Abstract
Objectives
The vaginal microbiota help protect the female genital tract from disease. We sought to describe the composition of the vaginal microbiota between pre-, peri- and postmenopausal women and to explore the association between the microbiota and vulvovaginal atrophy (VVA).
Methods
87 women (age 35–60) were classified as premenopausal (n=30), perimenopausal (n=29) or postmenopausal (n=28) according to STRAW guidelines. Mid-vagina bacterial community composition was characterized by 16S rRNA gene analysis.
Results
Bacterial communities clustered into six community state types (CSTs), of which four were dominated by Lactobacillus crispatus, L. gasseri, L. iners, or L. jensenii; and two (CST-IV-A and IV-B) had low relative abundance of Lactobacillus. CST IV-A was characterized by Streptococcus and Prevotella, whereas CST IV-B by Atopobium. There was a significant association between menopause stage and CST (p-value=0.004) and VVA and CST (p-value=0.002). Perimenopausal women were more likely to be classified as CST IV-A or the L. gasseri CST, whereas postmenopausal women were mostly CST IV-A. CSTs dominated by L. crispatus and L. iners were more prevalent in premenopausal women. Nineteen participants had signs of mild or moderate VVA. Compared to women with no VVA, the vaginal microbiota of women with mild or moderate atrophy had 25-fold greater odds of being classified as CST IV-A vs. L. crispatus CST (aOR: 25.89, 95% Credible Interval:2.98-406.79).
Conclusions
A distinct bacterial community state (CST IV-A) with low relative abundance of Lactobacillus was associated with VVA. Future studies recruiting a larger number of women are needed to replicate the findings. This study provides an impetus for future longitudinal studies designed to manage, modulate and restore vaginal microbiota homeostasis which would provide stronger evidence for a causal relationship with VVA and ultimately improve treatment and prevention of atrophic vaginitis in menopause.
Keywords: menopause, vaginal microbiota, vaginal atrophy, 16S rRNA gene analysis
INTRODUCTION
As women approach menopause, estrogen decline can lead to vulvovaginal atrophy (VVA).1 Several studies have estimated that 25–50% of postmenopausal women experience VVA which can include vulvovaginal symptoms of burning on urination, bleeding after intercourse, painful sexual intercourse, and vaginal discharge, soreness, itching or burning sensations.1–7 While topical estrogen is effective in treating symptoms of VVA in menopausal women3, 8, hormone therapy (HT) is contraindicated in some women, such as those with a history of breast cancer, known coronary artery disease, prior venous thromboembolic event or stroke, or active liver disease.6 Furthermore, potential side effects of estrogen therapy include vaginal bleeding, breast pain, and perineal pain.9 Optimal HT route, dosage and non-hormonal alternatives have not been well studied.10 Some vulvovaginal symptoms can be alleviated by use of vaginal lubricants3, however, emerging data suggest that lubricants may adversely affect the vaginal epithelium, lamina propria and the vaginal microbiota.11–14 New interventions are needed to reduce vulvovaginal symptoms caused by VVA in postmenopausal women.15
One approach to VVA that has not been well explored is harnessing the protective features of the vaginal microbiome.16, 17 The vaginal microbiota play an important role in preventing colonization by pathogenic organisms, including sexually transmitted and urinary tract infectious agents, and broadly act to maintain a woman’s gynecologic and reproductive health.18–20 The predominant connection between the vaginal microbiome and menopause is through the influential action of estrogen. Estrogen contributes to vaginal epithelium maturation through deposition of glycogen in the vaginal epithelium.3 Glycogen is metabolized by indigenous bacterial communities to produce organic acids (primarily lactate) which is thought to protect the genital tract.21 Prior studies of vaginal bacteria have largely been conducted using bacteriological culture techniques; however, a majority of microbial species (>90%) resist cultivation in the laboratory.22, 23 Complete characterization of the vaginal microbiota requires molecular approaches.24 To date, molecular studies (cultivation-independent censuses) of the vaginal microbiota have focused on reproductive-age women, mainly age 18–45 years.25–28 These studies have found a multitude of bacteria that could not be identified by traditional culture-based methods.29, 30 Molecular studies from our group and others31, 32 have shown that the diversity, composition, and relative abundance of microbial species in the vagina varies dramatically, but reproductive-age women can be grouped into five consistent groupings, referred to by Ravel et al.28 as community state types (CSTs). These five CSTs are often dominated by Lactobacillus crispatus (CST I), Lactobacillus gasseri (CST II), Lactobacillus iners (CST III), or Lactobacillus jensenii (CST V), whereas the fifth (CST IV) has lower proportions of Lactobacillus spp. and higher proportions of anaerobic organisms including Mobiluncus spp., and Atopobium vaginae. CST IV was recently divided into two sub-states, termed CST IV-A and IV-B, by Gajer and Brotman et al.32 CST IV-A is characterized by various species of anaerobic bacteria belonging to the genera Anaerococcus, Peptoniphilus, Prevotella and Streptococcus, while CST IV-B has higher proportions of the genera Atopobium and Megasphaera among others. Species-specific differences in vaginal microbiota have been shown to be important, such as in a recent study by Srinivasan et al.26, in which various bacterial species are associated with each of the four Amsel clinical signs for diagnosis of bacterial vaginosis (BV), suggesting links between specific vaginal bacteria and clinical signs.
In this study, we seek to determine whether the composition of vaginal microbiota differs between pre-, peri- and postmenopausal women using culture independent techniques and to explore the association between the vaginal microbiota and signs of VVA.
METHODS
Study population and specimen collection
Women included in this study were enrolled in a prospective cohort study assessing human papillomavirus in perimenopause (HIP study), which has been previously described.33–36 Briefly, 885 women aged 35–60 years, attending one of four outpatient gynecology clinics for routine examination in Baltimore, MD, were enrolled. Women were eligible for enrollment if they were not currently pregnant, had an intact uterus, and no history of organ transplantation or HIV infection. All medication use was also recorded. One woman in our study sample reported use of Prednisone and one reported Tamoxifen.
Menopausal stage was determined using the Stages of Reproductive Aging Workshop (STRAW) staging system37 which is based on day of the last menstrual cycle and self-reported cycle length variability in the past 12 months. Women were classified as premenopausal if they had a menstrual period in the past 12 months and reported no menstrual cycle variability. Currently menstruating women who reported menstrual cycle length variability were considered perimenopausal, and women without a menstrual cycle in the past 12 months were considered postmenopausal. The vaginal maturation index (VMI) to assess parabasal, intermediate and superficial vaginal epithelial cell types was not collected because it is not used in clinical practice1 and was not used in the context of the HIP study.
