Abstract
Patients suffering from inflammatory bowel disease are at a high risk of developing colorectal cancer. To assess the anti-cancer potential of botanicals, in this study, we evaluated the effects of Panax notoginseng on azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colitis. One week after A/J mice received AOM, the animals received DSS for 8 days, or were supplemented with P. notoginseng extract, at 30 or 90 mg/kg. DSS-induced colitis was scored with the disease activity index (DAI). The severity of the inflammatory lesions was evaluated by a colon tissue histological assessment. The expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) were also explored. We observed that the effects of P. notoginseng on the reduction of colon inflammation, expressed in DAI score, were in a dose-related manner (P < 0.01). P. notoginseng inhibited the reduction of the colon length and the loss of bodyweight in dose-related manner (all P < 0.05). The histological assessment of the colitis and inflammatory related immunohistochemical data also supported the pharmacological observations. Our data suggest that P. notoginseng is a promising candidate in preventing and treating colitis and inflammation-associated colon carcinogenesis.
Keywords: Panax notoginseng, notoginseng, inflammatory bowel disease, colitis, AOM/DSS model, colorectal carcinogenesis
INTRODUCTION
Ulcerative colitis and Crohn’s disease are two major forms of inflammatory bowel disease (IBD). The IBD is associated with colonic mucosal ulceration, shortening of the colon, reduced gastric motility and/or diarrhea, stool with blood and/or mucus, and body weight loss (Fiocchi, 1998; Kohn and Meddi, 2012). Patients suffering from this widespread and distressing medical problem are also at a high risk of developing colorectal cancer (Tanaka, 2009; Andersen et al., 2012), a very common form of cancer worldwide (Siegel et al., 2013).
Panax notoginseng is a Chinese herbal medicine that has a long history of use in China and other Asian countries for different medical conditions (Wang et al., 2006). This perennial botanical is distributed throughout southwest China, Burma, and Nepal. The portion of the plant commonly used in remedies is the root, which is dug up after the fruit has ripened. Previous studies have shown that P. notoginseng possessed potential anti-tumor activities (Chen et al., 2001; Wang et al., 2007b; Jin et al., 2010). Our group has previously reported that P. notoginseng extract increased the effects of 5-FU’s cancer chemotherapy, and this synergistic effect between P. notoginseng and 5-FU makes it possible to reduce the dose of 5-FU in combination with the herb and thereby decrease 5-FU dose-related toxicity (Wang et al., 2007a). We also demonstrated significant growth inhibitory effects of P. notoginseng and its constituents on human colorectal cancer cell lines (Wang et al., 2009b; Sun et al., 2011), although these observations were based only on in vitro preparations.
Chronic inflammation in the colon can lead to cancer. Experimentally, azoxymethane (AOM; a mutagenic agent) and/or dextran sodium sulfate (DSS; a pro-inflammatory reagent) have often been used in colorectal cancer chemoprevention animal studies (Tanaka et al., 2003; Neufert et al., 2007; Poudyal et al., 2012; Sliva et al., 2012). In this project, using an AOM/DSS mouse model, the colonic inflammation was established to mimic the inflamed colon and immunopathological disorders observed in humans (Murthy, 2006; Neufert et al., 2007). To evaluate P. notoginseng’s anti-colorectal cancer potential, the chemically induced experimental colitis was characterized by a change in stool consistency, blood in stool, reduction of colon length, and loss of body weight. Histological assessment of murine colitis and colon tissue immunohistochemical staining were also conducted to support the pharmacological observations. Our data suggest that as a natural product, P. notoginseng is a promising candidate in preventing and treating colitis.
MATERIALS AND METHODS
Chemicals and reagents
HPLC grade ethanol, n-butanol, acetonitrile, and DMSO were obtained from Fisher Scientific (Pittsburgh, PA). Milli Q water was supplied by a water purification system (US Filter, Palm Desert, CA). P. notoginseng saponin standards for ginsenosides Rb1, Rc, Rd, Re, and Rg1 were obtained from Indofine Chemical Company (Somerville, NJ); ginsenosides Rg2, Rg3, and notoginsenoside R1 were obtained from the Delta Information Center for Natural Organic Compounds (Xuancheng, Anhui, China). All saponin standards were of biochemical-reagent grade and at least 95% pure as confirmed by HPLC. Azoxymethane (AOM) was obtained from the NCI Chemical Carcinogen Reference Standard Repository, Midwest Research (Kansas City, MO). Dextran sodium sulfate (DSS, molecular weight of 36,000–50,000 Da) was obtained from MP Biomedicals (Solon, OH). Anti–inducible nitric oxide synthase (polyclonal, 1:500) was obtained from EMD Millipore (Billerica, MA). Anti–cyclooxygenase-2 (polyclonal, 1:500) was obtained from Cayman Chemical (Ann Arbor, MI). Avidin-biotin complex (ABC) kit and DAB peroxidase substrate kit were obtained from Vector Laboratories (Burlingame, CA). Hemoccult Sensa test strips were obtained from Beckman Coulter (Brea, CA).
Botanical material, preparation, and phytochemical analysis
The root of P. notoginseng (Panax notoginseng (Burk.) F.H. Chen), cultivated for 4 years, was obtained from Wenshan (Yunnan, China). Dr. Chong-Zhi Wang authenticated the plant materials and voucher specimen was deposited at the Tang Center for Herbal Medicine Research at University of Chicago (Chicago, IL). The air-dried root was extracted and lyophilized (Wang et al., 2008). Briefly, dried P. notoginseng roots were pulverized into fine powder with a pulverizer and passed through a 40-mesh screen. A 100 g of powdered P. notoginseng sample was extracted with 70% ethanol, and the solvent of the extract solution was evaporated under vacuum. The dried extract was dissolved in water, and then extracted with water-saturated n-butanol. The n-butanol phase was evaporated under vacuum and then lyophilized.
