Abstract
Recent studies have demonstrated that the small leucine-rich proteoglycans (SLRPs) biglycan and decorin impact tendon development, aging and healing in mature mice. However, despite the increased risk of tendon injury in the elderly, the role of SLRPs in tendon repair has not been investigated in aged animals. Therefore, our objective was to elucidate the influences of bigylcan and decorin on tendon healing in aged mice to relate our findings to previous work in mature mice. Since the processes of aging and healing are known to interact, our hypothesis was that aging mediates the role of biglycan and decorin on tendon healing. Patellar tendons from wild-type, biglycan-null and decorin-null mice were injured at 270 days using an established model. At 3 and 6 weeks post-surgery, structural, mechanical and biochemical analyses were performed and compared to uninjured controls. Early stage healing was inferior in biglycan-null and decorin-null mice as compared to wild type. However, tendons of all genotypes failed to exhibit improved mechanical properties between 3 and 6 weeks post-injury. In contrast, in a previous investigation of tendon healing in mature (i.e., 120 day-old) mice, only biglycan-null mice were deficient in early stage healing while decorin-null− mice were deficient in late-stage healing. These results confirm that the impact of SLRPs on tendon healing is mediated by age and could inform future age-specific therapies for enhancing tendon healing.
Keywords: Tendon, Injury, Aging, Proteoglycan, Biglycan, Decorin
1. Introduction
Tendon is a highly durable connective tissue comprised of a water-saturated network of uniaxially-aligned collagen fibrils. This specialized structure endows tendon with specific mechanical and structural properties that allow for proper transmission of force from muscle to bone. Thus, mechanical alterations produced by tendon injury adversely impact muscle performance and can lead to substantial pain and disability (Carroll et al., 2008). Unfortunately, advanced age is a risk factor for tendon injury (Yamamoto et al., 2010), and wound healing is impaired by the aging process (Kletsas et al., 2000). Therefore, understanding the constituent molecules involved in tendon repair in aged animals is an important step in the development of treatment strategies for tendon injury in the elderly
Recent investigations have implicated the class I small leucine-rich proteoglycans (SLRPs), biglycan and decorin, as important regulators of tendon development, aging and healing. Decorin, the predominant SLRP found in tendon, is comprised of a horseshoe-shaped decorin core protein and a sulfated chondroitin sulfate/dermatan sulfate (CS/DS) glycosaminglycan (GAG) (Orgel et al., 2009; Scott et al., 2004) while biglycan consists of a core protein with two sulfated (CS/DS) GAGs (Scott et al., 2006) and is generally expressed in tendon at lower levels (Zhang et al., 2006). The absence of either SLRP during development results in the formation of tendons with abnormal collagen fibril structures and altered mechanical properties (Dourte et al., 2012; Dourte et al., 2013; Robinson et al., 2005; Robinson et al., 2004; Zhang et al., 2006). Moreover, in mature (120 day-old) mice, the absence of decorin significantly impairs the long-term injury response of tendon while the absence of biglycan is detrimental only for early-stage healing (0-3 weeks post-injury) (Dunkman et al., 2013b). However, the post-developmental effects of SLRPs are not age-independent. In fact, the absence of decorin inhibits structural and mechanical alterations associated with the aging process (Dunkman et al., 2013a). Thus, the impact of SLRPs on tendon healing may also be influenced by the interacting process of aging. Nevertheless, the effects of biglycan and decorin on healing in aged mice have not yet been investigated. Therefore, the objective of this study was to investigate the impacts of bigylcan and decorin on the injury response of tendons in aged mice and to compare with previous work in mature mice. We hypothesized that the aging process mediates the specific effect of SLRPs on the repair response and that the deleterious effects of aging on tendon healing will be exacerbated by the absence of SLRPs
2. Results
2.1. Biomechanical Properties
In aged wild type (WT), decorin null (Dcn−/−) and biglycan null (Bgn−/−) mice, the dynamic modulus |E*| of injured tendons was significantly lower than in uninjured controls (Figure 1a, Supplemental Table 1). Thus, as expected, injured tendons produced lower stress in response to an induced cyclic strain and therefore exhibited inferior mechanical properties. However, improvements in this parameter during the healing process were not detected—that is, |E*| did not significantly increase from 3 and 6 weeks post-injury in any genotype group
Figure 1.