An interviewer-administered questionnaire collected detailed information on demographics, gynecologic and menstrual history, medication use, exogenous hormone use history, current and past sexual behaviors, sexual functioning, general medical history, and tobacco and alcohol use. Participants underwent a standardized clinical assessment and pelvic examination, which included clinician inspection for vaginal atrophy, including signs of vaginal pallor, dryness, rugosity, blanching of tissue, friability, and petechiae. The clinician performed a speculum-assisted exam and collected a cervical swab for HPV testing (Digene HPV sampler kit) and a mid-vaginal swab for microbiota analysis (Elution swab system with 1 ml of Amies transport media, Copan). Samples were frozen at −80°C immediately after collection until processed.
For the purposes of this sub-study on composition of vaginal bacterial communities, we randomly selected 90 women evenly split across pre, peri and post menopause, for a cross-sectional analysis at their baseline visit. Additional inclusion criteria for these women were as follows: human papillomavirus-negative, no self-reported history of sexually transmitted infection in the past 6 months, no diagnosis of BV or other sexually transmitted infections at the current visit, and no exogenous hormone use. The final dataset included 87 women with complete data on bacterial composition and VVA: n= 30 women in premenopause (STRAW -3), n=29 in perimenopause (STRAW -1 and STRAW-2), and n=28 in postmenopause (STRAW +1 and STRAW +2.
All study procedures were approved by the Johns Hopkins Bloomberg School of Public Health and the University of Maryland School of Medicine Institutional Review Boards.
Composition of vaginal bacterial communities
(i) DNA extraction, 16S rRNA gene amplification and pyrosequencing
DNA extraction from vaginal swabs, PCR amplification of the V1-V2 hypervariable regions of 16S rRNA genes using primers barcoded 27F and 338R38 and pyrosequencing were performed as previously described.28 Microbiome analysis was blinded to menopause status and age.
(ii) Sequence analysis
The split-library.pl script part of the QIIME software package28 was used for quality control of the sequence reads using the following criteria: 1) no base ambiguity 2) minimum and maximum read length of 220 bp and 400 bp; 3) an average read quality of q25 over a sliding window of 50 bp. If the read quality dropped below q25 it was trimmed at the first base pair of the window and then reassessed for length criteria; 5) a perfect match to a barcode sequence; and 6) presence of the 338R 16S primer sequence used for amplification. Sequences were binned based on sample-specific barcode sequences and trimmed by removal of the barcode and primer sequences (forward, if present, and reverse). High quality sequence reads were first de-replicated using 99% similarity using the UCLUST software package39 and detection of potential chimeric sequences was performed using the UCHIME component of UCLUST 40. Chimeric sequences were then removed prior to taxonomic assignments.
Genus level taxonomic assignments were performed by using the RDP Classifier41, and further species level assignments of Lactobacillus sp. were done using 126 HMM Lactobacillus species models using the software speciateIT (speciateIT.sourceforge.net).28 For each sample, vectors of phylotype proportions were clustered into CSTs as previously described by Gajer et al.32
Statistical analysis
We sought to describe the differences in vaginal bacterial community composition between pre-, peri- and postmenopausal women and to evaluate clinical associations with the vagina’s bacterial composition and abundance. Unadjusted associations were assessed using Fisher’s exact test. Bayesian multinomial logistic regression was utilized for modeling the microbiota because CST (the outcome) consisted of six categories and the data were too sparse for large-sample frequentist inference.42, 43 For all analyses, CST I (dominated by L. crispatus) was treated as the reference because it was the most common CST. Models were adjusted for the following confounders: sexual activity in prior 6 months, race/ethnicity, marital status, and lifetime number of sexual partners. Estimation was carried out using Markov-chain Monte Carlo sampling. Convergence of the Markov chain was assessed using the Geweke, Heidelberger-Welch, and Raftery diagnostics.44–46 Some CSTs were combined so that no categories had zero women in order to ensure model convergence. CST IVA and CST IVB were combined to overcome data sparseness and because they are both low-Lactobacillus states with a large degree of overlap on diversity of other anaerobes.32 CST II and CST V were also combined to overcome data sparseness (there were relatively few women in each, L. gasseri and L. jensenni-dominated) and because both of these Lactobacillus species are considered to be protective in epidemiologic studies based on BV, metabolomics and pH outcomes.28, 47
Among women who were classified to the non-Lactobacillus dominated CST IV-A and IV-B, a sensitivity analysis was performed by evaluating the abundance of 205 individual bacteria in their association with menopause status and clinical outcomes using redundancy analysis (RDA) controlling for confounding factors. RDA is a dimension-reduction approach based on principal components.48 Model inference is performed using MANOVA and tested using a permutation test. Analyses were performed in R version 2.15.1 using the Zelig, MCMCpack, CODA, and vegan packages.48–51 Statistical significance was defined as p< 0.05 or Bayesian credible intervals for odds ratios excluding 1.
