Abstract
Essentially nothing is known about the molecular underpinnings of crustacean circadian clocks. The genome of Daphnia pulex, the only crustacean genome available for public use, provides a unique resource for identifying putative circadian proteins in this species. Here, the Daphnia genome was mined for putative circadian protein genes using Drosophila melanogaster queries. The sequences of core clock (e.g. CLOCK, CYCLE, PERIOD, TIMELESS and CRYPTOCHROME 2), clock input (CRYPTOCHROME 1) and clock output (PIGMENT DISPERSING HORMONE RECEPTOR) proteins were deduced. Structural analyses and alignment of the Daphnia proteins with their Drosophila counterparts revealed extensive sequence conservation, particularly in functional domains. Comparisons of the Daphnia proteins with other sequences showed that they are, in most cases, more similar to homologs from other species, including vertebrates, than they are to those of Drosophila. The presence of both CRYPTOCHROME 1 and 2 in Daphnia suggests the organization of its clock may be more similar to that of the butterfly Danaus plexippus than to that of Drosophila (which possesses CRYPTOCHROME 1 but not CRYPTOCHROME 2). These data represent the first description of a putative circadian system from any crustacean, and provide a foundation for future molecular, anatomical and physiological investigations of circadian signaling in Daphnia.
Keywords: biological rhythm, circadian, Cladocera, clock, Crustacea, Daphnia pulex, Drosophila melanogaster, genome
1. Introduction
Virtually all organisms exhibit physiological and behavioral rhythms oscillating with a period of approximately 24-h. Regardless of species, these physiological/behavioral patterns, commonly referred to as circadian rhythms, are characterized by four basic properties (Allada and Chung, 2010): I. they persist under constant conditions (indicating the presence of a self-sustaining clock), II. the clock-driven activity reoccurs approximately every 24-h, III. the activity pattern is entrained by the solar day, and IV. the period of the activity, while sensitive to changes in environmental conditions, is stable over a wide range of temperatures. In addition, all circadian systems have three functional components (Allada and Chung, 2010): I. a core clock, which is responsible for time keeping, II. input pathways that act to synchronize the clock to the environment, and III. output pathways that transmit the timing information for the control of physiology and behavior (see Table 1 for the proteins in each category). Depending on the system in question, the cellular location of these components may be distinct or contained within a common locus.
Table 1.
Putative Daphnia pulex genes identified via genome mining
| Protein | Gene name* | Gene location/length | Homology to query | ||
|---|---|---|---|---|---|
|
| |||||
| Scaffold | Length (nucleotides) | Blast score | E-value | ||
|
| |||||
|
CORE CLOCK PROTEINS1
| |||||
| CASEIN KINASE II α-subunit (CKII α) | dappu-ckII α | 17 | 3693 | 576 | 1e-164 |
| CASEIN KINASE II β-subunit (CK II β) | dappu-ckII β | 41 | 2093 | 410 | 5e-115 |
|
| |||||
| CLOCK (CLK) | dappu-clk | 27 | 5939 | 407 | 2e-113 |
|
| |||||
| CLOCKWORK ORANGE (CWO) | dappu-cwo† | 19 | 3773 | 130 | 3e-30 |
|
| |||||
| CYCLE (CYC) | dappu-cyc | 1 | 6473 | 437 | 1e-122 |
|
| |||||
| DOUBLETIME (DBT) | dappu-dbt | 1 | 2124 | 550 | 1e-156 |
|
| |||||
| PAR DOMAIN PROTEIN 1e (PDP1e) | dappu-pdp1ε | 21 | 4794 | 139 | 3e-33 |
|
| |||||
| PERIOD (PER) | dappu-per | 47 | 5860 | 289 | 1e-77 |
|
| |||||
| PROTEIN PHOSPHATASE 1 (PP1) A | dappu-pp1 a | 145 | 2591 | 584 | 3e-167 |
| PROTEIN PHOSPHATASE 1 (PP1) B | dappu-pp1 b | 12 | 1972 | 566 | 1e-161 |
|
| |||||
| PROTEIN PHOSPHATASE 2A (PP2A) catalytic subunit – MICROTUBULE STAR (MTS) | dappu-mts | 13 | 2099 | 427 | 5e-120 |
| PROTEIN PHOSPHATASE 2A (PP2A) regulatory subunit – WIDERBORST (WBT) A | dappu-wbt a | 8 | 6199 | 746 | 0.0 |
| PROTEIN PHOSPHATASE 2A (PP2A) regulatory subunit – WIDERBORST (WBT) B | dappu-wbt b | 2 | 4882 | 614 | 5e-176 |
| PROTEIN PHOSPHATASE 2A (PP2A) regulatory subunit – TWINS (TWS) | dappu-tws | 43 | 4157 | 777 | 0.0 |
|
| |||||
| SHAGGY (SGG) | dappu-sgg | 76 | 5712 | 665 | 0.0 |
|
| |||||
| SUPERNUMERARY LIMBS (SLIMB) | dappu-slimb | 169 | 2880 | 807 | 0.0 |
|
| |||||
| TIMELESS 1 (TIM1) A | dappu-tim1 a | 24 | 14166 | 326 | 8e-89 |
| TIMELESS 1 (TIM1) B | dappu-tim1 b | 6 | 5052 | 282 | 2e-75 |
| TIMELESS 1 (TIM1) C | dappu-tim1 c | 10 | 3650 | 276 | 1e-73 |
| TIMELESS 1 (TIM1) D | dappu-tim1 d | 6 | 5438 | 270 | 5e-72 |
| TIMELESS 1 (TIM1) E | dappu-tim1 e | 6 | 5542 | 258 | 3e-68 |
| TIMELESS 1 (TIM1) F | dappu-tim1 f | 24 | 11674 | 235 | 2e-61 |
| TIMELESS 1 (TIM1) G | dappu-tim1 g | 75 | 2798 | 218 | 3e-56 |
| TIMELESS 1 (TIM1) H | dappu-tim1 h | 91 | 4537 | 181 | 5e-45 |
|
| |||||
| VRILLE (VRI) | dappu-vri | 92 | 2225 | 153 | 5e-37 |
|
| |||||
|
INPUT PATHWAY PROTEINS1
| |||||
| CRYPTOCHROME (CRY) A – (CRY1) | dappu-cry a≠ | 40 | 2706 | 474 | 6e-134 |
| CRYPTOCHROME (CRY) B – (CRY2) | dappu-cry bΔ | 18 | 4661 | 358 | 7e-99 |
| CRYPTOCHROME (CRY) C | dappu-cry c‡ | 10 | 3072 | 355 | 5e-98 |
| CRYPTOCHROME (CRY) D – (CRY DASH) | dappu-cry d+ | 7 | 2052 | 129 | 5e-30 |
|
| |||||
|
OUTPUT PATHWAY PROTEINS
| |||||
| PIGMENT DISPERSING HORMONE RECEPTOR (PDHR) | dappu-pdhr£ | 1 | 4621 | 362 | 5e-100 |
It should be noted that the group designations are based on the organization of the Drosophila melanogaster clock, whose proteins were used for querying the Daphnia genome. Unlike Drosophila, which possess only CRY1 (an input pathway protein), D. pulex appears to contain multiple CRY isoforms, including both CRY1 and CRY2 (a core clock protein), though in this table, all are presented under the “INPUT PATHWAY PROTEIN” heading.
All genes appear correctly annotated (Genes2010 Annotation) in wFleaBase (http://wfleabase.org/) unless otherwise noted.
Gene annotated as “conserved protein” in wFleaBase.
Gene annotated as “dna photolyase” in wFleaBase.
Gene annotated as “phr6-4, cryptochrome-1” in wFleaBase.
Gene annotated as “phr6-4, cryptochrome-1” in wFleaBase.
Gene annotated as “Cryptochrome-1” in wFleaBase.
Gene annotated as “class b secretin-like g-protein coupled receptor gprcal2” in wFleaBase.
While a number of species have been the subjects of investigations into the molecular mechanisms underlying the core circadian clock, perhaps the best studied are insects, and in particular, the fruit fly Drosophila melanogaster (Allada and Chung, 2010; Tomioka and Matsumoto, 2010). In Drosophila, work from many laboratories has elucidated several interacting molecular feedback loops, which form the core of a molecular clock. As recently reviewed by Allada and Chung (2010), a heterodimer formed by the CLOCK (CLK) and CYCLE (CYC) proteins binds to E-box elements in the promoter regions of the period (per) and timeless (tim) genes, activating their transcription (typically peaking late in the day). Due to this activation, PERIOD (PER) and TIMELESS (TIM) proteins are produced, accumulate and dimerize in the cytoplasm during the early evening hours, are translocated to the nucleus at approximately midnight, ultimately binding to the CLK/CYC heterodimer. The binding of the PER/TIM heterodimer to CLK/CYC inhibits this complex’s DNA binding to, and hence activation of, the per and tim genes during the late night.
In addition to the core clock proteins CLK, CYC, PER and TIM, a number of others are also involved in the control of the core clock feedback loop of Drosophila (Allada and Chung, 2010). Specifically, PER, TIM and CLK each exhibit rhythmic phosphorylation, with the peak in this state occurring in the late night or early day; PER is phosphorylated by casein kinase Iε (DOUBLETIME [DBT]) and CASEIN KINASE II (CKII), while TIM is phosphorylated by GLYCOGEN SYNTHASE KINASE 3B (SHAGGY [SGG]) and CKII. CLK is phosphorylated by a nuclear complex of PER and DBT. The phosphorylation of PER is known to enhance its repressor activity. In addition, phosphorylated PER and TIM are targets of the phosphatases PROTEIN PHOSPHATASE 2A (PP2A) and PROTEIN PHOSPHATASE 1 (PP1), the former of which is believed to be involved in generation of PER’s phosphorylation rhythm. The peak in phosphorylation of these proteins is known to precede their disappearance, which at least partially involves the ubiquitination of DBT-phosphorylated PER (and its resulting degradation via the ubiquitin-proteasome pathway) by the E3 ubiquitin ligase SUPERNUMERARY LIMBS (SLIMB); the proteolysis of PER removes repression of the CLK/CYC complex allowing for a new cycle of per and tim transcription. TIM is the target for CYPTOCHROME (CRY), a cell autonomous blue-light photoreceptor protein, which triggers its degradation (in the remainder of this paper Drosophila-type CRY is referred to as CRY1 to distinguish it from vertebrate-type CRY or CRY2, which is present both with and without CRY1 in non-drosophalid insects; e.g. Yuan et al., 2007).
In addition to their roles in regulating the PER-TIM feedback loop, the CLK/CYC heterodimer also activates several other interdependent feedback loops that are hypothesized to play roles in setting the phase and amplitude of the Drosophila core clock, as well as its rhythmic output (Allada and Chung, 2010). Specifically, CLK/CYC bind to E-box elements in the promoters of the par domain protein 1 (pdp1) and vrille (vri) genes, activating their transcription. In turn, the PAR DOMAIN PROTEIN 1 (PDP1) and VRILLE (VRI) proteins activate and repress, respectively, the transcription of the clock (clk) and cryptochrome (cry) genes. Because the accumulation of PDP1 is delayed relative to that of VRI, the rhythms of clk and cry activation are antiphase (peaking in early day) to those of per and tim. In addition the CLK/CYC heterodimer also activates transcription of the clockwork orange (cwo) gene. The CLOCKWORK ORANGE (CWO) protein, a basic helix-loop-helix (HLH) repressor, in turn targets the E-box elements of CLK/CYC target genes, repressing their activation.
Interestingly, while the Drosophila circadian system is arguably the best understood in the animal kingdom, it may not be stereotypical, even within insects (e.g. Zhu et al., 2005; Yuan et al., 2007; Zhu et al., 2008). Based on the complement of CRYs present, several models have been proposed for clock systems in insects (Yuan et al., 2007). Specifically, whereas D. melanogaster possesses a single CRY, in many insects, two CRYs have been identified, one similar to that of Drosophila, commonly referred to as dCRY or CRY1, and the other similar to that present in vertebrates, commonly referred to as CRY2; in several insects, only CRY2 has been found. In essentially all systems where it is present, CRY1 is proposed as a photosensitive input to the clock, providing a mechanism for entraining the clock to the solar day (Yuan et al., 2007). In contrast, CRY2 does not appear to play a role in photic entrainment, but rather appears to be a core clock protein, functioning as a repressor of CLK/CYC-mediated transcription (e.g. Zhu et al., 2005; Yuan et al., 2007; Zhu et al., 2008). Thus, in Drosophila, CRY likely functions solely as an input to the clock system, whereas in other insects members of the CRY family appear to serve both as inputs to the clock (CRY1) and as members of the core clock ensemble itself (CRY2); in insects with only CRY2, novel photic entrainment pathways are hypothesized, with CRY2 proposed to function primarily, perhaps solely, as a transcriptional repressor (e.g. Zhu et al., 2005; Yuan et al., 2007; Zhu et al., 2008). Evolutionary studies of CRY gene duplication and loss suggest that the clock system possessing both CRY1 and CRY2 is the most ancestral organization (Yuan et al., 2007).
As in other organisms, many crustaceans are known to display circadian patterns in physiology and behavior. As recently reviewed by Strauss and Dircksen (2010), known/postulated crustacean circadian behaviors include, but are not limited to, locomotion, feeding, moulting, reproduction, hatching/larval release, color change, and diel vertical migration. Interestingly, and despite the rich repertoire of circadian rhythms exhibited by crustaceans, essentially nothing is known about the molecular underpinnings of circadian clocks in these animals. While many laboratories have attempted to molecularly clone crustacean circadian proteins via reverse transcription polymerase chain reaction using degenerate primers, only two putative circadian proteins have thus far been identified and characterized from crustaceans, i.e. a putative homolog CLK from the freshwater prawn Macrobrachium rosenbergii (Yang et al., 2006) and a CRY homolog from the Antarctic krill Euphausia superba (Mazzotta et al., 2010).
The recent sequencing of the genome of the cladoceran crustacean Daphnia pulex provides an alternative avenue for identifying putative crustacean homologs of known insect circadian proteins, namely identification via genome mining; members of the genus Daphnia, like many other planktonic crustaceans, are known to exhibit pronounced diel migratory behaviors (e.g. Lampert, 1989; Loose, 1993; Loose and Dawidowicz, 1994). In the study presented here, we have used such a strategy to predict a large suite of D. pulex proteins that show significant homology to those that form the molecular underpinnings of the D. melanogaster circadian clock. Structural analysis of the identified proteins, which include, among others, putative homologs of PER, TIM, CLK and CYC, revealed that essentially all contain the domains known to be required for function in the fruit fly. Moreover, putative homologs of both CRY1 and CRY2 were identified, suggesting that the clock system of Daphnia is organized more like that proposed for lepidopterans and mosquitoes, than it is to the Drosophila system (Yuan et al., 2007), i.e. CRY1 acting as a circadian photoreceptor to the clock and CRY2 participating in the establishment of the core clock itself. In addition, a protein likely involved in mediating the output signaling of the clock, i.e. a receptor for pigment dispersing hormone, was identified and characterized. Taken collectively, the data presented here represent the first description of a putative circadian system from any crustacean, and provide a foundation for future molecular, anatomical and physiological investigations of circadian signaling in D. pulex and other crustacean species.