Before pharmacological evaluation, the P. notoginseng extract was analyzed using HPLC (Wang et al., 2009a; Mao et al., 2012). The HPLC system was a Waters 2960 instrument (Milford, MA) with a quaternary pump, an automatic injector, a photodiode array detector (Model 996), and Waters Millennium 32 software for peak identification and integration. The separation was carried out on an Alltech Ultrasphere C18 column (5 µ, 250 × 3.2 mm I.D.) (Deerfield, IL) with a guard column (7.5 × 3.2 mm I.D.). For HPLC analysis, a 20-µL sample was injected into the column and eluted at room temperature with a constant flow rate of 1.0 mL/min. For the mobile phase, acetonitrile (solvent A) and water (solvent B) were used. Gradient elution started with 17.5% solvent A and 82.5% solvent B. Elution solvents were then changed to 21% A for 20 min, then to 26% A for 3 min and held for 19 min, at 36% A for 13 min, at 50% A for 9 min, at 95% A for 2 min and held for 3 min. Lastly, eluting solvents were changed to 17.5% A for 3 min and held for 8 min. The detection wavelength was set at 202 nm.
Animal treatment
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Chicago. All experiments were carried out in male A/J mice, 6–8 weeks old, weighing between 18 and 22 g, obtained from Jackson Laboratories (Bar Harbor, ME). Mice were caged under controlled room temperature, humidity and light (12/12 h light/dark cycle) and allowed unrestricted access to standard mouse chow and tap water. The mice were allowed to acclimate to these conditions for at least 7 days before inclusion in the experiments.
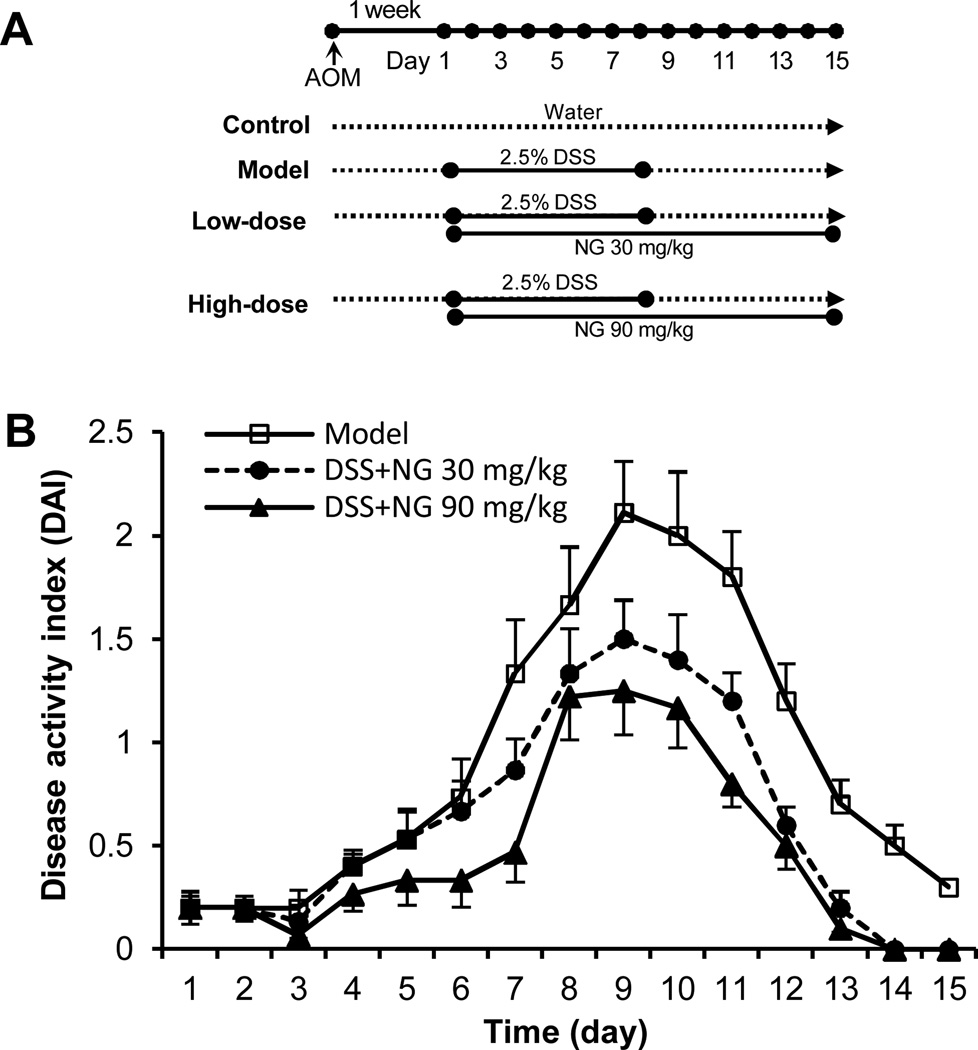
The experimental protocol is shown in Figure 2(A). There were 4 experimental groups (n = 10 per group). Group 1 was the control. Animals in Group 2 (model group), Group 3, and Group 4 initially received a single intraperitoneal injection of AOM (7.5 mg/kg). One week after the AOM injection (set as Day 1), these animals began to receive 2.5% DSS in drinking water for 8 consecutive days. The animals in Group 3 (low-dose group) and Group 4 (high-dose group) also received P. notoginseng in doses of 0.15 mg/ml and 0.45 mg/ml, respectively, in drinking water for 15 consecutive days. We calculated that the P. notoginseng daily dose was approximately 30 mg/kg and 90 mg/kg for low-dose and high-dose groups, respectively. The animals were sacrificed on Day 15, and tissue samples were collected for additional observations.
Figure 2.
Effects of P. notoginseng on acute experimental colitis in A/J mice. (A) Experimental protocol. (B) P. notoginseng attenuated the DSS-induced colitis, expressed as disease activity index (DAI). Data from the control group are all zeros from Day 1 to Day 15 (not shown). The dose-related effects of P. notoginseng is observed (P < 0.01). NG, P. notoginseng.
