Abstract
Purpose
The relationship between cerebral blood volume (CBV) and cerebral blood flow (CBF) underlies blood oxygenation level dependent functional MRI signal. This study investigates the potential for improved characterization of the CBV-CBF relationship in humans, and examines gender effects as well as spatial variations in the CBV-CBF relationship.
Methods
Healthy subjects were imaged non-invasively at rest and during visual stimulation, constituting the first MRI measurement of the absolute CBV-CBF relationship in humans with complete coverage of the functional areas of interest.
Results
CBV and CBF estimates were consistent with literature, and their relationship varied both spatially and with gender. In a region of interest (ROI) with stimulus induced activation in CBV and CBF at the P<0.05 significance level, a power function fit resulted in CBV = 2.1 CBF0.32 across all subjects, CBV = 0.8 CBF0.51 in females and CBV = 4.4 CBF0.15 in males. Exponents decreased in both genders as ROIs were expanded to include less significantly activated regions.
Conclusion
Consideration for potential gender related differences, as well as regional variations under a range of physiological states, may reconcile some of the variation across literature and advance our understanding of the underlying cerebrovascular physiology.
Keywords: Cerebral blood volume, Cerebral Blood Flow, Brain activation, Blood oxygenation level dependent, Functional MRI, Grubb’s Relationship, Visual Stimulation
Introduction
Blood oxygenation level dependent (BOLD) functional MRI (fMRI) is primarily sensitive to changes in deoxyhemoglobin concentration with activation; and calibrated fMRI aims to dissociate changes in CMRO2, the cerebral metabolic rate of oxygen, from changes in cerebral blood volume (CBV) and cerebral blood flow (CBF) (1,2). In lieu of CBV measurements, many studies have assumed that CBV is related to CBFα (1,3–7), with α=0.38 based on the absolute CBV-CBF relationship obtained by Grubb et al. in macaque brains under hypo and hypercapnia using PET (8), although, α may differ across functional challenges, brain regions and species (2,8–12).
The CBV-CBF relationship has been studied extensively in rats under anesthesia, and published exponents range from 0.18 to 0.64 across respiratory manipulations and functional activation, with spatial and temporal variations (11–16). Few results are available in humans: exponents of 0.29 and 0.64 during respiratory manipulation (17,18), and 0.3 during functional activation (10,19) were reported using PET measurements of absolute total CBV and CBF. Changes in arterial, capillary and venous compartments impact BOLD differently as well. Venous CBV is expected to have the largest impact on BOLD due to large oxygenation changes on the venous side (20). Optical measurements in animals indicate a complicated mechanism involving small venous CBV changes (21) as also supported by MRI in animals (14,22) and humans (23). Oxygenation changes have even been shown on the arterial side, accompanying large fractional volume changes (21,24). Significant capillary CBV changes have also been suggested (21,25–27), given that capillaries are the major source of oxygen extraction and closer to the activation site (27). Smaller exponents of the CBV-CBF relationship, of 0.18 during respiratory manipulation (28) and 0.23 during functional activation (23), were reported based on MRI measurements of relative contributions of venous blood to CBV. Although underlying hemodynamic parameters have been shown to vary (29–38), potential variations in the CBV-CBF relationship, spatially, with gender, and age for example have not yet been considered.
This study presents the first MRI measurement of the absolute CBV-CBF relationship in humans with complete multi-slice coverage of the functional areas of interest. Healthy subjects were non-invasively imaged at rest and during visual stimulation. Absolute total CBV and CBF were quantified in the steady-state using an Inversion Recovery (IR) based method with extended slice coverage (39), and Arterial Spin Labeling (ASL), respectively. Grubb et al. and a number of PET studies have reported exponents based on absolute total CBV measurements (8,10,17–19). The current non-invasive method with sensitivity to total CBV may not only enable more direct comparisons, but may also complement existing arterial or venous CBV weighted methods to improve our understanding of compartmental changes in different brain regions, stimulus types and durations in humans. Improved characterization of the CBV-CBF relationship in humans under various metabolic or functional challenges and conditions may have significant implications for fMRI calibration; advance physiological interpretation of BOLD and our understanding of the fundamental physiological relationship between neuronal activity, hemodynamic regulation and metabolism.
Methods
CBV Measurement
Absolute CBV was quantified non-invasively using a previously described method (39), as summarized below. A novel multi-slice IR-EPI pulse sequence (Figure 1a (39)) with varying contrast weightings and an efficient rotating slice acquisition order was used to acquire data at rest and during visual activation. Non-selective inversion is followed by the acquisition of multiple slices at inversion time TI. The slice acquisition order is shifted over successive TRs until each slice is acquired at each TI. Spoilers and a global saturation pulse ensure steady-state by enforcing identical recovery durations for all slices. For this multi-slice inversion-recovery experiment with inversion time TI (s), repetition time TR (s), an additional saturation pulse at time TS (saturation time, s) and longitudinal relaxation time constant T1 (s), longitudinal magnetization Mz at time TI is:
| Eq. [1] |
where M0 is the equilibrium magnetization (A/m) established by the external magnetic field B0 (T). The signal vs. TI curve differs between rest and activation conditions as summarized below.
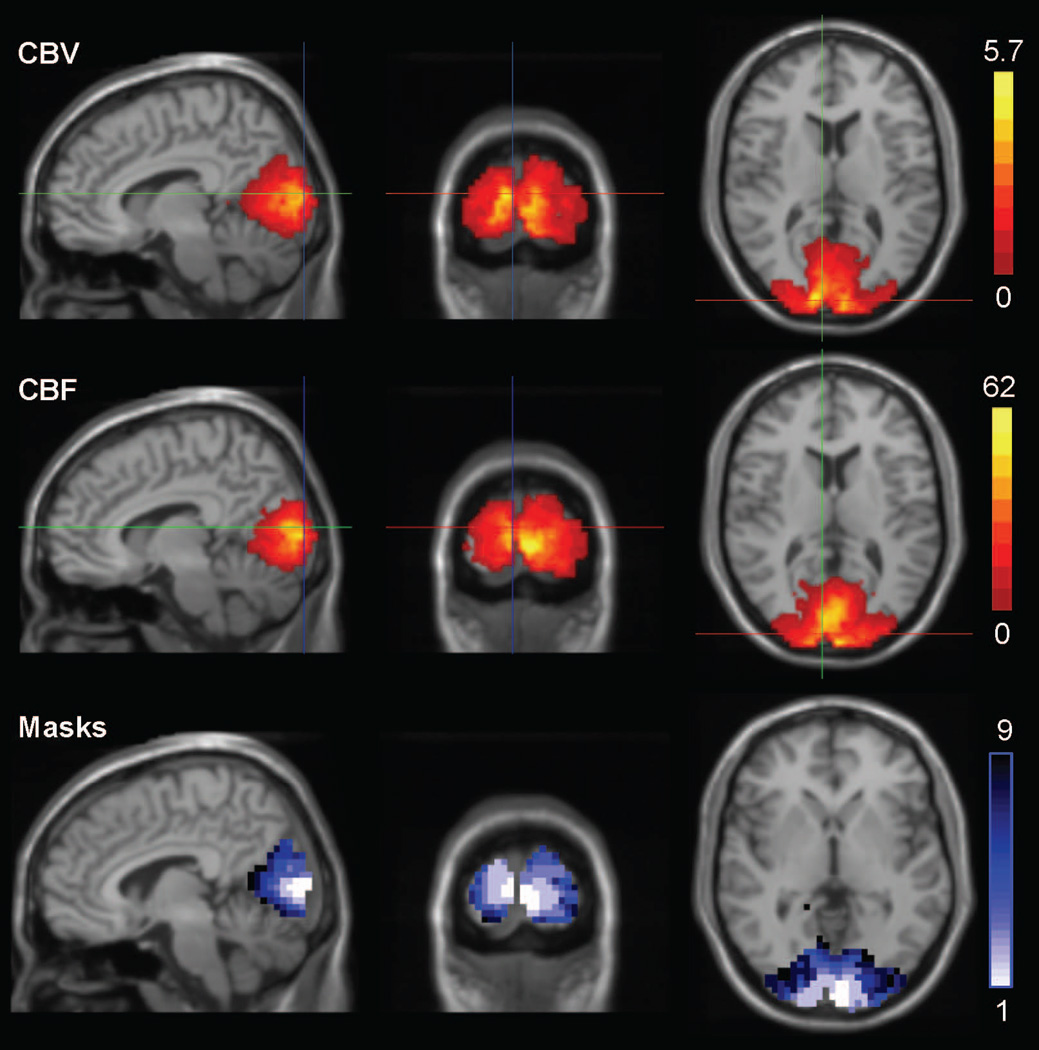
Figure 1.