2. Materials and methods
2.1. Genome sequencing and gene modeling
For current descriptions of the preparation, sequencing and modeling of the D. pulex genome, readers are referred to http://wfleabase.org/ (Colbourne et al., 2005; 2011), which is maintained by the Indiana University Genome Informatics Laboratory (Indiana University, Bloomington, IN, USA).
2.2. Genome mining
Genome mining was accomplished using BLAST+ 2.2.23 software (downloadable from the National Center for Biotechnology Information, Bethesda, MD, USA; ftp://ftp.ncbi.nlm.nih.gov/blast/executables/blast+/) and the beta-release of the D. pulex Genes 2010 frozen genome assembly (Indiana University Genome Informatics Laboratory, and Center for Genomics and Bioinformatics at Indiana University, Bloomington, IN, USA; http://wfleabase.org/) as described in several earlier publications (Christie et al., 2011; McCoole et al., 2011); D. melanogaster proteins were used to query the genome. For all searches, the BLAST score and BLAST-generated E-value for significant alignment are provided in Table 1. To strengthen our gene identifications, the sequence of the Daphnia protein deduced from each gene was reciprocally blasted against the Drosophila proteins curated in FlyBase (Tweedie et al., 2009); the results of these analyses are shown in Table 2. In addition, each protein was blasted against all non-redundant protein sequences curated at NCBI (excluding Daphnia proteins, obvious partial proteins, synthetic constructs and provisional protein sequences) using the online program protein blast (blastp algorithm used; http://blast.ncbi.nlm.nih.gov/Blast.cgi); the top five hits for each protein are shown in Table 3.
Table 2.
Reciprocal blasting of deduced Daphnia pulex proteins versus known Drosophila melanogaster protein sequences in FlyBase
| Daphnia protein | Top named protein hit in FlyBase
|
|||
|---|---|---|---|---|
| Protein name | FlyBase ID | Homology to query
|
||
| Blast score | E-value | |||
|
| ||||
|
CORE CLOCK PROTEINS1
| ||||
| Dappu-CKII α | CKIIalpha-PC | FBpp0070043 | 566.2 | 1.06e-161 |
| Dappu-CKII β | CKIIbeta-PE | FBpp0089135 | 409.1 | 9.91e-115 |
|
| ||||
| Dappu-CLK | CLK-PA | FBpp0076500 | 397.9 | 1.64e-110 |
|
| ||||
| Dappu-CWO | CWO-PA | FBpp0081723 | 106.3 | 7.84e-23 |
|
| ||||
| Dappu-CYC | CYC-PA | FBpp0074693 | 432.6 | 3.92e-73 |
|
| ||||
| Dappu-DBT | DCO-PC (DBT) | FBpp0085106 | 540.8 | 5.61e-154 |
|
| ||||
| Dappu-PDP1e | PDP1-PD | FBpp0076495 | 109.8 | 2.72e-24 |
|
| ||||
| Dappu-PER | PER-PA | FBpp0070455 | 264.6 | 3.30e-70 |
|
| ||||
| Dappu-PP1 A | PP1alpha-96A-PA | FBpp0084026 | 598.2 | 2.36e-171 |
| Dappu-PP1 B | FLW-PB (PP1) | FBpp0071382 | 599.7 | 8.31e-172 |
|
| ||||
| Dappu-PP2A MTS | PP4-19C-PE (MTS) | FBpp0077017 | 592.8 | 1.02e-169 |
| Dappu-PP2A WBT A | WDB-PE | FBpp0084579 | 625.9 | 1.67e-179 |
| Dappu-PP2A WBT B | PP2A-B′-PJ | FBpp0288759 | 744.2 | 0 |
| Dappu-PP2A TWS | TWS-PH | FBpp0081671 | 755.0 | 0 |
|
| ||||
| Dappu-SGG | SGG-PA | FBpp0070450 | 658.7 | 0 |
|
| ||||
| Dappu-SLIMB | SLMB-PA (SLIMB) | FBpp0083434 | 803.1 | 0 |
|
| ||||
| Dappu-TIM1 A | TIM-PJ | FBpp0291971 | 308.1 | 2.47e-83 |
| Dappu-TIM1 B | TIM-PJ | FBpp0291971 | 261.9 | 1.69e-69 |
| Dappu-TIM1 C | TIM-PJ | FBpp0291971 | 275.8 | 9.85e-74 |
| Dappu-TIM1 D | TIM-PC | FBpp0077254 | 251.1 | 3.03e-66 |
| Dappu-TIM1 E | TIM-PI | FBpp0291970 | 255.8 | 1.10e-67 |
| Dappu-TIM1 F | TIM-PC | FBpp0077254 | 212.2 | 2.43e-54 |
| Dappu-TIM1 G | TIM-PI | FBpp0291970 | 219.2 | 7.13e-57 |
| Dappu-TIM1 H | TIM-PI | FBpp0291970 | 193.7 | 5.21e-49 |
|
| ||||
| Dappu-VRI | VRI-PD | FBpp0289297 | 149.1 | 8.29e-36 |
|
| ||||
|
INPUT PATHWAY PROTEINS1
| ||||
| Dappu-CRY A | CRY-PA | FBpp0083150 | 476.1 | 5.56e-140 |
| Dappu-CRY B | PHR6-4-PB | FBpp0080935 | 495.0 | 2.59e-134 |
| Dappu-CRY C | PHR6-4-PB | FBpp0080935 | 594.7 | 5.15e-170 |
| Dappu-CRY D | PHR6-4-PB | FBpp0080935 | 167.5 | 2.12e-41 |
|
| ||||
|
OUTPUT PATHWAY PROTEINS
| ||||
| Dappu-PDHR | PDFR-PA | FBpp0099841 | 345.5 | 5.62e-95 |
Abbreviations: CKII, casein kinase II; CLK, CLOCK; CRY, CRYPTOCHROME; CWO, CLOCKWORK ORANGE; CYC, CYCLE; DBT, DOUBLETIME; MTS, MICROTUBULE STAR; PDFR, PIGMENT DISPERSING FACTOR RECEPTOR; PDHR, PIGMENT DISPERSING HOROMONE RECEPTOR; PDP1ε; PAR DOMAIN PROTEIN 1ε; PER, PERIOD; PHR, PHOTOLYASE; PP1, PROTEIN PHOSPHATASE 1; PP2A, PROTEIN PHOSPHATASE 2A; SGG, SHAGGY; SLIMB, SUPERNUMERARY LIMBS; TIM, TIMELESS; TWS, TWINS; VRI, VRILLE; WBT, WIDERBORST.
It should be noted that the group designations are based on the organization of the Drosophila melanogaster clock, whose proteins were used for querying the Daphnia genome. Unlike Drosophila, which possesses only CRY1 (an input pathway protein), D. pulex appears to contain multiple CRY isoforms, including both CRY1 and CRY2 (a core clock protein), though in this table, all are presented under the “INPUT PATHWAY PROTEIN” heading.
Table 3.
blastp analyses of putative Daphnia pulex circadian system proteins vs. all NCBI curated non-redundant protein sequences*
| Daphnia protein | Top five blastp protein hits
|
||||
|---|---|---|---|---|---|
| Accession no. | Species | Protein description | Blast score | E-value | |
|
| |||||
|
CASEIN KINASE II (CKII) – α subunit
| |||||
| Dappu-CKII α | 1NA7_A | Homo sapiens | Chain A, Catalytic Subunit of Protein Kinase Ck2 | 624 | 9e-177 |
| EFN84867 | Harpegnathos saltator | Casein kinase II subunit alpha | 621 | 6e-176 | |
| EFN62439 | Camponotus floridanus | Casein kinase II subunit alpha | 620 | 6e-176 | |
| EGI62250 | Acromyrmex echinatior | Casein kinase II subunit alpha | 619 | 2e-175 | |
| O76484 | Spodoptera frugiperda | Casein kinase II subunit alpha | 605 | 3e-171 | |
|
| |||||
|
CASEIN KINASE II (CKII) – β subunit
| |||||
| Dappu-CKII β | CAI18393 | Homo sapiens | casein kinase 2, beta polypeptide | 425 | 2e-117 |
| EDL26660 | Mus musculus | casein kinase II, beta subunit, isoform CRA_c | 424 | 3e-117 | |
| EDL83523 | Rattus norvegicus | casein kinase 2, beta subunit, isoform CRA_b | 424 | 4e-117 | |
| CBK38916 | Mytilus galloprovincialis | protein kinase CK2beta regulatory subunit | 422 | 2e-116 | |
| CAJ83806 | Xenopus tropicalis | casein kinase 2, beta subunit | 421 | 4e-116 | |
|
| |||||
|
CLOCK (CLK)
| |||||
| Dappu-CLK | BAJ16353 | Thermobia domestica | CLOCK | 467 | 5e-129 |
| AAR14936 | Antheraea pernyi | CLOCK | 461 | 2e-127 | |
| EGI62057 | Acromyrmex echinatior | Circadian locomoter output cycles protein kaput | 444 | 2e-122 | |
| EFN76178 | Hapregnathos saltator | Circadian locomoter output cycles protein kaput | 442 | 1e-121 | |
| EFN61630 | Camponotus floridanus | Circadian locomoter output cycles protein kaput | 441 | 3e-121 | |
|
| |||||
|
CLOCKWORK ORANGE (CWO)
| |||||
| Dappu-CWO | AAF54527 | Drosophila melanogaster | Clockwork orange | 132 | 3e-28 |
| AAF24476 | Drosophila melanogaster | Sticky ch1 | 132 | 3e-28 | |
| EFN67685 | Camponotus floridanus | Hairy/enhancer-of-split related with YRPW motif protein 2 | 131 | 4e-28 | |
| ACK77653 | Drosophila melanogaster | RE11081p | 131 | 5e-28 | |
| EGI62806 | Acromyrmex echinatior | Hairy/enhancer-of-split related with YRPW motif protein 1 | 130 | 9e-28 | |
|
| |||||
|
CYCLE (CYC)
| |||||
| Dappu-CYC | BAJ16354 | Thermobia domestica | CYCLE | 612 | 8e-173 |
| ABI21880 | Lutzomyia longipalpis | Cycle | 592 | 5e-167 | |
| EFN72014 | Camponotus floridanus | Aryl hydrocarbon receptor nuclear translocator-like protein 1 | 567 | 2e-159 | |
| AAW80970 | Xenopus laevis | BMAL1 | 483 | 5e-134 | |
| Q6YGZ5 | Tyto alba | Aryl hydrocarbon receptor nuclear translocator-like protein 1 | 474 | 3e-131 | |
|
| |||||
|
DOUBLETIME (DBT)/CASEIN KINASE Iε
| |||||
| Dappu-DBT | EFN64010 | Camponotus floridanus | Casein kinase I isoform epsilon | 590 | 9e-167 |
| EGI65788 | Acromyrmex echinatior | Casein kinase I isoform epsilon | 584 | 6e-165 | |
| Q5ZLL1 | Gallus gallus | Casein kinase I isoform epsilon | 582 | 4e-164 | |
| AAF65549 | Mesocricetus auratus | casein kinase I epsilon | 582 | 4e-164 | |
| EFB26732 | Ailuropoda melanoleuca | PANDA_002512 | 582 | 5e-164 | |
|
| |||||
|
PAR DOMAIN PROTEIN 1ε (PDP 1ε)
| |||||
| Dappu-PDP 1ε | EDM05669 | Rattus norvegicus | rCG33934 | 176 | 5e-42 |
| AAN12026 | Drosophila melanogaster | PAR-domain protein 1, isoform J | 175 | 7e-42 | |
| AAN12025 | Drosophila melanogaster | PAR-domain protein 1, isoform D | 175 | 8e-42 | |
| AAN12027 | Drosophila melanogaster | PAR-domain protein 1, isoform C | 174 | 2e-41 | |
| EFB19547 | Ailuropoda melanoleuca | PANDA_015107 | 174 | 2e-41 | |
|
| |||||
|
PERIOD (PER)
| |||||
| Dappu-PER | AAN02439 | Blattella germanica | circadian clock protein PERIOD | 397 | 4e-108 |
| BAG48878 | Gryllus bimaculatus | period | 370 | 6e-100 | |
| BAI47546 | Modicogryllus siamensis | period clock protein | 367 | 6e-99 | |
| Q25637 | Periplaneta americana | Period circadian protein | 366 | 1e-98 | |
| ADO24377 | Laupala paranigra | period | 353 | 7e-95 | |
|
| |||||
|
PROTEIN PHOSPHATASE 1 (PP1)
| |||||
| Dappu-PP1 A | EFN69572 | Camponotus floridanus | Serine/threonine-protein phosphatase alpha-1 isoform | 654 | 0.0 |
| ADY40510 | Ascaris suum | Serine/threonine-protein phosphatase PP1-beta | 643 | 0.0 | |
| EFA05189 | Tribolium castaneum | TcasGA2_TC015321 | 642 | 0.0 | |
| ACO15366 | Caligus clemensi | Serine/threonine-protein phosphatase PP1-beta | 641 | 0.0 | |
| BAJ85104 | Hordeum vulgare | predicted protein | 640 | 0.0 | |
| Dappu-PP1 B | CAD61270 | Danio rerio | similar to human protein phosphatase 1, catalytic subunit, beta isoform | 624 | 4e-177 |
| AAM88380 | Canis lupus familiaris | protein phosphatase type 1 catalytic subunit delta isoform | 622 | 2e-176 | |
| ABQ18261 | Carassius auratus | protein serine/threonine phosphatase-1 catalytic subunit beta isoform | 622 | 2e-176 | |
| EFN76901 | Harpegnathos saltator | Serine/threonine-protein phosphatase PP1-beta catalytic subunit | 622 | 2e-176 | |
| BAE38902 | Mus musculus | unnamed protein product | 622 | 3e-176 | |
|
| |||||
|
PROTEIN PHOSPHATASE 2A – catalytic subunit MICROTUBULE STAR (MTS)
| |||||
| Dappu-MTS | EFN85419 | Harpegnathos saltator | Serine/threonine-protein phosphatase 4 catalytic subunit | 605 | 3e-171 |
| AAD01262 | Takifugu rubripes | serine/threonine phosphatase | 601 | 4e-170 | |
| AAV38551 | Homo sapiens | protein phosphatase 4 (formerly X), catalytic subunit | 598 | 2e-169 | |
| ACO10717 | Caligus rogercresseyi | Serine/threonine-protein phosphatase 4 catalytic subunit | 595 | 3e-168 | |
| ACO10485 | Caligus rogercresseyi | Serine/threonine-protein phosphatase 4 catalytic subunit | 595 | 4e-168 | |
|
| |||||
|
PROTEIN PHOSPHATASE 2A – regulatory subunit WIDERBORST (WBT)
| |||||
| Dappu-WBT A | EFN66909 | Camponotus floridanus | Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit epsilon isoform | 769 | 0.0 |
| EGI66726 | Acromyrmex echinatior | Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit alpha isoform | 761 | 0.0 | |