Disease activity index
DSS induced colitis was scored as the disease activity index (DAI) as described previously (Ghia et al., 2006). In brief, the DAI was the combined scores of weight loss (0, none; 1, 0–5%; 2, 5–10%; 3, 10–20%; 4, >20%), stool consistency change (0, none; 2, loose stool; 4, diarrhea), and bleeding (0, none; 1, trace; 2, mild hemoccult; 3, obvious hemoccult; 4, gross bleeding), and then divided by 3. The animals were scored for the DAI at the same time of each day, blind to the treatment. The minimal score was 0 and the maximal score was 4.
Histological assessment
Paraffin-embedded tissue samples were serially sectioned, and some sections were stained with hematoxylin and eosin (H&E). The stained sections were subsequently examined for histopathological changes by a gastrointestinal pathologist.
The histology score was determined by multiplying the percent involvement of each of the three following histologic features by the percent area of involvement (Konoshima et al., 1999): inflammation severity (0, none; 1, minimal; 2, moderate; 3, severe), inflammation extent (0, none; 1, mucosa; 2, mucosa and submucosa; 3, transmural), crypt damage (0, none; 1, one third of crypt damaged; 2, two thirds of crypt damaged; 3, crypts lost, surface epithelium intact; 4, crypts lost, surface epithelium lost), percent area involvement (0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–75%; 4, 76–100%).
Immunohistochemical staining
Some sections of mouse colon tissue were incubated with antibodies against inducible nitric oxide synthase (iNOS; rabbit polyclonal; diluted 1:500; EMD Millipore, Billerica, MA), cyclooxygenase-2 (COX-2; rabbit polyclonal; diluted 1:100; Cayman Chemical, Ann Arbor, MI).
The avidin-biotin indirect immunoperoxidase method was used for immunohistochemistry of paraffin-embedded sections. The Vectastain Elite ABC kit was used according to the manufacturer’s instruction (Vector Laboratories, Burlingame, CA). The samples were incubated with biotin/avidin horseradish peroxidase conjugates and chromogen DAB (3, 3’-diaminobenzidine) (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin. One set of sections was incubated in phosphate-buffered saline (PBS) as a primary antibody to serve as a negative control. The staining was accomplished by rocking slides using the Antibody Amplifier to ensure consistent, sensitive, and reproducible staining.
Statistical analysis
Data were presented as mean ± standard error (S.E.). Data were analyzed using analysis of variance (ANOVA) for repeated measures and Student’s t-test. The level of statistical significance was set at P < 0.05.
RESULTS
HPLC analysis of P. notoginseng saponin profile
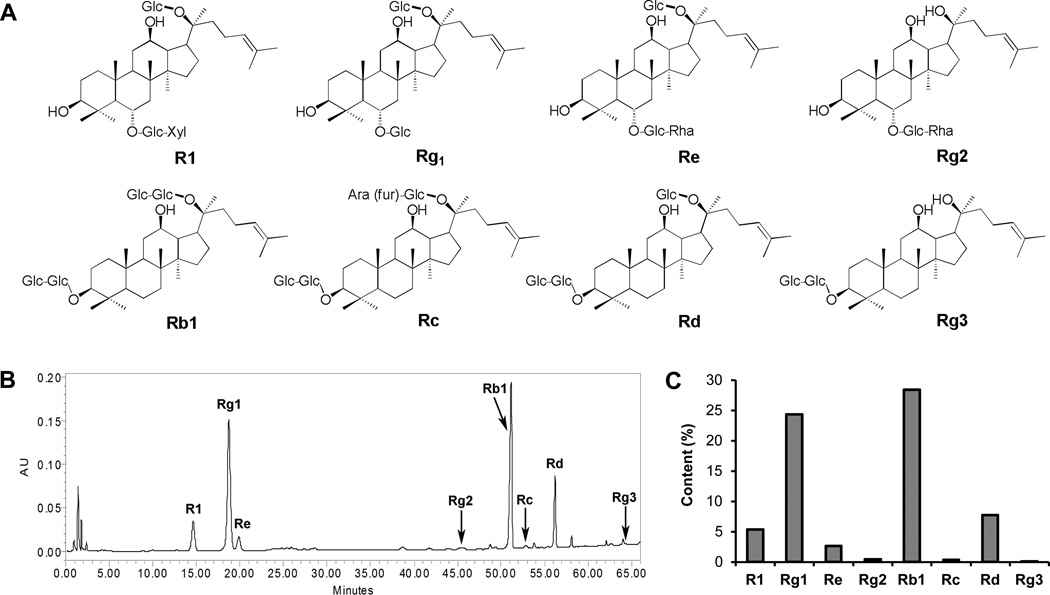
The linearity of the analytical method was assayed by analyzing standard solutions in the ranges of 2–400 µg/mL for ginsenoside Rb1 and Rg1, 1–200 µg/mL for notoginsenoside R1, ginsenosides Rc, Rd, Re, Rg2 and Rg3. Calibration curves were constructed from the measured peak areas and the related amount of ginsenosides. For all 8 saponins (Figure 1A), the calibration curves showed good linearity (R2 > 0.9990). P. notoginseng saponins in the extract were identified by comparison of their retention times with those obtained from the chromatograms of mixed ginsenoside standards. From the chromatogram of the P. notoginseng extract (Figure 1B), four main peaks of notoginsenoside R1, ginsenosides Rg1, Rb1 and Rd were observed. The content of saponins in the extract was calculated using standard curves of P. notoginseng saponins. As shown in Figure 1C, the contents of two ginsenosides Rg1 and Rb1, reached over 20% (24.4% and 28.4%, respectively). The contents of other six saponins are: R1, 5.3%; Re, 2.6%; Rg2; 0.4%; Rc, 0.3%; Rd, 7.8%; Rg3, 0.1%. Compared to the total saponin content in the extract (69.3%), the proportion of Rg1 and Rb1 accounted for 76.2% of the total saponin.