Increases in CBV (mL/100 mL) and CBF (mL/100 mL/min) in the occipital cortex with visual stimulation (composite image over all subjects); and ROI masks used in analyses (Masks 1–4 are the intersections and masks 5–8 are the unions of CBV and CBV activations at significance levels P<0.01, P<0.05, P<0.1, and P<0.2, respectively, while mask 9 is the anatomical Brodmann area definition of the primary occipital cortex. Masks 1–9 cover approximately 4, 16, 27, 41, 29, 45, 55, 69, and 89cm3, respectively.)
In a voxel containing CSF and brain parenchyma, where CBV is the blood fraction in brain parenchyma, MR signal magnitude includes the following contributions:
| Eq. [2] |
where K is a calibration factor accounting for equilibrium magnetization with transmit and receive sensitivity effects assuming uniform coil profiles and M0 (a.u.), OBV and DBV are the oxygenated and deoxygenated blood volumes, respectively. Each compartment contributes signal according to its volume fraction F in the voxel (dimensionless), water proton density C (dimensionless), effective transverse relaxation time constant T2* (s), and longitudinal magnetization, Mz, at the time of excitation including longitudinal relaxation effects. Signal at inversion time TI is:
| Eq. [3] |
for i = CSF, OBV, and DBV, where TE is the echo time (s), with:
| Eq. [4] |
for i = CSF, OBV, DBV, and TISSUE. CSF fraction changes with activation were found to be negligible in the occipital cortex (40,41). This allows limiting the number of parameters in the current study, however, CSF may have larger effects in other brain areas (41,42) and it would be desirable to extend the model to consider such changes elsewhere (39). Extravascular tissue signal is modified by the influence of deoxygenated blood and its oxygenation level (43–45), as follows:
| Eq. [5] |
| Eq. [6] |
where T2,TISSUE is the transverse relaxation time constant (s), FDBV is the fraction of DBV in the voxel (%), YDBV is the DBV oxygenation fraction (%), Hct is the microvascular hematocrit estimate (%), J0 is the zero-order Bessel function, γ is the gyromagnetic ratio (42.576 MHz/T), Δx is the susceptibility difference between fully oxygenated and deoxygenated blood (ppm).
T1 of blood varies slightly with hematocrit and blood oxygenation, decreasing with increased hematocrit and reduced oxygenation (46,47), as follows:
| Eq. [7] |
where Yb is the average blood oxygenation fraction (%, Yb = 98% for OBV, Yb = YDBV for DBV, Yb = (YOBV․FOBV + YDBV․FDBV) / (FOBV+FDBV) for CBV), a = 2.4084 (s−1), b = 0.708 (s−1), c = −1.9998 (s−1), d = −0.2892 (s−1), based on interpolation of published results (46–48). For instance, the commonly used blood T1s of 1624–1627ms correspond to Yb = 81% (average of measurements at arterial Yb = 92% and venous Yb = 69%), and a T1 of 1612ms corresponds to Yb = 77%, approximately, both at Hct of 42% (average of male and female macrovascular Hct). Considering a lower microvascular Hct of 37.4% (85% of macrovascular Hct), blood T1s of 1747ms and 1703ms correspond to an arterial oxygenation fraction of Yb = 98% and venous Yb = 61%, respectively (48).
T2* of blood also varies with hematocrit and blood oxygenation, decreasing with increasing hematocrit and decreasing oxygenation (49,50). At 3T under physiological conditions (49):
| Eq. [8] |
where A*, B*, and C* depend on hematocrit. For Hct = 34%, (females, for 85% of macrovascular Hct = 40%) a*: 16.1957 (s−1), b* = 36.5348 (s−1), c* = 91.3478 (s−1), and for Hct = 38.25% (males, for 85% of macrovascular Hct = 45%) a* = 16.75 (s−1), b* = 37.625 (s−1), c* = 103.1 (s−1) based on interpolation of published results (48,49).
Functional challenges influence the voxel signal S through changes in compartment fractions F (Eq. [3] and [5]) as well as blood oxygenation Yb (Eq. [3] and [5–8]). A slight curve shift occurs upon stimulation (19,51), and CBV:
| Eq. [9] |
is estimated by fitting the fractional signal change between rest and activation, (Sact−Srest)/Srest, over multiple TI times.
CBF Measurement
Absolute CBF was quantified using Q2TIPS Pulsed ASL (PASL) imaging (52) at baseline and during stimulation. The EPISTAR (echo-planar imaging with signal targeting using alternating radio frequency) sequence was modified by adding a train of thin-slice saturation RF pulses, applied at a post-labeling delay inversion time after the inversion RF pulse, to control the bolus delivery and suppress intravascular signal from large vessels (52). Interleaved labeling and control images were acquired using gradient echo-planar imaging in an ascending order. A slab-selective hypersecant inversion RF pulse was applied to slabs inferior and superior to the imaging slab for ASL (inferior slab) and as control (superior slab) for magnetization transfer effects, respectively. A bipolar gradient was applied to the imaging slices to suppress signal contamination from labeled arterial water within large vessels.
MRI Experiments
Twelve healthy subjects provided written informed consent and participated in this Yale University Institutional Review Board approved, Health Insurance Portability and Accountability Act compliant study (mean age ± sd: 31.8±6 years, 5 females). Previously acquired data (39) has been re-analyzed to evaluate regional and gender related variations in CBV, CBF, and the CBV-CBF relationship. Experiments were performed on a 3T whole body scanner (Tim Trio, Siemens Medical Systems, Erlangen Germany) with a 32-channel receive-only phased-array head coil and body coil transmission. 3D high-resolution (MPRAGE, 1mm isotropic, 176×202×179mm FOV, TR/TI/TE:1500ms/800ms/2.83ms) acquisition was followed by multi-slice 2D high-resolution (FLASH, 1mm in-plane, TR/TE: 300/3.69ms), T1 mapping, PD-weighted, CBV and CBF sequences with the same slice prescription. Multi-slice prescriptions consisted of 20 transverse slices covering the whole brain including the calcarine fissure with 4mm slice thickness and 2mm gap. The CBV sequence parameters were: TE/TS/TR=11ms/1.2s/3s, gradient-echo EPI, 192×256mm FOV, 4×4mm in-plane. Images were acquired at the following 60 TI values: TIs(n,s) = TIstart + (n−1) × TIshift + (s−1) × TIgap, where TIstart=400ms, TIshift=13ms, TIgap=38.51ms (acquisition duration for one slice), n=1 to 3, and s=1 to 20, covering the TI range of 400–1158ms with 13ms resolution using 3 sets of 20 TI values. For T1 mapping, TI values covering the TI range of 120–2400ms were acquired in steps of 120ms with TR/TE/TS =6s/11ms/2.5s and 3 repetitions. The CBF parameters were: TR/TE/TI1/sliceTR: 3s/20ms/1.4s/52.3ms, post-labeling delays TI(s) = 1400ms + 52.3ms × (s−0.5) for slices s=1 to 20, 10cm adiabatic inversion of slabs 2cm inferior and superior to the imaging slab for labeling and control, a bipolar gradient of 5cm/s was applied to suppress signal contamination from labeled arterial water within large vessels. PD parameters were the same as the CBF parameters except TR/ TI/ TD: 8s/ 6.05s/ 0.
