Abstract
Glucose-regulated protein 78 (GRP78), a molecular chaperone widely elevated in human cancers, is critical for endoplasmic reticulum (ER) protein folding, stress signaling and PI3K/AKT activation. Genetic knockout models of GRP78 revealed that GRP78 maintains homeostasis of metabolic organs, including liver, pancreas and adipose tissues. Hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC) are the most common liver cancers. There is a lack of effective therapeutics for HCC and CC, highlighting the need to further understand liver tumorigenic mechanisms. PTEN, a tumor suppressor that antagonizes the PI3K/AKT pathway, is inactivated in a wide range of tumors, including 40–50% of human liver cancers. To elucidate the role of GRP78 in liver cancer, we created a mouse model with biallelic liver-specific deletion of Pten and Grp78 mediated by Albumin-Cre-recombinase (cPf/f78f/f). Interestingly, in contrast to PTEN, deletion of GRP78 was progressive but incomplete. At 3 months, cPf/f78f/f livers showed hepatomegaly, activation of lipogenic genes, exacerbated steatosis and liver injury, implying that GRP78 protects the liver against PTEN-null mediated pathogenesis. Furthermore, in response to liver injury, we observed increased proliferation and expansion of bile duct and liver progenitor cells in cPf/f78f/f livers. Strikingly, bile duct cells in cPf/f78f/f livers maintained wild-type (WT) GRP78 level while adjacent areas showed GRP78 reduction. Analysis of signaling pathways revealed selective JNK activation, β-catenin downregulation, along with PDGFRα upregulation, which was unique to cPf/f78f/f livers at 6 months. Development of both HCC and CC was accelerated and evident in cPf/f78f/f livers at 8–9 months, coinciding with intense GRP78 expression in the cancer lesions, and GRP78 expression in adjacent normal areas reverted back to the WT level. In contrast, c78f/f livers showed no malignancy even at 14 months. These studies reveal GRP78 is a novel regulator for PTEN-loss mediated liver injury and cancer progression.
Keywords: Liver cancer, PTEN, GRP78, genetic model, steatosis, signaling pathway
INTRODUCTION
Glucose-regulated protein 78 (GRP78), also known as BiP/HSPA5, belongs to the HSP70 family. GRP78 is a major endoplasmic reticulum (ER) chaperone protein critical for protein quality control of the ER as well as a master regulator of the unfolded protein response (UPR).1,2 Under ER stress, GRP78 is titrated away by the accumulation of malfolded proteins, releasing and activating the UPR sensors to restore ER homeostasis. In response to prolonged and severe ER stress, the UPR triggers apoptotic pathways leading to cell death.3 Accumulating evidence indicates that a subfraction of GRP78 localizes at the cell surface under pathological conditions, such as in cancer cells.2,4 Cell surface GRP78 acts as a multifunctional receptor to regulate signaling pathways, such as activating the PI3K/AKT signaling for cancer survival and proliferation.5,6
Hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC) are the most common primary liver cancers and are associated with poor prognosis. Currently, a paucity of effective treatments for advanced liver cancers highlights the need to understand liver tumorigenic mechanisms. The tumor suppressor, PTEN (phosphatase and tenson homolog deleted on chromosome 10), which encodes for a non-redundant plasma membrane lipid phosphatase that antagonizes the PI3K signaling pathway,7 is mutated or deleted in 40–50% of human liver cancers.8 Loss of PTEN activates PI3K/AKT pathway, leading to cancer progression in a wide variety of human cancers. Liver-specific PTEN knockout in mice causes fatty liver and liver injury which activates progenitor cells to become tumor-initiating cells and over time (~12 months) leads to both HCC and CC development.9,10 Liver progenitor cells are bi-potential and quiescently reside in the stem cell niche. During chronic or massive liver injury, liver progenitor cells are activated to proliferate and differentiate into hepatocytes and cholangiocytes (bile duct cells). However, deregulated liver progenitors can give rise to liver cancer.11
GRP78 is commonly upregulated in various tumors due to intrinsic alterations of cancer cell metabolism and hyperproliferation, as well as extrinsic factors in the tumor microenvironment, such as glucose deprivation and hypoxia leading to ER stress.4,12 Increased GRP78 is generally associated with greater risk for cancer recurrence and poorer patient outcome. In cancer cell lines and mouse models, GRP78 has been shown to promote tumor survival, progression and resistance to therapy.4,12,13 For instance, GRP78 deficiency in the mouse prostate epithelium potently suppresses prostate tumorigenesis and AKT activation mediated by the loss of PTEN.14 Similarly, GRP78 haploinsufficiency blocks Pten-null mediated-leukemia and AKT signaling.15 In view of these studies, GRP78 might be required for PTEN-loss driven tumorigenesis. However, several recent studies suggest that in metabolic organs, the effect of GRP78 deletion is complex with both primary and compensatory consequences resulting from partial loss of GRP78. For example, Grp78 heterozygosity protects against high fat diet-induced obesity, type 2 diabetes and pancreatitis in the exocrine pancreas through compensatory mechanisms, whereby ER chaperones and other protective ER stress pathways are upregulated.16,17 In the case of adipose tissues, GRP78 is required for adipogenesis and glucose homeostasis.18 In the liver, breeding of Grp78 floxed/floxed mice14,19 with the Albumin-Cre transgenic mice generated offspring (Alb-Cre; Grp78f/f) with incomplete deletion of GRP78.20 Primary hepatocytes isolated from these mice retained 30% wild-type (WT) level of GRP78. These mice exhibited impaired insulin signaling and exacerbated liver injury resulting from alcohol and high fat diet, and were sensitized to a variety of acute and chronic hepatic disorders,20 consistent with a protective role of GRP78 in tissues in general.
Taken together, the effects of GRP78 deletion could be tissue-specific and complex. Moreover, the role of GRP78 on PTEN-loss driven liver cancer development has yet to be determined. Thus, for the present study, we took advantage of the viability of mice with partial hepatic GRP78 deficiency to create a liver-specific biallelic (Ptenf/fGrp78f/f) deletion mouse model mediated by Alb-Cre, referred to below as cPf/f78f/f. Previous reports demonstrated that Alb-Cre is activated at embryonic day 9.5 in fetal hepatoblasts which are common progenitors of hepatocytes and bile duct cells. However, postnatally, Alb-Cre activity is maintained in hepatocytes and liver progenitor cells but not in adult bile duct cells.21,22 Here we characterized the phenotypes of cPf/f78f/f mice. Our studies revealed that in the context of PTEN deletion, concomitant GRP78 reduction in hepatocytes led to increased hepatomegaly, steatosis, liver injury and progenitor cell proliferation. GRP78 expression in bile duct cells was intact in these mice and bile duct proliferation was readily observed. At 6 months, we detected selective JNK activation, β-catenin downregulation, along with PDGFRα upregulation in cPf/f78f/f livers. At 8–9 months, HCC and CC were clearly identified in cPf/f78f/f livers, associating with strong GRP78 re-expression. In contrast and despite the development of fatty liver and inflammation, there was no malignancy detected in livers of Alb-Cre; Grp78f/f (c78f/f) mice even at 12–14 months. Overall, these results reveal a novel role of GRP78 in regulating PTEN-loss mediated liver injury and cancer progression.