Dynamic modulus (|E*|) and tangent of the phase angle (tanδ) (a-b) as a function of injury state for each genotype group and (c-d) as a function genotype for each injury state (n=12-19 specimens per genotype per injury state, mean ± standard deviation). Results are shown for a 1 Hz deformation at a strain level of 8%, but data were similar at other frequencies and strain levels Improved mechanical properties (increased |E*| and decreased tanδ) were not measured for any genotype group between 3 and 6 weeks post-injury. However, |E*| was highest and tanδ was lowest in the WT group at 3 weeks-post injury, indicating that these tendons exhibited superior early-stage healing. Solid horizontal bars denote p<0.05/2 while dashed bars denote p<0.01/2.
In aged WT mice, tanδ was generally unchanged following injury, although tanδ increased slightly at low frequencies and low strains (Figure 1b, Supplemental Table 1). In contrast, aged Bgn−/− and Dcn−/− tendon became more fluid-like (increased tanδ) after injury. In other words, these tendons became more dissipative and were less able to store and release elastic energy during cyclic loading. During the healing process, tanδ decreased slightly at high strains and low frequencies in the mutant genotypes
Comparing across genotypes, |E*| in uninjured tendons was generally highest in the Bgn−/− group (Figure 1, Supplemental Table 2). However, at 3 weeks post-injury, |E*| in WT tendons was significantly higher than both Dcn−/− and Bgn−/−. Similarly, tanδ in uninjured tendons was highest in the WT group. But at 3 weeks post-injury, tanδ was lowest in the WT group. At 6 weeks post-injury, |E*| was highest in the WT group while no differences among genotypes were found in tanδ. However, as mentioned previously, |E*| did not change from 3 to 6 weeks in any genotype group. Taken together, these findings demonstrate that WT tendons exhibited superior mechanical properties compared to the null genotypes (increased |E*| and decreased tanδ) at 3 weeks post-injury but not prior to injury. That is, improvements in mechanical properties from 0-3 weeks post-injury were greater in WT tendons than in the mutant genotypes
2.2. SLRP Expression
Aged, uninjured Dcn−/− mouse tendons expressed more biglycan than uninjured WT mouse tendons (Figure 2a). Biglycan expression was upregulated after injury in aged WT tendons while expression of biglycan in Dcn−/− mice was constant at all pre- and post-injury time points. Aged, uninjured biglycan-null mice exhibited a trend towards higher decorin expression than WT (Figure 2b). Differences in decorin expression after injury were not detected in any genotype groups. No changes in fibromodulin expression between injury states or genotype groups were found (data not shown)
Figure 2.
RT-qPCR analysis for gene expression of biglycan and decorin (n=8-12 specimens per genotype per injury state, mean ± standard deviation). (a) As expected, biglycan and (b) decorin expression were completely suppressed in Bgn−/− and Dcn−/− tendons. Expression of biglycan increased in WT tendons 3 weeks post-injury and was significantly higher than in Dcn−/− tendons. Decorin expression was unaffected by injury in all genotype groups.
2.3. Tenocytes and Fiber Alignment
Uninjured Bgn−/− tendons were significantly more cellular than WT (Figure 3a). After injury, across all genotypes, there was a pronounced and significant increase in cellular density marking proliferation and repair (Figure 3a,d-l). Similarly, more rounded cells dominated in all genotypes after injury (Figure 3b,d-l). Moreover, at 6 weeks after injury, Dcn−/− tendons were less cellular than WT (Figure 3a). Finally, changes in collagen fiber alignment following injury were not detected (Figure 3c)
Figure 3.
Histological analysis of (a-b) tenocytes and (c) fiber alignment (n=4-5 specimens per genotype, mean ± standard deviation). Also shown are (d-l) representative histological images from tendons each genotype at each stage of healing (in each image, auto levels, auto contrast and auto color filters were applied in Adobe Photoshop). Cell density and aspect ratio increased after injury, while collagen alignment remained constant. Uninjured Bgn−/− tendons were more cellular than WT; however, cell density was lower in Dcn−/− tendons at 6-weeks post injury than in WT.