Figure 1.
Chemical structures and HPLC analysis of saponins in P. notoginseng extract. The structures of determined saponins are shown in (A). HPLC chromatogram of P. notoginseng extract recorded at 202 nm is shown in (B). HPLC conditions are described in the Materials and Methods. Saponin content of determined compounds is shown in (C). Eight P. notoginseng saponins were determined: notoginsenoside R1 (R1), and ginsenosides Rg1, Re, Rg2, Rb1, Rc, Rd and Rg3.
P. notoginseng suppressed experimental colitis
After DSS treatments, animals in the model group showed apparent diarrhea and rectal bleeding, starting from Day 4. As the treatment continued, the presence and development of inflammation manifested clearly. The disease severity, scored by the disease activity index (DAI), reached its highest level on Day 9 (Figure 2). Figure 2(B) shows the effects of P. notoginseng on the reduction of the DAI score in a dose-related manner (P < 0.01). This suppression of the experimental colitis was not only evident during DSS treatment, but also very obvious after the cessation of its administration, suggesting that P. notoginseng significantly promoted recovery from the colitis.
P. notoginseng attenuated colitis-induced colon length reduction
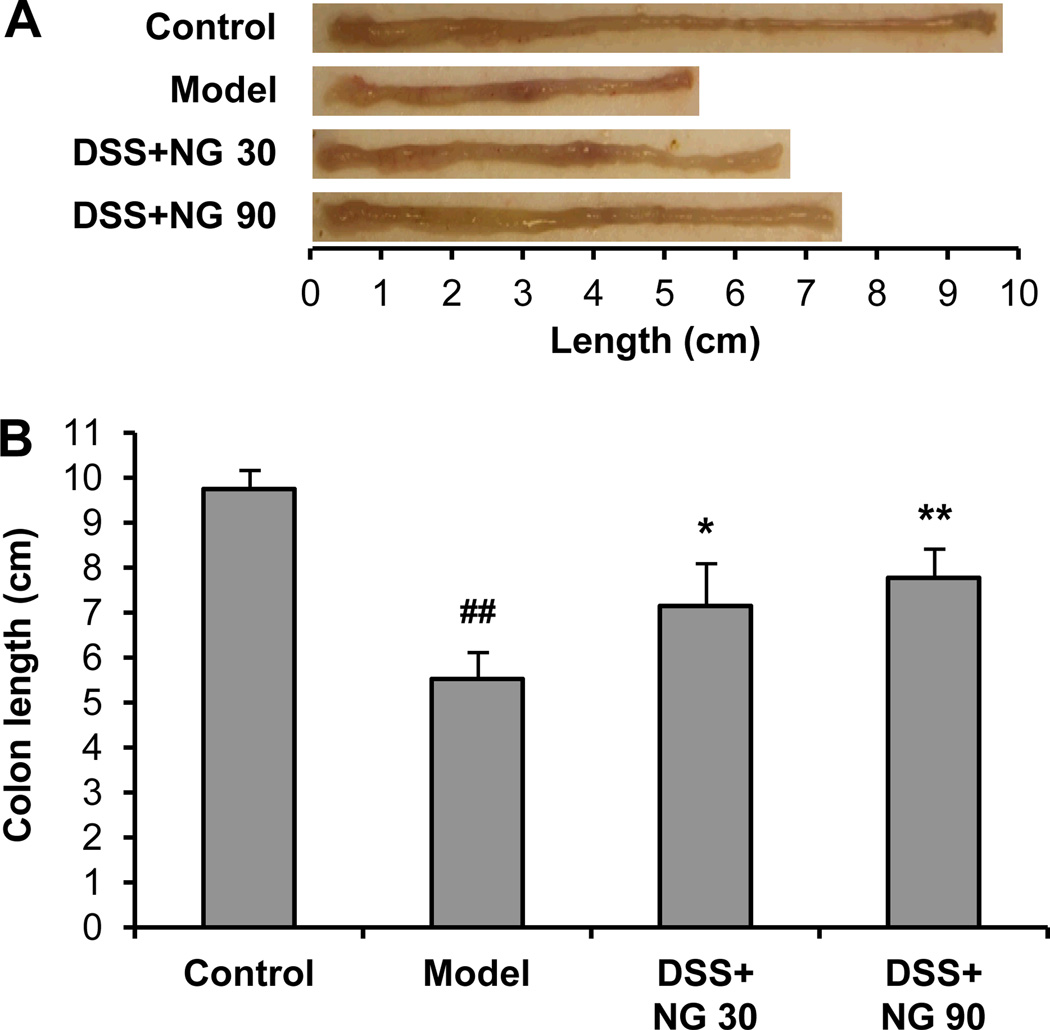
As an objective measure of the severity of inflammation, colon length in different groups was measured (Figure 3). The control group animals had an average colon length of 9.8 ± 0.5 cm. The DSS treatment lead to a very significant reduction of colon length, and the average length in the model group animals was reduced to 5.5 ± 0.4 cm (P < 0.01 compared to control). Treatment with low-dose and high-dose P. notoginseng inhibited the reduction of the colon in as dose-related manner (6.8 ± 0.6 and 7.5 ± 0.7 cm, P < 0.05 and P < 0.01) compared to the model group, respectively.
Figure 3.
Mouse colon length changes in different groups. (A) Representative photographic images of the colon. (B) For the model group, DSS treatment very significantly reduced the colon length in comparison with the control group (#, P < 0.01). P. notoginseng low-dose and high-dose treatment significantly decreased the colon length reduction compared to the model group, respectively (*, P < 0.05 and **, P < 0.01). NG 30, P. notoginseng 30 mg/kg; NG 90, P. notoginseng 90 mg/kg.