RESULTS
Creation of the mouse model with biallelic deletion of GRP78 and PTEN in the liver
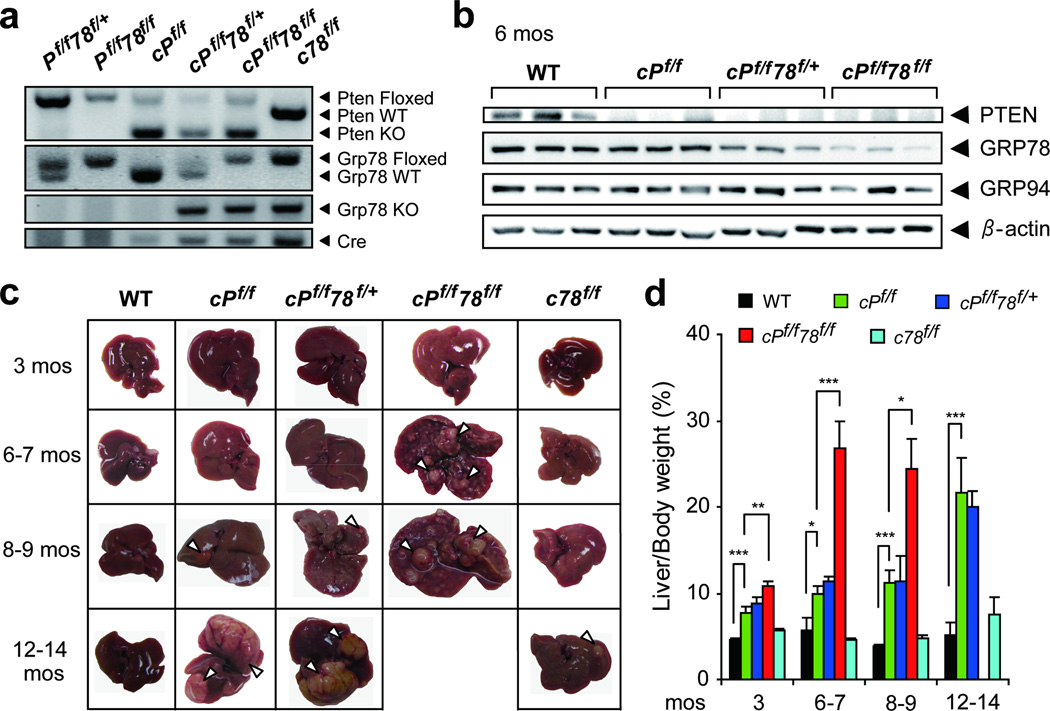
The breeding scheme for the generation of the following five groups of mouse cohorts is shown in Figure S1. They include: 1) single homozygous knockout of Pten with Alb-Cre (cPf/f); 2) homozygous knockout of Pten and heterozygous knockout of Grp78 with Alb-Cre (cPf/f78f/+); 3) homozygous knockout of Pten and Grp78 with Alb-Cre (cPf/f78f/f); 4) single homozygous knockout of Grp78 with Alb-Cre (c78f/f); and 5) WT derived from littermates without the Alb-Cre transgene serving as normal controls. In this set of breeding, a different strain of Alb-Cre mice was used from a previous study.20 This allowed us to test if the residual GRP78 observed in c78f/f livers was due to variability of the Cre activity among different transgenic mouse strains.
PCR performed with genomic DNA extracted from the livers of the mouse cohorts validated that the various alleles were excised accordingly. We detected a Pten knockout band in cPf/f mice, a Grp78 knockout band in c78f/f mice, and both Pten and Grp78 knockout bands in cPf/f78f/+ and cPf/f78f/f mice but not in WT mice (Figure 1a). Western blot analysis of liver lysates isolated from 6 month-old mice showed that PTEN knockout was efficient in cPf/f, cPf/f78f/+ and cPf/f78f/f mice (around 95% reduction compared to the WT) (Figure 1b). As observed previously,20 GRP78 level only decreased gradually. At 3 months, we detected about 50% reduction of GRP78 in cPf/f78f/f and c78f/f livers and about 70% reduction by 6 months, compared to the WT (Figure S2). cPf/f78f/+ livers showed intermediate GRP78 level (Figure 1b). In all the mouse cohorts, no compensatory upregulation of the ER chaperone GRP94 was observed. Morphologically, the size of livers with PTEN deficiency increased significantly over WT and c78f/f livers (Figure 1c and d). This is likely due in part to enhanced insulin signaling in PTEN-null livers resulting in redistribution of body fat to the liver as previously reported.9 Indeed, fat redistribution was also observed in our mouse cohorts, with cPf/f78f/f mice exhibiting even lower body (gonadal) fat than cPf/f mice (Figure S3a and b).
Figure 1.
Liver-specific PTEN deletion and GRP78 reduction accelerated hepatomegaly. (a) Representative PCR genotyping results from livers isolated from wild-type (WT, littermates without the Cre transgene such as Pf/f78f/+ and Pf/f78f/f mice), cPf/f, cPf/f78f/+, cPf/f78f/f and c78f/f mice. (b) Western blot analysis of PTEN, GRP78 and GRP94 levels in livers from mice of the indicated genotypes, with β-actin serving as the loading control. (c) Representative liver pictures from the indicated genotypes aged from 3 to 14 months. Arrowheads point to nodules on the liver surface. (d) Liver weight to body weight (percentage) in five types of mice (n=4 to 11) at the age indicated. Data are presented as mean ± S.E. *p<0.05; **p<0.01; ***p<0.001.
Livers of cPf/f78f/f mice exhibited multiple nodules on the surface as early as 6–7 months, while cPf/f and cPf/f78f/+ mice started from 8–9 months and c78f/f mice from 12–14 months (Figure 1c). At 6–7 months, the weight of cPf/f78f/f livers normalized over body weight, increased 5-fold compared to WT livers, and 2.5-fold compared to PTEN-null livers (Figure 1d). We observed that cPf/f78f/f mice were not viable beyond 9 months. In contrast, the normalized liver weight of c78f/f mice remained similar to the WT and these mice were fully viable. Therefore, hepatic GRP78 reduction accelerated PTEN-null driven hepatomegaly and abnormal nodule growth.