RESULTS
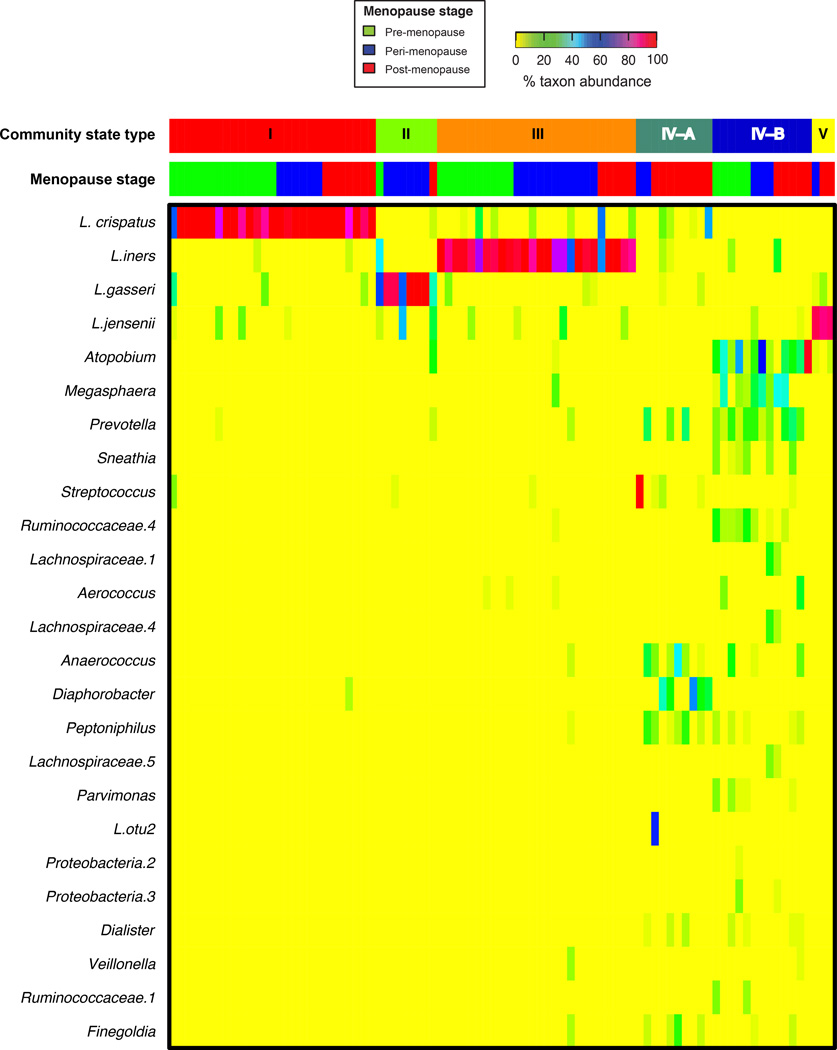
By using methods we have described previously32, the bacterial communities of the 87 women were classified into one of six CSTs based on differences in species composition and their relative abundances. The 25 most abundant bacteria (of 205 observed) are displayed in Figure 1. Lactobacillus spp. were the dominant members of the vaginal microbiota in each stage of menopausal transition (83% in premenopausal women, 83% in perimenopause and 54% in postmenopause).
Figure 1. Heatmap of relative percent abundance of bacterial taxa found in the vaginal bacterial communities of 87 women in pre-, peri- and post-menopause.
Vaginal microbiota clustered into six community state types; CST-I, II, III and V were dominated by Lactobacillus crispatus, L. gasseri, L. iners, or L. jensenii, respectively. Two CSTs lacked significant numbers of lactobacilli (CST-IV-A and IV-B). This heatmap lists the top 25 most abundant bacteria; however data is available on more than 200 bacteria found in the female genital tract of these participants. CSTs are defined using Jensen-Shannon divergence between all pairs of community states and Ward linkage hierarchical clustering as described by Gajer et al.32. Menopause stage is indicated at the top of the heatmap.
Demographic and clinical factors at baseline are presented in Table 1. Among postmenopausal women, 29% were classified to CST IV-A, 25% to CST I (L. crispatus-dominated) and 18% to CST III (L. iners dominated). Forty-six percent of premenopausal women were dominated by L. crispatus (CST I) while 38% of perimenopausal women were dominated by L. iners (CST III). Nineteen women were found on clinical exam to have mild or moderate vaginal atrophy, 14 of which were postmenopausal. Distribution of CSTs, signs of vaginal atrophy, and age were the only significant differences observed between pre-, peri- and postmenopausal women. Table 2 displays the distribution of women by CST and signs of VVA. Women classified to CST IV-A were noted by the clinicians to have the most severe signs of VVA.
Table 1.
Demographic and clinical data by menopausal transition status, Baltimore, MD (n=87)
| Pre- menopause |
Peri- menopause |
Post- menopause |
P- valueb |
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Community state type (CST)a, dominant bacterial species | 0.004 | ||||||
| CST I (Lactobacillus crispatus) | 14 | 46.7 | 6 | 20.7 | 7 | 25.0 | |
| CST II (L. gasseri) | 1 | 3.3 | 6 | 20.7 | 1 | 3.6 | |
| CST III (L. iners) | 10 | 33.3 | 11 | 37.9 | 5 | 17.9 | |
| CST IV-A (Low-lactobacillus) | 0 | 0.0 | 2 | 6.9 | 8 | 28.6 | |
| CST IV-B (Low-lactobacillus) | 5 | 16.7 | 3 | 10.3 | 5 | 17.9 | |
| CST V (L. jensenii) | 0 | 0.0 | 1 | 3.5 | 2 | 7.1 | |
| Signs of vulvovaginal atrophy | 0.000 | ||||||
| None | 27 | 93.1 | 21 | 87.5 | 12 | 46.2 | |
| mild | 2 | 6.9 | 2 | 8.3 | 9 | 34.6 | |
| moderate | 0 | 0.0 | 1 | 4.2 | 5 | 19.2 | |
| Signs of vaginal dryness | 0.003 | ||||||
| none | 28 | 96.6 | 23 | 92.0 | 17 | 60.7 | |
| mild | 1 | 3.5 | 2 | 8.0 | 5 | 17.9 | |
| moderate | 0 | 0.0 | 0 | 0.0 | 5 | 17.9 | |
| severe | 0 | 0.0 | 0 | 0.0 | 1 | 3.6 | |
| Atrophic vaginitis requiring treatment | 0 | 0.0 | 0 | 0.0 | 2 | 8.3 | 0.186 |
| Age | 0.000 | ||||||
| 35–39 | 4 | 13.3 | 1 | 3.5 | 1 | 3.6 | |
| 40–44 | 9 | 30.0 | 5 | 17.2 | 0 | 0.0 | |
| 45–49 | 13 | 43.3 | 12 | 41.4 | 1 | 3.6 | |
| 50–54 | 4 | 13.3 | 10 | 34.5 | 8 | 28.6 | |
| 55–60 | 0 | 0.0 | 1 | 3.5 | 18 | 64.3 | |
| BMI | 0.432 | ||||||
| underweight | 0 | 0.0 | 0 | 0.0 | 1 | 3.6 | |
| normal | 13 | 43.3 | 10 | 34.5 | 11 | 39.3 | |