A visual stimulation paradigm consisting of a full-field black-and-white flashing checkerboard (frequency 10Hz) was presented on a back-projection screen viewed from a mirror mounted on the head coil. A block paradigm was generated in Eprime (Psychology Software Tools, Sharpsburg PA) with ON and OFF blocks of 78s duration each, where a generous 18s after each ON/OFF transition was allowed for settling of the hemodynamic response. CBV data were acquired on 12 subjects (5 female, 7 male) with three OFF/ON cycles such that each CBV acquisition lasted 7min 48sec (6 blocks of 78 s), which was repeated for three sets of TI values each with three repetitions. CBF data were acquired on 10 subjects (5 female, 5 male) with four OFF/ON cycles such that each CBF acquisition lasted 10min 24sec (8 blocks of 78 s).
Data Processing
Time series images were grouped into volumes with the same contrast (same TI times), and motion corrected using SPM (Statistical Parametric Mapping, www.fil.ion.ucl.ac.uk/spm/). Motion correction involved registering all images via a six-parameter affine transformation initially to the middle image in each time series, finding the average of this registration, and then registering to this average. Linear drift correction was applied, and data were averaged over blocks and repetitions.
As customary, absolute CBF was calculated from the difference between interleaved labeled and control image pairs, averaged over multiple acquisitions (53–55). Hematocrit differences between males and females (8,56) were taken into account in CBF calculations by assuming a linear decrease in hematocrit from macro to microvasculature (from 45% in males and 40% in females to 85% of these values (8,56)) over five pre-capillary compartments of equal volume (57), leading to arterial blood T1 of 1728 ms in females and 1673 ms in males.
Data were transformed to a common whole-brain template defined by the Montreal Neurological Institute (MNI) using BioimageSuite (www.bioimagesuite.org) using a combination of three transformations: linear transformations that co-register each subject’s functional images (average over TI values) to the same subject’s high-resolution 2D, then 3D images, and a non-linear transformation that co-registers these 3D images to the MNI brain. Tri-linear interpolation was employed for re-gridding and all subsequent analyses were performed in MNI space at 4×4×4mm resolution.
CBV was calculated following the procedure described in (39), by fitting the relative signal change between rest and activation, (Sact−Srest)/Srest, to the biophysical model: CSF and tissue T1s were measured while previous publications and models provided estimates of the T1 and T2* of blood, and T2* of tissue as functions of oxygenation and compartment fractions (Eq. [3] and [5–8], Table 1), enabling estimation of oxygenation during activation, CSF fractions, CBV at rest and during activation. CSF and tissue T1s for each subject were obtained from T1 maps generated by least-squares fitting to signal over multiple TIs, leading to CSF T1 of 4166 ± 0.377 ms (mean ± sd across subjects, vs. published values of 4300 ms (58) and 3700 ± 500 ms (59)), and occipital GM T1 of 1267 ± 52 ms (mean ± sd across subjects, vs. published values of 1283 ± 37ms to 1356 ± 26 ms (60) and 1122 ± 117 ms (58)). Microvascular hematocrit was assumed to be 85% of macrovascular hematocrit (8) of 45% in males and 40% in females (56). OBV was assumed to be nearly fully oxygenated (YOBV = 98%), occupying 21% of CBV at rest (considering baseline CBV consisting of 21% arterial/arteriolar, 33% capillary, 46% venous contributions based on microvascular morphometry (57,61)). DBV consists of the remaining capillaries and venules at rest, with Ycapillary=77% and Yvenule=61% typically assumed at rest considering an exponential drop in oxygen saturation from arterioles to venules (49,62), such that YDBV,rest=68.78% (vs. published values of Yv,rest=68.7% (23) and Yv,rest=69% (63)). No assumptions were made regarding OBV vs. DBV fractions, or YDBV, during activation. Parameters used in CBV fitting are listed in Table 1. Errors in the assumed parameters (i.e. of −10% to 10%) have been shown to result in a comparable range of errors in the fitted parameters: CBV estimates were most strongly influenced by errors in Hct (up to 15%) and resting oxygenation (up to 12%), while errors in T2 and T2* values had less influence on all estimates (up to 6%). Overestimation of CBV resulted from overestimation of Hct or underestimation of resting oxygenation. Over and underestimation of parameters had fairly symmetric effects on error, except for resting oxygenation whose overestimation resulted in slightly smaller errors in all parameters other than CSF fraction (39).
Table 1.
Parameters used in CBV model fitting
| Parameter | Description | Value |
|---|---|---|
| HctMALE | Microvascular hematocrit in males (%) | 45%×85% (8,56) |
| HctFEMALE | Microvascular hematocrit in females (%) | 40%×85% (8,56) |
| CBLOOD | Blood water proton density (mL water / mL blood) | 0.95–0.22×Hct (42) |
| CCSF | CSF water proton density (mL water / mL CSF) | 1 (42) |
| CGM | GM water proton density (mL water / mL GM) | 0.89 (42) |
| Δx | Susceptibility difference between oxygenated and deoxygenated blood (ppm) | 0.2 (43) |
| CSF T2* | CSF effective transverse relaxation time constant (ms) | 1442 (66) |
| GM T2 | GM transverse relaxation time constant (ms) | 71.1 (40,49) |
| (OBV/CBV)REST | Oxygenated blood volume fraction at rest (%) | 21% (57,61) |
| YOBV | OBV oxygenation fraction (%) | 98% (89) |
| YDBV,REST | DBV oxygenation fraction at rest (%) | 68.78% (49,57,61,62) |
The CBV-CBF relationship was evaluated across nine region-of-interest (ROI) masks covering a range of sizes (Figure 1). Areas with significant activation in the primary visual cortex (Brodmann areas 17 and 18 defined on the MNI brain (64)) were identified using t-tests of task-induced changes between rest and activation in CBV and CBF, at four levels of significance (P<0.01, P<0.05, P<0.1, and P<0.2). ROI masks were generated from areas showing activation in both CBV and CBF (four intersections, ROI masks 1–4), from areas showing activation in CBV or CBF (four unions, ROI masks 5–8), and the Brodmann area definition (one anatomical, ROI mask 9). Mean CBV and CBF values were calculated within each ROI mask (voxels with CBV>30 mL/100 g were excluded as vessels). This resulted in one data point for each subject at baseline and activation states in each ROI. The CBV-CBF relationship was estimated by fitting to a power function (CBV = a ․ CBFb) as well as a linear function (CBV = c + d ․ CBF). Matlab (Mathworks, Natick MA) functions for linear and nonlinear regression were used for fitting, student's t-tests (two-tailed) were used for comparisons (across rest vs. activation states, ROIs, ages and gender), analysis of covariance (ANCOVA, separate lines for each gender, no constraints) and multiple comparison tests were used for comparisons across genders over multiple ROIs.