GRP78 reduction in PTEN-null livers promoted liver steatosis, liver injury and bile duct proliferation
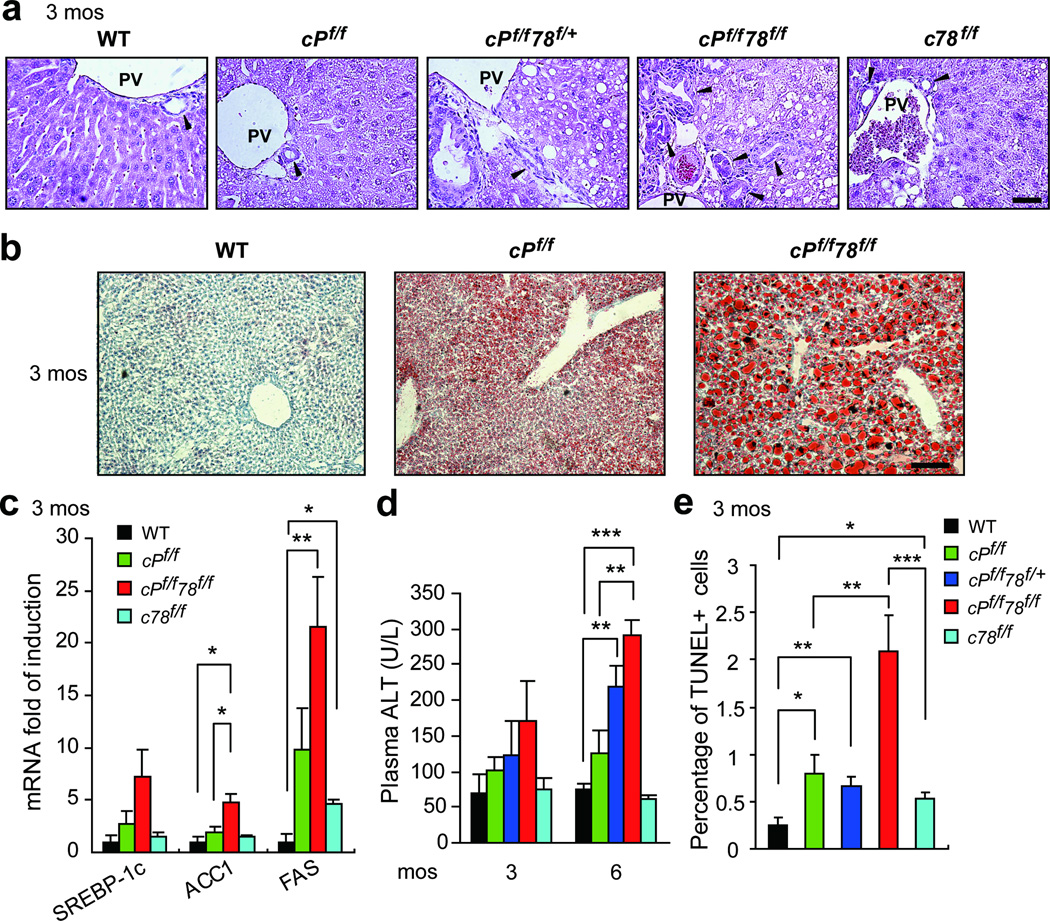
Consistent with previous reports,9,20 histological analysis of livers showed mild lipid accumulation in both cPf/f and c78f/f mice at 3 months (Figure 2a). In contrast, fat droplets were readily evident in cPf/f78f/+ and cPf/f78f/f livers (Figure 2a). Oil Red O staining further confirmed more severe steatosis in cPf/f78f/f livers (Figure 2b). Additionally, bile duct proliferation was detected in 2 out 5 cPf/f78f/f livers at 3 months (Figure 2a). In agreement with increased lipid accumulation, mRNA levels of lipogenic genes SREBP-1c, ACC1 and FAS were upregulated in cPf/f78f/f livers, compared to WT and cPf/f mice (Figure 2c). Recent evidence suggests that the transcription factor XBP-1 is a regulator of hepatic lipogenesis.23 In the present study, we observed mild Xbp-1 mRNA splicing in c78f/f livers which was consistent with previous data.20 However, Xbp-1 splicing was minimal in cPf/f78f/f livers (Figure S4a). ATF4 has also been implicated in lipogenic gene expression,24 but we did not detect increased ATF4 protein level in cPf/f78f/f livers (Figure S4b).
Figure 2.
Enhanced fat accumulation, bile-duct proliferation, injury and apoptosis in cPf/f78f/f livers. (a) H&E staining of livers from the indicated genotypes at 3 months. Scale bar shows 50 µm. PV: portal vein. Black arrowheads denote examples of bile ducts. (b) Oil Red O staining of livers from the indicated genotypes at 3 months. Scale bar shows 100 µm. (c) Real-time quantitative PCR analysis of lipogenic genes SREBP-1c, ACC1 and FAS in livers of the indicated genotypes at 3 months (n=3 to 7 per genotype per gene). Expression levels of each gene were normalized to the levels of 18S RNA. (d) Liver injury of mice aged 3 months and 6 months was measured by plasma ALT test (n=3 to 5). (e) Quantification of TUNEL-positive cells on liver sections of the indicated genotypes at 3 months. Data are presented as mean ± S.E. *p<0.05, **p<0.01, ***p<0.001.