| EFA06323 | Tribolium castaneum | TcasGA2_TC009194 | 747 | 0.0 | |
| AAN14114 | Drosophila melanogaster | widerborst, isoform C | 742 | 0.0 | |
| AAH64358 | Homo sapiens | PPP2R5E protein | 678 | 0.0 | |
| Dappu-WBT B | EFN69797 | Camponotus floridanus | Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit delta isoform | 855 | 0.0 |
| EFN83893 | Harpegnathos saltator | Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit delta isoform | 848 | 0.0 | |
| BAG61599 | Homo sapiens | unnamed protein product | 836 | 0.0 | |
| AAQ01559 | Mus musculus | protein phosphatase 2A B56 delta subunit | 830 | 0.0 | |
| BAF84486 | Homo sapiens | unnamed protein product | 828 | 0.0 | |
|
| |||||
|
PROTEIN PHOSPHATASE 2A – regulatory subunit TWINS (TWS)
| |||||
| Dappu-TWS | EFA10095 | Tribolium castaneum | TcasGA2_TC012273 | 823 | 0.0 |
| AAN13458 | Drosophila melanogaster | twins, isoform E | 777 | 0.0 | |
| AAF54498 | Drosophila melanogaster | twins, isoform A | 776 | 0.0 | |
| AAA99871 | Drosophila melanogaster | phosphoprotein phosphatase 2A 55 lDa regulatory subunit | 775 | 0.0 | |
| P56932 | Rattus norvegicus | Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B delta isoform | 761 | 0.0 | |
|
| |||||
|
SHAGGY (SGG)/GLYCOGEN SYNTHASE KINASE 3B
| |||||
| Dappu-SGG | ABO61882 | Rhipicephalus microplus | glycogen synthase kinase | 676 | 0.0 |
| EFN78376 | Harpegnathos saltator | Protein kinase shaggy | 676 | 0.0 | |
| EGI58906 | Acromyrmex echinatior | Protein kinase shaggy | 673 | 0.0 | |
| Q5YJC2 | Spermophilus citellus | Glycogen synthase kinase-3 beta | 670 | 0.0 | |
| P18266 | Rattus norvegicus | Glycogen synthase kinase-3 beta | 666 | 0.0 | |
|
| |||||
|
SUPERNUMERARY LIMBS (SLIMB)/E3 UBIQUITIN LIGASE
| |||||
| Dappu-SLIMB | BAE26547 | Mus musculus | unnamed protein product | 840 | 0.0 |
| EAW49771 | Homo sapiens | beta-transducin repeat containing, isoform CRA_b | 839 | 0.0 | |
| EDL94307 | Rattus norvegicus | beta-transducin repeat containing | 839 | 0.0 | |
| DAA14816 | Bos taurus | beta-tranducin repeat containing protein | 838 | 0.0 | |
| BAG36210 | Homo sapiens | unnamed protein product | 838 | 0.0 | |
|
| |||||
|
TIMELESS (TIM)
| |||||
| Dappu-TIM A | AAR15505 | Danaus plexippus | timeless | 468 | 3e-129 |
| BAB85487 | Sarcophaga bullata | timeless | 344 | 4e-92 | |
| AAB94890 | Drosophila melanogaster | circadian clock protein | 344 | 6e-92 | |
| AAF51098 | Drosophila melanogaster | timeless, isoform B | 343 | 7e-92 | |
| AAN10371 | Drosophila melanogaster | timeless, isoform D | 343 | 7e-92 | |
|
| |||||
| Dappu-TIM B | AAF66996 | Antheraea pernyi | timeless | 394 | 4e-107 |
| AAR15505 | Danaus plexippus | timeless | 383 | 7e-104 | |
| AAB94930 | Drosophila virilis | circadian clock protein | 313 | 1e-82 | |
| ABW71828 | Chymomyza costata | timeless | 303 | 1e-79 | |
| EFA04644 | Tribolium castaneum | timeless | 302 | 2e-79 | |
|
| |||||
| Dappu-TIM C | EFA04644 | Tribolium castaneum | timeless | 397 | 4e-108 |
| AAR15505 | Danaus plexippus | timeless | 371 | 3e-100 | |
| AAN10371 | Drosophila melanogaster | timeless, isoform D | 302 | 1e-79 | |
| P49021 | Drosophila melanogaster | Protein timeless | 302 | 1e-79 | |
| AAB94890 | Drosophila melanogaster | circadian clock protein | 302 | 2e-79 | |
|
| |||||
| Dappu-TIM D | EFA04644 | Tribolium castaneum | timeless | 387 | 4e-105 |
| AAF66996 | Antheraea pernyi | timeless | 386 | 1e-104 | |
| AAR15505 | Danaus plexippus | timeless | 382 | 2e-103 | |
| AAB94890 | Drosophila melanogaster | circadian clock protein | 293 | 8e-77 | |
| AAF51098 | Drosophila melanogaster | timeless, isoform B | 293 | 8e-77 | |
|
| |||||
| Dappu-TIM E | ADV36936 | Drosophila melanogaster | timeless, isoform I | 282 | 2e-73 |
| AAB94890 | Drosophila melanogaster | circadian clock protein | 282 | 2e-73 | |
| AAF51098 | Drosophila melanogaster | timeless, isoform B | 282 | 2e-73 | |
| AAN10372 | Drosophila melanogaster | timeless, isoform C | 281 | 3e-73 | |
| AAL13507 | Drosophila melanogaster | GH03106p | 281 | 3e-73 | |
|
| |||||
| Dappu-TIM F | P49021 | Drosophila melanogaster | Protein timeless | 236 | 2e-59 |
| AAN10371 | Drosophila melanogaster | timeless, isoform D | 236 | 2e-59 | |
| AAF51098 | Drosophila melanogaster | timeless, isoform B | 236 | 2e-59 | |
| AAB94890 | Drosophila melanogaster | circadian clock protein | 236 | 2e-59 | |
| AAF51097 | Drosophila melanogaster | timeless, isoform K | 236 | 3e-59 | |
|
| |||||
| Dappu-TIM G | O17482 | Drosophila virilis | Protein timeless | 236 | 6e-60 |
| AAB94930 | Drosophila virilis | tim | 236 | 9e-60 | |
| BAB85487 | Sarcophaga bullata | timeless | 232 | 2e-58 | |
| AAY40757 | Aedes aegypti | TIMELESS | 231 | 3e-58 | |
| AAF51098 | Drosophila melanogaster | timeless, isoform B | 231 | 4e-58 | |
|
| |||||
| Dappu-TIM H | BAJ16356 | Gryllus bimaculatus | TIMELESS | 216 | 1e-53 |
| O17482 | Drosophila virilis | Protein timeless | 214 | 6e-53 | |
| AAB94930 | Drosophila virilis | tim | 213 | 9e-53 | |
| BAB85471 | Sarcophaga crassipalpis | TIMELESS | 212 | 3e-52 | |
| BAB85487 | Sarcophaga bullata | timeless | 211 | 7e-52 | |
|
| |||||
|
VRILLE (VRI)
| |||||
| Dappu-VRI | AAT86041 | Danaus plexippus | vrille | 169 | 2e-39 |
| AAS92609 | Antheraea pernyi | vrille | 166 | 1e-38 | |
| CAX37108 | Acyrthosiphon pisum | vrille | 159 | 1e-36 | |
| CAA72535 | Drosophila melanogaster | bZIP transcription factor | 155 | 3e-35 | |
| AAF52237 | Drosophila melanogaster | vrille, isoform A | 155 | 3e-35 | |
|
| |||||
|
CRYPTOCHROME (CRY)
| |||||
| Dappu-CRY A | BAF45421 | Dianemobius nigrofasciatus | cryptochrome precursor | 523 | 2e-146 |
| AAK11644 | Antheraea pernyi | cryptochrome | 516 | 2e-144 | |
| AAX58599 | Danaus plexippus | cryptochrome | 511 | 1e-142 | |
| BAI67362 | Bactrocera cucurbitae | cryptochrome | 506 | 5e-141 | |
| BAI67363 | Bactrocera cucurbitae | cryptochrome | 504 | 9e-141 | |
|
| |||||
| Dappu-CRY B | EFR20390 | Anopheles darlingi | AND_20159 | 882 | 0.0 |
| ABB29887 | Anopheles gambiae | cryptochrome 2 | 877 | 0.0 | |
| ABO31112 | Bombus impatiens | cryptochrome 2 protein | 851 | 0.0 | |
| EFN85258 | Harpegnathos saltator | Cryptochrome-1 | 850 | 0.0 | |
| BAG07408 | Riptotus pedestris | cryptochrome-m | 844 | 0.0 | |
|
| |||||
| Dappu-CRY C | AAI66277 | Xenopus tropicalis | LOC100144974 protein | 669 | 0.0 |
| EFR25332 | Anopheles darlingi | AND_09443 | 620 | 1e-175 | |
| ACN10565 | Salmo salar | Cryptochrome-1 | 612 | 5e-173 | |
| CAG02357 | Tetraodon nigroviridis | unnamed protein product | 612 | 6e-173 | |
| AAL90322 | Drosophila melanogaster | RE11660p | 595 | 8e-168 | |
|
| |||||
| Dappu-CRY D | CBN81995 | Dicentrarchus labrax | Cryptochrome DASH | 634 | 1e-179 |
| Q4KML2 | Danio rerio | Cryptochrome DASH | 623 | 2e-176 | |
| AAH98514 | Danio rerio | Cryptochrome DASH | 619 | 3e-175 | |
| CAO90052 | Microcystis aeruginosa | unnamed protein product | 475 | 8e-132 | |
| P77967 | Synechocystis sp. | Cryptochrome DASH | 452 | 5e-125 | |
|
| |||||
|
PIGMENT DISPERSING HORMONE RECEPTOR (PDHR)
| |||||
| Dappu-PDHR | BAH85843 | Marsupenaeus japonicus | pigment dispersing hormone receptor | 385 | 8e-105 |
| ADG46049 | Drosophila melanogaster | MIP16931p | 380 | 3e-103 | |
| AAF45788 | Drosophila melanogaster | PDF receptor | 380 | 3e-103 | |
| CAB72288 | Drosophila melanogaster | EG:BACR25B3.3 | 347 | 2e-93 | |
| CAP34509 | Caenorhabditis briggsae | CBG_16586 | 227 | 7e-71 | |
excluding Daphnia proteins, partial proteins, synthetic constructs and provisional protein sequences.
2.3. Analyses of protein structure
Analyses of protein structural motifs were accomplished using the online program SMART (http://smart.embl-heidelberg.de/ [Schultz et al., 1998; Letunic et al., 2009]) and homology to the structural motifs of previously described insect circadian proteins, predominantly ones from D. melanogaster. Alignment of all proteins shown in our figures was done using the online program MAFFT version 6 (http://align.bmr.kyushu-u.ak.jp/mafft/online/server/; [Katoh and Toh, 2008]). Amino acid identity was calculated as number of identical amino acids (denoted by [*]) divided by the total number of amino acids in the longest sequence, while amino acid similarity was calculated as number of identical and similar amino acids (the latter denoted by the [:] and [.] symbols in the protein alignments) divided by the total number of amino acids in longest sequence.
2.4. Figure production
Alignments generated in MAFFT were copied and pasted into Microsoft Word, and the structural domains identified by SMART analyses, colored using this program. For all figures, a common coloring scheme was used to highlight each structural domain: serine/threonine kinase catalytic, red; HLH, green; PAS, light blue; PAC, blue; coiled-coil, pink; orange, yellow; basic region leucine zipper, dark blue; protein phosphatase 2A, dark green; WD40, dark red; FBOX, dark gray; transmembrane, light gray; hormone receptor, black.
3. Results
As stated in Section 1, all known circadian systems are composed of three functional components: a core clock, input pathways that act to synchronize the clock to the environment, and output pathways that transmit timing information. With one exception, the results of genome searches and protein analyses are grouped according their putative role within the theoretical Daphnia circadian system, i.e. core clock proteins, input pathway proteins, or output pathway proteins; the results for CRY are presented under “input pathway proteins”, though, in some species, family members also serve as key components of the core clock as well. Within each of these grouping, data are presented in alphabetical order based on the Drosophila protein name. It should be noted that the Daphnia protein sequences reported in this study are based on the Genes 2010 gene model algorithm, which, in a previous study (Christie et al., 2011), was found to typically have the best fit with the extant D. pulex transcriptome data, at least for peptide precursor protein genes. This said, other gene model algorithms (i.e. JGI, Gnomon, PASA and SNAP) did in some cases predict slightly different protein sequences from those shown here, and readers should take heed of this and treat the sequences presented as theoretical rather than biochemically-confirmed.
3.1. Core clock proteins
3.1.1. CASEIN KINASE II (CKII)
3.1.1.1. CKII α-subunit
A single D. pulex gene (dappu-ckII α) was identified as encoding a putative CKII α-subunit protein via a query using a D. melanogaster CKII α (Accession no. AAN11415; Adams et al., 2000). The Genes 2010 gene model shows dappu-ckII α to be located on Scaffold 17 of the genome, with a predicted length of 3693 nucleotides (Table 1).
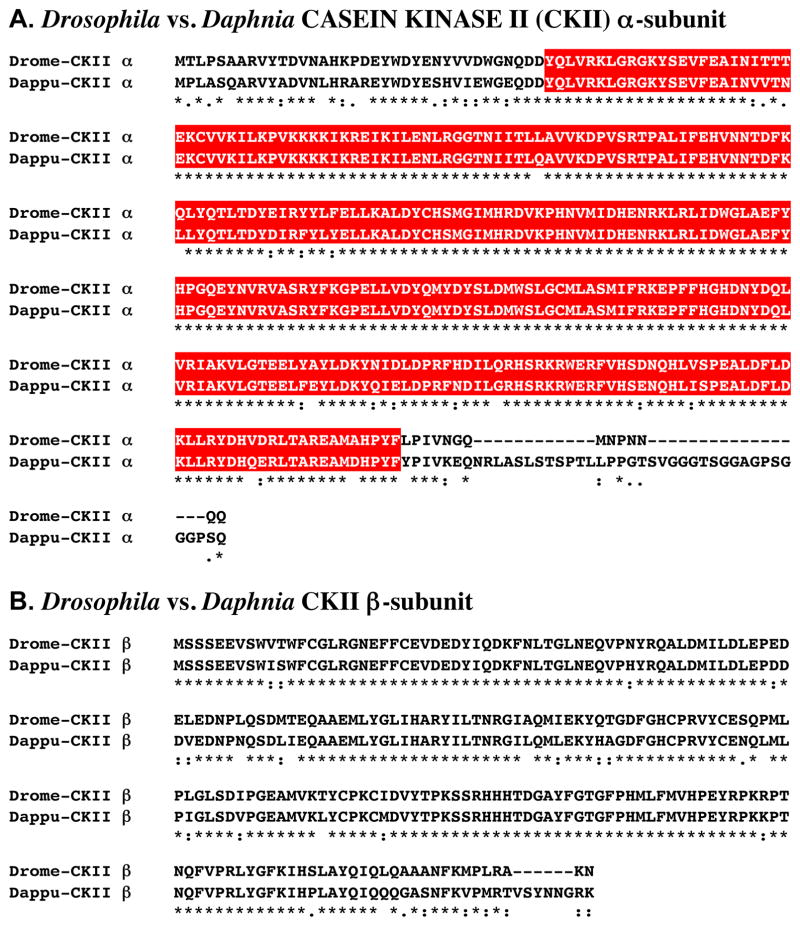
Figure 1A shows the alignment of the protein deduced from dappu-ckII α (Dappu-CKII α; 365 amino acids in overall length) with that of the Drosophila query (336 amino acids long).
Figure 1.