| overweight | 4 | 13.3 | 10 | 34.5 | 7 | 25.0 | |
| obese | 13 | 43.3 | 9 | 31.0 | 9 | 32.1 | |
| Ethnicity | 0.288 | ||||||
| white | 20 | 66.7 | 24 | 82.8 | 22 | 81.5 | |
| black | 8 | 26.7 | 2 | 6.9 | 4 | 14.8 | |
| other | 2 | 6.7 | 3 | 10.3 | 1 | 3.7 | |
| Education | 0.538 | ||||||
| high school | 4 | 13.3 | 5 | 17.2 | 5 | 17.9 | |
| some college | 10 | 33.3 | 3 | 10.3 | 6 | 21.4 | |
| college graduate | 9 | 30.0 | 12 | 41.4 | 8 | 28.6 | |
| postgraduate | 7 | 23.3 | 9 | 31.0 | 9 | 32.1 | |
| Marital status | 0.052 | ||||||
| married | 19 | 63.3 | 16 | 55.2 | 21 | 75.0 | |
| widowed | 1 | 3.3 | 0 | 0.0 | 0 | 0.0 | |
| separated | 3 | 10.0 | 0 | 0.0 | 0 | 0.0 | |
| divorced | 1 | 3.3 | 8 | 27.6 | 4 | 14.3 | |
| single | 6 | 20.0 | 5 | 17.2 | 3 | 10.7 | |
| Income | 0.698 | ||||||
| <40K | 1 | 3.3 | 2 | 6.9 | 2 | 7.1 | |
| 40–80K | 4 | 13.3 | 5 | 17.2 | 4 | 14.3 | |
| 80–120K | 3 | 10.0 | 8 | 27.6 | 7 | 25.0 | |
| >120K | 14 | 46.7 | 8 | 27.6 | 8 | 28.6 | |
| unknown | 8 | 26.7 | 6 | 20.7 | 7 | 25.0 | |
| Cigarette smoking, current | 1 | 3.3 | 3 | 10.3 | 2 | 7.1 | 0.600 |
| History of abnormal Papanicolaou smear | 16 | 53.3 | 13 | 46.4 | 10 | 38.5 | 0.538 |
| Lifetime sex partners, male | |||||||
| 0–5 | 20 | 69.0 | 17 | 58.6 | 19 | 67.9 | |
| 6–11 | 6 | 20.7 | 7 | 24.1 | 8 | 28.6 | |
| 11–20 | 3 | 10.3 | 4 | 13.8 | 1 | 3.6 | |
| 21+ | 0 | 0.0 | 1 | 3.5 | 0 | 0.0 | |
| New sex partner in the past 6 months | 0.099 | ||||||
| no sex | 5 | 16.7 | 12 | 41.4 | 6 | 21.4 | |
| sex, no new sex partner | 24 | 80.0 | 17 | 58.6 | 22 | 78.6 | |
| new sex partner | 1 | 3.3 | 0 | 0.0 | 0 | 0.0 | |
| Ever use hormone replacement therapy | 0.207 | ||||||
| never | 30 | 100.0 | 28 | 96.6 | 26 | 92.9 | |
| former | 0 | 0.0 | 1 | 3.5 | 2 | 7.1 | |
Community state types defined by Gajer et al.32 and described in the text.
Fisher's exact p-value
Table 2.
Number of women with signs of vulvovaginal atrophy by bacterial community state type
| community state type | CST I | CST II | CST III | CST-IVA | CST-IVB | CST V | p-valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dominant bacterial species | L. crispatus | L. gasseri | L. iners | low- lactobacillus, diverse |
Atopobium, Gardnerella |
L. jensenni | |||||||
| vaginal atrophy | N | % | N | % | N | % | N | % | N | % | N | % | 0.002 |
| none | 21 | 84.0 | 6 | 100.0 | 21 | 91.3 | 2 | 22.2 | 8 | 61.5 | 2 | 66.7 | |
| mild | 3 | 12.0 | 0 | 0.0 | 2 | 8.7 | 3 | 33.3 | 4 | 30.8 | 1 | 33.3 | |
| moderate | 1 | 4.0 | 0 | 0.0 | 0 | 0.0 | 4 | 44.4 | 1 | 7.7 | 0 | 0.0 | |
| vaginal dryness | |||||||||||||
| none | 23 | 92.0 | 6 | 100.0 | 21 | 91.3 | 5 | 55.6 | 10 | 76.9 | 2 | 66.7 | 0.011 |
| mild | 1 | 4.0 | 0 | 0.0 | 2 | 8.7 | 0 | 0.0 | 2 | 15.4 | 1 | 33.3 | |
| moderate | 1 | 4.0 | 0 | 0.0 | 0 | 0.0 | 4 | 44.4 | 0 | 0.0 | 0 | 0.0 | |
| severe | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 7.7 | 0 | 0.0 | |
Fisher's exact p-value
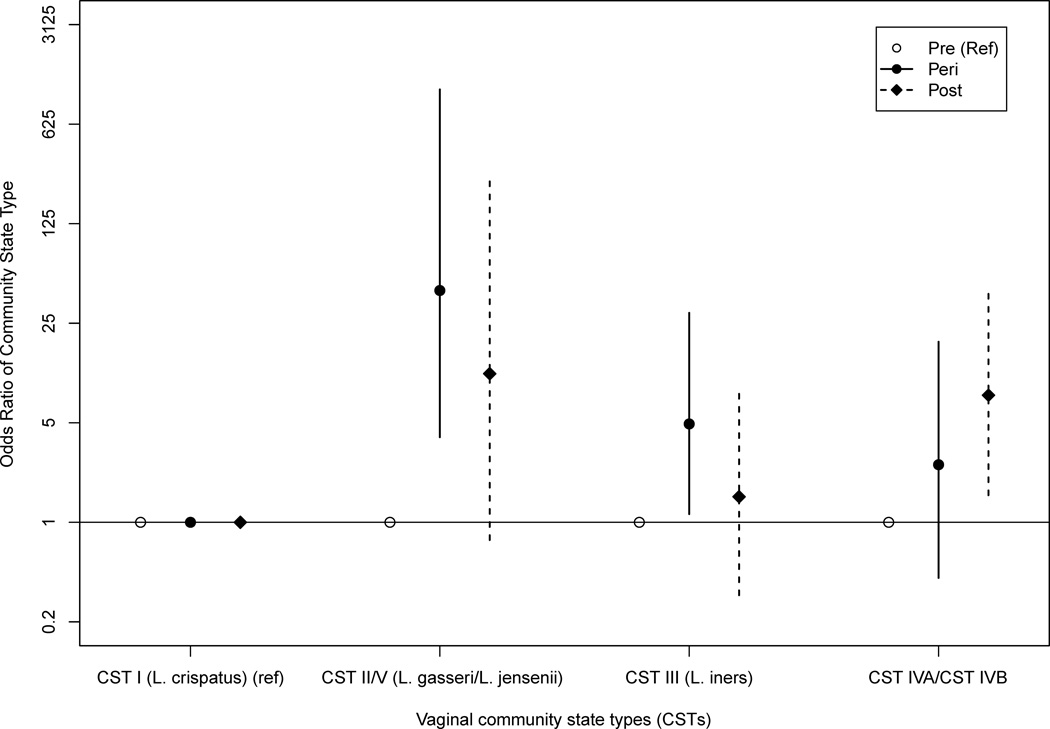
In order to model the association between menopause stage and CST (Figure 2 and Table 3), we combined CST IV-A with CST IV-B into one category and also CST II (L. gasseri-dominated) with CST V (L. jensenii-dominated) into a category to address data sparseness. Postmenopausal women had a 7.80 greater odds (95% Credible Interval (CrI) 1.55–41.50) of being classified as CST IV-A or CST IV-B versus the L. crispatus-dominated CST I than pre-menopausal women. Perimenopausal women had a 42.43 greater odds (95% CrI: 3.95–1096) of being classified as CST II/V (L. gasseri or L. jensenii-dominated) versus CST I (L. crispatus-dominated) than premenopausal women.