Results
Stimulation resulted in bilateral activation in all subjects. Increases in CBV and CBF in the occipital cortex are shown in Figure 1 in a composite image across all subjects, along with the ROI masks used in the analyses. ROIs with highly significant activation were smaller as expected (ROI1<ROI2<ROI3<ROI4 & ROI5<ROI6<ROI7<ROI8). Intersection ROIs (ROIs 1–4), only including voxels activated in both methods were generally smaller; union ROIs (ROIs 5–8), also including voxels activated in only one of the methods were generally larger. Only the largest intersection ROI (ROI 4) was slightly larger than the smallest union ROI (ROI 5), resulting in an ascending size order of: ROI1, ROI2, ROI3, ROI5, ROI4, ROI6, ROI7, ROI8, ROI9. CBV and CBF values at rest and activation across all ROIs are provided in Table 2. Smaller ROIs were associated with larger CBV values and larger activation induced increases in CBV. The smallest CBV increase was observed in the largest ROI (ROI 9, anatomical mask) where CBV increased by 22.2%, from 5.4 mL/100 mL at rest to 6.7 mL/100 mL during activation. The largest CBV increase was observed in the smallest ROI (ROI 1, activation mask, P<0.01 significance level in both CBV and CBF) where CBV increased by 39.4%, from 7.9 mL/100 mL at rest to 11 mL/100 mL during activation. CSF fractions ranged from 6% to 18%, covering approximately 9% of ROIs on average and with slightly larger CSF fractions in smaller ROIs (ranging from 7–18% in the smallest ROI, to 6–12% in the largest ROI). Smaller ROIs were also associated with larger increases in CBF, and CBF responses were higher than CBV responses overall. The smallest CBF increase was observed in the largest ROI (ROI 9, anatomical mask) where CBF increased by 23.1%, from 72.8 mL/min/100 mL at rest to 88.9 mL/min/100 mL during activation. The largest CBF increase was observed in the smallest ROI (ROI 1, activation mask, P<0.01 significance level in both CBV and CBF) where CBF increased by 65.6%, from 72.9 mL/min/100 mL at rest to 120.7 mL/min/100 mL during activation. Within group standard deviations were smaller at rest (0.5 to 1 mL/100 mL in CBV; 22.9–24.5 mL/min/100 mL in CBF) than during activation (0.7–1.3 mL/100 mL in CBV; 26–35.7 mL/min/100 mL in CBF), and decreased with increasing ROI volume (1–1.3 mL/100mL to 0.5–0.7 mL/100 mL in CBV; 24.5–35.7 to 22.9–26 mL/min/100 mL in CBF).
Table 2.
CBV and CBF values at rest, during activation, and percentage changes with activation across all ROIs, over all subjects, female subjects, or male subjects.
| ROI | Rest | CBV Activation |
Change | Rest | CBF Activation |
Change | |
|---|---|---|---|---|---|---|---|
| All | 1 | 7.9 ± 1.0 | 11.0 ± 1.3 | 39.4 | 72.9 ± 24.5 | 120.7 ± 35.7 | 65.6 |
| 2 | 7.3 ± 0.8 | 10.0 ± 1.2 | 37.6 | 72.8 ± 25.1 | 112.1 ± 35.2 | 54.1 | |
| 3 | 6.9 ± 0.7 | 9.3 ± 1.1 | 34.7 | 71.2 ± 25.2 | 105.0 ± 33.9 | 47.4 | |
| 4 | 6.7 ± 0.7 | 8.8 ± 1.0 | 31.1 | 71.2 ± 25.0 | 98.9 ± 31.6 | 39.0 | |
| 5 | 6.7 ± 0.5 | 9.0 ± 0.9 | 33.9 | 71.5 ± 24.9 | 100.5 ± 32.5 | 40.6 | |
| 6 | 6.5 ± 0.6 | 8.4 ± 0.9 | 30.7 | 73.9 ± 25.0 | 99.5 ± 31.0 | 34.6 | |
| 7 | 6.2 ± 0.6 | 8.0 ± 0.8 | 28.7 | 73.6 ± 24.6 | 96.6 ± 29.8 | 31.2 | |
| 8 | 5.8 ± 0.5 | 7.3 ± 0.7 | 26.4 | 72.7 ± 23.7 | 92.8 ± 28.1 | 27.8 | |
| 9 | 5.4 ± 0.5 | 6.7 ± 0.7 | 22.2 | 72.8 ± 22.9 | 88.9 ± 26.0 | 23.1 | |
| Female | 1 | 8.1 ± 1.2 | 11.6 ± 1.4 | 44.5 | 90.1 ± 15.1 | 143.9 ± 29.0 | 60.1 |
| 2 | 7.5 ± 0.9 | 10.6 ± 1.2 | 41.2 | 90.3 ± 17.2 | 134.2 ± 31.1 | 48.5 | |
| 3 | 7.1 ± 0.8 | 9.8 ± 1.0 | 37.8 | 88.8 ± 18.5 | 126.5 ± 31.0 | 42.2 | |
| 4 | 6.8 ± 0.7 | 9.1 ± 1.0 | 33.7 | 88.3 ± 19.8 | 118.6 ± 29.6 | 34.4 | |
| 5 | 6.8 ± 0.5 | 9.3 ± 0.8 | 36.9 | 87.9 ± 20.1 | 120.0 ± 30.3 | 36.7 | |
| 6 | 6.6 ± 0.6 | 8.8 ± 0.8 | 33.3 | 90.6 ± 20.8 | 118.4 ± 29.2 | 30.9 | |
| 7 | 6.3 ± 0.6 | 8.3 ± 0.8 | 31.0 | 89.8 ± 20.9 | 114.7 ± 28.4 | 27.8 | |
| 8 | 6.0 ± 0.6 | 7.7 ± 0.7 | 28.5 | 88.0 ± 20.9 | 109.6 ± 27.1 | 24.8 | |
| 9 | 5.6 ± 0.5 | 7.0 ± 0.7 | 24.6 | 86.9 ± 21.3 | 103.9 ± 25.3 | 20.0 | |
| Male | 1 | 7.7 ± 0.7 | 10.4 ± 1.0 | 35.1 | 55.6 ± 19.5 | 97.6 ± 25.2 | 83.9 |
| 2 | 7.0 ± 0.7 | 9.4 ±1.0 | 34.2 | 55.3 ± 18.9 | 90.0 ± 23.0 | 69.4 | |
| 3 | 6.7 ± 0.7 | 8.9 ±0.9 | 31.7 | 53.6 ± 17.6 | 83.5 ± 20.5 | 61.4 | |
| 4 | 6.5 ± 0.6 | 8.4 ±0.8 | 28.5 | 54.1 ± 16.4 | 79.1 ± 18.7 | 50.5 | |
| 5 | 6.6 ± 0.6 | 8.6 ±0.8 | 31.0 | 55.0 ± 17.1 | 80.9 ± 20.7 | 51.3 | |
| 6 | 6.3 ± 0.5 | 8.1 ±0.7 | 28.2 | 57.2 ± 16.2 | 80.5 ± 18.9 | 43.6 | |
| 7 | 6.0 ± 0.5 | 7.6 ±0.7 | 26.4 | 57.5 ± 15.7 | 78.6 ± 17.9 | 39.1 | |
| 8 | 5.6 ± 0.4 | 7.0 ±0.6 | 24.4 | 57.3 ± 14.8 | 76.1 ± 16.9 | 34.6 | |
| 9 | 5.2 ± 0.4 | 6.4 ±0.5 | 21.5 | 58.6 ± 13.8 | 73.9 ± 16.9 | 27.2 | |
CBV values are in mL/100 mL; CBF values are in mL/min/100 mL; Change refers to increase (or decrease) relative to rest as a percentage (%).
CBV and CBF at rest and during activation are also provided in Table 2 across all ROIs for each gender. Gender data generally showed the same trends as the combined data: Smaller ROIs were associated with larger CBV values and larger CBV increases. The smallest CBV increase was in the largest ROI (ROI 9, anatomical mask) where CBV increased by 24.6% in females (from 5.6 mL/100 mL to 7.0 mL/100 mL) and by 21.5% in males (from 5.2 mL/100 mL to 6.4 mL/100 mL). The largest CBV increase was in the smallest ROI (ROI 1) where CBV increased by 44.5% in females (from 8.1 mL/100 mL at rest to 11.6 mL/100 mL during activation) and by 35.1% in males (from 7.7 mL/100 mL at rest to 10.4 mL/100 mL during activation). Smaller ROIs were also associated with larger CBF increases. The smallest CBF increase was in the largest ROI (ROI 9) where CBF increased by 20% in females (from 86.9 mL/min/100 mL to 103.9 mL/min/100 mL) and by 27.2% in males (from 58.6 mL/min/100 mL to 73.9 mL/min/100 mL). The largest CBF increase was in the smallest ROI (ROI 1) where CBF increased by 60.1% in females (from 90.1 mL/min/100 mL to 143.9 mL/min/100 mL) and by 83.9% in males (from 55.6 mL/min/100 mL to 97.6 mL/min/100 mL). CBF increases were higher than CBV increases in all ROIs for all subjects, in all ROIs for males, and in smaller ROIs in females (except for larger ROIs in females, where CBV increases exceeded CBF increases).