2.4. Fibril Structure
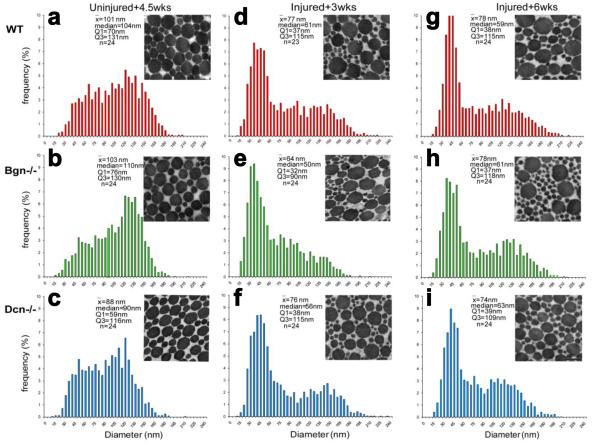
In uninjured tendons, fibril diameter distributions appeared bimodal for all genotypes (Figure 4a-c). In Bgn−/− tendons, the large diameter subpopulation exhibited a distinct peak that was more than twice as high as the peak of the small diameter subpopulation (Figure 4b). Thus, large diameter fibrils were substantially more frequent than smaller diameter fibrils. In contrast, the large diameter subpopulations in uninjured WT and Dcn−/− tendons were only slightly larger than the corresponding small diameter subpopulations (Figure 4a,c). Moreover, the larger diameter subpopulation in Dcn−/− tendons was shifted to smaller diameters.
Figure 4.
TEM analysis of fibril diameter distribution (n=4-6 per genotype per injury state) for (a-c) uninjured tendons, (d-f) tendons assessed 3 week post-injury and (g-i) tendons assessed 6 weeks post-injury. In all groups, a population of small fibrils appeared after injury. However, differences between genotypes at 6 weeks post-injury were negligible.
After injury, there was a marked increase in the number of smaller diameter fibrils, attributable to the assembly of new fibrils (Figure 4d-i). At both 3 and 6 weeks post-injury, WT and Dcn−/− tendons exhibited bimodal fibril diameter distributions with little overlap between subpopulations. However, the large diameter subpopulation in the Dcn−/− group was shifted to larger diameters (Figure 4f,i). In contrast, both the small and large diameter subpopulations in Bgn−/− tendons were shifted to smaller diameters and substantial overlap between subpopulations was apparent at 3 weeks post-injury (Figure 4e). However, this overlap was no longer evident at 6 weeks post-injury (Figure 4h). Furthermore, differences between genotypes at 6 weeks post-injury and changes in WT and Dcn−/− fibril distributions between 3 and 6 weeks were not evident.
3. Discussion
To our knowledge, this study is the first investigation of the specific effects of biglycan and decorin on tendon healing in aged mice. The absence of these molecules was detrimental to healing in aged patellar tendons from 0-3 weeks post-injury. However, mechanical and structural improvements were negligible in all genotype groups between 3 and 6 weeks post-injury. Since a recent study demonstrated reduced early-stage healing in mature (i.e., 120 day-old) Bgn−/− mouse tendons and reduced late-stage healing in mature Dcn−/− mouse tendons (Dunkman et al., 2013b), the present findings confirm our hypothesis that the influence of biglycan and decorin on tendon healing is age-dependent. Specifically, while biglycan and decorin are critical for proper tendon healing at younger ages, in aged mice, the presence of these molecules is beneficial only in early-stage healing and is insufficient to produce adequate late-stage healing.
The improved functional properties of WT tendons at 3 and 6 weeks-post injury were not fully corroborated by structural and biochemical assays. For example, the fibril diameter distribution and collagen alignment of these tendons were not distinct from the mutant genotypes after 6 weeks of healing. Therefore, it is likely that other structural changes (e.g., collagen cross-linking) were responsible for the measured genotype-dependent mechanical alterations in the early stages of healing. A number of recent investigations have demonstrated marked changes in the decorin molecule in aged skin (Carrino et al., 2011; Carrino et al., 2000; Li et al., 2013; Nomura, 2006). In particular, the size of the decorin glycosaminoglycan is reduced in older individuals. It is conceivable that such structural alterations in decorin with aging impact its interaction with type I collagen and underlie the inability of this molecule to promote late-stage healing in aged mouse tendons (in contrast to its beneficial effects in young mice).