Body weight changes
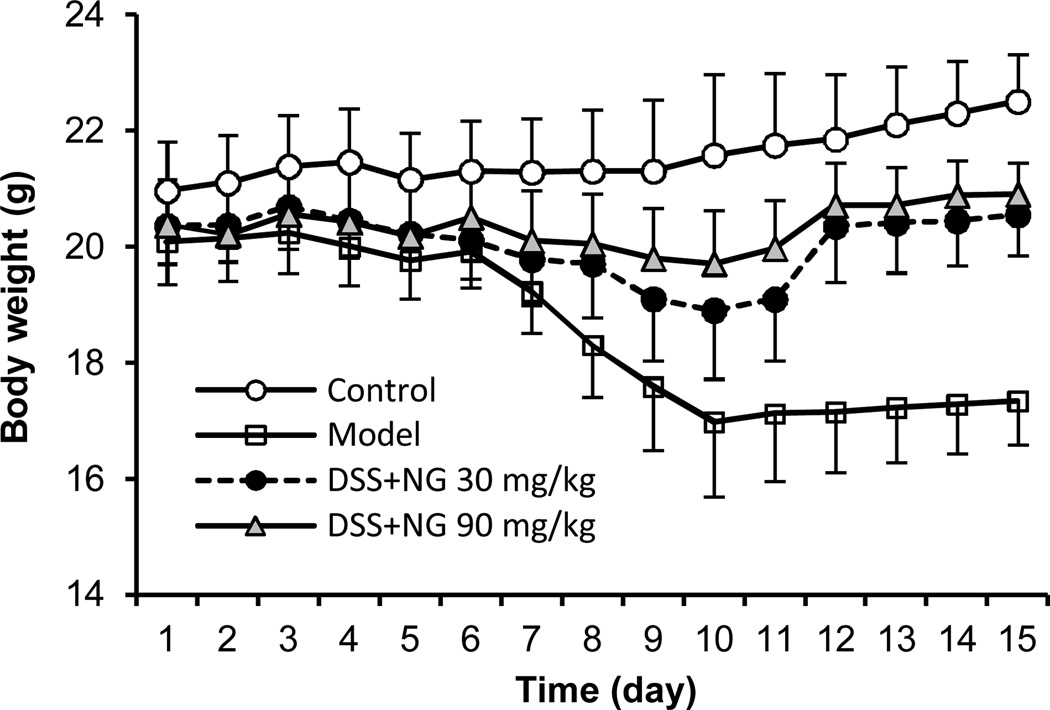
Figure 4 shows the body weight changes in different experimental groups. Compared to the control group, which had a slow weight gain, the model group had significant weight reduction beginning on Day 7. This reduced weight remained for over 7 days after cessation of the DSS. However, both low-dose and high-dose P. notoginseng groups significantly reduced the body weight reduction (P < 0.05 and P < 0.01 compared to the model group, respectively).
Figure 4.
Effects of P. notoginseng on mouse body weight changes. While the model group showed significant weight reduction from Day 7, P. notoginseng low-dose and high-dose treatment significantly reduced the DSS-induced weight reduction. (P < 0.05 and P < 0.01 compared to the model group, respectively).
Histological characterization
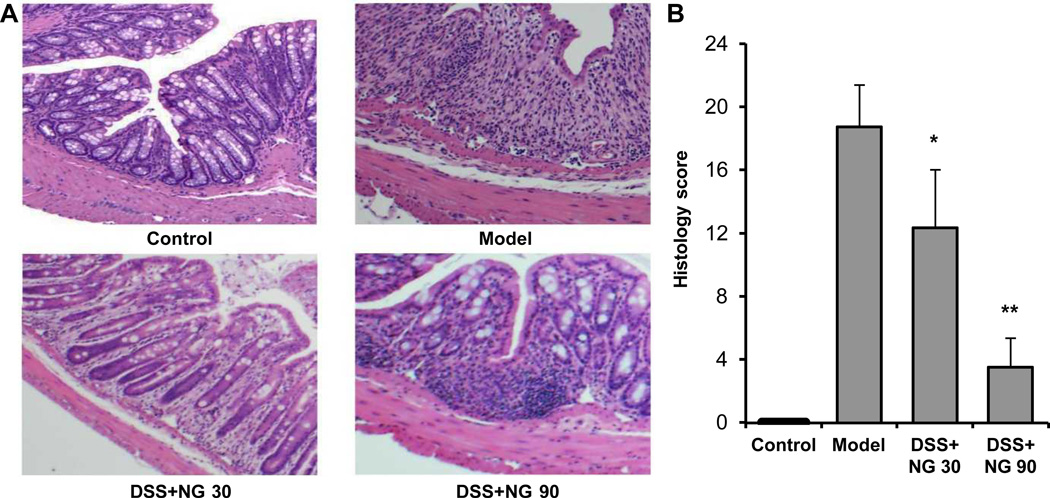
From H&E staining histological sections, control mouse colon sections showed intact epithelium, well-defined crypt length, no neutrophil infiltration in mucosa and submucosa, and no ulcers or erosions. In contrast, colon tissue from model animals showed severe inflammatory lesions, characterized by complete loss of crypts, surface erosion and ulcerations, lamina propria fibrosis and acute and chronic transmural inflammatory infiltrate. For mice treated with P. notoginseng, there was a significant reduction in inflammatory injury. Colon tissue from the low-dose group showed moderate inflammation, patchy architectural distortion, and mild lamina propria fibrosis. From the high-dose group, colon mucosa had tightly packed glands with a normal amount of goblet cells. The lamina propria contains mild patchy neutrophilic infiltrate. Figure 5 shows representative H&E staining histological sections (A), and overall histology scores are provided (B).
Figure 5.
Effects of P. notoginseng on the histological characterization in DSS-induced mouse colitis. (A) Representative H&E staining histological sections of control, DSS only, DSS plus low-dose P. notoginseng (NG 30), and DSS plus high-dose P. notoginseng (NG 90). (B) Overall histology scores in these different groups. *, P < 0.05; **, P < 0.01 compared to the model group.