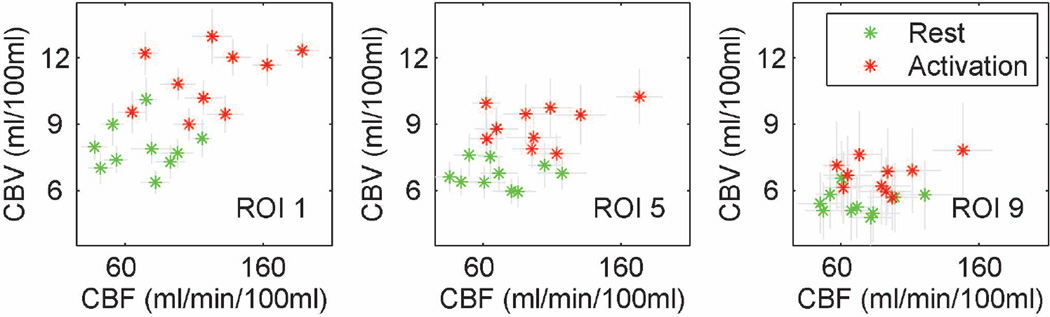
CBV and CBF values at rest and during activation, and their activation induced increases were compared across genders, and ROIs, as summarized in Table 3. In all ROIs, CBV at rest, CBV during activation, CBV increases, CBF at rest and CBF during activation were higher in females than in males, whereas CBF increases were higher in males than in females. With this small cohort, differences between genders did not reach significance for CBV at rest (P >0.05), with only a slight trend for differences in CBV during activation and CBV increases (P~0.2). On the other hand, differences between genders were significant for CBF both at rest and during activation (P<0.05), with only a slight trend for differences in CBF increases (P~0.2). All possible combinations of ROIs were compared to evaluate differences between CBV and CBF values across ROIs. There were significant differences in CBV at rest and during activation (P<0.05) in 97% of comparisons across ROIs. CBF during activation also varied significantly (P<0.05) in 94% of comparisons across ROIs, while CBF at rest, CBV increases and CBF increases showed significant differences (P<0.05) in 19%, 31% and 39% of comparisons across ROIs, respectively. CBV and CBF values across three sample ROIs are shown in Figure 2.
Table 3.
Summary of comparisons across genders and ROIs, of CBV and CBF values at rest, during activation, and percentage changes with activation.
| Comparison | Rest | CBV Activation |
Change | Rest | CBF Activation |
Change |
|---|---|---|---|---|---|---|
| Gender | NS | P ~ 0.2 | P ~ 0.2 | P < 0.05 | P < 0.05 | P ~ 0.2 |
| Spatial | P < 0.051 | P < 0.051 | NS2 | NS3 | P < 0.053 | NS4 |
(NS: not significant;
Spatial: across all ROI combinations, P<0.05 in 197%, 231%, 319%, 494%, 39% of cases);
Age difference between genders was NS, P=0.897).
Figure 2.
CBV (mL/100 mL) and CBF (mL/100 mL/min) across ROIs 1, 5, and 9. Error bars represent one standard deviation; green: rest; red: activation.
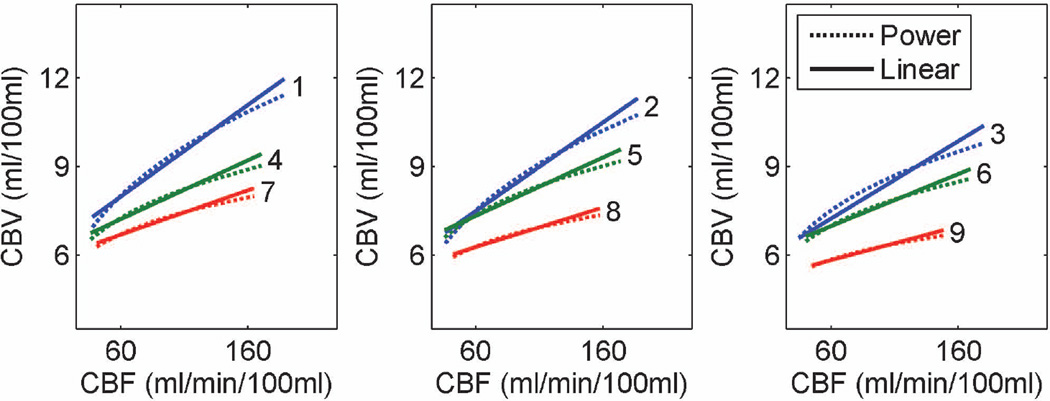
Based on these underlying differences, the CBV-CBF relationship was fitted to both a power function, CBV = a CBFb, and a linear function, CBV = c + d․ CBF, in all subjects as well as in females and males separately, in each ROI. Fitting to a power function resulted in the expression CBV = 2.1 CBF0.32 while fitting to a linear function resulted in the expression CBV = 5.7 + 0.03 CBF in ROI 2, which corresponds to stimulus induced activation in CBV and CBF at the P<0.05 significance level. Both power and linear fits showed spatial variation, with power exponents decreasing from 0.31 ± 0.08 to 0.14 ± 0.10, and slopes decreasing from 0.031 ± 0.008 to 0.012 ± 0.007, going from the smallest ROI to the largest ROI. Power and linear fitting results across all ROIs and subjects are depicted in Figure 3. Fitting results across all ROIs using all subjects, as well as male and female subjects separately are provided in Table 4.
Figure 3.
Power and linear function fits to the CBF-CBV relationship across all ROIs. Each curve or line was obtained from 20 data points (10 subjects in 2 conditions, rest or activation, each). Both power exponents and slopes consistently decrease with increasing ROI sizes.
Table 4.
Results of fitting the CBV-CBF relationship to power and linear functions over different ROIs, over all subjects, female subjects, or male subjects. ROI 2 exponents, corresponding to stimulus induced activation in CBV and CBF at the P<0.05 significance level, are highlighted.