Putative Daphnia pulex CASEIN KINASE II (CKII) α- and β-subunit proteins. (A) Alignment of Drosophila melanogaster CKII α-subunit (Drome-CKII α) with D. pulex CKII α-subunit (Dappu-CKII α). (B). Alignment of D. melanogaster CKII β-subunit (Drome-CKII β) with D. pulex CKII β-subunit (Dappu-CKII β). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, serine/threonine kinase catalytic domains predicted by SMART analyses are highlighted in red.
Comparison of the sequence of Dappu-CKII α with that of Drome-CKII α revealed 81.1% amino acid identity/89.6 % amino acid similarity between the two proteins. SMART analyses of Dappu-CKII α and Drome-CKII α identified a single, highly conserved (93.4% identical/97.9% similar) serine/threonine kinase catalytic domain within each protein (Fig. 1A).
Reciprocal blasting of Dappu-CKII α against all proteins curated in FlyBase revealed CKII α (Flybase no. FBpp0070043) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Interestingly, blastp comparison of Dappu-CKII α with all non-redundant protein sequences curated by NCBI revealed the catalytic subunit of human CK II (1NA7_A) to be the most similar protein match (Table 3); the remaining top five blastp hits are all insect CKII α proteins, though Drosophila CKII α is not among them (Table 3).
3.1.1.2. CKII β-subunit
A single D. pulex gene (dappu-ckII β) was identified as encoding a putative CKII β-subunit protein via a query using a D. melanogaster CKII β (AAF48093; Adams et al., 2000). The Genes 2010 gene model shows dappu-ckII β to be located on Scaffold 41 of the genome, with a predicted length of 2093 nucleotides (Table 1).
Figure 1B shows the alignment of the protein deduced from dappu-ckII β (Dappu-CKII β; 221 amino acids in overall length) with that of the Drosophila query (215 amino acids long). Comparison of the sequence of Dappu-CKII β with that of Drome-CKII β revealed 84.2% amino acid identity/94.6 % amino acid similarity between the two proteins. No functional domains were identified in either Dappu-CKII β or Drome-CKII β via SMART analysis.
Reciprocal blasting of Dappu-CKII β against all proteins curated in FlyBase identified CKII β (Flybase No. FBpp0089135) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Interestingly, and similar to the result found for Dappu-CKII α, a human CKII β protein (CAI18393) was found to have the highest homology score when Dappu-CKII β was blasted against all non-redundant protein sequences curated by NCBI (Table 3). Perhaps even more surprising was the finding that none of the remaining top five blastp hits from the NCBI database were from insects. In fact, only one invertebrate protein, a CKII β from the bivalve mollusc Mytilus galloprovincialis (CBK3891), was among these hits (Table 3).
3.1.2. CLOCK (CLK)
A single D. pulex gene (dappu-clk) was identified as encoding a putative CLK protein via a query using a D. melanogaster CLK (AAC62234; Bae et al., 1998). The Genes 2010 gene model shows dappu-clk to be located on Scaffold 27 of the genome, with a predicted length of 5939 nucleotides (Table 1).
Figure 2A shows the alignment of the protein deduced from dappu-clk (Dappu-CLK; 890 amino acids in overall length) with that of the Drosophila query (1027 amino acids long). Comparison of the sequence of Dappu-CLK with that of Drome-CLK revealed 30.8% amino acid identity/57.4% amino acid similarity between the two proteins. SMART analyses of Dappu-CLK and Drome-CLK identified similar, though not identical, sets of structural domains within each protein. Specifically, both Dappu-CLK and Drome-CLK are predicted to contain a single HLH domain, two PAS domains, and a single PAC domain (Fig. 2A). In addition, Drome-CLK is predicted to contain three coiled-coil regions; this motif is absent in Dappu-CLK (Fig. 2A). For those domains that are shared between Dappu-CLK and Drome-CLK, extensive amino acid conservation is evident: HLH, 60.8% identity/96.1 % similarity; PAS1, 43.3 % identity/80.6 % similarity; PAS2, 73.1 % identity/92.5 % similarity; PAC, 70.5 % identity/93.2 % similarity. Essentially no conservation of sequence is seen between the two proteins in any of the coiled-coil regions (Fig. 2A).
Figure 2.
Putative Daphnia pulex CLOCK (CLK) protein. (A) Alignment of Drosophila melanogaster CLK (Drome-CLK) with D. pulex CLK (Dappu-CLK). (B). Alignment of Macrobrachium rosenbergii CLK (Macro-CLK) with Dappu-CLK. In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, helix-loop-helix, PAS, PAC and coiled-coil domains identified by SMART analyses are highlighted in green, light blue, blue, and pink, respectively.
As stated in Section 1, CLK is one of the few circadian proteins for which a putative crustacean family member has been identified, i.e. M. rosenbergii CLK (Yang et al., 2006). Comparison of the sequences of Dappu-CLK and Macro-CLK (AAX44045; Yang et al., 2006), revealed a level of amino acid identity/similarity similar to that seen for Dappu-CLK and Drome-CLK (i.e. 30.4 % identity/56.0 % similarity; Fig. 2B). As for Dappu-CLK and Drome-CLK, SMART analysis of Macro-CLK identified single HLH domain, two PAS domains, and a single PAC domain within this protein (Fig. 2B). In addition, this analysis identified a single coiled-coil region in Macro-CLK (Fig. 2B). Comparison of the HLH, PAS and PAC domains of Macro-CLK and Dappu-CLK revealed slightly higher levels of conservation to those reported above for Drome-CLK and Dappu-CLK (HLH, 62.7% identity/100 % similarity; PAS1, 60.0% identity/85.0% similarity; PAS2, 80.0% identity/95.0% similarity; PAC, 81.8% identity/93.2% similarity). Little sequence conservation is seen between the Macrobrachium and Daphnia CLKs in the coiled-coil region of the former protein.
Reciprocal blasting of Dappu-CLK against the proteins curated in FlyBase identified CLK (Flybase no. FBpp0076500) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Comparison of Dappu-CLK with all non-redundant protein sequences curated by NCBI revealed the top five blastp hits to be insect CLK sequences, with a CLK from the firebrat Thermobia domestica (BAJ16353) showing the highest similarity (Table 3); Drosophila CLK was not among these proteins (Table 3).
3.1.3. CLOCKWORK ORANGE (CWO)
A single D. pulex gene (dappu-cwo) was identified as encoding a putative CWO protein via a query using a D. melanogaster CWO (AAF54527; Adams et al., 2000). The Genes 2010 gene model shows dappu-cwo to be located on Scaffold 19 of the genome, with a predicted length of 3773 nucleotides (Table 1).
Figure 3 shows the alignment of the protein deduced from dappu-cwo (Dappu-CWO; 809 amino acids in overall length) with that of the Drosophila query (698 amino acids long). Comparison of the sequence of Dappu-CWO with that of Drome-CWO revealed 23.9% amino acid identity/51.4% amino acid similarity between the two proteins. SMART analyses of Dappu-CWO and Drome-CWO identified a single HLH and a single orange domain within each protein (Fig. 3); high levels of amino acid conservation were evident when the Daphnia domains were compared to their Drosophila counter parts (HLH, 78.2% identity/92.7% similarity; ORANGE, 37.5% identity/85% similarity).
Figure 3.
Putative Daphnia pulex CLOCKWORK ORANGE (CWO) protein. Alignment of Drosophila melanogaster CWO (Drome-CWO) with D. pulex CWO (Dappu-CWO). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, helix-loop-helix and orange domains identified by SMART analyses are highlighted in green and yellow, respectively.
Reciprocal blasting of Dappu-CWO against all proteins curated in FlyBase identified CWO (Flybase No. FBpp0081723) as the D. melanogaster protein most similar to the Daphnia query. Moreover, blastp comparison of Dappu-CWO with all non-redundant protein sequences curated by NCBI revealed D. melanogaster CWO (AAF54527) as most similar to this protein as well (Table 3); three of the top five blastp hits are D. melanogaster sequences, the remaining two are also insect proteins (Table 3).
3.1.4. CYCLE (CYC)
A single D. pulex gene (dappu-cyc) was identified as encoding a putative CYC protein via a query using a D. melanogaster CYC (AAF49107; Adams et al., 2000). The Genes 2010 gene model shows dappu-cyc to be located on Scaffold 1 of the genome, with a predicted length of 6473 nucleotides (Table 1).
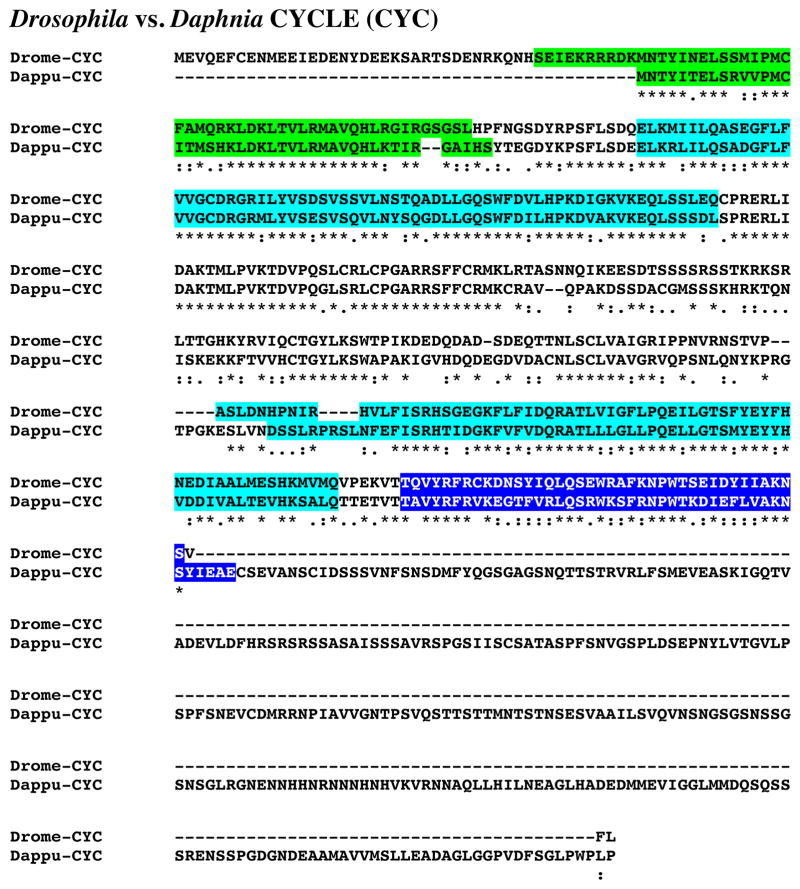
Figure 4 shows the alignment of the protein deduced from dappu-cyc (Dappu-CYC; 654 amino acids in overall length) with that of the Drosophila query (413 amino acids long). Comparison of the sequence of Dappu-CYC with that of Drome-CYC revealed 33.9% amino acid identity/49.7% amino acid similarity between the two proteins. SMART analyses of Dappu- and Drome-CYC identified a single HLH domain, two PAS domains and a single PAC domain in each protein (Fig. 4). Comparisons of the D. pulex domains with those of D. melanogaster show high levels of amino acid conservation in these portions of the proteins: HLH, 71.4 % identity/95.2 % similarity; PAS1, 75.0 % identity/94.1 % similarity; PAS2, 59.7 % identity/91.9 % similarity; PAC, 53.8% identity/94.9 % similarity.
Figure 4.
Putative Daphnia pulex CYCLE (CYC) protein. Alignment of Drosophila melanogaster CYC (Drome-CYC) with D. pulex CYC (Dappu-CYC). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, helix-loop-helix, PAS and PAC domains identified by SMART analyses are highlighted in green, light blue and blue respectively.
Reciprocal blasting of Dappu-CYC against all proteins curated in FlyBase identified CYC (Flybase no. FBpp0074693) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Comparison of Dappu-CYC with all non-redundant protein sequences curated by NCBI identified a CYC from the firebrat T. domestica (BAJ16354) to be the most similar protein match (Table 3); no Drosophila proteins were among the top five blastp hits (Table 3).
3.1.5. DOUBLETIME (DBT)
A single D. pulex gene (dappu-dbt) was identified as encoding a putative DBT protein via a query using a D. melanogaster DBT (AAF57110; Adams et al., 2000). The Genes 2010 gene model shows dappu-dbt to be located on Scaffold 1 of the genome, with a predicted length of 2124 nucleotides (Table 1).
Figure 5 shows the alignment of the protein deduced from dappu-dbt (Dappu-DBT; 409 amino acids in overall length) with that of the Drosophila query (440 amino acids long). Comparisons of the sequence of Dappu-DBT with Drome-DBT revealed 61.4% amino acid identity/78.4% amino acid similarity between the two proteins. SMART analyses of Dappu-DBT and Drome-DBT identified a serine/threonine kinase domain in each protein, the sequences of which were nearly identical, i.e. 82.6% amino acid identity/96.2% amino acid similarity (Fig. 5).
Figure 5.
Putative Daphnia pulex DOUBLETIME (DBT) protein. Alignment of Drosophila melanogaster DBT (Drome-DBT) with D. pulex DBT (Dappu-DBT). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, serine/threonine kinase domains identified by SMART analyses are highlighted in red.
Reciprocal blasting of Dappu-DBT against all proteins curated in FlyBase identified DBT (Flybase no. FBpp0085106) as the most similar D. melanogaster protein to the Daphnia query (Table 2). Comparison of Dappu-DBT with all non-redundant protein sequences curated by NCBI revealed a casein kinase Iε (an alternative name for DBT) from the ant Camponotus floridanus (EFN64010) to be most similar to Daphnia DBT (Table 3); no Drosophila proteins were among the top five blastp hits (Table 3).
3.1.6. PAR DOMAINE PROTEIN 1ε (PDP1ε)
A single D. pulex gene (dappu-pdp1ε) was identified as encoding a putative PDP1ε protein via a query using a D. melanogaster PDP1ε (AAF04509; Lin et al., 1997). The Genes 2010 gene model shows dappu-pdp1ε to be located on Scaffold 21 of the genome, with a predicted length of 4794 nucleotides (Table 1).
Figure 6 shows the alignment of the protein deduced from dappu-pdp1ε (Dappu-PDP1ε; 350 amino acids in overall length) with that of the Drosophila query (351 amino acids long). Comparisons of the sequence of Dappu-PDP1ε with that of Drome-PDP1ε revealed 39.6% amino acid identity/63.5% amino acid similarity between the two proteins. SMART analyses of Dappu-PDP1ε and Drome-PDP1ε identified a single basic region leucine zipper domain in each protein, which were 67.7% identical/81.5% similar in amino acid composition (Fig. 6).
Figure 6.
Putative Daphnia pulex PAR DOMAIN PROTEIN 1ε (PDP1ε) protein. Alignment of Drosophila melanogaster PDP1ε (Drome-PDP1ε) with D. pulex PDP1ε (Dappu-PDP1ε). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, basic region leucine zipper domains identified by SMART analyses are highlighted in dark blue.
Reciprocal blasting of Dappu-PDP1ε against all proteins curated in FlyBase identified PDP1 (Flybase No. FBpp0076495) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Interestingly, comparison of Dappu-PDP1ε with all non-redundant protein sequences curated by NCBI identified a rat protein (EDM05669), likely a PDP1, to be most similar to Dappu-PDP1ε (Table 3), though the next three of the top five blastp hits were D. melanogaster PDP1 isoforms (Table 3).