Immunohistochemical staining
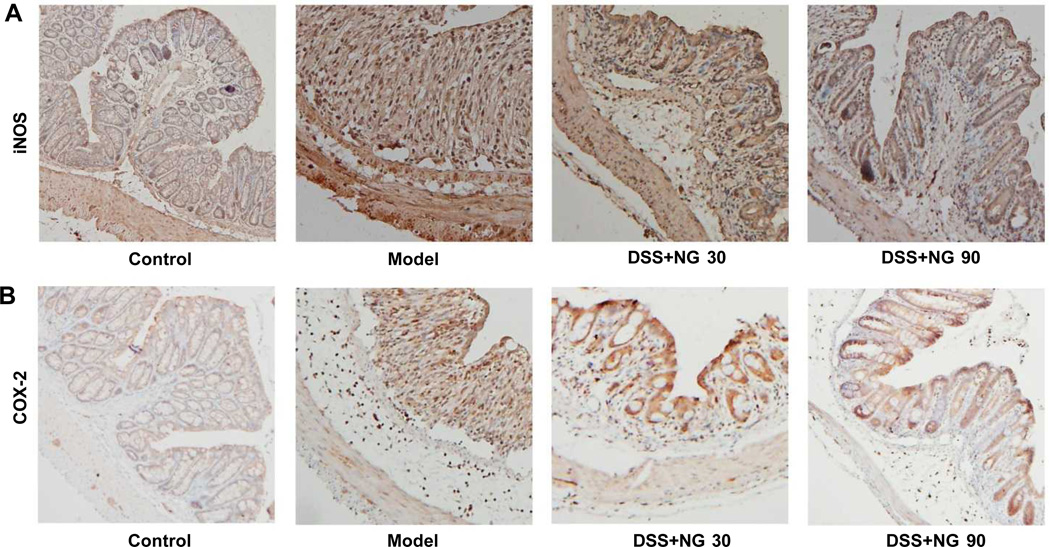
To explore the impact of P. notoginseng on inflammatory markers in vivo, we examined iNOS and COX-2 expression using colon tissue immunohistochemical staining. Figure 6(A) shows representative sections of the expression of iNOS, and Figure 6(B) shows expression of COX-2. The immunohistochemical images show that iNOS and COX-2 levels were elevated in DSS treated mice, with most of the staining appearing in the inflammatory cells. Compared to the model group, the expression of iNOS and COX-2 in colon tissue was markedly decreased after P. notoginseng treatment, supporting the DAI data and histological evaluation.
Figure 6.
Mouse colon tissue immunohistochemical staining with expression of (A) iNOS and (B) COX-2. Representative sections of control, DSS only, DSS plus low-dose P. notoginseng (NG 30), and DSS plus high-dose P. notoginseng (NG 90). DSS treatment induces iNOS and COX-2 expressions. Positive staining is brown colored. Magnification ×200.
DISCUSSION
Colorectal cancer remains a leading cause of morbidity and mortality worldwide. It is a significant contributor to the burden of disease for the American public. In 2013 alone, there is an estimated 142,820 new colorectal cancer cases and 50,830 the cancer-related deaths in the U.S. (Siegel et al., 2013). In fact, 1 in 21 American men and women are at risk for developing invasive colorectal cancer in their lifetime.
Targeting inflammatory pathways has been shown to be effective in preventing the formation of colonic tumors and their malignant progression in both animal and human studies (Madka and Rao, 2013). Non-steroidal anti-inflammatory drugs (NSAIDS) can reduce colorectal cancer tumorigenesis, but concerns and long-term risks of NSAIDs make this form of chemoprevention unsuitable as a general recommendation (Garcia Rodriguez et al., 2013; Zhou et al., 2013). Given the limitations of today’s standards of practice, there is strong motivation for exploring alternative strategies for colorectal cancer and its associated inflammation. In this study, the effects of P. notoginseng were evaluated using an AOM/DSS mouse model.
Previously, the in vitro anti-proliferative effects of major saponins in P. notoginseng were evaluated using human colorectal cancer cell lines. We observed that P. notoginseng could arrest colorectal cancer cells in S and G2/M phases. In addition, the remarkable apoptosis induction activities of P. notoginseng were shown (Wang et al., 2009b; Sun et al., 2011). From our encouraging in vitro data to in vivo studies, human colorectal cancer cells can be implanted in immunodeficient nude mice for tumor growth and observation. However, this xenograft mouse model is not specifically designed to investigate colorectal cancer.
Several animal models have been used for colorectal cancer studies, especially mouse models for inflammation related colon cancer. IBD is a group of chronic dysregulated inflammatory conditions in the large and small intestines in humans, and it is well known that chronic inflammation in the colon can lead to cancer (Hanauer, 1996; Geier et al., 2007). In the traditional medical system, anti-inflammatory botanicals had protective effects against DSS-induced colitis (Kim et al., 2011). DSS was used alone to induce colitis in early studies, however, recently published studies often used AOM plus DSS together, since AOM enhanced DSS induced colitis (Tanaka et al., 2003; Long et al., 2013). AOM is a genotoxic, colonic carcinogen routinely used to induce colon tumors in mice (Poole et al., 2004; Neufert et al., 2007). In this study, the mouse colitis was chemically induced by AOM/DSS (Tanaka et al., 2003; Neufert et al., 2007; Poudyal et al., 2012; Sliva et al., 2012). Our inflammation-related mouse model of colorectal carcinogenesis combines AOM and DSS to induce colon lesions with positive inducible nitric oxide synthase and COX-2 (Maltzman et al., 1997; Kohno et al., 2006).
In this study, we showed that, P. notoginseng inhibited experimental colitis, in a dose-related manner, resulting in overall attenuation of inflammation DAI, including colon length and body weight changes. The effects of P. notoginseng were further confirmed by the H&E staining histological characterization in mouse acute colitis and colon tissue, and supported by our preliminary immunohistochemical staining data, such as expression of iNOS and COX-2.