| Power Fit: CBV = a CBF b | Linear Fit: CBV = c + d․ CBF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | a | b | RMSE | R | c | d | RMSE | R | |
| All | 1 | 2.3 ± 0.9 | 0.31 ± 0.08 | 1.36 | 0.68 | 6.1 ± 0.8 | 0.031 ± 0.008 | 1.29 | 0.72 |
| 2 | 2.1 ± 0.9 | 0.32 ± 0.09 | 1.37 | 0.63 | 5.7 ± 0.8 | 0.030 ± 0.008 | 1.32 | 0.66 | |
| 3 | 2.8 ± 1.2 | 0.24 ± 0.10 | 1.39 | 0.50 | 5.7 ± 0.8 | 0.026 ± 0.008 | 1.20 | 0.63 | |
| 4 | 3.1 ± 1.3 | 0.21 ± 0.10 | 1.25 | 0.47 | 6.0 ± 0.8 | 0.020 ± 0.009 | 1.23 | 0.47 | |
| 5 | 3.1 ± 1.4 | 0.21 ± 0.10 | 1.27 | 0.45 | 6.1 ± 0.8 | 0.020 ± 0.009 | 1.25 | 0.48 | |
| 6 | 3.1 ± 1.4 | 0.20 ± 0.10 | 1.18 | 0.42 | 5.9 ± 0.8 | 0.018 ± 0.008 | 1.16 | 0.44 | |
| 7 | 3.3 ± 1.5 | 0.17 ± 0.10 | 1.10 | 0.37 | 5.8 ± 0.7 | 0.015 ± 0.008 | 1.08 | 0.40 | |
| 8 | 3.2 ± 1.4 | 0.16 ± 0.10 | 0.98 | 0.35 | 5.5 ± 0.7 | 0.013 ± 0.008 | 0.97 | 0.38 | |
| 9 | 3.2 ± 1.5 | 0.14 ± 0.10 | 0.86 | 0.31 | 5.1 ± 0.6 | 0.012 ± 0.007 | 0.85 | 0.34 | |
| Female | 1 | 0.6 ± 0.6 | 0.57 ± 0.18 | 0.29 | 0.74 | 4.2 ± 1.7 | 0.046 ± 0.01 | 0.28 | 0.74 |
| 2 | 0.8 ± 0.7 | 0.51 ± 0.17 | 1.43 | 0.72 | 4.5 ± 1.6 | 0.040 ± 0.01 | 1.43 | 0.72 | |
| 3 | 1.0 ± 0.8 | 0.47 ± 0.17 | 1.31 | 0.69 | 4.6 ± 1.5 | 0.035 ± 0.01 | 1.30 | 0.69 | |
| 4 | 1.3 ± 1.1 | 0.40 ± 0.18 | 1.25 | 0.61 | 4.9 ± 1.4 | 0.030 ± 0.01 | 1.24 | 0.62 | |
| 5 | 1.3 ± 1.0 | 0.40 ± 0.17 | 1.23 | 0.63 | 5.0 ± 1.4 | 0.030 ± 0.01 | 1.22 | 0.64 | |
| 6 | 1.5 ± 1.3 | 0.35 ± 0.18 | 1.22 | 0.54 | 5.1 ± 1.4 | 0.025 ± 0.01 | 1.21 | 0.56 | |
| 7 | 2.0 ± 1.7 | 0.29 ± 0.19 | 1.18 | 0.46 | 5.2 ± 1.4 | 0.021 ± 0.01 | 1.16 | 0.48 | |
| 8 | 2.2 ± 2.0 | 0.25 ± 0.19 | 1.09 | 0.40 | 5.1 ± 1.3 | 0.017 ± 0.01 | 1.07 | 0.43 | |
| 9 | 2.9 ± 2.5 | 0.17 ± 0.19 | 0.98 | 0.30 | 5.1 ± 1.2 | 0.013 ± 0.01 | 0.96 | 0.35 | |
| Male | 1 | 3.4 ± 1.9 | 0.23 ± 0.13 | 0.28 | 0.53 | 7.3 ± 1.4 | 0.023 ± 0.02 | 1.62 | 0.44 |
| 2 | 4.4 ± 3.0 | 0.15 ± 0.16 | 1.57 | 0.32 | 7.2 ± 1.4 | 0.014 ± 0.02 | 1.60 | 0.26 | |
| 3 | 4.6 ± 3.2 | 0.13 ± 0.16 | 1.45 | 0.27 | 7.0 ± 1.4 | 0.012 ± 0.02 | 1.47 | 0.21 | |
| 4 | 4.9 ± 3.4 | 0.10 ± 0.17 | 1.28 | 0.21 | 6.9 ± 1.3 | 0.009 ± 0.02 | 1.30 | 0.16 | |
| 5 | 3.8 ± 2.3 | 0.17 ± 0.15 | 1.18 | 0.42 | 6.9 ± 1.3 | 0.010 ± 0.02 | 1.34 | 0.18 | |
| 6 | 4.8 ± 3.5 | 0.10 ± 0.17 | 1.20 | 0.20 | 6.7 ± 1.3 | 0.008 ± 0.02 | 1.21 | 0.15 | |
| 7 | 3.7 ± 2.5 | 0.15 ± 0.16 | 0.98 | 0.35 | 6.5 ± 1.2 | 0.005 ± 0.02 | 1.10 | 0.10 | |
| 8 | 5.0 ± 3.7 | 0.06 ± 0.18 | 0.96 | 0.12 | 6.1 ± 1.1 | 0.003 ± 0.02 | 0.96 | 0.07 | |
| 9 | 5.9 ± 4.3 | 0.00 ± 0.17 | 0.80 | 0.00 | 5.9 ± 1.0 | 0.002 ± 0.02 | 0.80 | 0.04 | |
RMSE: root mean squared error; R2: Coefficient of determination; ROIs 1– 4: intersections and ROIs 5–8: unions of CBV and CBV activations at significance levels P<0.01, P<0.05, P<0.1 and P<0.2, respectively; ROI 9: Brodmann area definition.
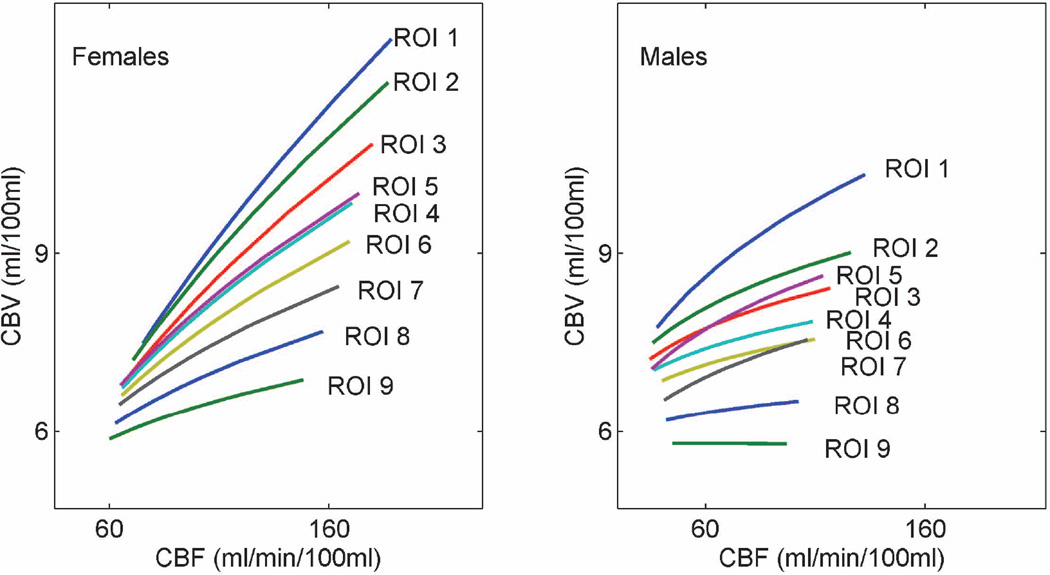
Both power and linear function fits showed variations with gender, as well as across ROIs, consistent with the observed differences in the underlying hemodynamic parameters. Fitting to a power function resulted in the expression CBV = 0.8 CBF0.51 in females vs. the expression CBV = 4.4 CBF0.15 in males, in ROI 2 (stimulus induced activation in CBV and CBF at the P<0.05 significance level). Fitting to a linear function resulted in the expression CBV = 4.5 + 0.04 CBF in females vs. the expression CBV = 7.2 + 0.014 CBF in males, in the same ROI. Both power and linear fits continued to show spatial variation within individual genders, with power exponents decreasing from 0.57 ± 0.18 to 0.17 ± 0.19 in females, and from 0.23 ± 0.13 to 0.00 ± 0.17 in males, going from the smallest ROI to the largest ROI. Slopes from linear fits decreased from 0.046 ± 0.01 to 0.013 ± 0.01 in females, and from 0.023 ± 0.02 to 0.002 ± 0.02 in males, across the same ROI range. Power fits to the CBF-CBV relationship across all ROIs, for female and male subjects are depicted in Figure 4.
Figure 4.
Power fits to the CBF-CBV relationship across all ROIs, for female and male subjects. Each gender curve was obtained from 10 data points (5 subjects of each gender, in 2 conditions, rest or activation, each).