Figure 2.
Odds ratio for community state type (CST) by menopausal stage adjusted for sexual activity in prior 6 months, race/ethnicity, marital status, and lifetime number of sexual partners
Table 3.
Association between vaginal bacterial community state type and menopausal status
| Community state type (CST) | CST I | IVA or IVBa | II or Va | III | |||
|---|---|---|---|---|---|---|---|
| Dominant bacterial species | L. crispatus | Low lactobacillus | L. gasseri or L. jensenni | L. iners | |||
| Menopausal status | ORb | aORc | OR | aOR | OR | aOR | |
| Post | 1 | 5.79 (1.51, 26.40) | 7.80 (1.55, 41.50) | 7.64 (0.66, 183.69) | 11.05 (0.75, 280.50) | 0.99 (0.23, 4.10) | 1.51 (0.35, 8.38) |
| Peri | 1 | 2.45 (0.47, 14.44) | 2.54 (0.41, 18.54) | 23.29 (2.70, 465.68) | 42.43 (3.95, 1096) | 2.73 (0.79, 11.20) | 4.91 (1.14, 29.60) |
| Pre | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
We combined CST IVA with IVB and CST II and V to address data sparseness.
OR (odds ratio) with 95% credible interval
aOR, adjusted odds ratio. Model adjusted for recent sex, race/ethnicity, marital status, and lifetime number of sexual partners
Although there were only two cases of atrophic vaginitis which required prescription treatment, which is too few for regression modeling, it is notable that both were assigned to CST IV-A, a state with relatively low Lactobacillus abundance. Both cases were postmenopausal women. (Table 1 and 4). Modeling VVA required combining CST II (L. gasseri-dominated) with CST V (L. jensenii-dominated) into a single category to address data sparseness. Compared to women with no signs of VVA, the vaginal microbiota of women with mild or moderate signs of VVA had 25-fold greater odds of being classified as CST IV-A (adjusted odds ratio [aOR]: 25.89, 95% CrI: 2.98–406.79) versus CST I (L. crispatus). Although not statistically significant, VVA tended to be more common among women classified as CST IV-B (aOR: 5.24 95% CrI: 0.85–40.42) or L. gasseri / L. jensenni-dominated (aOR: 11.05 95% CrI: 0.75—280.50) than CST I.
Table 4.
Association between vaginal bacterial community state type, menopausal status and signs of vulvovaginal atrophy, adjusted for confounding factorsa
| Community state type | (CST) CST I | CST II or Vb | CST III | CST IVA | CST IVB | ||||
|---|---|---|---|---|---|---|---|---|---|
| Dominant bacterial species | L. crispatus | L. gasseri or L. jensenni | L. iners | Low-lactobacillus | Low-lactobacillus, Atopobium | ||||
| Vulvovaginal atrophyd | OR (95% CrI)c | aOR (95% CrI) | OR (95% CrI) | aOR (95% CrI) | OR (95% CrI) | aOR (95% CrI) | OR (95% CrI) | aOR (95% CrI) | |
| Mild or Moderate | 1 | 0.53 (0.01, 5.08) | 0.32 (0.01, 3.61) | 0.46 (0.05, 2.94) | 0.46 (0.05, 3.01) | 23.58 (3.64, 234.05) | 25.89 (2.98, 406.79) | 3.50 (0.74, 18.82) | 5.24 (0.85, 40.42) |
| None | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Vaginal drynesse | |||||||||
| Mild, Moderate, or Severe | 1 | 1.20 (0.03, 12.86) | 0.96 (0.02, 15.10) | 1.10 (0.10, 17.92) | 1.09 (0.10, 11.12) | 11.15 (1.55, 122.82) | 9.92 (0.98, 117.82) | 3.89 (0.48, 42.05) | 3.71 (0.41, 40.50) |
| None | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Analysis adjusted for sexual activity in prior 6 months, race, marital status, and lifetime number of sexual partners
CSTs combined due to data sparseness.
Odds ratio (OR) and adjusted odds ratio (aOR) with 95% credible interval (CI).
Mild and moderate vulvovaginal atrophy combined due to data sparseness.
Mild, moderate and severe vaginal dryness combined due to data sparseness.
Because CST IV-A and IV-B have higher microbial diversity and both were associated with signs of VVA, we sought to determine if specific bacteria contribute to the association in a higher resolution analysis which restricted to these two state types. Among all women (all menopausal stages) assigned to CST IV-A and IV-B, there were no statistically significant associations between the clinical findings (menopause, VVA, vaginal dryness) and any individual bacteria comprising these two CSTs with adjustment for confounding variables.