In our previous studies using a xenograft nude mouse model, the botanical test compound was intraperitoneally injected for accurate dose control (Jin et al., 2012). However, like other herbal medicines, P. notoginseng is almost always taken orally (Ushiroyama et al., 2012). In the present study, P. notoginseng was administered orally, which is a more safe and practical way to deliver the treatment, and we carefully calculated the ingested dosage. For extended, long-term observation, it would be better to mix the botanical extract with mouse chow, which could be standard chow or chow formulated with high fat to mimic a western diet in humans (Huang et al., 2012; Ohnishi et al., 2012).
The bioactive constituents in P. notoginseng are believed as a group of triterpenoids or dammarane saponins. The antioxidant potential of P. notoginseng saponins has been reported (He et al., 2012). It is known that oxidant stress plays a role in chemical induced colitis, and the pathological severity can be reduced by antioxidant botanicals (Yasui et al., 2011; Oz et al., 2013). Whether the beneficial effect of P. notoginseng on AOM/DSS-induced colitis is linked to the antioxidant activities of saponins in the herb will be conducted in future studies.
Using HPLC analysis, we identified the major constituents of P. notoginseng in our study. However, after oral administration, P. notoginseng saponins could be metabolized extensively by intestinal microbiota (Wang et al., 2011; Wang et al., 2012a). Previous animal pharmacokinetic evaluations of ginseng compounds have focused on their parent constituents, but their metabolites, especially those transformed by intestinal microbiota, have largely not been carefully evaluated. In addition, the liver plays a major role in drug metabolism. Compounds, including those constituents from P. notoginseng, and their metabolites are excreted into the bile duct. Hepatic disposition, biliary excretion, and enterohepatic circulation may affect the pharmacokinetic profile of administered compounds. To obtain a complete pharmacokinetic profile of P. notoginseng, we recently developed a dynamic microdialysis sampling method to sensitively analyze the P. notoginseng metabolite profile in rat bile, and identified both parent compounds (notoginsenoside R1, ginsenosides Rg1, Rb1, and Rd) and metabolites (ginsenosides Rg2, Rh2, and compound K) (Wen et al., 2012). Our P. notoginseng anti-colitis observation should extend to gut microbiome and pharmacokinetic studies.
In this study, we observed that P. notoginseng attenuated experimental colitis in a mouse model. In future studies, we should extend the observation time for direct assessment of AOM/DSS-induced, colitis-associated colorectal carcinogenesis. Our study should also include other colorectal cancer animal models, especially the APC mutant Min (multiple intestinal neoplasia) mice with detailed mechanisms of actions (Yamada and Mori, 2007; Velmurugan et al., 2010).
Traditional medicine has been practiced for thousands of years based on clinical experience. It is necessary to integrate existing traditional knowledge of diseases with modern biomedical technologies (Wang et al., 2012b). The results from this study provided evidence-based information on complementary and alternative medicine intervention with an important clinical significance. P. notoginseng, as a candidate of botanical-based colorectal cancer chemoprevention, should be further tested for the unmet medical needs.
Acknowledgements
This work was supported in part by the NIH/NCCAM grants P01 AT 004418 and K01 AT005362.
Footnotes
Conflict of Interest
The authors have no conflict of interest to report.
REFERENCES
- Andersen V, Halfvarson J, Vogel U. Colorectal cancer in patients with inflammatory bowel disease: can we predict risk? World J Gastroenterol. 2012;18:4091–4094. doi: 10.3748/wjg.v18.i31.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FD, Wu MC, Wang HE, et al. Sensitization of a tumor, but not normal tissue, to the cytotoxic effect of ionizing radiation using Panax notoginseng extract. Am J Chin Med. 2001;29:517–524. doi: 10.1142/S0192415X0100054X. [DOI] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Cea-Soriano L, Tacconelli S, Patrignani P. Coxibs: pharmacology, toxicity and efficacy in cancer clinical trials. Recent Results Cancer Res. 2013;191:67–93. doi: 10.1007/978-3-642-30331-9_4. [DOI] [PubMed] [Google Scholar]
- Geier MS, Butler RN, Howarth GS. Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int J Food Microbiol. 2007;115:1–11. doi: 10.1016/j.ijfoodmicro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Hanauer SB. Inflammatory bowel disease. N Engl J Med. 1996;334:841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- He NW, Zhao Y, Guo L, Shang J, Yang XB. Antioxidant, antiproliferative, and pro-apoptotic activities of a saponin extract derived from the roots of Panax notoginseng (Burk.) F.H. Chen. J Med Food. 2012;15:350–359. doi: 10.1089/jmf.2011.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yu J, Jia X, Xiong L, Li N, Wen X. Zhenqing recipe improves glucose metabolism and insulin sensitivity by repressing hepatic FOXO1 in type 2 diabetic rats. Am J Chin Med. 2012;40:721–733. doi: 10.1142/S0192415X12500541. [DOI] [PubMed] [Google Scholar]
- Jin HR, Zhao J, Zhang Z, et al. The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis. 2012;3:e376. doi: 10.1038/cddis.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Hofseth AB, Cui X, et al. American ginseng suppresses colitis through p53-mediated apoptosis of inflammatory cells. Cancer Prev Res. 2010;3:339–347. doi: 10.1158/1940-6207.CAPR-09-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim KW, Kim DS, et al. The protective effect of Cassia obtusifolia on DSS-induced colitis. Am J Chin Med. 2011;39:565–577. doi: 10.1142/S0192415X11009032. [DOI] [PubMed] [Google Scholar]