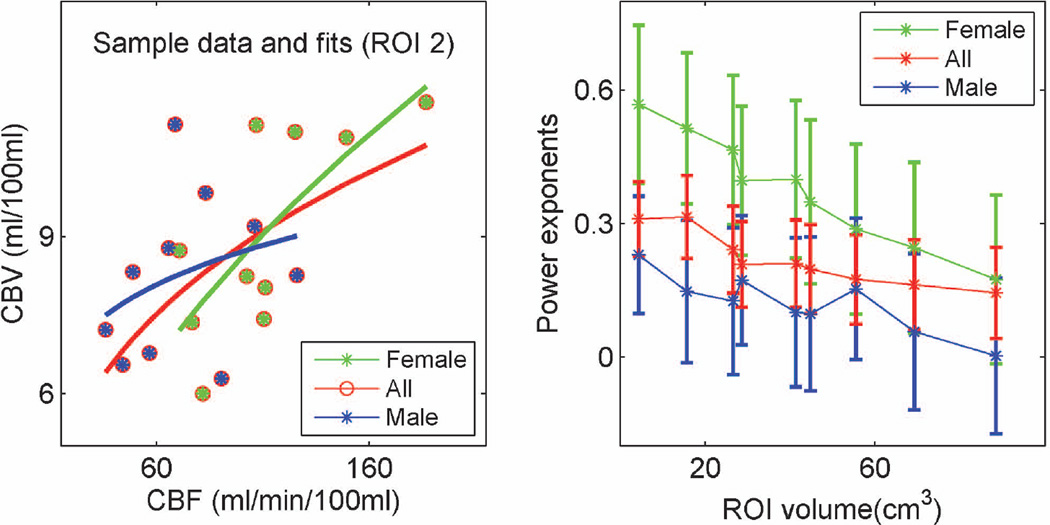
The left side of Figure 5 depicts experimental data and power fits in a sample ROI (ROI 2, stimulus induced activation in CBV and CBF at the P<0.05 significance level). Substantial inter-subject variations and small sample sizes resulted in large standard errors and wide confidence intervals (i.e. in ROI 2, exponents across all volunteers, females and males were 0.32, 0.51, and 0.15, with standard errors of 0.09, 0.17 and 0.16, and 90% confidence intervals of 0.17 to 0.47, 0.23 to 0.79 and −0.11 to 0.41, respectively). Despite these limitations, gender related variations in the CBV-CBF relationship were statistically significant when data over multiple ROIs was considered. Analysis of the covariance of the power exponents over multiple ROI sizes for females vs. males led to significantly different slopes (P<0.05), intercepts (P<0.05), and population marginal means (P<0.05) in multiple comparison tests, as well as significantly different exponents in females vs. males after removal of trends across ROI sizes (P<0.05). The right side of Figure 5 shows the CBV-CBF relationship power exponent estimates as a function of ROI size, for all subjects, for females and for males, demonstrating higher exponents in females and decreasing exponents with increasing ROI size.
Figure 5.
Left: Sample data and power fits across all subjects, female subjects or male subjects in ROI 2 (stimulus induced activation in CBV and CBF at the P<0.05 significance level). Right: Power exponents of the CBV-CBF relationship as a function of ROI size, for all subjects, female subject and male subjects (vertical bars represent standard errors of the estimates).
Discussion
Absolute CBV and CBF were quantified non-invasively in healthy subjects at rest and during visual stimulation. CBV and CBF estimates agree well with the literature showing both spatial and gender related variations.
Average CBV across all subjects and ROIs was 6.6 ± 0.75 mL/100 mL (with a range of 5.4 ± 0.5 to 7.9 ± 1.0 mL/100 mL across ROIs) at rest. Resting GM arterial CBV (CBVa) was previously reported as 1.605 mL/100 mL (65) and 2.04 ± 0.27 to 0.76 ± 0.17 mL/100 mL (66) using iVASO. Considering 21% arterial/arteriolar contribution to baseline CBV (57,61), our resting CBV corresponds to CBVa of 1.386 mL/100 mL, well within the range of iVASO results. Values near the upper end of our range were reported in occipital cortical GM of 6.67 ± 1.07 mL/100 g of tissue (7mL/100 mL with a brain tissue density of 1.05 g/mL) using bolus tracking (67), as well as 7.8 ± 1.1 mL/100 mL in the visual area using PET (68,69). Values near the lower end of our range, of 5.0 ± 1.5, 5.5 ± 0.2 and 5.6 ± 0.3 mL/100 mL were reported in (19,51,70), respectively (note that, in (51), one of the five subjects had a resting CBV of only 2.5 mL/100 mL while others averaged 5.7 mL/100 mL). Our observation of variations in resting CBV across ROIs is also in good agreement with the range of results from different approaches for ROI selection (anatomical descriptions, freehand tracings, circles, ellipses, activation based ROIs, T1 or SNR based thresholds (10,19,51,65,67–73) …) and spatial heterogeneities seen in (17,67,74,75). During activation, average CBV across all subjects and ROIs was 8.7 ± 1.3 mL/100 mL (with a range of 6.7 ± 0.7 to 11 ± 1.3 mL/100 mL across ROIs), with activation induced CBV increases ranging between 22.2% in the Brodmann ROI (ROI 9), to 37.6% and 39.4 % in the smallest most significantly activated ROIs (CBV and CBF activation, ROI 2: P<0.05, ROI 1: P<0.01). CBV increases during visual activation in humans have previously been reported as 19 ± 3 % and 22 ± 4% to 35 ± 4% (73) using bolus tracking; 32 ± 12% (51) and 42 ± 5% (19) using a biophysical model; 56± 1% using multi-echo VASO (76). CBV increases in our study are well within the range of previous publications and in closer agreement with bolus tracking results.
Average CBF across all subjects and ROIs was 72.5 ± 1 mL/min/100 mL (with a range of 71.2 ± 25 to 73.9 ± 25 mL/100 mL across ROIs) at rest. More pronounced inter-subject variability was observed in CBF as evidenced by large inter subject variations, consistent with reports of age and gender related variations in CBF. CBF slowly decreases with age, by ~0.5–1% per year (33,34,37,38). Although there was a significant difference in age, of ~10 years, between the younger and older halves of our subjects (P=0.0014, younger half: 27.1 ± 2.1 vs. older half: 37.2 ± 4.2 years); this difference was not large enough to observe any significant age related CBF or CBV differences across our subjects (P>0.05). Higher CBF has been reported in females across PET, SPECT, and MRI methods (29–36), with largest differences observed in the occipital lobe (31), in good agreement with the current study. We were able to readily observe these gender differences (with 50% female subjects, and no difference in age between genders, P=0.897), and our resting CBF measurements agree well with reports of increasing CBF values with increasing fractions of female subjects, including: whole-brain GM PET results of 65.1 ± 18.9 mL/min/100 mL with 27% females (n=11, ages: 23–29) (18) and continuous ASL (CASL) results of 92.0±15.1 mL/min/100 mL with 69% females (n=26, ages: 26±3) (77), 64.4 ± 6.5 ml /min/100 mL with all males (n=28), and 76.3 ± 10.7 mL/min/100 mL with all females (n=28) (31). During activation, average CBF across all subjects and ROIs was 102 ± 10 mL/min/100 mL (with a range of 88.9 ± 26 to 120.7 ± 35.7 mL/100 mL across ROIs). This corresponds to an activation induced CBF increase of 40.4 ± 13% on average (with a range of 23.1 to 65.5% across ROIs) in close agreement with literature on visual activations, including: 37 ± 2.5% with FAIR and 39 ± 2.6 % with PET (78), 38.5 ± 7.6% with FAIR and 36.9±8.8% with bolus tracking (72). Our observation of larger CBF increases with smaller activation areas also agrees well with literature: 55 ± 6% (23) and 55 ± 0.6% (79) increases in CBF were observed within an activation mask at the P<0.05 significance level using PASL, similar to our P<0.05 activation ROI CBF increase of 54.1%, and 58% to 64% increases using FAIR (32). Larger increases of 83±4% (from a baseline value of 98.7±13.6 mL/min/100 mL) using a combination of FAIR and Look-Locker EPI (80), and of 68% using PET (10) were reported using smaller ROIs, and these studies are in good agreement with our smallest ROI CBF increase of 65.6%.