We measured liver injury by examining plasma ALT. cPf/f78f/f mice displayed 1.7- and 2.3-fold increase in ALT level compared to cPf/f mice at 3 and 6 months, respectively, whereas the ALT level remained at the WT level for c78f/f mice (Figure 2d). Consistent with high level of injury, cPf/f78f/f livers exhibited a 4-fold increase in apoptosis compared to other genotypes (Figure 2e). Nonetheless, the percentage of apoptosis only reached 2% in cPf/f78f/f livers, thus, overall apoptosis in all genotypes was mild at 3 months. Furthermore, none of the genotypes analyzed showed an increase in hepatic expression of the transcription factor CHOP,3 which is often used as an indicator of ER stress induced apoptosis (Figure S4c). This result was consistent with the recent observation that PTEN deletion inhibits thapsigargin-induced CHOP induction in hepatocytes isolated from these mice.25
cPf/f78f/f livers showed increased proliferation, progenitor cell expansion and GRP78 re-expression in bile duct cells
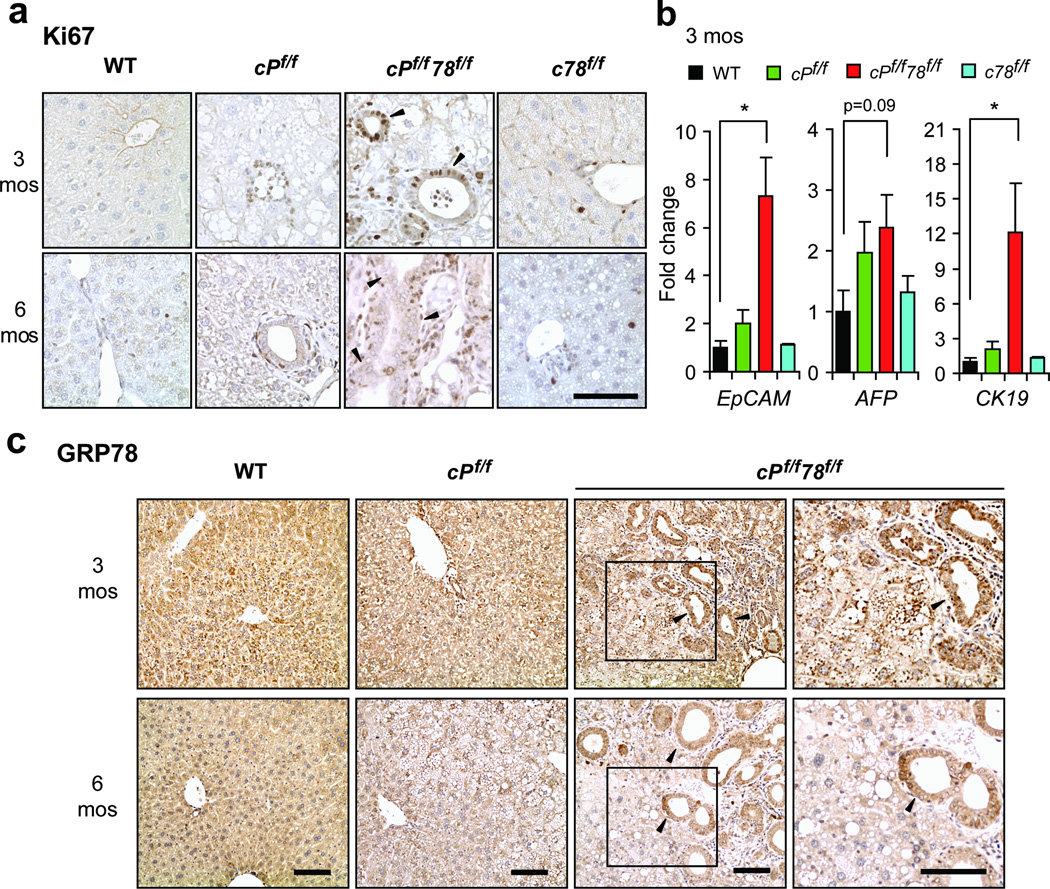
Liver injury generally elicits regeneration through proliferation.11 To ascertain liver proliferation, we examined Ki67 expression in mouse livers. We observed substantial increase in Ki67-positive cells in cPf/f78f/f livers, compared to cPf/f and c78f/f mice at both 3 and 6 months, whereas Ki67-positive cells were absent in WT livers (Figure 3a). Additionally, some Ki67-positive cells with liver progenitor cell morphology were detected around bile ducts in cPf/f78f/f livers (Figure 3a). Liver progenitor markers EpCAM, CK19 and AFP were also upregulated in cPf/f78f/f livers compared to WT, cPf/f and c78f/f livers, which further suggested proliferation (Figure 3b).
Figure 3.
cPf/f78f/f livers exhibited increased proliferation, progenitor cell expansion and GRP78 expression in bile duct cells. (a) Immunohistochemistry staining of cell proliferation marker Ki67 (brown nuclei) on livers of the indicated genotypes at 3 and 6 months. (b) Real-time quantitative PCR analysis of liver progenitor markers EpCAM, AFP and CK19 in livers of the indicated genotypes at 3 months. Expression levels of each gene were normalized to 18S RNA levels. Data are presented as mean ± S.E. *p<0.05. (c) Immunohistochemistry staining of GRP78 on livers from the indicated genotypes aged 3 and 6 months. Far right panels represent higher magnification of the boxed regions in cPf/f78f/f livers. Black arrowheads in panels a and c denote examples of bile ducts. Scale bars show 100 µm and are applicable to all panels.
As demonstrated by Western blot, there was residual GRP78 in cPf/f78f/f livers. Thus, to gain insight into the distribution pattern of GRP78 in cPf/f78f/f mice, we performed immunohistochemical (IHC) staining of the liver sections. Consistent with our Western blot results (Figure 1b and Figure S2a), GRP78 expression progressively decreased between 3 and 6 months of age. Strikingly, the cells of the expanded bile ducts in cPf/f78f/f livers showed robust GRP78 staining at both 3 and 6 months (Figure 3c). This suggests that some hepatoblasts with incomplete Cre-mediated recombination differentiated into bile ducts, where Alb-Cre was inactive. Moreover, some GRP78-positive cells around bile ducts resembled liver progenitors morphologically (Figure 3c).
Perturbation of selective signaling pathways in cPf/f78f/f livers
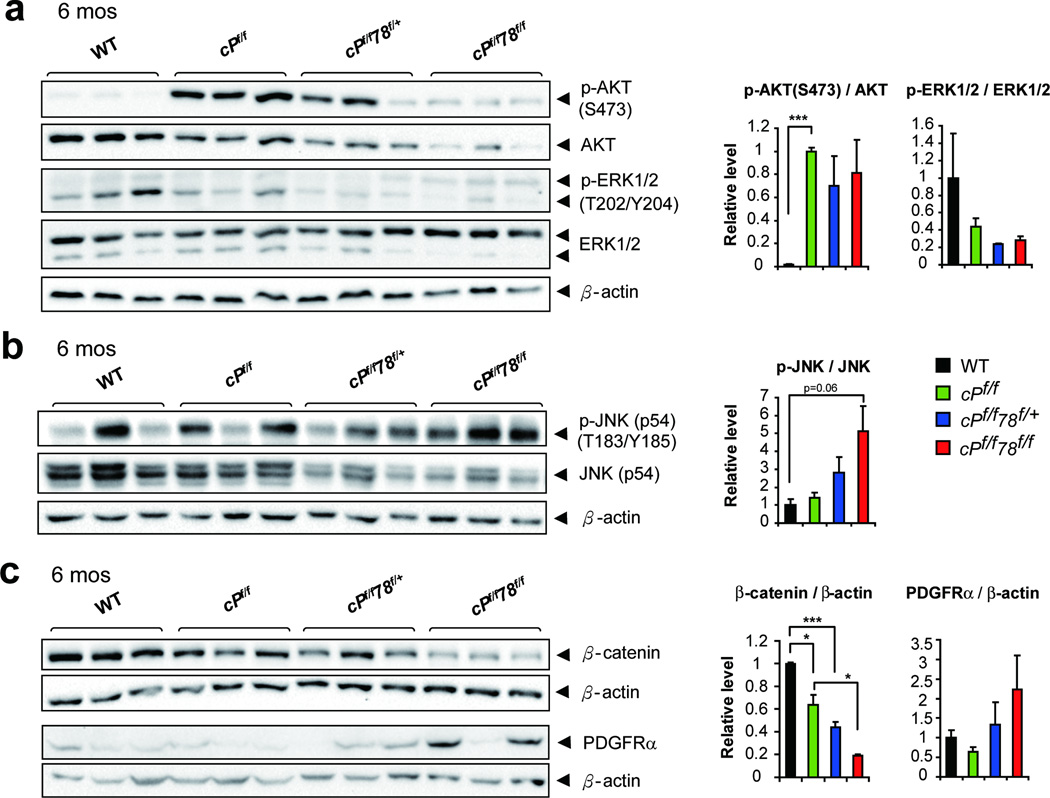
GRP78 deficiency has been demonstrated to suppress PI3K/AKT signaling.14,15 As expected, p-AKT(S473) was highly activated in cPf/f mice, consistent with the established role of PTEN as a negative regulator of PI3K/AKT (Figure 4a). At 6 months, both p-AKT(S473) and total AKT were dramatically reduced in cPf/f78f/f livers, and cPf/f78f/+ livers were also partially affected (Figure 4a). Because of the reduction in total AKT, the functional reduction in p-AKT was not statistically captured through the ratio p-AKT/AKT (Figure 4a). We also detected similar decrease in both p-S6K and S6K, downstream of AKT pathway, in cPf/f78f/f livers (Figure S5). In the case of ERK, similar levels of p-ERK1 and total ERK1 were observed in all the genotypes, whereas the levels of p-ERK2 and total ERK2 decreased in cPf/f78f/f livers. The level of p-JNK was moderately elevated in cPf/f78f/f livers, whereas total JNK level was slightly decreased in cPf/f78f/+ and cPf/f78f/f livers (Figure 4b). In contrast, β-catenin level was significantly reduced in cPf/f78f/f livers (Figure 4c). It has been reported that in vitro suppression of β-catenin in hepatoma cells leads to PDGFRα upregulation,26 which is associated with liver tumorigenesis.27 Consistent with this finding, we detected elevated PDGFRα expression in cPf/f78f/f livers (Figure 4c).