- Kohn A, Meddi P. How to manage IBD in patients with infections or malignancies? Dig Dis. 2012;30:420–424. doi: 10.1159/000338145. [DOI] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Curini M, et al. Dietary administration with prenyloxycoumarins, auraptene and collinin, inhibits colitis-related colon carcinogenesis in mice. Int J Cancer. 2006;118:2936–2942. doi: 10.1002/ijc.21719. [DOI] [PubMed] [Google Scholar]
- Konoshima T, Takasaki M, Tokuda H. Anti-carcinogenic activity of the roots of Panax notoginseng. II. Biol Pharm Bull. 1999;22:1150–1152. doi: 10.1248/bpb.22.1150. [DOI] [PubMed] [Google Scholar]
- Long TM, Chakrabarti A, Ezelle HJ, et al. RNase-L deficiency exacerbates experimental colitis and colitis-associated cancer. Inflamm Bowel Dis. 2013;19:1295–1305. doi: 10.1097/MIB.0b013e318281f2fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madka V, Rao CV. Anti-inflammatory Phytochemicals for Chemoprevention of Colon Cancer. Curr Cancer Drug Targets. 2013;13:542–557. doi: 10.2174/15680096113139990036. [DOI] [PubMed] [Google Scholar]
- Maltzman T, Whittington J, Driggers L, Stephens J, Ahnen D. AOM-induced mouse colon tumors do not express full-length APC protein. Carcinogenesis. 1997;18:2435–2439. doi: 10.1093/carcin/18.12.2435. [DOI] [PubMed] [Google Scholar]
- Mao Q, Yang J, Cui XM, et al. Target separation of a new anti-tumor saponin and metabolic profiling of leaves of Panax notoginseng by liquid chromatography with eletrospray ionization quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2012;59:67–77. doi: 10.1016/j.jpba.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Murthy S. Animal models of inflammatory bowel disease. In Vivo Model Inflamm. 2006;2:137–174. [Google Scholar]
- Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- Ohnishi R, Matsui-Yuasa I, Deguchi Y, et al. 1'-acetoxychavicol acetate inhibits adipogenesis in 3T3-L1 adipocytes and in high fat-fed rats. Am J Chin Med. 2012;40:1189–1204. doi: 10.1142/S0192415X12500887. [DOI] [PubMed] [Google Scholar]
- Oz HS, Chen T, de Villiers WJ. Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front Immunol. 2013;4:132. doi: 10.3389/fimmu.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AJ, Heap D, Carroll RE, Tyner AL. Tumor suppressor functions for the Cdk inhibitor p21 in the mouse colon. Oncogene. 2004;23:8128–8134. doi: 10.1038/sj.onc.1207994. [DOI] [PubMed] [Google Scholar]
- Poudyal D, Le PM, Davis T, et al. A hexane fraction of American ginseng suppresses mouse colitis and associated colon cancer: anti-inflammatory and proapoptotic mechanisms. Cancer Prev Res. 2012;5:685–696. doi: 10.1158/1940-6207.CAPR-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Sliva D, Loganathan J, Jiang J, et al. Mushroom Ganoderma lucidum prevents colitis-associated carcinogenesis in mice. PLoS ONE. 2012;7:e47873. doi: 10.1371/journal.pone.0047873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Qi LW, Du GJ, Mehendale SR, Wang CZ, Yuan CS. Red notoginseng: higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chem. 2011;125:1299–1305. doi: 10.1016/j.foodchem.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiroyama T, Yokoyama N, Hakukawa M, Sakuma K, Ichikawa F, Yoshida S. Clinical efficacy of macrophage-activating Chinese mixed herbs (MACH) in improvement of embryo qualities in women with long-term infertility of unknown etiology. Am J Chin Med. 2012;40:1–10. doi: 10.1142/S0192415X12500012. [DOI] [PubMed] [Google Scholar]
- Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia. 2010;12:95–102. doi: 10.1593/neo.91718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Aung HH, Zhang B, et al. Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res. 2008;28:2545–2551. [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Calway T, Yuan CS. Herbal medicines as adjuvants for cancer therapeutics. Am J Chin Med. 2012a;40:657–669. doi: 10.1142/S0192415X12500498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Luo X, Zhang B, et al. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother Pharmacol. 2007a;60:69–79. doi: 10.1007/s00280-006-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, McEntee E, Wicks S, Wu JA, Yuan CS. Phytochemical and analytical studies of Panax notoginseng (Burk.) F.H. Chen. J Nat Med. 2006;60:97–106. [Google Scholar]
- Wang CZ, Ni M, Sun S, et al. Detection of adulteration of notoginseng root extract with other Panax species by quantitative HPLC coupled with PCA. J Agric Food Chem. 2009a;57:2363–2367. doi: 10.1021/jf803320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Xie JT, Fishbein A, et al. Antiproliferative effects of different plant parts of Panax notoginseng on SW480 human colorectal cancer cells. Phytother Res. 2009b;23:6–13. doi: 10.1002/ptr.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Xie JT, Zhang B, et al. Chemopreventive effects of Panax notoginseng and its major constituents on SW480 human colorectal cancer cells. Int J Oncol. 2007b;31:1149–1156. [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Qi LW, Wang CZ, Li P. Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. Am J Chin Med. 2011;39:1103–1115. doi: 10.1142/S0192415X11009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang A, Sun H, Wang P. Systems biology technologies enable personalized traditional chinese medicine: a systematic review. Am J Chin Med. 2012b;40:1109–1122. doi: 10.1142/S0192415X12500826. [DOI] [PubMed] [Google Scholar]
- Wen XD, Yang J, Ma RH, et al. Analysis of Panax notoginseng metabolites in rat bile by liquid chromatography-quadrupole time-of-flight mass spectrometry with microdialysis sampling. J Chromatogr B. 2012;895–896:162–168. doi: 10.1016/j.jchromb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Mori H. Multistep carcinogenesis of the colon in Apc(Min/+) mouse. Cancer Sci. 2007;98:6–10. doi: 10.1111/j.1349-7006.2006.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Hosokawa M, Mikami N, Miyashita K, Tanaka T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem Biol Interact. 2011;193:79–87. doi: 10.1016/j.cbi.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf. 2013 doi: 10.1002/pds.3463. (In press) [DOI] [PubMed] [Google Scholar]