Biglycan expression increased at 3 weeks post-injury (relative to controls) only in WT tendons, post-injury expression levels of this molecules were comparable to younger (mature) mice (Dunkman et al., 2013b). Therefore, age-dependent changes in the effect of biglycan and decorin on tendon healing are not associated with age-dependent changes in gene expression at 3 and 6 weeks post-injury and may instead reflect altered SLRP expression at early time points (< 3 weeks post-injury) or alterations in baseline tendon structure due to the absence of SLRPs during development, growth and maturation.
Between 3 and 6 weeks post-injury, the stress response as measured by the parameter |E*| did not improve in any of the tested genotype groups. However, energy dissipation (i.e., fluid-like behavior) as measured by the parameter tanδ did significantly recover at several strain levels and oscillation frequencies in all genotype groups (Figure 1). These findings suggest that tanδ may be a more sensitive measure of functional alterations in tendon during the healing process than |E*|. That is, subtle structural alterations during the repair response may affect viscoelastic dissipation before changes in modulus are evident.
Collagen fibril diameter distributions were consistent with previous studies that found an increased number of small diameter fibrils in uninjured Dcn−/− patellar tendons as compared to wild type (Dourte et al., 2012; Dunkman et al., 2013a, b). However, it is important to note that the impact of decorin on fibril size is tendon-specific. For example, mean fibril diameters in the flexor digitorum longus tendon of Dcn−/− mice are larger than in wild type mice (Zhang et al., 2006).
Histologic analyses demonstrated a decreased density of cells in Dcn−/− tendons versus wild type at 6 weeks post-injury. This finding is surprising given that proliferation and migration are reduced in Dcn−/− embryonic fibroblasts in vitro (Ferdous et al., 2008; Ferdous et al., 2010; Ferdous et al., 2007). However, these apparent contrasting findings may be due to differences in the effect of decorin on cells from mice of different ages (e.g., embryonic versus aged) and in different environments (e.g., in vitro versus in vivo).
This study was not without limitations. Structural, mechanical and biochemical measurements were not conducted until 3 weeks after surgery. Therefore, the initial repair response of tendon was not assessed. Similarly, the actual protein content of biglycan and decorin in the injured tendons was not assessed due to their small size. Therefore, post-translational regulation of biglycan and decorin was not taken into account. However, our mechanical assays demonstrate clear differences between genotype groups at 3 weeks post injury; these data indicate an altered repair response in WT tendons. In addition, long-term healing (> 6 weeks post-injury) was not assessed in the current study and may be impacted by SLRPs. However, mechanical changes between 3 and 6 weeks post-injury were found in mature mice (Dunkman et al., 2013b). Thus, healing in aged patellar tendons is at best significantly slower than in their mature counterparts. Future work will investigate how decorin and biglycan affect tendon healing over longer time scales and assessment will begin earlier in the post-injury healing process.
Previous studies have demonstrated the potential use of SLRPs in treatments for injured or pathological tendons. For example, inhibition of decorin with anti-decorin shRNA improved healing outcomes in a rat patellar tendon model (Lu et al., 2013). However, the current study demonstrates that the effects of biglycan and decorin on tendon healing are age-dependent. Thus, our findings could be instructive for the design and implementation of novel therapeutic approaches for enhancing tendon healing that account for the age of a patient and optimize the treatment accordingly.
4. Experimental Procedures
4.1. Animals, injury model and sample collection
C57BL/6 wild-type (WT), biglycan transgenic null (Bgn−/−) and decorin transgenic null (Dcn−/−) female mice were used in this study with IACUC approval (Danielson et al., 1997; Xu et al., 1998). Wild-type mice were obtained from Jackson Laboratories and housed at the University of Pennsylvania. Transgenic mice were bred at the University of South Florida and shipped the University of Pennsylvania. Pilot studies demonstrated that murine patellar tendon mechanical properties decline significantly between 120 and 270 days post-natal (data not shown). Therefore, 120-day old mice were considered “mature” while 270-day old mice were considered “aged.” At 270 days post-natal, 30-35 aged animals of each genotype (WT: n=30, Bgn−/−:n=31, Dcn−/−:n=35) underwent bilateral surgery on their patellar tendons as previously described (Beason et al., 2012; Buckley et al., 2013; Dunkman et al., 2013b; Lin et al., 2006). Briefly, after cutting the skin, incisions were made along the side of the tendon to allow for a rubber coating backing to slip under the tendon. A 0.75 mm biopsy punch was then used to create a full thickness, partial width defect injury in the center of the patellar tendon. Finally, the skin was sutured and the animals were allowed to return to normal cage activity. Animals were sacrificed at 3 or 6 weeks after surgery. To serve as uninjured controls, 10-15 additional mice of each genotype (WT: n=15, Bgn−/−:n=15, Dcn−/−:n=20) were sacrificed at 300 days of age.
