA (2010) Insect overwintering in a changing climate. J Exp Biol 213: 980–994. [DOI] [PubMed] [Google Scholar]
- 18.Christophers SR (1960) Aedes aegypti (L.) The yellow fever mosquito. Its life history, bionomics and structure Chambridge: Cambridge at the University Press. 739 p. [Google Scholar]
- 19. Chen CS, Huang CC (1988) Ecological studies on Aedes aegypti and Ae.albopictus. I. Comparison of development threshold and life tables. Yushania 5: 1–15. [Google Scholar]
- 20. Ofuji K (1963) Possibility of establishment of yellow fever mosquito, Aedes aegypti (L.) in Japan. II. Cold- and dry-resistance of eggs, ecological zero point of larvae, development of larvae in early spring, and general summary. Endemic Dis Bull Nagasaki Univ 5: 209–222. [Google Scholar]
- 21.Leather SR, Walters KFA, Bale JS (1995) The ecology of insect overwintering. Cambridge: Cambridge University Press.
- 22. Khatchikian CE, Dennehy JJ, Vitek CJ, Livdahl TP (2010) Environmental effects on bed hedging in Aedes mosquito egg hatch. Evol Ecol 24: 1159–1169. [Google Scholar]
- 23. Ming JG, Jin H, Riley JR, Reynolds DR, Smith AD, et al. (1993) Autumn southward ‘return’ migration of the mosquito Culex tritaeniorhynchus in China. Med Vet Entomol 7: 323–327. [DOI] [PubMed] [Google Scholar]
- 24. Muir LE, Kay BH (1998) Aedes aegypti survival and dispersal estimated by mark-release-recapture in northern Australia. Am J Trop Med Hyg 58: 277–282. [DOI] [PubMed] [Google Scholar]
- 25. Reiter P, Amador MA, Anderson RA, Clark GG (1995) Short report: dispersal of Aedes aegypti in an urban area after blood feeding as demonstrated by rubidium-marked eggs. Am J Trop Med Hyg 52: 177–179. [DOI] [PubMed] [Google Scholar]
- 26. Service MW (1997) Mosquito (Diptera: Culicidae) dispersal–the long and short of it. J Med Entomol 34: 579–588. [DOI] [PubMed] [Google Scholar]
- 27. Trpis M, Hausermann W (1986) Dispersal and other population parameters of Aedes aegypti in an African village and their possible significance in epidemiology of vector-borne diseases. Am J Trop Med Hyg 35: 1263–1279. [DOI] [PubMed] [Google Scholar]
- 28. Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, et al. (2005) Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg 72: 209–220. [PubMed] [Google Scholar]
- 29. McDonald PT (1977) Population characteristics of domestic Aedes aegypti (diptera: culicidae) in villages on the Kenya coast. II. Dispersal within and between villages. J Med Entomol 14: 49–53. [DOI] [PubMed] [Google Scholar]
- 30. Lacroix R, Delatte H, Hue T, Reiter P (2009) Dispersal and survival of male and female Aedes albopictus (Diptera: Culicidae) on Reunion Island. J Med Entomol 46: 1117–1124. [DOI] [PubMed] [Google Scholar]
- 31. Maciel-de-Freitas R, Neto RB, Goncalves JM, Codeco CT, Lourenco-de-Oliveira R (2006) Movement of dengue vectors between the human modified environment and an urban forest in Rio de Janeiro. J Med Entomol 43: 1112–1120. [DOI] [PubMed] [Google Scholar]
- 32. Gould DJ, Mount GA, Scanlon JE, Ford HR, Sullivan MF (1970) Ecology and control of dengue vectors on an island in the Gulf of Thailand. J Med Entomol 7: 499–508. [DOI] [PubMed] [Google Scholar]