3.1.7. PERIOD (PER)
A single D. pulex gene (dappu-per) was identified as encoding a putative PER protein via a query using a D. melanogaster PER (AAF45804; Adams et al., 2000). The Genes 2010 gene model shows dappu-per to be located on Scaffold 47 of the genome, with a predicted length of 5860 nucleotides (Table 1).
Figure 7 shows the alignment of the protein deduced from dappu-per (Dappu-PER; 1286 amino acids in overall length) with that of the Drosophila query (1218 amino acids long). Comparisons of the sequence of Dappu-PER with Drome-PER revealed 27.4% amino acid identity/59.6% amino acid similarity between the two proteins. SMART analyses of Dappu-PER and Drome-PER identified two PAS domains in each protein (Fig. 7), both of which showed considerable amino acid conservation between the two proteins: PAS-1, 50.0% amino acid identity/82.3% amino acid similarity; PAS-2, 47.3% amino acid identity/84.2% amino acid similarity. In addition, SMART analysis identified a PAC domain within the Drome-PER (Fig. 7) but not in Dappu-PER. Interestingly, the portion of Dappu-PER that overlaps with the Drosophila PAC domain is 72.7% identical/93.2% similar in amino acid composition to that of the Drosophila protein (Fig. 7).
Figure 7.
Putative Daphnia pulex PERIOD (PER) protein. Alignment of Drosophila melanogaster PER (Drome-PER) with D. pulex PER (Dappu-PER). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, PAS and PAC domains identified by SMART analyses are highlighted in light blue and blue, respectively.
Reciprocal blasting of Dappu-PER against the proteins curated in FlyBase identified PER (Flybase No. FBpp0070455) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Moreover, blastp comparison of Dappu-PER with all non-redundant protein sequences curated by NCBI showed the top five hits to be insect PER proteins (Table 3), with an isoform from the cockroach Blattella germanica (AAN02439; Table 3) exhibiting the highest similarity.
3.1.8. PROTEIN PHOSPHATASE 1 (PP1)
Two D. pulex genes (dappu-pp1 a and dappu-pp1 b) were identified as encoding putative PP1 proteins via a query using a D. melanogaster PP1 (CAA39820; Dombradi et al., 1990). The Genes 2010 gene model shows dappu-pp1 a and dappu-pp1 b to be located on Scaffolds 145 and 12 of the genome, respectively, with lengths of 2591 and 1972 nucleotides (Table 1).
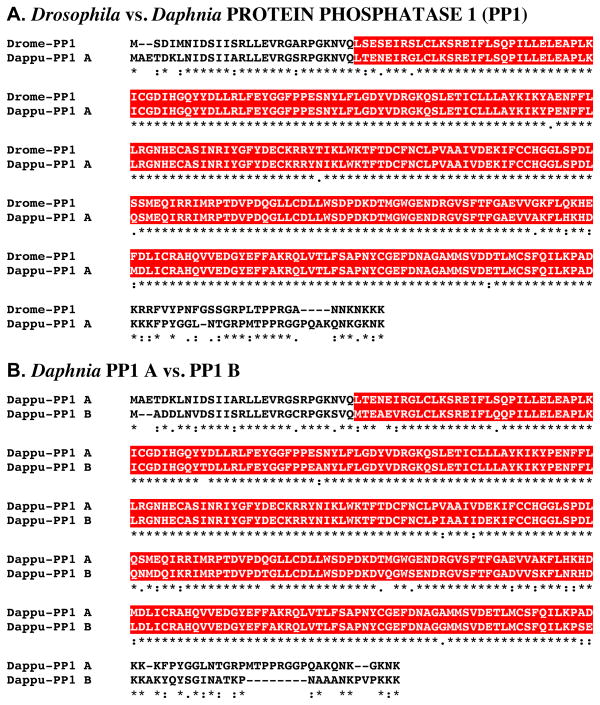
Figure 8A shows the alignment of the protein deduced from dappu-pp1 a (Dappu-PP1 A; 332 amino acids in overall length) with that of the Drosophila query (327 amino acids long). Comparison of the sequences of Dappu-PP1 A with Drome-PP1 revealed 84.0% amino acid identity/90.7% amino acid similarity between the two proteins. Comparison of the sequence of Dappu-PP1 B (325 amino acids in overall length) with that of the Drosophila query with that of Drome-PP1 revealed a similar level of amino acid conservation, i.e. 81.3% identity/89.6% similarity (alignment not shown). Figure 8B shows the alignment of the two Daphnia PP1s with one another. As can be seen from this panel, the two proteins are nearly identical in amino acid sequences (84.3% identity/96.9% similarity in amino acid composition). SMART analyses of Dappu-PP1 A, Dappu-PP1 B, and Drome-PP1 identified a single serine/threonine protein kinase domain in each protein (Fig. 8A–B). The serine/threonine protein kinase domain in each of the Daphnia proteins is nearly identical to that present in their Drosophila counterpart: Dappu-PP1 A vs. Drome-PP1, 95.9% amino acid identity/100% amino acid similarity; Dappu-PP1 B vs. Drome-PP1, 89.6% amino acid identity/99.3% amino acid similarity. Similarly, this domain is highly conserved between the two Daphnia proteins (91.5% identity/98.5% similarity).
Figure 8.
Putative Daphnia pulex PROTEIN PHOSPHATASE 1 (PP1) proteins. (A) Alignment of Drosophila melanogaster PP1 (Drome-PP1) with D. pulex PP1 A (Dappu-PP1 A). (B). Alignment of Dappu-PP1 A and PP1 B. In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, serine/threonine kinase domains identified by SMART analyses are highlighted in red.
Reciprocal blasting of Dappu-PP1 A and B against all proteins curated in FlyBase identified isoforms of PP1 (Flybase nos. FBpp0084026 and FBpp0071382, respectively; Table 2) as the most similar D. melanogaster proteins to the Daphnia queries. Comparison of the Dappu-PP1s with all non-redundant protein sequences curated by NCBI identified a serine/threonine-protein phosphatase alpha-1 isoform (of which PP1 is a family member) from the ant C. floridanus (EFN69572) to possess the highest similarity score to Dappu-PP1 A (Table 3), with a PP1 from the zebra fish Danio rerio (CAD61270) being most similar to Dappu-PP1 B (Table 3); no Drosophila proteins were among the top five blastp hits for either of the Dappu-PP1s (Table 3).
3.1.9. PROTEIN PHOSPHATASE 2A (PP2A)
3.1.9.1. PP2A catalytic subunit – MICROTUBULE STAR (MTS)
A single D. pulex gene (dappu-mtr) was identified as encoding a putative PP2A catalytic subunit protein (MTS) via a query using a D. melanogaster MTS sequence (AAF52567; Adams et al., 2000). The Genes 2010 gene model shows dappu-mts to be located on Scaffold 13 of the genome, with a predicted length of 2099 nucleotides (Table 1).
Figure 9A shows the alignment of the protein deduced from dappu-mts (Dappu-MTS; 308 amino acids in overall length) with that of the Drosophila query (309 amino acids long). Comparisons of the sequence of Dappu-MTS with Drome-MTS revealed 64.4% amino acid identity/90.3% amino acid similarity between the two proteins. SMART analyses of Dappu-MTS and Drome-MTS identified a single protein phosphatase 2A catalytic domain in each protein (Fig. 9A). Comparison of the sequences of these domains revealed 66.9% amino acid identity/92.6% amino acid similarity between these two regions of the proteins (Fig. 9A).
Figure 9.
Putative Daphnia pulex PROTEIN PHOSPHATASE 2A (PP2A) proteins. (A) Alignment of Drosophila melanogaster PP2A catalytic subunit MICROTUBULE STAR (MTS) protein (Drome-MTS) with D. pulex MTS (Dappu-MTS). (B1). Alignment of D. melanogaster PP2A regulatory subunit WIDERBORST (WBT) protein (Drome-WBT) with D. pulex WBT A (Dappu-WBT A). (B2). Alignment of Dappu-WBT A and Dappu-WBT B. (C). Alignment of D. melanogaster PP2A regulatory subunit TWINS (TWS) protein (Drome-WBT) with D. pulex TWS (Dappu-TWS). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, protein phosphatase 2A catalytic, coiled-coil and WD40 domains identified by SMART analyses are highlighted in dark green, pink and dark red, respectively.
Reciprocal blasting of Dappu-MTS against all proteins curated in FlyBase identified MTS (Flybase no. FBpp0077017) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Comparison of Dappu-MTS with all non-redundant protein sequences curated by NCBI revealed a serine/threonine-protein phosphatase 4 catalytic subunit from the ant Harpegnathos saltator (EFN85419) to be most similar to Daphnia MTS (Table 3); no Drosophila proteins were among the top five blastp hits (Table 3).
3.1.9.2. PP2A regulatory subunit
3.1.9.2.1. WIDERBORST (WBT)
Two D. pulex genes (dappu-wbt a and dappu-wbt b) were identified as encoding putative PP2A regulatory subunit proteins (WBTs) via a query using a D. melanogaster WBT sequence (AAF56720; Adams et al., 2000). The Genes 2010 gene model shows dappu-wbt a and b to be located on Scaffolds 8 and 2 of the genome, respectively, with a predicted lengths of 6199 and 4882 nucleotides (Table 1).
Figure 9B1 shows the alignment of the protein deduced from dappu-wbt a (Dappu-WBT A; 481 amino acids in overall length) with that of the Drosophila query (524 amino acids long). Comparisons of the sequence of Dappu-WBT with Drome-WBT revealed 73.7% amino acid identity/85.5% amino acid similarity between the two proteins. Alignment of Dappu-WBT B with Drome-WBT revealed a lower level of amino acid conservation between these two proteins, 52.6% identity/74.1% similarity (alignment not shown). Figure 9B2 shows the alignment of the two Daphnia WBTs; these proteins are 50.5% identical/69.7% similar in amino acid sequence. SMART analyses of Dappu-WBT A identified a single coiled-coil domain; this domain was not predicted by SMART analyses in either Dappu-WBT B or Drome-WBT, though in the former protein this region is 45.5% identical/69.7% similar in amino acid composition to that of Dappu-WBT A and in the latter protein 72.7%identical/97.0% similar to that of Dappu-WBT A (Fig. 9).
Reciprocal blasting of Dappu-WBT A and B against all proteins curated in FlyBase identified PP2A regulatory subunit isoforms (Flybase nos. FBpp0084579 and FBpp0288759, respectively; Table 2) as the most similar D. melanogaster proteins to the Daphnia queries. Comparison of the Dappu-WBTs with all non-redundant protein sequences curated by NCBI identified serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit isoforms from the ant C. floridanus to show the highest similarity scores to each of Dappu-WBTs (Accession nos. EFN66909 and EFN69797, respectively; Table 3); while a Drosophila isoform of WBT was among the top five blastp hits for Dappu-WBT A, none were among the top hits for Dappu-WBT B (Table 3).
3.1.9.2.2. TWINS (TWS)
A single D. pulex gene (dappu-tws) was identified as encoding a putative PP2A regulatory subunit protein (TWS) via a query using a D. melanogaster TWS sequence (AAF54498; Adams et al., 2000). The Genes 2010 gene model shows dappu-tws to be located on Scaffold 43 of the genome, with a predicted length of 4157 nucleotides (Table 1).
Figure 9C shows the alignment of the protein deduced from dappu-tws (Dappu-TWS; 443 amino acids in overall length) with that of the Drosophila query (499 amino acids long). Comparisons of the sequence of Dappu-TWS with Drome-TWS revealed 71.5% amino acid identity/83.6% amino acid similarity between the two proteins. SMART analyses of Dappu-TWS and Drome-TWS identified six and seven WD40 domains in these proteins, respectively (Fig. 9C). Comparison of the sequences of the shared WD40 domains, as well as the region of the Daphnia protein corresponding to the sixth of the seven Drosophila domains, revealed high degrees of amino acid conservation in these regions of the two proteins: WD40 1, 87.2% identity/100% similarity; WD40 2, 90.2% identity/100% similarity; WD40 3, 90.0% identity/95.0% similarity; WD40 4, 89.5% identity/94.7% similarity; WD40 5, 87.2% identity/100 similarity; WD40 6 (no domain formally identified by SMART in Daphnia), 75% identity/100% similarity; WD40 7, 94.7% identity/100% similarity (Fig. 9C).
Reciprocal blasting of Dappu-TWS against all proteins curated in FlyBase identified TWS (Flybase No. FBpp0081671) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Comparison of Dappu-TWS to all non-redundant protein sequences curated by NCBI identified a beetle Tribolium castaneum protein (EFA10095) as showing the highest similarity to the Daphnia query (Table 3); three Drosophila TWS isoforms were among the top five blastp hits (Table 3).
3.1.10. SHAGGY (SGG)
A single D. pulex gene (dappu-sgg) was identified as encoding a putative SGG protein via a query using a D. melanogaster SGG (AAN09084; Adams et al., 2000). The Genes 2010 gene model shows dappu-sgg to be located on Scaffold 76 of the genome, with a predicted length of 5712 nucleotides (Table 1).
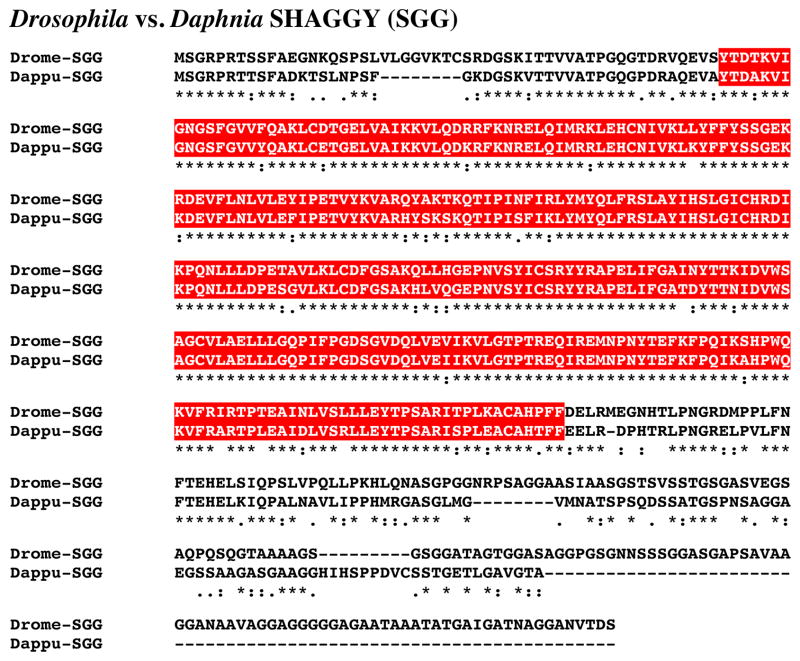
Figure 10 shows the alignment of the protein deduced from dappu-sgg (Dappu-SGG; 439 amino acids in overall length) with that of the Drosophila query (514 amino acids long). Comparison of the sequence of Dappu-SGG with Drome-SGG revealed 64.8% amino acid identity/77.6% amino acid similarity between the two proteins. SMART analyses of Dappu-SGG and Drome-SGG identified a single serine/threonine kinase domain in each protein (Fig. 10); the two serine/threonine kinase domains are nearly identical in amino acid sequence, i.e. 89.5% identity/98.2% similarity. Reciprocal blasting of Dappu-SGG against all proteins curated in FlyBase identified SGG (Flybase no. FBpp0070450) as D. melanogaster protein most similar to the Daphnia query (Table 2). Comparison of Dappu-SGG with all non-redundant protein sequences curated by NCBI revealed a glycogen synthase kinase (of which SGG is family member) from the tick Rhipicephalus microplus (ABO61882) to be the most similar protein match (Table 3); no Drosophila proteins were among the top five blastp hits (Table 3).