Immediately after sacrifice, a randomly selected hind limb (left or right) was removed, wrapped in gauze, soaked in PBS, and frozen for the future mechanical testing. The tendon of the contralateral limb was bisected through the scar tissue and prepared for two of three additional assays: PCR, histology, and TEM.
4.2. Mechanical Testing
Prior to testing, the tibia-patellar tendon-patella complex was isolated by fine dissection. The tendon was stamped into a “dog-bone” shape and the cross-sectional area measured with a custom laser device. The tibia was potted in acrylic and secured with a staple. The pot and the patella were gripped with custom fixtures and loaded into a PBS bath on an Instron 5848 universal testing system for mechanical testing. Throughout preparation, the tendon was kept hydrated with PBS. The dynamic testing protocol consisted of 1) preconditioning, 2) stress relaxation at strain levels of 4%, 6% and 8%, 3) a sinusoidal frequency sweep (10 cycles at 0.01, 0.1, 1, 5, and 10 Hz) at each strain level, 4) return to gauge length, and 5) ramp to failure(Buckley et al., 2013; Dourte et al., 2012; Dourte et al., 2013; Dunkman et al., 2013a, b; Lujan et al., 2009).
From the load and grip-to-grip displacement data, the dynamic modulus |E*|— equal to the ratio of the stress and strain amplitudes—was calculated at each frequency and strain. |E*| is a measure of the overall resistance to deformation in a material under oscillatory loading that accounts for both elastic and viscous resistance. Because tendons are viscoelastic, the stress lags the strain under sinusoidal deformation. This lag, known as the phase angle δ, was also measured. The tangent of δ—a parameter proportional to the ratio of dissipated and stored energy—was calculated and reported. For a solid, tanδ is zero while tanδ approaches infinity for a pure fluid.
At each strain level and frequency, t-tests were performed to compare across injury states within each genotype and across genotypes within each injury state. Bonferroni corrections were applied to adjust for multiple comparisons. Significance was set at and p≤0.025 and 0.025≤p≤0.05 was considered a trend. Inferences were made based upon consistent results, as described previously (Dunkman et al., 2013a, b).
4.3. RT-qPCR
Expression levels were quantified by real-time quantitative polymerase chain reaction assay (RT-qPCR) for biglycan, decorin and fibromodulin relative to beta-actin as previously described (Dunkman et al., 2013b). Briefly, individual tissue samples were homogenized in QIAzol reagent (Qiagen, Valencia, CA). Total RNA was isolated using a RNeasy Micro Kit (Qiagen) as previously adapted (Mienaltowski et al., 2009). To analyze expression from each individual sample, mRNA was amplified by Single Primer Isothermal Amplification (SPIA) (Dafforn et al., 2004) The mRNA of 25 ng of total RNA was reverse transcribed into cDNA and amplified using the WT-Ovation RNA Amplification System (NuGEN, San Carlos, CA). The resulting SPIA product was purified with a QIAquick PCR Purification Kit (Qiagen) and the amplified cDNA was eluted with 30 μl RNase-free water (Dunkman et al., 2013b). For qPCR analysis, 1 μl of amplified cDNA template was added to a reaction volume of 20 μl per well in an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) with a Fast SYBR Green Master Mix (Applied Biosystems). Mouse specific primers for β-actin (Actb: F— AGATGACCCAGATCATGTTTGAGA; R— CACAGCCTGGATGGCTACGT), decorin (Dcn: F— GCTGCGGAAATCCGACTTC; R— TTGCCGCCCAGTTCTATGAC), and biglycan (Bgn: F— CCTTCCGCTGCGTTACTGA; R— GCAACCACTGCCTCTACTTCTTATAA) were used. Amplified cDNA for each individual uninjured or injured patellar tendon sample was analyzed in triplicate with a single negative RT control (0.83 ng total RNA per well) for each sample and each gene. Gene specific efficiencies were calculated using LinRegPCR v7.5 software for each qPCR plate and the relative quantity of mRNA for each gene of interest was computed using the relative gene expression ratios formula (Ramakers et al., 2003; Schefe et al., 2006). Between 8 and 12 specimens were analyzed per genotype per group, and outliers were removed if their measured expression was greater than two standard deviations from the mean. According to this criterion, at most only 1 outlier per genotype and injury status was identified and eliminated. To test for differences in gene expression, Mann-Whitney tests were used and significance was set at and p≤0.05/2 and 0.05/2≤p≤0.1/2 was considered a trend.