Figure 4.
Perturbation of selective signaling pathways in cPf/f78f/f livers. (a) Representative Western blots of p-AKT(S473), total AKT, p-ERK1/2(T202/Y204) and total ERK1/2 levels from livers of the indicated genotypes at 6 months. (b) Representative Western blots of p-JNK (p54, T183/Y185) and total JNK (p54) levels. (c) Representative Western blots of β-catenin and PDGFRα expression. β-actin was used as the loading control and corresponding quantifications were shown on the right. n=3 for each genotype. Data are presented as mean ± S.E. *p<0.05, ***p<0.001.
Accelerated HCC and CC development and repopulation of GRP78-positive cells in cPf/f78f/f livers
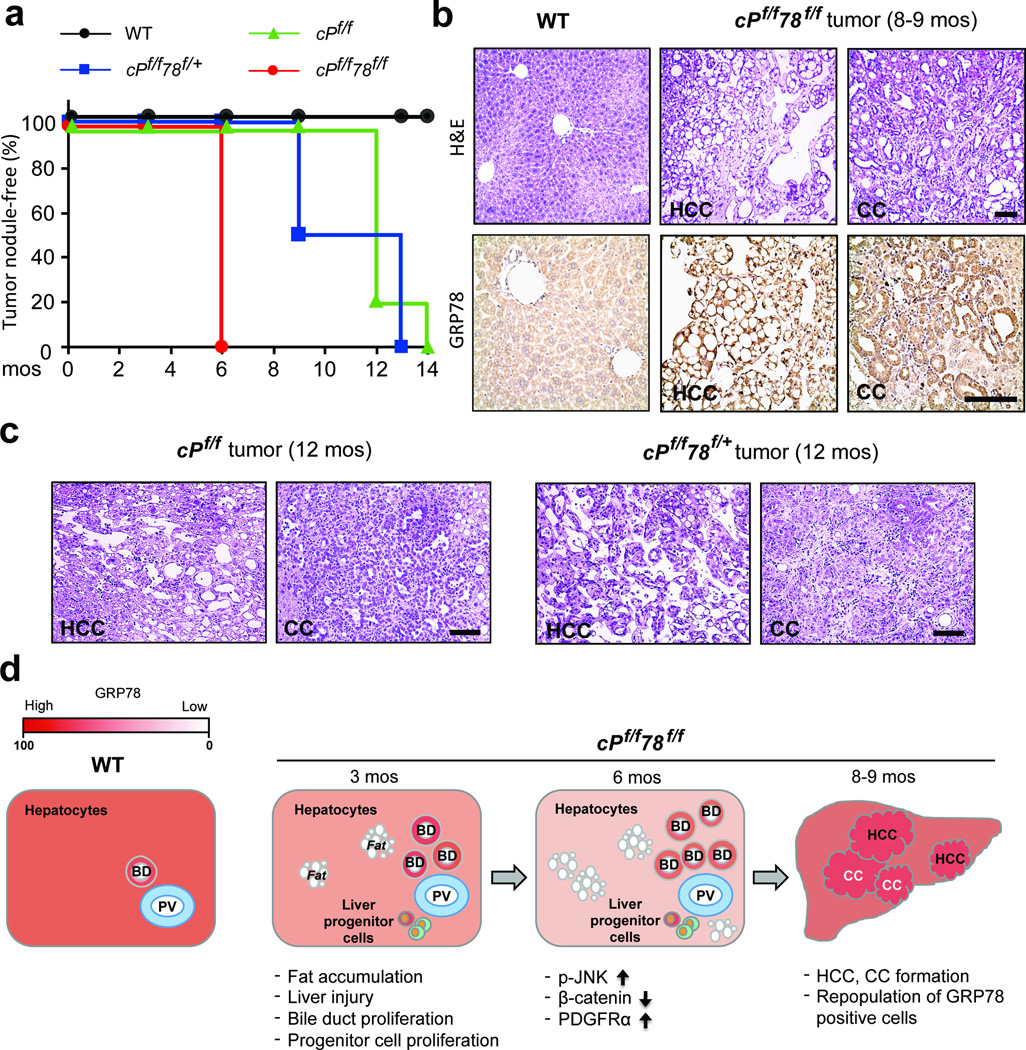
In following liver morphology of the mouse cohorts, at the time of sacrifice, all cPf/f78f/f mice developed visible tumor-like nodules by 6 months whereas for cPf/f78f/+ mice, 50% exhibited nodules by 9 months and 100% by 13 months, and for cPf/f mice, liver nodules were not apparent until 12 months (Figure 5a). c78f/f livers displayed very few and small nodules at 12–14 months (Figure 1c) and H&E staining showed fatty liver accompanied by inflammation with no sign of malignancy (data not shown). While cPf/f78f/f livers showed extensive bile duct proliferation at 6 months (Figure 3c), H&E staining revealed no malignancy (data not shown), however, both HCC and CC were evident starting from 8–9 months (Figure 5b). Intense GRP78 staining was detected in HCC and CC areas in cPf/f78f/f livers and GRP78 level in adjacent normal areas was comparable to the WT level (Figure 5b). We further observed that both cPf/f and cPf/f78f/+ mice developed HCC and CC starting from 12 months (Figure 5c). Taken together, these results suggest that cPf/f78f/f mice had accelerated HCC and CC development. Furthermore, HCC and CC areas in the liver were associated with the repopulation with GRP78-positive cells, which may confer selective advantage for the cancer cells.