DISCUSSION
Evidence is mounting that the human microbiome in later stages of life is very different from that of younger adults.52, 53 Little is known about the composition of the vaginal microbiota across the stages of menopausal transition, interactions with estrogen, or the associations with gynecologic complaints and sexual functioning in menopause. In this study, we found significant associations between vaginal bacterial composition with both menopause stage and signs of vaginal atrophy. We identified a novel community assemblage (CST IV-A), which was highly associated with signs of VVA and was predominantly found among postmenopausal women. CST IV-A is a low Lactobacillus state and is typified by a higher abundance of genera including Anaerococcus, Peptoniphilus and Prevotella, which could be playing a putative role in the clinical presentation of VVA. The transition to CST IV-A may begin in perimenopausal women who tended to be dominated by either L. iners or CST IV-A. L. iners is unusual among lactobacilli in that some strains may have pathogenic effects and current thought is that some strains may not be as protective and are less efficient at maintaining a stable vaginal microenvironment.32, 54 Rampersaud et al. have hypothesized that inerolysin, a cholesteroal dependent cytolysin produced by L. iners, may play a role in disease pathogenesis and also has an effect on the vaginal microbiota.55 Future work should follow women as they transition through menopause and document individual-level changes in the vaginal microbiota and associations with clinical outcomes. Such work could lead to interventions that maintain a woman’s premenopausal vaginal microbiota through menopause and could evaluate if the vaginal microbiota protects the female reproductive tract in menopause. Interventions might include prebiotics and probiotics alone or in conjunction with antibiotics17, 56 and changes in behaviors which are thought to negatively affect the vaginal microbiota, for example smoking57, 58 or lubricant use14, 59.
It is well known that estrogen therapy is effective for the treatment of VVA and concomitantly, hormonal replacement increases the abundance of vaginal Lactobacillus sp.60–62 However, the independent contribution of the increase in Lactobacillus to improving VVA symptoms is not known. One study by Petricevic et al.17 found orally administered probiotic L. rhamnosus GR-1 and L. reuteri RC-14 improved Nugent Gram stain scores of vaginal smears which is suggestive of increased abundance of Lactobacillus spp., but the authors did not report on change in atrophic symptoms. Gram stain scoring is based on morphologic assessment and therefore the bacterial assignments may be inaccurate.20 Future work could consider vaginal specimen assessments using molecular techniques.
The results of our study based on 16S gene analysis are consistent with prior work which was conducted using bacteriological cultivation techniques. In 1997, Hillier et al.63 reported on the vaginal bacteria of 73 postmenopausal women who had not received estrogen replacement therapy. Using biochemical assays to characterize bacteria, the authors found 49% of women harbored Lactobacillus sp., and among those that did, concentrations were 10- to 100-fold less than those observed in premenopausal women. Prevotella bivia (33% of women), Gardnerella vaginalis (27%), Ureaplasma urealyticum (13%), and Candida albicans (1%) were also reported. In a 2003 study that characterized the bacteria in the vaginal introitus using cultivation techniques, Pabich et al.64 found Lactobacillus sp. were present in the majority of postmenopausal women (60%), although more commonly found in women taking hormone replacement therapy. In 2004, Burton et al.65 reported Atopobium vaginae as a common member of the vaginal microbiota of postmenopausal women. Our study of 87 women expands the 2011 work of Hummelen et al.16 who utilized 16S rRNA gene sequencing to characterize the vaginal microbiota of 32 postmenopausal women and found an inverse correlation between Lactobacillus and dryness as well as increased bacterial diversity in women experiencing moderate to severe vaginal dryness. Among participants not reporting symptoms, L. iners and L. crispatus were generally the most abundant. Our study overcame some of the limitations of prior studies by recruiting women in various phases of menopause and by utilizing molecular techniques which can characterize the complete bacterial composition of the microbiome.
Of interest for future research is the association between the vaginal microbiota and aerobic vaginitis (AV) described by Donders et al.66 Donders designates AV as a less severe form of desquamative inflammatory vaginitis. AV may be common in menopausal women and is distinct from BV. Its diagnosis is based on microscopy (bacterial composition), host response (elevated levels of pro-inflammatory cytokines IL-6 and IL-1-β) and a yellow discharge and foul (but not fishy) odor. Based on our preliminary bacterial findings, it is possible that CST IV-A is common in AV.
Strengths of this study include the largest sample size to date across pre-, peri and postmenopausal women evaluating the vaginal microbiota in all phases of menopause transition using cultivation-independent methods. In addition, the study used standardized clinical exams, and VVA signs could be individually evaluated.
There are several limitations to the study design. The HIP study was not designed to recruit women with atrophic vaginitis, and therefore there were only two cases that required treatment in the sub-study. However, we present data on 19 women whom the clinicians described as having mild to moderate vaginal atrophy on pelvic exam. The overall sample size of 87 is still a relatively limited sample size, which resulted in wide credible intervals. These wide intervals underscore the uncertainty of the results and the need for larger studies. However, the findings will fuel hypothesis-driven work on the role of CST IV-A microbiota in VVA. In addition, this analysis was a cross-sectional study of women at different stages of menopause. Future research will characterize the dynamics of the vaginal microbiota in women as they are prospectively followed during the stages of menopause. Gardnerella vaginalis, a bacterium known to be key member in the vagina, could be under represented in this study because of the use of the primer set 27F and 338R, however there are known biases with any primer set. There may be other unknown confounding factors that need to be controlled for in the analysis. Self-reported symptoms of vaginal atrophy were not collected at the baseline visit, however vaginal symptoms, sexual activity and sexual functioning were queried at follow-up visits, hence were not included in this analysis. Lastly, there may be misclassification of STRAW staging due to errors in self-report of menstrual cycle. However, results were consistent when evaluated by age categories (data not shown).
CONCLUSION
Our data provide evidence that there are differences in the bacterial communities between pre, peri and post-menopause and we hypothesize that these anaerobes may play a role in signs and symptomatology of VVA. Probiotic pessaries may not necessarily be the answer, as it is likely a complex undertaking to shift the vaginal microbiome and host responses. We hypothesize that use of generic lactobacilli-containing probiotics in women where the Lactobacillus species in the product are not indigenous may upset homeostatic mechanisms. Personalized treatments should rationally select probiotics based on a better appreciation of species-specific28 and temporal differences32 of vaginal ecology.
It is expected that this and future work will lead to the development of studies designed to manage, modulate and restore vaginal microbiota homeostasis in an effort to improve treatment and prevention of VVA.
Acknowledgments
Sources of financial support: This study was supported by National Institutes of Health grants K01-AI080974 (Brotman), K25-AG034216 (Shardell), U01-AI070921 (Ravel), UH2-AI083264 (Ravel and Forney), and R01-CA123467 (Gravitt).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest / financial disclosure: The authors report no conflict of interest.