Figure 10.
Putative Daphnia pulex SHAGGY (SGG) protein. Alignment of Drosophila melanogaster SGG (Drome-SGG) with D. pulex SGG (Dappu-SGG). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, serine/threonine kinase domains identified by SMART analyses are highlighted in red.
3.1.11. SUPERNUMERARY LIMBS (SLIMB)
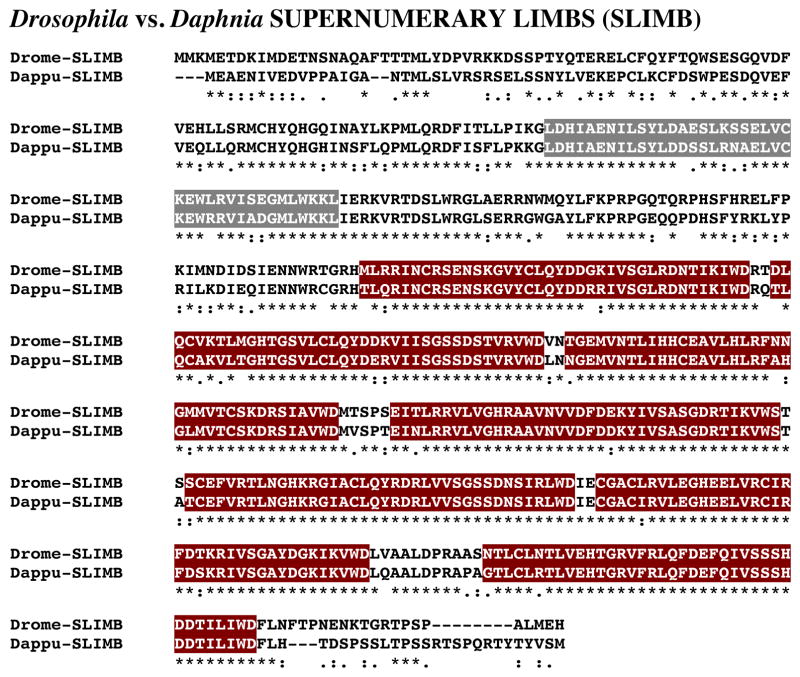
A single D. pulex gene (dappu-slimb) was identified as encoding a putative SLIMB protein via a query using a D. melanogaster SLIMB (AAF55853; Adams et al., 2000). The Genes 2010 gene model shows dappu-slimb to be located on Scaffold 169 of the genome, with a predicted length of 2880 nucleotides (Table 1).
Figure 11 shows the alignment of the protein deduced from dappu-slimb (Dappu-SLIMB; 510 amino acids in overall length) with that of the Drosophila query (510 amino acids long). Comparison of the sequence of Dappu-SLIMB with Drome-SLIMB revealed 76.3% amino acid identity/91.8% amino acid similarity between the two proteins. SMART analyses of Dappu-SLIMB and Drome-SLIMB identified an FBOX domain and seven WD40 domains in each protein (Fig. 11); the amino acid sequences of each of these domains is highly conserved between the two species: FBOX domain, 80.0% amino acid identity/95.0% similarity; WD40-1, 89.5% amino acid identity/94.7% amino acid similarity; WD40-2, 84.2% amino acid identity/94.7% amino acid similarity; WD40-3, 89.5% amino acid identity/97.4% amino acid similarity; WD40-4, 94.7% amino acid identity/100% amino acid similarity; WD40-5, 97.4% amino acid identity/100% amino acid similarity; WD40-6, 94.7% amino acid identity/100% amino acid similarity; WD40-7, 94.7% amino acid identity/100% amino acid similarity.
Figure 11.
Putative Daphnia pulex SUPERNUMERARY LIMBS (SLIMB) protein. Alignment of Drosophila melanogaster SLIMB (Drome-SLIMB) with D. pulex SLIMB (Dappu-SLIMB). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, FBOX and WD40 domains identified by SMART analyses are highlighted in dark gray and dark red, respectively.
Reciprocal blasting of Dappu-SLIMB against all proteins curated in FlyBase identified SLIMB (Flybase No. FBpp0083434) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Interestingly, comparison of Dappu-SLIMB to all non-redundant protein sequences curated by NCBI identified vertebrate proteins, all apparent members of the E3 ubiquitin ligase family, as the top five blastp hits, with a mouse protein (BAE26547) being the best match with the Daphnia sequence (Table 3).
3.1.12. TIMELESS
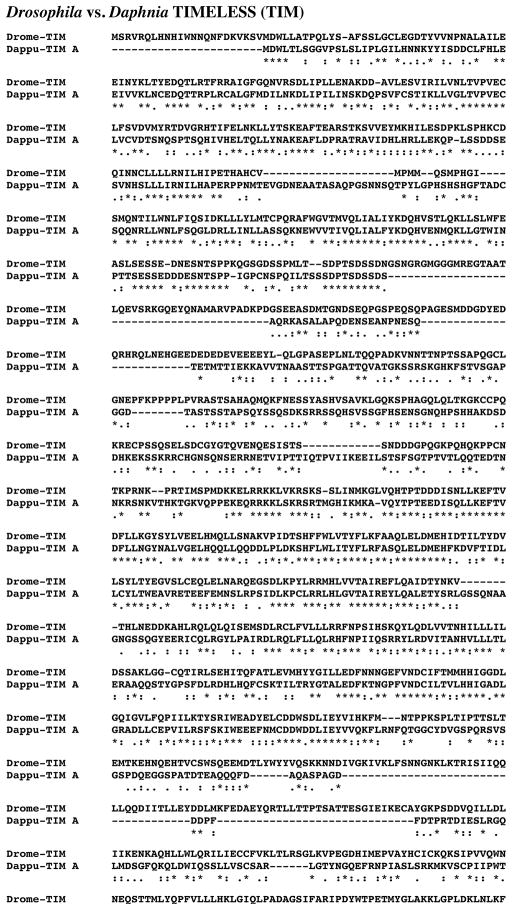
Eight D. pulex genes (dappu-tim a, dappu-tim b, dappu-tim c, dappu-tim d, dappu-tim e, dappu-tim f, dappu-tim g, and dappu-tim h) were identified as encoding putative TIM proteins via a query using a D. melanogaster TIM (AAN10371; Adams et al., 2000). The Genes 2010 gene model shows dappu-tim a, dappu-tim b, dappu-tim c, dappu-tim d, dappu-tim e, dappu-tim f, dappu-tim g, and dappu-tim h to be located on Scaffolds 24, 6, 10, 6, 6, 24, 75, and 91 of the genome, respectively, with lengths of 14166, 5052, 3650, 5438, 5542, 11674, 2798, and 4537 nucleotides (Table 1).
Figure 12 shows the alignments of the protein deduced from dappu-tim a (Dappu-TIM A; 1197 amino acids in overall length) with that of the Drosophila query (1421 amino acids in length). Comparison of the amino acid sequences of these two proteins revealed 29.8% identity/60.1% similarity between the two TIMs. Comparisons of the other Daphnia TIMs with Drome-TIM showed varying levels of amino acid conservation, with some nearly as high as that seen between Dappu-TIM A and Drome-TIM, and others considerably lower: Dappu-TIM B, 23.6% amino acid identity/52.0% amino acid similarity; Dappu-TIM C, 23.7% amino acid identity/47.8% amino acid similarity; Dappu-TIM D, 26.4% amino acid identity/55.9% amino acid similarity; Dappu-TIM E, 23.4% amino acid identity/47.9% amino acid similarity; Dappu-TIM F, 22.3% amino acid identity/51.1% amino acid similarity; Dappu-TIM G, 14.2% amino acid identity/30.8% amino acid similarity; and Dappu-TIM H, 21.3% amino acid identity/45.6% amino acid similarity (alignments not shown). Alignment of the eight Daphnia TIM proteins to one another shows considerable variation in sequence composition between the proteins (in the interest of space, this alignment is provided only as an online supplemental figure [Supplemental Figure 1]). Table 3 provides pairwise comparisons of the amino acid identity/similarity of Dappu-TIM A-H. No functional domains were identified by SMART analyses in Drome-TIM or any of the Dappu-TIMs. Reciprocal blasting of eight Dappu-TIMs against all proteins curated in FlyBase identified an isoform of TIM as the D. melanogaster protein most similar to each Daphnia query (Flybase nos. FBpp0291971, FBpp0291971, FBpp0291971, FBpp0077254, FBpp0291970, FBpp0077254, FBpp0291970 and FBpp0291970, respectively; Table 2). Comparison of the Dappu-TIMs with all non-redundant protein sequences curated by NCBI revealed each to be most similar to an insect TIM isoform (i.e. the butterfly Danaus plexippus [AAR15505], the moth Antheraea pernyi [AAF66996], the beetle T. castaneum [EFA04644], T. castaneum [EFA04644], D. melanogaster [ADV36936], D. melanogaster [P49021], the fruit fly Drosophila virilis [O17482], and the cricket Gryllus bimaculatus [BAJ16356] for Dappu-TIM A-H, respectively; Table 3). In fact, the top five hits for each of the Daphnia proteins were insect isoforms of TIM (Table 3).
Figure 12.
Putative Daphnia pulex TIMELESS (TIM) protein. Alignment of Drosophila melanogaster TIM (Drome-TIM) with D. pulex TIM A (Dappu-TIM A). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure.
3.1.13. VRILLE (VRI)
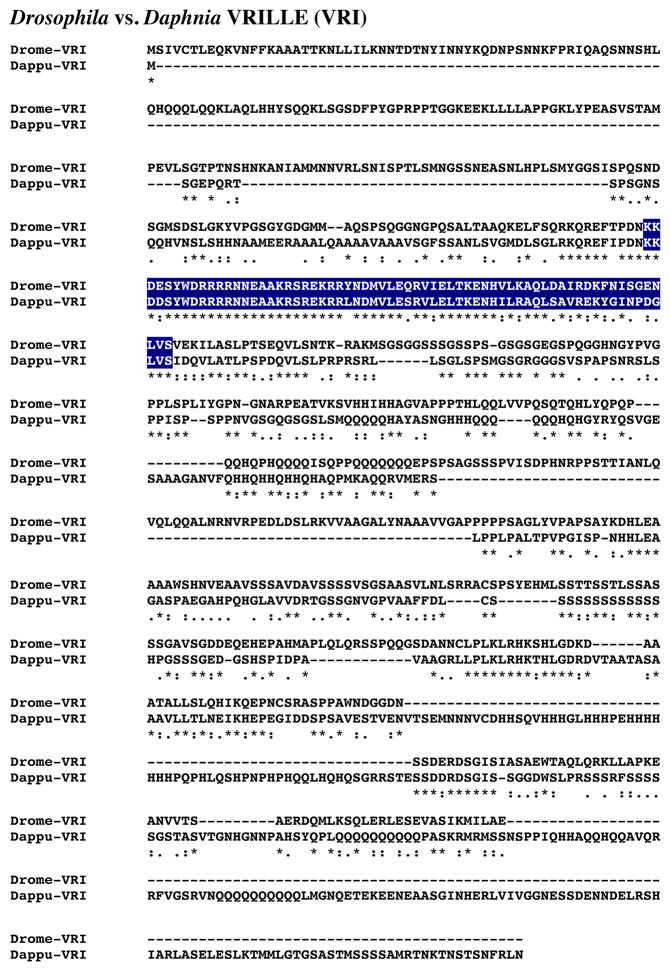
A single D. pulex gene (dappu-vri) was identified as encoding a putative VRI protein via a query using a D. melanogaster VRI (AAF52237; Adams et al., 2000). The Genes 2010 gene model shows dappu-vri to be located on Scaffold 92 of the genome, with a predicted length of 2225 nucleotides (Table 1).
Figure 13 shows the alignment of the protein deduced from dappu-vri (Dappu-VRI; 676 amino acids in overall length) with that of the Drosophila query (729 amino acids long). Comparison of the sequences of Dappu-VRI and Drome-VRI revealed 27.7% amino acid identity/49.5% amino acid similarity between the two proteins. SMART analyses of Dappu-VRI and Drome-VRI identified a single basic region leucine zipper domain in each protein (Fig. 13); the amino acid sequence of this domain is highly conserved between the two VRIs, i.e. 76.9% amino acid identity and 96.9% amino acid similarity.
Figure 13.
Putative Daphnia pulex VRILLE (VRI) protein. Alignment of Drosophila melanogaster VRI (Drome-VRI) with D. pulex VRI (Dappu-VRI). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, basic region leucine zipper domains identified by SMART analyses are highlighted in dark blue.
Reciprocal blasting of Dappu-VRI against all proteins curated in FlyBase identified VRI (Flybase no. FBpp0289297) as the most similar D. melanogaster protein to the Daphnia query (Table 2). Comparison of Dappu-VRI with all non-redundant protein sequences curated by NCBI revealed a VRI from the butterfly D. plexippus (ATT86041) to be most similar to Daphnia VRI (Table 3); two Drosophila VRIs are among the top five blastp hits for this query (Table 3).
3.2. Input pathway proteins
3.2.1. CRYPTOCHROME (CRY)
Four D. pulex genes (dappu-cry a, dappu-cry b, dappu-cry c, and dappu-cry d) were identified as encoding putative CRY proteins via a query using a D. melanogaster CRY (AAC83828; Emery et al., 1998). The Genes 2010 gene model shows that these genes are located on Scaffolds 40, 18, 10, and 7 of the genome, respectively, with lengths of 2706, 4661, 3072 and 2052 nucleotides (Table 1).
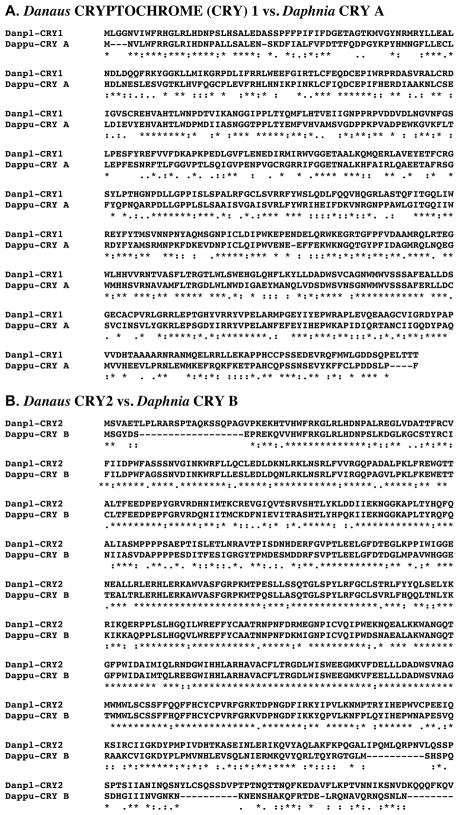
Figure 14A shows the alignment of the protein deduced from dappu-cry a (Dappu-CRY A; 525 amino acids in overall length) with that of the Drosophila query (542 amino acids long). Comparison of the sequence of Dappu-CRY A with Drome-CRY revealed 44.8% amino acid identity/76.4% amino acid similarity between the two proteins (Figure 14A). Alignments of Dappu-CRY B, Dappu-CRY C and Dappu-CRY D with Drome-CRY also revealed high levels of structural homology between the proteins: Dappu-CRY B vs. Drome-CRY, 37.6% amino acid identity/69.1%; Dappu-CRY C vs. Drome-CRY, 38.2% amino acid identity/69.9% amino acid similarity; Dappu-CRY D vs. Drome-CRY, 24.4% amino acid identity/59.6% amino acid similarity (alignments not shown). Figure 14B shows the alignment of the four Daphnia CRYs with one another. As this panel shows, the four proteins show considerable variation in amino acid composition. Table 4 provides pairwise comparisons of the amino acid identity/similarity of Dappu-CRY A-D. No functional domains were identified by SMART analyses in Drome-CRY or any of the Dappu-CRYs.