Figure 5.
Accelerated HCC and CC formation and GRP78 re-expression in cPf/f78f/f livers. (a) Kaplan-Meier plot of WT, cPf/f, cPf/f78f/+ and cPf/f78f/f mice showing tumor nodule-free percentage over time. n=3 to 11 for each genotype at each time point. (b) Upper: H&E staining of liver sections from WT and cPf/f78f/f mice at 8–9 months. cPf/f78f/f mice showed histological features of HCC and CC. Lower: immunohistochemistry staining of GRP78 on liver sections of WT mice and tumor sections from cPf/f78f/f mice aged 8–9 months. (c) H&E staining of liver tumor sections from cPf/f and cPf/f78f/+ mice at 12 months, which showed the presence of both HCC and CC. Scale bars show 100 µm and are applicable to panels b and c. (d) Schematic model of tumor development in cPf/f78f/f mice. Hepatic GRP78 expression level at each stage corresponds to the color gradient bar on the upper left corner. The major phenotypes at each stage are indicated below. BD: bile duct; PV: portal vein.
DISCUSSION
Mutation or deletion of PTEN is commonly observed in liver cancer and GRP78 is required for tumorigenesis in a variety of cancers.13 In studying the role of GRP78 in liver cancer development using the loss of PTEN as a model system, we made several unexpected discoveries which expand our understanding on how GRP78 regulates tumorigenesis in a metabolic organ. In the case of liver, injury leads to hepatocyte death that in turn activates proliferation of residual hepatocytes and/or liver progenitor cells, which may eventually promote liver cancer.10,11 We noted that unlike other tissue-specific knockout models where GRP78 can be nearly completely eliminated,14,28,29 the c78f/f and cPf/f78f/f livers maintained about 30% of WT level of GRP78. This is consistent with a previous study using a different Alb-Cre transgenic mouse strain.20 Interestingly, both residual white and brown adipose tissues maintain 40% GRP78 expression in adipose Grp78 knockout mice.18 This suggests that GRP78 may be indispensable for survival in liver and adipose tissues. While male c78f/f mice maintained in these studies for 14 months showed no malignancy, GRP78 reduction combined with loss of PTEN led to onset of HCC and CC at 8–9 months. Based on our findings, we propose the following model which is summarized in Figure 5D. In a normal liver, GRP78 is constitutively expressed and maintains liver homeostasis. However, in cPf/f78f/f livers, there is loss of PTEN and gradual depletion of GRP78 following Alb-Cre-mediated recombination at 3 months. The loss of GRP78 protection at this stage exacerbates fat accumulation caused by PTEN deficiency. Fatty liver leads to liver injury, which activates bile duct and liver progenitor cell proliferation. At 6 months, JNK activation, coupled with β-catenin loss and PDGFRα upregulation could also contribute to liver injury.30,31 At the same time, GRP78 cannot be ablated completely in hepatocytes. Bile duct and progenitor cells that escape Cre exhibit growth advantage and repopulate the liver with GRP78-positive cells. At 8–9 months, HCC and CC growth is evident in cPf/f78f/f livers, correlating with strong GRP78 expression, and GRP78 level in adjacent normal areas is comparable to the WT level. While the molecular mechanisms leading to these events await future investigations, our results raise several interesting questions.
First, why does GRP78 reduction combined with PTEN deletion in the liver accelerate liver tumorigenesis, whereas GRP78 knockout in other tissues usually inhibits tumor formation? Liver cancer is unique in that cancer arises as a result of injury and regeneration. In the context of PTEN loss, we observed that GRP78 reduction fueled liver steatosis, apparently via a mechanism independent of XBP-1, which led to injury that induced progenitor cell proliferation. If GRP78 expression is eliminated in the tumor cells, tumor development may be arrested as seen in the other cancer models. However, in the case of cPf/f78f/f livers, GRP78-positive bile duct and progenitor cells that escaped Alb-Cre repopulated the liver, thus the developed liver tumors were not handicapped by the lack of GRP78. Consistent with this explanation are the findings that β-catenin-positive cells repopulate β-catenin-null livers due to a progressive loss of Alb-Cre expression,32 and GRP78-positive cells rapidly repopulate the intestinal epithelium upon genetic knockout in AhCre1-Grp78f/f mice.33
With regard to the origin of the HCC and CC in cPf/f78f/f livers, HCC and CC could be derived from liver progenitor cells.11 Our immunohistochemistry data indicated strong GRP78 expression in HCC and CC tissues, and that some cells around proliferating bile ducts exhibited liver progenitor cell morphology and also expressed GRP78. These observations suggest the possibility that liver progenitor cell is the origin of malignancies. Interestingly, transdifferentiation of hepatocytes to bile duct epithelial cells has been demonstrated.34 Conversely, some biliary epithelial cells appear capable of differentiating to hepatocytes.35 Thus, future studies will be required to address whether the GRP78-positive bile duct cells give rise to GRP78-positive liver cancers.
GRP78 overexpression has been reported in various tumors, including HCC.36,37 As the major chaperone with potent anti-apoptotic properties and a signaling regulator, GRP78 protects tumor cells from ER stress and host cell defense, thereby promoting tumor growth. This is consistent with our observation that GRP78 was upregulated in cPf/f liver tumors (Figure S6). GRP78 has been reported to protect liver cancer cells from ER stress-induced apoptosis and promote their invasion in a metastasis model.37,38 Thus, robust GRP78 expression may indeed be required for liver cancer progression. Since several therapeutics against GRP78 are in development,13 it will be interesting to treat liver cancer with these agents to see if they can block the re-expression of GRP78 and suppress liver cancer progression, while maintaining essential liver functions. In treatment of other cancers with anti-GRP78 agents, since the adult liver can function normally with partial GRP78 level whereas cancer requires high level of GRP78, the damage to normal liver may be limited. As reported recently, antibodies against cell surface GRP78 were well tolerated in pharmacokinetic/toxicology studies in mice, monkeys and patients.39,40
JNK signaling pathway has been linked to the growth of carcinogen-induced HCC and about 50–60% of human HCC shows strong activation of JNK1.41 The function of sustained JNK activation in hepatocyte death and subsequent inflammation and carcinogenesis is recapitulated in various genetic mouse models developing HCC.30 Our results revealed that p-JNK was moderately elevated in cPf/f78f/f livers at 6 months, accompanied by increased inflammation (data not shown) prior to HCC and CC formation, suggesting that JNK might partially contribute to accelerated liver tumorigenesis. While this requires validation, the extent of apoptosis remained low (below 2%) in cPf/f78f/f livers at 6 months (data not shown), thus moderate activation of JNK in these livers did not majorly affect apoptosis linked to sustained activation of JNK.