Data deposition: The bacterial 16S rRNA gene sequences were deposited in the National Center for Biotechnology Information Short Read Archive (SRA accession: SRA090069, study accession: SRP025158).
REFERENCES
- 1.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal Atrophy. Mayo Clinic Proceedings. 2010;85(1):87–94. doi: 10.4065/mcp.2009.0413. [10.4065/mcp.2009.0413] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon JA, Reape KZ. Understanding the menopausal experiences of professional women. Menopause. 2009;16(1):73–76. doi: 10.1097/gme.0b013e31817b614a. [DOI] [PubMed] [Google Scholar]
- 3.Sturdee DW, Panay N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. 2010;13(6):509–522. doi: 10.3109/13697137.2010.522875. [DOI] [PubMed] [Google Scholar]
- 4.Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA) - results from an international survey. Climacteric : the journal of the International Menopause Society. 2012;15(1):36–44. doi: 10.3109/13697137.2011.647840. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 5.Tan O, Bradshaw K, Carr BR. Management of vulvovaginal atrophy-related sexual dysfunction in postmenopausal women: an up-to-date review. Menopause. 2012;19(1):109–117. doi: 10.1097/gme.0b013e31821f92df. [Review] [DOI] [PubMed] [Google Scholar]
- 6.The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257–271. doi: 10.1097/gme.0b013e31824b970a. [Practice Guideline Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kingsberg SA, Krychman ML. Resistance and Barriers to Local Estrogen Therapy in Women with Atrophic Vaginitis. J Sex Med. 2013 doi: 10.1111/jsm.12120. [DOI] [PubMed] [Google Scholar]
- 8.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;(4):CD001500. doi: 10.1002/14651858.CD001500.pub2. [Meta-Analysis Review] [DOI] [PubMed] [Google Scholar]
- 9.Nyirjesy P. Postmenopausal vaginitis. Current infectious disease reports. 2007;9(6):480–484. doi: 10.1007/s11908-007-0073-5. [DOI] [PubMed] [Google Scholar]
- 10.Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(Suppl 1):S46–S52. doi: 10.1016/j.maturitas.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Brotman RM, Ravel J, Cone RA, Zenilman JM. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex TransmInfect. 2010;86(4):297–302. doi: 10.1136/sti.2009.040592. [86/4/297 pii ;10.1136/sti.2009.040592 doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorbach PM, Weiss RE, Fuchs E, et al. The slippery slope: lubricant use and rectal sexually transmitted infections: a newly identified risk. Sex Transm Dis. 2012;39(1):59–64. doi: 10.1097/OLQ.0b013e318235502b. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex TransmDis. 2008;35(5):512–516. doi: 10.1097/OLQ.0b013e3181644669. [DOI] [PubMed] [Google Scholar]
- 14.Wolf LK. Chemical and Engineering News. 2012. Dec 10, Studies Raise Questions About Safety Of Personal Lubricants; pp. 46–47. ed. http://cen.acs.org/articles/90/i50/Studies-Raise-Questions-Safety-Personal.html2012. [Google Scholar]
- 15.Hansen KA, Eyster KM. What happened to WHI: menopausal hormonal therapy in 2012. Clinical obstetrics and gynecology. 2012;55(3):706–712. doi: 10.1097/GRF.0b013e31825cab41. [DOI] [PubMed] [Google Scholar]
- 16.Hummelen R, Macklaim JM, Bisanz JE, et al. Vaginal Microbiome and Epithelial Gene Array in Post-Menopausal Women with Moderate to Severe Dryness. PLoS ONE. 2011;6(11):e26602. doi: 10.1371/journal.pone.0026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petricevic L, Unger FM, Viernstein H, Kiss H. Randomized, double-blind, placebo-controlled study of oral lactobacilli to improve the vaginal flora of postmenopausal women. European journal of obstetrics, gynecology, and reproductive biology. 2008;141(1):54–57. doi: 10.1016/j.ejogrb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9(6):e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 2010;202(12):1907–1915. doi: 10.1086/657320. [10.1086/657320 doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin DH. The Microbiota of the Vagina and Its Influence on Women's Health and Disease. AmJ MedSci. 2011 doi: 10.1097/MAJ.0b013e31823ea228. [10.1097/MAJ.0b013e31823ea228 doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruickshank R. The conversion of the glycogen of the vagina into lactic acid. The Journal of Pathology and Bacteriology. 1934 [Google Scholar]
- 22.Bomar L, Maltz M, Colston S, Graf J. Directed Culturing of Microorganisms Using Metatranscriptomics. mBio. 2011;2(2):e00012-11-e-11. doi: 10.1128/mBio.00012-11. [10.1128/mBio.00012-11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59(1):143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakken LR. Separation and Purification of Bacteria from Soil. Applied and Environmental Microbiology. 1985;49(6):1482–1487. doi: 10.1128/aem.49.6.1482-1487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronaghi M. Pyrosequencing sheds light on DNA sequencing. Genome Res. 2001;11(1):3–11. doi: 10.1101/gr.11.1.3. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brotman R, Gajer P, Sakamoto J, et al. International Society for Sexually Transmitted Disease Research. Canada: Qu,bec City; 2011. Jul 10 to 13, Temporal Dynamics of Vaginal Bacterial Communities. [Google Scholar]
- 28.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. ProcNatlAcadSciUSA. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [1002611107 pii ;10.1073/pnas.1002611107 doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferris MJ, Masztal A, Aldridge KE, Fortenberry JD, Fidel PL, Jr, Martin DH. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC infectious diseases. 2004;4:5. doi: 10.1186/1471-2334-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. The New England journal of medicine. 2005;353(18):1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan S, Liu C, Mitchell CM, et al. Temporal Variability of Human Vaginal Bacteria and Relationship with Bacterial Vaginosis. PLoS ONE. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Science translational medicine. 2012;4(132):132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gravitt PERE, Silver MI, Marks M, Chang K, Burke AE, Visicidi R. A cohort effect of the sexual revolution may be masking an increase in HPV detection at menopause in the US. J Infect Dis. doi: 10.1093/infdis/jis660. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rositch AF, Burke AE, Viscidi RP, Silver MI, Chang K, Gravitt PE. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: acquisition and reactivation in older women. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rositch AF, Silver MI, Burke A, et al. The Correlation Between Human Papillomavirus Positivity and Abnormal Cervical Cytology Result Differs by Age Among Perimenopausal Women. J Low Genit Tract Dis. 2012 doi: 10.1097/LGT.0b013e3182503402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marks MA, Viscidi RP, Chang K, et al. Differences in the concentration and correlation of cervical immune markers among HPV positive and negative perimenopausal women. Cytokine. 2011 doi: 10.1016/j.cyto.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) 5 ed. pp. 874–878. [DOI] [PubMed] [Google Scholar]