Figure 14.
Putative Daphnia pulex CRYPTOCHROME (CRY) proteins. (A) Alignment of Drosophila melanogaster CRY (Drome-CRY) with D. pulex CRY A (Dappu-CRY A). (B). Alignment of Dappu-CRY A–D. In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure.
Table 4.
Matrix of percent amino acid identity/similarity between putative Daphnia pulex TIMELESS (TIM) proteins
| TIM-A | TIM-B | TIM-C | TIM-D | TIM-E | TIM-F | TIM-G | TIM-H | |
|---|---|---|---|---|---|---|---|---|
| TIM-A | ||||||||
| TIM-B | 47.1/70.9 | |||||||
| TIM-C | 47.7/68.3 | 44.1/71.2 | ||||||
| TIM-D | 48.6/73.9 | 46.0/70.3 | 41.7/67.8 | |||||
| TIM-E | 45.0/65.8 | 45.0/68.8 | 44.0/72.4 | 58.7/75.8 | ||||
| TIM-F | 31.5/55.2 | 26.5/49.3 | 25.8/44.6 | 27.0/50.3 | 22.8/42.4 | |||
| TIM-G | 28.8/45.9 | 31.9/53.1 | 33.9/54.2 | 26.6/46.4 | 28.3/50.5 | 16.7/29.9 | ||
| TIM-H | 37.8/62.2 | 36.9/63.4 | 37.8/68.5 | 36.5/64.2 | 38.1/68.3 | 19.8/40.1 | 26.8/48.9 | |
Values shown are percent amino acid identity/similarity.
Percent identity = number of amino acids identically conserved between the two proteins divided by the total number of amino acids in the longer protein.
Percent similarity = number of identical and similar amino acids in the two proteins divided by the total number of amino acids in the longer protein
As discussed in Section 1, along with CLK, CRY is the only other circadian protein for which a crustacean family member is known, i.e. an isoform from the Antarctic krill E. superba (Mazzotta et al., 2010). Alignments of the Daphnia CRYs with Eupsu-CRY, show similar levels of amino acid conservation to that seen for alignments with the Drosophila protein: Dappu-CRY A vs. Eupsu-CRY, 36.0% identity/69.5% similarity; Dappu-CRY B vs. Eupsu-CRY, 67.1% identity/88.6% similarity; Dappu-CRY C vs. Eupsu-CRY, 47.2% identity/76.5% similarity; Dappu-CRY D vs. Eupsu-CRY, 26.8% identity/58.9% similarity (alignments not shown).
Reciprocal blasting of four Dappu-CRYs against all proteins curated in FlyBase identified members of the CRY/6-4 photolyase family as the most similar D. melanogaster proteins to the Daphnia queries. For Dappu-CRY A, CRY (Flybase no. FBpp0083150) was found to be the most similar Drosophila protein to the query sequence, while the remaining three sequences were found to be most similar to 6-4 photolyase (Flybase no. FBpp0080935). Comparison of the Dappu-CRYs with all non-redundant protein sequences curated by NCBI revealed each to be most similar to a CRY protein, though all are more similar to isoforms from other species than they are to Drosophila proteins (i.e. the cricket Dianemobius nigrofasciatus [BAF45421], the mosquito Anopheles darlingi [EFR20390], the clawed frog Xenopus tropicalis [AAI66277], and the European seabass Dicentrarchus labrax [CBN81995]) for Dappu-CRY A-D, respectively). Based on the results of our blastp analyses (Table 3), it would appear that Dappu-CRY A is a homolog of the Drosophila-type or CRY1 subfamily, with Dappu-CRY B being a homolog of the vertebrate-type or CRY2 subfamily; alignments of Dappu-CRY A and B with CRY1 and CRY2 of the butterfly D. plexippus, respectively, are shown in Figure 15. Dappu-CRY D appears most similar to members of the CRY DASH subfamily (Table 3). It is unclear as to which subfamily of the CRY/6-4 photolyase superfamily Dappu-CRY C is a member (Table 3).
Figure 15.

Alignment of Daphnia pulex CRYPTOCHROME (CRY) A and B with their Danaus plexippus homologs. (A) Alignment of D. plexipus CRY1 (Danpl-CRY1) with D. pulex CRY A (Dappu-CRY A). (B). Alignment of D. plexipus CRY2 (Danpl-CRY2) with D. pulex CRY B (Dappu-CRY B). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure.
3.3. Output pathway proteins
3.3.1. PIGMENT DISPERSING HORMONE RECEPTOR (PDHR)
A single D. pulex gene (dappu-pdhr) was identified as encoding a putative PDHR via a query using a D. melanogaster pigment dispersing factor receptor (PDFR; AAF45788; Adams et al., 2000). The Genes 2010 gene model shows dappu-pdhr to be located on Scaffold 1 of the genome, with a predicted length of 4621 nucleotides (Table 1).
Figure 16 shows the alignment of the protein deduced from dappu-pdhr (Dappu-PDHR; 516 amino acids in overall length) with that of the Drosophila query (669 amino acids long). Comparison of the sequence of Dappu-PDHR with that of Drome-PDFR revealed 32.7% amino acid identity/55.0% amino acid similarity between the two proteins. SMART analyses of Dappu-PDHR and Drome-PDFR identified seven transmembrane domains (TMDs) in each protein (Fig. 16). The amino acid sequences of these TMDs are highly conserved between the two proteins: TMD1, 52.2% amino acid identity and 87.0% amino acid similarity; TMD2, 72.2% amino acid identity and 94.4% amino acid similarity; TMD3, 72.7% amino acid identity and 95.5% amino acid similarity; TMD4, 47.4% amino acid identity and 78.9% amino acid similarity; TMD5, 55.5% amino acid identity and 95.0% amino acid similarity; TMD6, 72.2% amino acid identity and 83.3% amino acid similarity; TMD7, 81.8% amino acid identity and 100% amino acid similarity. In addition, a single hormone receptor domain was identified in Dappu-PDHR; this domain is absent in Drome-PDFR, though the corresponding region of this protein is 35.8% identical/58.9% similar to its Daphnia counterpart (Fig. 16).
Figure 16.
Putative Daphnia pulex PIGMENT DISPERSING HORMONE RECEPTOR (PDHR) protein. Alignment of Drosophila melanogaster pigment dispersing factor receptor (Drome-PDFR) with D. pulex PDHR (Dappu-PDHR). In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure. In this figure, hormone receptor and transmembrane domains identified by SMART analyses are highlighted in black and light gray, respectively.
Reciprocal blasting of Dappu-PDHR against all proteins curated in FlyBase identified PDFR (Flybase No. FBpp0099841) as the D. melanogaster protein most similar to the Daphnia query (Table 2). Comparison of Dappu-PDHR to all non-redundant protein sequences curated by NCBI identified a PDHR from the penaeid shrimp Marsupenaeus japonicus (BAH85843) as the top protein match for the query; three Drosophila proteins were also among the top five blastp hits identified via the Daphnia protein (Table 3).
4. Discussion
4.1. Genome mining identifies a putative set of putative circadian proteins in Daphnia pulex
Over the last decade, genome mining has become a major method for protein discovery in both vertebrates and invertebrates. At present, the only crustacean genome that has been fully sequenced and is available for public use is that of the cladoceran D. pulex (Colbourne et al., 2005; Bauer, 2007; Stollewerk, 2010; Colbourne et al., 2011; Tautz, 2011). Recently, this resource has been used for protein discovery in this species, providing detailed information on the structures of molecules involved in many physiological/behavioral processes, for example, steroid biosynthesis and innate immunity (Rewitz and Gilbert, 2008; McTaggart et al., 2009).
In the study presented here, we have used the D. pulex genome to mine for proteins that may be involved in the control of circadian rhythmicity in this species. Specifically, we used the sequences of known Drosophila circadian proteins to query the Daphnia genome for putative ortholog genes and their encoded proteins. Using this strategy, a number of putative D. pulex circadian genes and their proteins were identified and characterized, including those likely involved in the establishment of the core clock, i.e. PER, TIM, CLK, CYC and CRY2, as well as proteins in their post-translational modifications and degradation, i.e. DBT, CK2, SGG, PP2A, PP1 and SLIMB. Moreover, genes and proteins putatively involved in setting the phase and amplitude of the core clock were identified, i.e. PDP1, VRI and CWO, as were orthologs of the blue-light receptor protein CRY1, which likely function as input pathways to the core clock, synchronizing it to the solar day, and PDHR, which may serve to transduce one of the clock’s output signals. Taken collectively, this collection of genes/proteins represents the first putative set of circadian proteins thus far described from any crustacean.
4.2. Several Daphnia circadian genes appear to exhibit extensive gene duplications
Overall, Daphnia have an unusually high level of gene duplication in comparison with the other arthropods for which genomic databases exist (Colbourne et al., 2011). In fact, only in aphids has a similar level of gene duplication been noted (Huerta-Cepas et al., 2010; Ollivier et al., 2010; Shigenobu et al., 2010); both Daphnia and aphids are cyclical parthenogens (Cortés et al., 2008). The purpose of gene redundancy in these and other species is generally not well understood. For some genes, the encoded protein isoforms may be nonfunctional, may have different kinetic properties for the same substrate, or may have novel functions (Force et al., 1999). It is also possible that the protein isoforms may be expressed in a life stage-specific manner (development, diapause, reproduction), or that their expression is tissue-specific. Multiple genes, and hence protein isoforms, may help an organism adjust to shifting environmental conditions, e.g. changes in salinity, oxygen levels, or temperature.
As discussed in Section 1, a defining parameter of circadian rhythms is temperature compensation, the molecular basis of which is not well understood (Salomé and McClung, 2005; Salomé et al, 2010). Tomaiuolo et al. (2008) constructed a mathematical model based on two splice variant isoforms of the β-subunit of Drosophila CKII that differ in kinetic rates of PER phosphorylation. This model suggests that through dynamic regulation of the proportions of the two β-subunit isoforms expressed, an increase in robustness of the circadian clock can be predicted. Two per alleles exist in wild populations of D. melanogaster, with population differences in frequency that vary by latitude (Sawyer et al., 1997). The PER proteins have different thermokinetic properties that may be involved in the circadian clock temperature compensation. Likewise, in a northern-latitude Drosophila population, the tim mutation, ls-tim, has been shown to adjust photoresponsiveness in this more seasonally-variable environment (Sandrelli et al., 2007). The multiple Daphnia PP1, PP2A-WBT and TIM variants predicted here may likewise possess different kinetic parameters to offset temperature, salinity, oxygen, and/or other environmental variables.
4.3. Conservation of structural domains suggests Daphnia possesses an insect-like molecular clock, but organized more like that of butterflies and mosquitoes than of Drosophila
In our study, putative homologs to most of the known Drosophila circadian proteins were identified in D. pulex. Structural domains in both the Daphnia and Drosophila proteins were analyzed via the online program SMART (Schultz et al., 1998; Letunic et al., 2009). While we realize that not all of the domains/functional regions that have been reported for the Drosophila circadian proteins are detected via this program (e.g. Saez and Young, 1996; Ousley et al., 1998; Chang and Reppert, 2003; Lin and Todo, 2005), those that were, for the most part, appear to be highly conserved between the two species’ putative homologs; significant amino acid variation was noted outside of functional regions for several proteins. Even where discrepancies were noted, e.g. a PAC domain identified in Drome-PER but not in Dappu-PER, the corresponding regions of the two proteins were often very similar in amino acid composition (in the case of the PER PAC domain 72.7% identical/93.2% similar). Interestingly, blast analyses of the Daphnia sequences show them to be, for the most part, more similar to proteins identified from other species than they are to Drosophila. Moreover, the presence of both CRY1 and CRY2 in Daphnia suggests that its molecular clock is likely organized more similar to that recently described for butterfly and mosquito (e.g. Zhu et al., 2005; Yuan et al., 2007; Zhu et al., 2008) where CRY1 is proposed as a photosenstive input to the clock and CRY2 is core clock protein (functioning to repress of CLK/CYC-mediated transcription), than it is to Drosophila (which possesses only CRY1).
4.4. Potential cellular locus and output signals of a Daphnia neuronal clock
All circadian systems have three functional components: a core clock, which is responsible for time keeping, input pathways that act to synchronize the clock to the environment, and output pathways that transmit the timing information from the clock for the control of physiology and behavior. Here we have identified a protein that may function as the input to a Daphnia clock, i.e. an isoform of CRY1, as well as proteins that may act to establish the core molecular clock itself, i.e. PER, TIM, CLK, CYC, CRY2, DBT, SGG, VRI, etc. To be determined, however, are possible output pathways from the Daphnia clock that would signal the timing information necessary to establish circadian rhythms in physiology and behavior in this species.
As the cellular location of the core circadian clock (or clocks) in D. pulex is unknown, it is difficult to postulate how output signals would be generated in, and transmitted from, this timekeeper. This said, work conducted on other species (both invertebrate and vertebrate) would suggest that circulating hormones are an important part of the Daphnia clock’s output pathway, mediating the expression of the overt circadian rhythms present in this species. One possibility is that hormones are released directly from the clock cells themselves; alternatively, the clock cells may project to and innervate relay sites, likely endocrine organs, which are the sources of the hormonal signals. Regardless of locus, in insects, several peptide hormones have been shown to be key components of circadian signaling systems, particularly pigment dispersing factor (PDF), a member of the pigment dispersing hormone (PDH) family; PDF is present in a number of known circadian clock neurons in Drosophila (for review see: Allada and Chung, 2010; Tomioka and Matsumoto, 2010).
Recent transcriptome and genome mining in D. pulex has identified a homolog of PDF/PDH in this species, NSELINSLLGLPRFMKVVamide (Gard et al., 2009; Christie et al., 2011). Moreover, immunohistochemistry using an antibody generated against β-PDH (NSELINSILGLPKVMNDAamide) labels a small set of neurons (~8 somata) that are distributed throughout the brain/optic ganglia of D. pulex (Gard et al., 2009). While currently speculation, the role of PDF as a signaling agent, and hence marker, for some clock cells in the brain of Drosophila suggests that PDH-immunopositive neurons in the brain of D. pulex may represent at least a subset of the cellular loci for a circadian neuronal pacemaker in this species, a hypothesis recently strengthened by the finding of circadian patterns of activity in at least some of these cells (Strauβ et al., 2011).