β-catenin signaling, the central effector of canonical Wnt signaling pathway and a recognized oncogene, is also implicated in HCC. However, overexpressing β-catenin in transgenic mouse models has not exhibited spontaneous HCC.31 Paradoxically, hepatic β-catenin loss impairs the ability of the liver to counteract DEN-induced oxidative stress and enhances HCC through PDGFRα/PI3K/AKT(Thr308 phosphorylation) signaling.26 Furthermore, hepatic β-catenin deletion increases fatty liver and steatohepatitis under diet-induced metabolic stress.42 These results suggest that β-catenin loss can sensitize livers to injury and eventually tumorigenesis, which mimics the predominant scenario of human HCC where tumors often occur in steatohepatitis or cirrhosis background.31 cPf/f78f/f livers exhibited enhanced injury compared to cPf/f livers, and we observed β-catenin downregulation along with PDGFRα upregulation and significantly increased p-AKT(Thr308) (data not shown) in the liver at 6 months. Oxidative stress and overexpression of PDGF have been reported in PTEN-null livers.10 In combination, these factors might promote PDGFRα activation. Therefore, it is tempting to speculate that increased PDGFRα may be one of the mechanisms in our model to accelerate liver tumors, and hepatic β-catenin loss might also sensitize the liver to the injury induced by PTEN and GRP78 double knockout, further promoting tumorigenesis. Liver fat accumulation, injury and tumorigenesis can be also promoted by loss of autophagy, which can be monitored by increase in P62.43 Interestingly, we observed a trend of increased P62 level in cPf/f78f/f livers, suggesting perturbation of autophagy (Figure S7). Future studies are required to address whether these altered pathways are causative factors for the HCC and CC formation in cPf/f78f/f livers. In summary, our studies reveal new mechanisms affected by GRP78 reduction in PTEN-null induced tumorigenesis. The role of GRP78 in different forms of liver cancer and other metabolic cancers warrants further investigation.
MATERIALS AND METHODS
Mice
Grp78f/f mice on a mixed C57BL/6;129/Sv background14,19 and Ptenf/fGrp78f/f mice on a C57BL/6;6xDBA2;129 background were previously described.14 Ptenf/fGrp78f/f mice were crossed with the transgenic Alb-Cre; Ptenf/f (cPf/f) mice on a C57BL/6;J129svj background10 to generate Alb-Cre; Ptenf/fGrp78f/f (cPf/f78f/f) and Alb-Cre; Ptenf/fGrp78f/+ (cPf/f78f/+) mice. Grp78f/f mice were crossed with cPf/f78f/f mice to generate Alb-Cre; Grp78f/f (c78f/f) mice (Figure S1). Littermates that were negative for the Cre transgene were used as WT controls. Male mice were used in all experiments. Genotyping was performed by PCR using genomic DNA extracted from mouse tails and liver biopsies as previously described.14 Blood samples were collected through retro-orbital bleeding prior to tissue collection. All protocols for animal use were reviewed and approved by the USC Institutional Animal Care and Use Committee.
Histology
Mice were euthanized and their livers were removed and rinsed in PBS. Samples collected from the livers were immediately frozen for RNA and protein extraction, fixed in 10% zinc formalin, or frozen in OCT compound (Tissue-Tek Sakura, Torrance, CA). Formalin-fixed tissue samples were embedded in paraffin, sectioned at 4 µm and stained with H&E for morphology analysis. Frozen tissue samples were sectioned at 10 µm for Oil Red O staining.
Immunohistochemistry staining
The staining procedures of paraffin sections were performed as described.14 Polyclonal rabbit anti-GRP78 (H-129, 1:100, Santa Cruz Biotechnology, Dallas, TX) antibody was used to determine GRP78 levels in the liver. Cell proliferation was evaluated by Ki67 staining (Ab-4, 1:200, Thermo Scientific, Fremont, CA).
Oil Red O staining
Frozen liver sections were briefly fixed in cold 10% formalin. Slides were stained in Oil Red O solution (3 mg/ml) and hematoxylin for counterstain.
Plasma alanine aminotransferase (ALT) quantitation
Plasma ALT was determined using ALT Reagent (Raichem, San Diego, CA).
TUNEL assay
Apoptosis was determined using TUNEL staining (Roche Diagnostics, Mannheim, Germany).
Western blot analyses
Western blot analyses were performed as described.14 Liver samples were homogenized in RIPA buffer with added protease and phosphatase inhibitor cocktail (Pierce, Rockford, IL) and 25 µg cell lysate was subjected to SDS-PAGE. The immunoblot membranes were incubated with primary antibodies at 4°C overnight and the protein signals were detected with ECL reagent or SuperSignal West Femto stable peroxide buffer (Pierce, Rockford, IL) after reacting with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX). The primary antibodies used are as follows: Monoclonal mouse anti-GRP78 (1:2000) is a gift of Parkash Gill (Keck School of Medicine of USC). Monoclonal rat anti-GRP94 (1:5000) is from Enzo Life Sciences (Farmingdale, NY). Mouse anti-PTEN (26H9, 1:1000), rabbit anti-AKT (1:1000), rabbit anti-p-AKT(Ser473) (1:1000), rabbit anti-ERK1/2 (1:1000) and mouse anti-p-ERK1/2 (Thr202/Tyr204) (E10, 1:1000) are from Cell Signaling (Danvers, MA). Rabbit anti-JNK (FL, 1:1000), rabbit anti-p-JNK (G-7, 1:1000) and rabbit anti-β-catenin (H-102, 1:3000) are from Santa Cruz Biotechnology. Rabbit anti-PDGFRα (N2C2, internal, 1:1000) is from GeneTex (Irvine, CA). Mouse anti-β-actin (1:5000) is from Sigma (St. Louis, MO).
Real-time quantitative PCR
RNA was extracted from mouse livers and reverse-transcription and real-time PCR were performed as previously described.44 Primers used for AFP, EpCAM, CK19 and 18S RNA have been described.10,45 The following primers were used for lipogenic genes: SREBP-1c, 5’-GTTACTCGAGCCTGCCTTCAGG-3’ and 5’-CAAGCTTTGGACCTGGGTGTG-3’; ACC1, 5’-GGACAGACTGATCGCAGAGAAAG-3’ and 5’-TGGAGAGCCCCACACACA-3’; FAS, 5’-GCTGCGGAAACTTCAGGAAAT-3’ and 5’-AGAGACGTGTCACTCCTGGACTT-3’.