- 38.McKenna P, Hoffmann C, Minkah N, et al. The Macaque Gut Microbiome in Health, Lentiviral Infection, and Chronic Enterocolitis. PLoSPathog. 2008;4(2):e20. doi: 10.1371/journal.ppat.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 40.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–200. doi: 10.1093/bioinformatics/btr381. [Research Support, N.I.H, Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 43.Imai K, King G, Lau O. Toward a Common Framework for Statistical Analysis and Development. J Comput Graph Stat. 2008;17(4):892–913. [Google Scholar]
- 44.Geweke JF. Evaluating the accuracy of sampling-based approaches to the calculation of posterior moments. Federal Reserve Bank of Minneapolis, Research Department staff report 148. Minneapolis, Minn.: Federal Reserve Bank of Minneapolis. 1991 [Google Scholar]
- 45.Heidelberger P, Welch PD. Simulation Run Length Control in the Presence of an Initial Transient. Operations Research. 1983;31(6):1109–1144. [Google Scholar]
- 46.Raftery AE, Lewis SM. [Practical Markov Chain Monte Carlo]: Comment: One Long Run with Diagnostics: Implementation Strategies for Markov Chain Monte Carlo. Statistical Science. 1992;7(4):493–497. [Google Scholar]
- 47.Bai G, Gajer P, Nandy M, et al. Comparison of storage conditions for human vaginal microbiome studies. PLoS One. 2012;7(5):e36934. doi: 10.1371/journal.pone.0036934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Legendre P, Anderson MJ. DISTANCE-BASED REDUNDANCY ANALYSIS: TESTING MULTISPECIES RESPONSES IN MULTIFACTORIAL ECOLOGICAL EXPERIMENTS. Ecological Monographs. 1999;69(1):1–24. [Google Scholar]
- 49.Plummer M, Best N, Cowles K, Vines K. CODA: convergence diagnosis and output analysis for MCMC. R News. 2006;6(1):7–11. [Google Scholar]
- 50.Martin AD, Quinn KM, Park JH. MCMCpack: Markov Chain Monte Carlo in R. J Stat Softw. 2011;42(9):1–21. [Google Scholar]
- 51.Imai K. Gary King and Olivia Lau. Zelig: Everyone's Statistical Software. 2006 [Google Scholar]
- 52.Biagi E, Candela M, Franceschi C, Brigidi P. The aging gut microbiota: new perspectives. Ageing research reviews. 2011;10(4):428–429. doi: 10.1016/j.arr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Kinross J, Nicholson JK. Gut microbiota: Dietary and social modulation of gut microbiota in the elderly. Nature reviews Gastroenterology & hepatology. 2012;9(10):563–564. doi: 10.1038/nrgastro.2012.169. [DOI] [PubMed] [Google Scholar]
- 54.Ferris MJ, Norori J, Zozaya-Hinchliffe M, Martin DH. Cultivation-independent analysis of changes in bacterial vaginosis flora following metronidazole treatment. JClinMicrobiol. 2007;45(3):1016–1018. doi: 10.1128/JCM.02085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rampersaud R, Planet PJ, Randis TM, et al. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J Bacteriol. 2011;193(5):1034–1041. doi: 10.1128/JB.00694-10. [JB.00694-10 pii ;10.1128/JB.00694-10 doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reid G. Probiotic and prebiotic applications for vaginal health. Journal of AOAC International. 2012;95(1):31–34. doi: 10.5740/jaoacint.sge_reid. [DOI] [PubMed] [Google Scholar]
- 57.Tankó LB, Christiansen C. An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause (New York, NY) 2004;11(1):104–109. doi: 10.1097/01.GME.0000079740.18541.DB. [10.1097/01.GME.0000079740.18541.DB] [DOI] [PubMed] [Google Scholar]
- 58.Brotman Rebecca M, Ravel Jacques, Gajer Pawel, et al. The International Society for Sexually Transmitted Disease Research, 20th Biennial Congress. Austria: Vienna; 2013. Biomarkers of cigarette smoking and association with the vaginal microbiota. [Google Scholar]
- 59.Fuchs EJ, Lee LA, Torbenson MS, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. The Journal of Infectious Diseases. 2007;195(5):703–710. doi: 10.1086/511279. [10.1086/511279] [DOI] [PubMed] [Google Scholar]
- 60.Burton JP, Reid G. Evaluation of the bacterial vaginal flora of 20 postmenopausal women by direct (Nugent score) and molecular (polymerase chain reaction and denaturing gradient gel electrophoresis) techniques. JInfectDis. 2002;186(12):1770–1780. doi: 10.1086/345761. [DOI] [PubMed] [Google Scholar]
- 61.Heinemann C, Reid G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. CanJMicrobiol. 2005;51(9):777–781. doi: 10.1139/w05-070. [DOI] [PubMed] [Google Scholar]
- 62.Ozkinay E, Terek MC, Yayci M, Kaiser R, Grob P, Tuncay G. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG. 2005;112(2):234–240. doi: 10.1111/j.1471-0528.2004.00329.x. [DOI] [PubMed] [Google Scholar]
- 63.Hillier SL, Lau RJ. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997;25(Suppl 2):S123–S126. doi: 10.1086/516221. [DOI] [PubMed] [Google Scholar]
- 64.Pabich WL, Fihn SD, Stamm WE. Prevalence and determinants of vaginal flora alterations in postmenopausal women. Journal of infectious Dis. 2003 doi: 10.1086/378203. [DOI] [PubMed] [Google Scholar]
- 65.Burton JP, Devillard E, Cadieux PA, Hammond JA, Reid G. Detection of Atopobium vaginae in postmenopausal women by cultivation-independent methods warrants further investigation. JClinMicrobiol. 2004;42(4):1829–1831. doi: 10.1128/JCM.42.4.1829-1831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donders GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG. 2002;109(1):34–43. doi: 10.1111/j.1471-0528.2002.00432.x. [DOI] [PubMed] [Google Scholar]