In addition to PDF, a number of other hormones, primarily peptides, have been implicated in circadian signaling in insects. For example, corazonin, crustacean cardioactive peptide (CCAP) and diapause hormone have all been suggested as possible output signals from insect clock systems (e.g. Sehadová et al., 2007); isoforms of both corazonin and CCAP have been predicted from the D. pulex transcriptome and/or genome (Gard et al., 2009; Christie et al., 2011). Likewise, several peptide hormones have been shown to, or are postulated to show, circadian rhythms in their cycling in decapod crustaceans (Strauss and Dircksen, 2010), i.e. red pigment concentrating hormone and crustacean hyperglycemic hormone. Transcriptome and genome mining in D. pulex suggests that these hormonal systems too are present in this species (Gard et al., 2009; Christie et al., 2011). In fact, via transcriptome and genome mining over 100 peptide hormones have recently been identified in D. pulex (Gard et al., 2009; Christie et al., 2011). Here we have identified a putative PDH receptor protein, which, if we are correct in the peptide being a circadian signal in Daphnia, may function to transduce at least one of the core clocks output signals for the control of physiology and behavior in this species.
5. Conclusions and future directions
Circadian rhythms in physiology and behavior have been documented in numerous crustacean species; however, little is known about the molecular and/or cellular machinery underlying them in any member of this arthropod subphylum (Strauss and Dircksen, 2010). This said, their well-mapped nervous systems and amenability to in-depth electrophysiological and molecular investigations make them an optimal group of animals for studying circadian biology. Moreover, the fact that many intertidal crustaceans exhibit both circadian and circatidal rhythms make these animals an ideal model to explore possible interactions between circadian and circatidal signaling systems, including whether these two timekeeping systems use common or distinct molecular and/or cellular components.
Clearly the first step toward understanding circadian signaling in any species is obtaining knowledge of the molecules required for the establishment of the core clock. Here, we have achieved this milepost for the cladoceran crustacean D. pulex using a strategy combining genome mining and phylogenetic comparisons to known previously identified circadian proteins. D. pulex is now the only crustacean for which a putative set of circadian genes and proteins are known. With these data, we are now positioned to begin functional studies directed at determining if the mRNAs of the identified genes cycle in a circadian fashion and, if so, whether these rhythms are similar to those seen in insects. Similarly, the proteins deduced from these identified genes now allows for the generation of Daphnia-specific antibodies to these molecules, which will be useful both for mapping the distribution of these proteins and for determining if they cycle in manners similar to their insect counterparts. Finally, the identification of the D. pulex circadian genes described here now provide targets for knockdown experiments designed to elucidate the functional roles their encoded proteins play in the establishment of circadian signaling in this and other crustacean species.
Supplementary Material
Supplemental Figure 1. Alignment of Dappu-TIM A-H. In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure.
Table 5.
Matrix of percent amino acid identity/similarity between putative Daphnia pulex CRYPTOCHROME (CRY) proteins
| CRY-A | CRY-B | CRY-C | CRY-D | |
|---|---|---|---|---|
| CRY-A | ||||
| CRY-B | 37.4/70.7 | |||
| CRY-C | 38.3/71.2 | 48.1/78.7 | ||
| CRY-D | 26.3/61.0 | 26.4/58.8 | 27.7/64.3 | |
Values shown are percent amino acid identity/similarity.
Percent identity = number of amino acids identically conserved between the two proteins divided by the total number of amino acids in the longer protein.
Percent similarity = number of identical and similar amino acids in the two proteins divided by the total number of amino acids in the longer protein
Acknowledgments
Joseph Shaw (Indiana University) and Benjamin King (MDIBL) are thanked for assisting us in developing a strategy to mesh the BLAST+ 2.2.23 software with the beta-release of the Daphnia pulex genome assembly. Financial support for this work was provided by: NIH P20 RR046463-10 from the INBRE Program of the National Center for Research Resources (Patricia Hand, Ph.D., Principal Investigator) and through institutional funds provided by MDIBL (to AEC). The authors acknowledge that the sequencing of the genome and portions of the analyses of it was performed at the DOE Joint Genome Institute under the auspices of the U.S. Department of Energy’s Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Livermore National Laboratory under Contract No. W-7405-Eng-48, Lawrence Berkeley National Laboratory under Contract No. DE-AC02-05CH11231, Los Alamos National Laboratory under Contract No. W-7405-ENG-36, and in collaboration with the Daphnia Genomics Consortium (DGC; http://daphnia.cgb.indiana.edu). Additional analyses were performed by wFleaBase, developed at the Genome Informatics Laboratory of Indiana University with support to Don Gilbert from the National Science Foundation and the National Institutes of Health. Coordination infrastructure for the DGC is provided by The Center for Genomics and Bioinformatics at Indiana University, which is supported in part by the METACyt Initiative of Indiana University, funded in part through a grant from the Lilly Endowment, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke Z, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai Z, Lasko P, Lei Y, Levitsky AA, Li J, Li Z, Liang Y, Lin X, Liu X, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RD, Scheeler F, Shen H, Shue BC, Sidén-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Woodage T, Worley KC, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang G, Zhao Q, Zheng L, Zheng XH, Zhong FN, Zhong W, Zhou X, Zhu S, Zhu X, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae K, Lee C, Sidote D, Chuang KY, Edery I. Circadian regulation of a Drosophila homolog of the mammalian Clock gene: PER and TIM function as positive regulators. Mol Cell Biol. 1998;18:6142–6151. doi: 10.1128/mcb.18.10.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DJ. The Daphnia genomics consortium meeting: the genome biology of the model crustacean Daphnia. Expert Rev Proteomics. 2007;4:601–602. doi: 10.1586/14789450.4.5.601. [DOI] [PubMed] [Google Scholar]
- Chang DC, Reppert SM. A novel C-terminal domain of Drosophila PERIOD inhibits dCLOCK:CYCLE-mediated transcription. Current Biol. 2003;13:758–762. doi: 10.1016/s0960-9822(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Christie AE, McCoole MD, Harmon SM, Baer KN, Lenz PH. Genomic analyses of the Daphnia pulex peptidome. Gen Comp Endocrinol. 2011;171:131–150. doi: 10.1016/j.ygcen.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Cáceres CE, Carmel L, Casola C, Choi JH, Detter JC, Dong Q, Dusheyko S, Eads BD, Fröhlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kültz D, Laforsch C, Lindquist E, Lopez J, Manak JR, Muller J, Pangilinan J, Patwardhan RP, Pitluck S, Pritham EJ, Rechtsteiner A, Rho M, Rogozin IB, Sakarya O, Salamov A, Schaack S, Shapiro H, Shiga Y, Skalitzky C, Smith Z, Souvorov A, Sung W, Tang Z, Tsuchiya D, Tu H, Vos H, Wang M, Wolf YI, Yamagata H, Yamada T, Ye Y, Shaw JR, Andrews J, Crease TJ, Tang H, Lucas SM, Robertson HM, Bork P, Koonin EV, Zdobnov EM, Grigoriev IV, Lynch M, Boore JL. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne JK, Singan VR, Gilbert DG. wFleaBase: the Daphnia genome database. BMC Bioinformatics. 2005;6:45. doi: 10.1186/1471-2105-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés T, Tagu D, Simon JC, Moya A, Martínez-Torres D. Sex versus parthenogenesis: a transcriptomic approach of photoperiod response in the model aphid Acyrthosiphon pisum (Hemiptera: Aphididae) Gene. 2008;408:146–156. doi: 10.1016/j.gene.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Dombrádi V, Axton JM, Brewis ND, da Cruz e Silva EF, Alphey L, Cohen PT. Drosophila contains three genes that encode distinct isoforms of protein phosphatase 1. Eur J Biochem. 1990;194:739–745. doi: 10.1111/j.1432-1033.1990.tb19464.x. [DOI] [PubMed] [Google Scholar]
- Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan Y, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard AL, Lenz PH, Shaw JR, Christie AE. Identification of putative peptide paracrines/hormones in the water flea Daphnia pulex (Crustacea; Branchiopoda; Cladocera) using transcriptomics and immunohistochemistry. Gen Comp Endocrinol. 2009;160:271–287. doi: 10.1016/j.ygcen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Huerta-Cepas J, Marcet-Houben M, Pignatelli M, Moya A, Gabaldón T. The pea aphid phylome: a complete catalogue of evolutionary histories and arthropod orthology and paralogy relationships for Acyrthosiphon pisum genes. Insect Mol Biol. 2010;19(Suppl 2):13–21. doi: 10.1111/j.1365-2583.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- Lampert W. The adaptive significance of diel vertical migration of zooplankton. Funct Ecol. 1989;3:21–27. [Google Scholar]
- Letunic I, Doerks T, Bork P. SMART 6: recent updates and new developments. Nucleic Acids Res. 2009;37:D229–D232. doi: 10.1093/nar/gkn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Todo T. The cryptochromes. Genome Biol. 2005;6:220. doi: 10.1186/gb-2005-6-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Lin MH, Horváth P, Reddy KL, Storti RV. PDP1, a novel Drosophila PAR domain bZIP transcription factor expressed in developing mesoderm, endoderm and ectoderm, is a transcriptional regulator of somatic muscle genes. Development. 1997;124:4685–4696. doi: 10.1242/dev.124.22.4685. [DOI] [PubMed] [Google Scholar]
- Loose C. Daphnia diel vertical migration behaviour: response to vertebrate predator abundance. Arch Hydrobiol Heih Ergebn Limnol. 1993;39:29–36. [Google Scholar]
- Loose C, Dawidowicz P. Trade-offs in diel vertical migration by zooplankton: the costs of predator avoidance. Ecology. 1994;75:2255–2263. [Google Scholar]
- Mazzotta GM, De Pittà C, Benna C, Tosatto SC, Lanfranchi G, Bertolucci C, Costa R. A cry from the krill. Chronobiol Int. 2010;27:425–445. doi: 10.3109/07420521003697494. [DOI] [PubMed] [Google Scholar]
- McCoole MD, Baer KN, Christie AE. Histaminergic signaling in the central nervous system of Daphnia and a role for it in the control of phototactic behavior. J Exp Biol. 2011;214:1773–1782. doi: 10.1242/jeb.054486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart SJ, Conlon C, Colbourne JK, Blaxter ML, Little TJ. The components of the Daphnia pulex immune system as revealed by complete genome sequencing. BMC Genomics. 2009;10:175. doi: 10.1186/1471-2164-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollivier M, Legeai F, Rispe C. Comparative analysis of the Acyrthosiphon pisum genome and expressed sequence tag-based gene sets from other aphid species. Insect Mol Biol. 2010;19:33–45. doi: 10.1111/j.1365-2583.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- Ousley A, Zafarullah K, Chen Y, Emerson M, Hickman L, Sehgal A. Conserved regions of the timeless (tim) clock gene in Drosophila analyzed through phylogenetic and functional studies. Genetics. 1998;148:815–825. doi: 10.1093/genetics/148.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz KF, Gilbert LI. Daphnia Halloween genes that encode cytochrome P450s mediating the synthesis of the arthropod molting hormone: evolutionary implications. BMC Evol Biol. 2008;8:60. doi: 10.1186/1471-2148-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez L, Young MW. Regulation of nuclear entry of the Drosophila clock proteins Period and Timeless. Neuron. 1996;17:979–990. doi: 10.1016/s0896-6273(00)80222-6. [DOI] [PubMed] [Google Scholar]
- Salomé PA, McClung CR. PSEUDO-RESPONSE REGULATOR 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell. 2005;17:791–803. doi: 10.1105/tpc.104.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé PA, Weigel D, McClung CR. The role of Arabidopsis morning loop components CCA1, LHY, PRR7, and PRR9 in temperature compensation. Plant Cell. 2010;22:3650–3661. doi: 10.1105/tpc.110.079087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrelli F, Tauber E, Pegoraro M, Mazzotta G, Cisotto P, Landskron J, Stanewsky R, Piccin A, Rosato E, Zordan M, Costa R, Kyriacou CR. Selection at the timeless locus in Drosophila melanogaster. Science. 2007;316:1898–1900. doi: 10.1126/science.1138426. [DOI] [PubMed] [Google Scholar]
- Sawyer LA, Hennessy JM, Peixoto AA, Rosato E, Parkinson H, Costa R, Kyriacou CP. Natural variation in a Drosophila clock gene and temperature compensation. Science. 1997;278:2117–2120. doi: 10.1126/science.278.5346.2117. [DOI] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a mimple modular achitecture research tool: identification of signaling domains. Proc Natl Acad Sci USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehadová H, Shao QM, Sehnal F, Takeda M. Neurohormones as putative circadian clock output signals in the central nervous system of two cricket species. Cell Tissue Res. 2007;328:239–255. doi: 10.1007/s00441-006-0339-5. [DOI] [PubMed] [Google Scholar]
- Shigenobu S, Bickel RD, Brisson JA, Butts T, Chang CC, Christiaens O, Davis GK, Duncan EJ, Ferrier DE, Iga M, Janssen R, Lin GW, Lu HL, McGregor AP, Miura T, Smagghe G, Smith JM, van der Zee M, Velarde RA, Wilson MJ, Dearden PK, Stern DL. Comprehensive survey of developmental genes in the pea aphid, Acyrthosiphon pisum: frequent lineage-specific duplications and losses of developmental genes. Insect Mol Biol. 2010;19:47–62. doi: 10.1111/j.1365-2583.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- Stollewerk A. The water flea Daphnia – a ‘new’ model system for ecology and evolution? J Biol. 2010;9:21. doi: 10.1186/jbiol212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J, Dircksen H. Circadian clocks in crustaceans: identified neuronal and cellular systems. Front Biosci. 2010;15:1040–1074. doi: 10.2741/3661. [DOI] [PubMed] [Google Scholar]
- Strauß J, Zhang Q, Verleyen P, Huybrechts J, Neupert S, Predel R, Pauwels K, Dircksen H. Pigment-dispersing hormone in Daphnia interneurons, one type homologous to insect clock neurons displaying circadian rhythmicity. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-011-0636-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D. Not just another genome. BMC Biol. 2011;9:8. doi: 10.1186/1741-7007-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo M, Bertram R, Houle D. Enzyme isoforms may increase phenotypic robustness. Evolution. 2008;62:2884–2893. doi: 10.1111/j.1558-5646.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka K, Matsumoto A. A Comparative view of insect circadian clock systems. Cell Mol Life Sci. 2010;67:1397–1406. doi: 10.1007/s00018-009-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie S, Ashburner M, Falls K, Leyland P, McQuilton P, Marygold S, Millburn G, Osumi-Sutherland D, Schroeder A, Seal R, Zhang H, FlyBase Consortium. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 2009;37:D555–D559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Dai ZM, Yang F, Yang WJ. Molecular cloning of Clock cDNA from the prawn, Macrobrachium rosenbergii. Brain Res. 2006;1067:13–24. doi: 10.1016/j.brainres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24:948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- Zhu H, Sauman I, Yuan Q, Casselman A, Emery-Le M, Emery P, Reppert SM. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 2008;6:e4. doi: 10.1371/journal.pbio.0060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Yuan Q, Briscoe AD, Froy O, Casselman A, Reppert SM. The two CRYs of the butterfly. Curr Biol. 2005;15:R953–954. doi: 10.1016/j.cub.2005.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Alignment of Dappu-TIM A-H. In the line immediately below each sequence grouping, stars indicated amino acids that are identically conserved, while single and double dots denote amino acids that are similar in structure.