Statistical analysis
Statistical significance was assayed by 2-tailed Student’s t test, and the error bars reflect standard error (S.E.).
Supplementary Material
ACKNOWLEDGEMENTS
We like to thank Dr. Louis Dubeau and members of the Lee laboratory for helpful discussions and Dr. Chengyu Liang for the gift of antibodies. We also like to thank the USC Norris Comprehensive Cancer Center Cell and Tissue Imaging and Translational Pathology Core Facilities supported by NCI grant P30 CA014089 for histology and the Tissue Imaging Core Facility of the USC Research Center for Liver Diseases (P30 DK048522) for microscopy. This work was supported in part by funding from the National Institutes of Health (R01 CA027607 and P01 AG034906) to ASL.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–156. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 4.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299–2306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- 6.Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181–188. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 8.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 9.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected] Proc Natl Acad Sci USA. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galicia VA, He L, Dang H, Kanel G, Vendryes C, French BA, et al. Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2. Gastroenterology. 2010;139:2170–2182. doi: 10.1053/j.gastro.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 12.Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71:289–297. [PubMed] [Google Scholar]
- 13.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, et al. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wey S, Luo B, Tseng CC, Ni M, Zhou H, Fu Y, et al. Inducible knockout of GRP78/BiP in the hematopoietic system suppresses Pten-null leukemogenesis and AKT oncogenic signaling. Blood. 2012;119:817–825. doi: 10.1182/blood-2011-06-357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye R, Jung DY, Jun JY, Li J, Luo S, Ko HJ, et al. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye R, Mareninova OA, Barron E, Wang M, Hinton DR, Pandol SJ, et al. Grp78 heterozygosity regulates chaperone balance in exocrine pancreas with differential response to cerulein-induced acute pancreatitis. Am J Pathol. 2010;177:2827–2836. doi: 10.2353/ajpath.2010.100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu G, Ye R, Jung DY, Barron E, Friedline RH, Benoit VM, et al. GRP78 plays an essential role in adipogenesis and postnatal growth in mice. FASEB J. 2013;27:955–964. doi: 10.1096/fj.12-213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229–239. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Itoh T, Tanimizu N, Miyajima A. Liver stem/progenitor cells: their characteristics and regulatory mechanisms. J Biochem. 2011;149:231–239. doi: 10.1093/jb/mvr001. [DOI] [PubMed] [Google Scholar]
- 23.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Huang Z, Du Y, Cheng Y, Chen S, Guo F. ATF4 regulates lipid metabolism and thermogenesis. Cell Res. 2010;20:174–184. doi: 10.1038/cr.2010.4. [DOI] [PubMed] [Google Scholar]
- 25.Zeng N, Li Y, He L, Xu X, Galicia V, Deng C, et al. Adaptive basal phosphorylation of eIF2alpha is responsible for resistance to cellular stress-induced cell death in Pten-null hepatocytes. Mol Cancer Res. 2011;9:1708–1717. doi: 10.1158/1541-7786.MCR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang XF, Tan X, Zeng G, Misse A, Singh S, Kim Y, et al. Conditional beta-catenin loss in mice promotes chemical hepatocarcinogenesis: role of oxidative stress and platelet-derived growth factor receptor alpha/phosphoinositide 3-kinase signaling. Hepatology. 2010;52:954–965. doi: 10.1002/hep.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stock P, Monga D, Tan X, Micsenyi A, Loizos N, Monga SP. Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther. 2007;6:1932–1941. doi: 10.1158/1535-7163.MCT-06-0720. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Ye R, Barron E, Baumeister P, Mao C, Luo S, et al. Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ. 2010;17:488–498. doi: 10.1038/cdd.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wey S, Luo B, Lee AS. Acute inducible ablation of GRP78 reveals its role in hematopoietic stem cell survival, lymphogenesis and regulation of stress signaling. PLoS One. 2012;7:e39047. doi: 10.1371/journal.pone.0039047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikolaou K, Sarris M, Talianidis I. Molecular pathways: the complex roles of inflammation pathways in the development and treatment of liver cancer. Clin Cancer Res. 2013;19:2810–2816. doi: 10.1158/1078-0432.CCR-12-1961. [DOI] [PubMed] [Google Scholar]
- 31.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson MD, Wickline ED, Bowen WB, Lu A, Singh S, Misse A, et al. Spontaneous repopulation of beta-catenin null livers with beta-catenin-positive hepatocytes after chronic murine liver injury. Hepatology. 2011;54:1333–1343. doi: 10.1002/hep.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering M, et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 2013;3:1128–1139. doi: 10.1016/j.celrep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract. 1995;191:513–524. doi: 10.1016/s0344-0338(11)80870-8. [DOI] [PubMed] [Google Scholar]
- 36.Luk JM, Lam CT, Siu AF, Lam BY, Ng IO, Hu MY, et al. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics. 2006;6:1049–1057. doi: 10.1002/pmic.200500306. [DOI] [PubMed] [Google Scholar]
- 37.Su R, Li Z, Li H, Song H, Bao C, Wei J, et al. Grp78 promotes the invasion of hepatocellular carcinoma. BMC Cancer. 2010;10:20. doi: 10.1186/1471-2407-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Jiang K, Gao D, Kang X, Sun C, Zhang Q, et al. Clusterin protects hepatocellular carcinoma cells from endoplasmic reticulum stress induced apoptosis through GRP78. PLoS One. 2013;8:e55981. doi: 10.1371/journal.pone.0055981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hensel F, Eckstein M, Rosenwald A, Brandlein S. Early development of PAT-SM6 for the treatment of melanoma. Melanoma Res. 2013 doi: 10.1097/CMR.0b013e328362cbc8. (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 40.Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra S, et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth and metastasis. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-1106. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa H, Maeda S. Molecular mechanisms of liver injury and hepatocarcinogenesis: focusing on the role of stress-activated MAPK. Patholog Res Int. 2012;2012:172894. doi: 10.1155/2012/172894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744–753. doi: 10.2353/ajpath.2010.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding WX. Role of autophagy in liver physiology and pathophysiology. World J Biol Chem. 2010;1:3–12. doi: 10.4331/wjbc.v1.i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni M, Zhou H, Wey S, Baumeister P, Lee AS. Regulation of PERK signaling and leukemic cell survival by a novel cytosolic isoform of the UPR regulator GRP78/BiP. PLoS One. 2009;4:e6868. doi: 10.1371/journal.pone.0006868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo B, Lam BS, Lee SH, Wey S, Zhou H, Wang M, et al. The endoplasmic reticulum chaperone protein GRP94 is required for maintaining hematopoietic stem cell interactions with the adult bone marrow niche. PLoS One. 2011;6:e20364. doi: 10.1371/journal.pone.0020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.