4.4. Transmission Electron Microscopy
Tendon were prepared for transmission electron microscopy as previously described (Dunkman et al., 2013a). Tendons were fixed with 2.5% glutaraldehyde/4% formaldehyde fixative, post-fixed with osmium tetroxide, dehydrated with ethanol, embedded in Epon 812and polymerized at 60 °C. Ultra-thin cross-sections were imaged on JEOL 1400 transmission electron microscope (JEOL Ltd., Tokyo, Japan) equipped with a Gatan Orius widefield side mount CC Digital camera (Gatan Inc., Pleasanton, CA). An analysis of tendon diameter was done as previously described (Dunkman et al., 2013a). Pooled data from 4 to 6 mice of the same genotype and injury state was used. Five or six digital images from each tendon were taken at 60,000x. Images were analyzed using an RM Biometrics-Bioquant Image Analysis System (Nashville, TN). A region of interest (ROI) of appropriate size was determined within the image so that a minimum of 80 fibrils were measured from each image. Fibril diameters were measured along the minor axis of the fibril cross-section. Tendon diameter measurements were pooled into groups by genotype and injury state.
4.5. Histology and Polarized Light Microscopy
The entire knee joint with a bisected tendon was preserved in formalin immediately after sacrifice and dissection. Samples were decalcified and embedded within paraffin according to standard histological techniques. Sections (~7 μm) were cut and stained with hematoxylin and eosin. Polarized light images were taken and analyzed with custom software to obtain values of circular standard deviation to quantify collagen alignment (with higher values representing less aligned tendon) (Thomopoulos et al., 2003). Four images were taken at the injury site with a traditional light microscope. Each image was analyzed with BioQuant software to obtain cell density and the average aspect ratio of the cells. Mean values for each sample were used for comparison and statistics. Mann Whitney tests were used for comparisons of circular standard deviation—a parameter which is not normally distributed—and t-tests were used for comparisons of cell density and aspect ratio.
Supplementary Material
Highlights.
We studied how the effect of proteoglycans on tendon healing is impacted by aging.
Early stage healing of aged tendons was inferior without biglycan or decorin.
Healing from 3-6 weeks post-injury was absent in aged tendons of all genotypes.
The impact of biglycan and decorin on tendon healing is mediated by age.
Acknowledgments
This study was funded by NIH/NIAMS grant 5R01AR055543 and the Penn Center for Musculoskeletal Disorders (NIH/NIAMS, P30 AR050950). None of the authors have any conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beason DP, Kuntz AF, Hsu JE, Miller KS, Soslowsky LJ. Development and evaluation of multiple tendon injury models in the mouse. J Biomech. 2012;45:1550–1553. doi: 10.1016/j.jbiomech.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MR, Dunkman AA, Reuther KE, Kumar A, Pathmanathan L, Beason DP, Birk DE, Soslowsky LJ. Validation of an Empirical Damage Model for Aging and In Vivo Injury of the Murine Patellar Tendon. Journal of Biomechanical Engineering. 2013;135:0410051–0410057. doi: 10.1115/1.4023700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrino DA, Calabro A, Darr AB, Dours-Zimmermann MT, Sandy JD, Zimmermann DR, Sorrell JM, Hascall VC, Caplan AI. Age-related differences in human skin proteoglycans. Glycobiology. 2011;21:257–268. doi: 10.1093/glycob/cwq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrino DA, Sorrell JM, Caplan AI. Age-related changes in the proteoglycans of human skin. Arch Biochem Biophys. 2000;373:91–101. doi: 10.1006/abbi.1999.1545. [DOI] [PubMed] [Google Scholar]
- Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol. 2008;105:1907–1915. doi: 10.1152/japplphysiol.00059.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafforn A, Chen P, Deng G, Herrler M, Iglehart D, Koritala S, Lato S, Pillarisetty S, Purohit R, Wang M, Wang S, Kurn N. Linear mRNA amplification from as little as 5 ng total RNA for global gene expression analysis. BioTechniques. 2004;37:854–857. doi: 10.2144/04375PF01. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourte LM, Pathmanathan L, Jawad AF, Iozzo RV, Mienaltowski MJ, Birk DE, Soslowsky LJ. Influence of Decorin on the Mechanical, Compositional, and Structural Properties of the Mouse Patellar Tendon. J Biomech Eng-T Asme. 2012:134. doi: 10.1115/1.4006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourte LM, Pathmanathan L, Mienaltowski MJ, Jawad AF, Birk DE, Soslowsky LJ. Mechanical, compositional, and structural properties of the mouse patellar tendon with changes in biglycan gene expression. J Orthop Res. 2013;31:1430–1437. doi: 10.1002/jor.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013a;32:3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ. The Tendon Injury Response is Influenced by Decorin and Biglycan. Ann Biomed Eng. 2013b doi: 10.1007/s10439-013-0915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous Z, Lazaro LD, Iozzo RV, Hook M, Grande-Allen KJ. Influence of cyclic strain and decorin deficiency on 3D cellularized collagen matrices. Biomaterials. 2008;29:2740–2748. doi: 10.1016/j.biomaterials.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous Z, Peterson SB, Tseng H, Anderson DK, Iozzo RV, Grande-Allen KJ. A role for decorin in controlling proliferation, adhesion, and migration of murine embryonic fibroblasts. J Biomed Mater Res A. 2010;93:419–428. doi: 10.1002/jbm.a.32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous Z, Wei VM, Iozzo R, Hook M, Grande-Allen KJ. Decorin-transforming growth factor- interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282:35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- Kletsas D, Pratsinis H, Zervolea I, Handris P, Sevaslidou E, Ottaviani E, Stathakos D. Fibroblast responses to exogenous and autocrine growth factors relevant to tissue repair. The effect of aging. Ann N Y Acad Sci. 2000;908:155–166. doi: 10.1111/j.1749-6632.2000.tb06644.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu Y, Xia W, Lei D, Voorhees JJ, Fisher GJ. Age-dependent alterations of decorin glycosaminoglycans in human skin. Scientific reports. 2013;3:2422. doi: 10.1038/srep02422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39:61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lu P, Zhang GR, Cai YZ, Heng BC, Ren H, Wang LL, Ji J, Zou XH, Ouyang HW. Lentiviral encoded shRNA silencing of proteoglycan decorin enhances tendon repair and regeneration within a rat model. Cell transplantation. 2013;22:1507–1517. doi: 10.3727/096368912X661292. [DOI] [PubMed] [Google Scholar]
- Lujan TJ, Underwood CJ, Jacobs NT, Weiss JA. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J Appl Physiol. 2009;106:423–431. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienaltowski MJ, Huang L, Frisbie DD, McIlwraith CW, Stromberg AJ, Bathke AC, Macleod JN. Transcriptional profiling differences for articular cartilage and repair tissue in equine joint surface lesions. BMC medical genomics. 2009;2:60. doi: 10.1186/1755-8794-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y. Structural change in decorin with skin aging. Connect Tissue Res. 2006;47:249–255. doi: 10.1080/03008200600846606. [DOI] [PubMed] [Google Scholar]
- Orgel JP, Eid A, Antipova O, Bella J, Scott JE. Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PLoS One. 2009;4:e7028. doi: 10.1371/journal.pone.0007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng. 2004;126:252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel "gene expression's CT difference" formula. Journal of molecular medicine. 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- Scott PG, Dodd CM, Bergmann EM, Sheehan JK, Bishop PN. Crystal structure of the biglycan dimer and evidence that dimerization is essential for folding and stability of class I small leucine-rich repeat proteoglycans. J Biol Chem. 2006;281:13324–13332. doi: 10.1074/jbc.M513470200. [DOI] [PubMed] [Google Scholar]
- Scott PG, McEwan PA, Dodd CM, Bergmann EM, Bishop PN, Bella J. Crystal structure of the dimeric protein core of decorin, the archetypal small leucine-rich repeat proteoglycan. Proc Natl Acad Sci U S A. 2004;101:15633–15638. doi: 10.1073/pnas.0402976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nature genetics. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, Kobayashi T. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.