The variations in CBV and CBF values translated into spatial and gender related variations in both power and linear function representations of the CBV-CBF relationship. The exponents or slopes of the CBV-CBF relationship reflect the degree to which rising perfusion is accommodated through blood velocity increases vs. volume increases (diameter changes or recruitment effects). Spatial variation of the CBV-CBF relationship is driven by the combination of spatial variations in CBV at rest and during activation, as well as CBF during activation (Table 3). Fairly uniform perfusion appears to be maintained across the brain at rest despite variations in vascular density, while areas of higher vascular density respond to activation with larger volume responses (Figure 2, Table 4). In addition to publications supporting variations in the underlying CBV and CBF values in humans, experiments on anesthetized rat models also support spatial variations of the CBV-CBF relationship (12,81). The limited human literature available appears to also support our observation of gender related variations in the CBV-CBF relationship. Fitting to PET measurements of absolute CBV and CBF during respiratory manipulation yielded power exponents of 0.29 using all male subjects (17) while higher power exponents of 0.46–0.64 were found when 27% of the subjects were female (18), in line with our observation of a steeper curve or line representing females. Similarly, during activation, a power exponent of 0.16 was suggested as appropriate for BOLD modeling of the venous contributions to total CBV in a cohort where 55% of the subjects were female (79), while the relationship was described by a higher power exponent of 0.23 in a cohort where 87.5% of the subjects were female (23) using the VERVE (venous refocusing for volume estimation) method. While exponents reported in these papers reflect the relationship between relative changes in CBF and relative changes in the venous contribution to CBV, preventing a direct comparison with our findings or PET results, these smaller values are consistent with an expected 36% to 46% venous contribution to CBV (14,61), and our observation of a steeper CBV-CBF relationship in females compared to males. Fitting the absolute CBV-CBF relationship during visual stimulation in males produced an exponent of 0.30 in a manually traced ROI of ~0.7cm3 using PET (Ito 2001), while it produced an exponent of 0.23 in our smallest ROI of 4cm3. Although a direct comparison is difficult without matching ROIs, this agrees well with our findings of decreasing exponents with increasing ROI volume.
The reasons for the observed gender differences are unclear, and there is little evidence for gender-based anatomical differences in the visual system but some of the proposed explanations include differences in visual physiology, differences in the vascular response to focal brain activation (i.e. to vasoactive substances that may be part of the neuronal activity and blood flow coupling mechanism), and hormonal influences (32). Hematocrit levels differ between males and females (~12% lower in females) (56). Lower hematocrit has been suggested to result in reduced oxygen carrying capacity, such that an adequate supply of oxygen to the brain may require higher CBF in females (33). Lower hematocrit also increases blood relaxation times (46,47,49,50) and has been considered in the current study (see Eqs. [3] and [5]). Our use of gender specific T1s (instead of an average value for both genders) decreased CBF in females and increased CBF in males by 1–2%, slightly de-emphasizing the observed gender differences. Lower hematocrit also corresponds to reduced blood viscosity, which is consistent with the observation of shorter transit times in women (33,82). The largest transit time gender differences were seen in younger subjects and diminished with age. Considering this worst case scenario, failing to account for ~110ms transit time difference between males and females would lead to CBF overestimation in females and underestimation in males by 7–8%. CBF gender differences persist not only after accounting for these differences in MRI, but also across other modalities (29–36), and more studies are required to better understand these differences in brain hemodynamics.
Areas showing activation in both CBV and CBF (intersection ROIs) corresponded generally to smaller ROIs, while areas showing activation in either CBV or CBF (union ROIs) corresponded generally to larger ROIs. As a result, spatial effects resulting from the use of intersection vs. union ROIs cannot be distinguished from spatial effects resulting from differences in ROI sizes. Both the power and linear functions appear equally descriptive within this physiological range of changes in normal humans, given the limited number of subjects, limited SNR and the described intrinsic inter-subject variability, in accord with the conclusions of (23,83).
One of the primary utilities of the CBV-CBF relationship exponent, α, is in calibrated fMRI, and altering the assumed value of α could have a notable impact on the estimation of CMRO2. In simulations, its overestimation by ~50% resulted in ~30% underestimation of CMRO2 and its underestimation by ~50% resulted in up to ~40% overestimation of CMRO2 (23). Since the CBV-CBF relationship is nonlinear, the exact severity of the errors depends on the true value of α. When the intermediate parameter M, the maximum achievable BOLD signal, was also estimated using calibrated fMRI (i.e. hypercapnic calibration) instead of assumed based on literature, similar biases resulted in the estimation of M; these reduced the final bias in CMRO2, however, at the cost of introducing a dependency of error magnitude on the CBF change (larger errors with larger changes in CBF) (23). Further studies are desirable to better characterize the CBV-CBF relationship with more subjects across more brain regions, species, gender, or functional challenges. In the meantime, benefits of increased accuracy from measuring each parameter on each subject or such additional calibration scans should be carefully weighed against the requirements of increased scan time and potential reductions in the number of subjects, as the answer may differ across studies.
Hemodynamic parameters have been reported using many different approaches for ROI selection, including manually placed circles or ellipses of various diameters on one or more slices or averaged across distinct regions (10,17,37); circles centered on significant CBF activation regions (84); freehand tracings on average BOLD, CBF and CBV activation maps (85); a fixed number of pixels centered over anatomical definitions (86); as well as other activation, T1 or SNR based thresholds (19,51,65,67–73). Our use of activation based ROIs may align well with functional areas across subjects, however, could also bias measurements towards higher values, with potentially larger contributions from small arteries. To limit signal contribution from small arteries, we used a modified QUIPSS II ASL implementation (Q2TIPS) which was proposed to suppress in-flowing arterial signal (52). Additionally, a bi-polar gradient was inserted between RF excitation and EPI data acquisition as a crusher to further suppress the confounding effects from larger vessels. Both CBF and CBV measurements were based on this sequence. Furthermore, CBV increases in different vascular compartments have been shown to depend on stimulus duration among other factors (87): arterial CBV changes dominate during shorter stimuli (21,22,24), while capillary (25) and venous contributions (88) develop more slowly, such that longer stimulus durations, such as the 78s stimulus used in this study, may be more appropriate for quantification of BOLD (88) which has more vein bias. However, despite such efforts to limit potential confounds, caution should always be used in interpretation of results and comparisons across methods.
Conclusion
We report on the first MRI measurement of the absolute CBV-CBF relationship in healthy human subjects with complete multi-slice coverage of the functional areas of interest. Estimates of absolute CBV and CBF at rest and during visual stimulation were consistent with prior publications. The CBV-CBF relationship varied both spatially and with gender, with CBV = 2.1 CBF0.32 across all volunteers, CBV = 0.8 CBF0.51 in females and CBV = 4.4 CBF0.15 in males, in a commonly used ROI corresponding to stimulus induced activation in CBV and CBF at the P<0.05 significance level. Exponents also decreased in both genders as ROIs were expanded to include less significantly activated regions, reconciling some of the variation across prior publications. Such improved characterization of the CBV-CBF relationship in humans under various metabolic or functional challenges may have significant implications for fMRI calibration; and advance our understanding of the fundamental physiological relationship between neuronal activity, hemodynamic regulation and metabolism under normal, pathological and neuronally active conditions. These findings highlight the importance of considering potential gender related differences, as well as regional variations under a range of physiological states in the interpretation of results.
Acknowledgements
This research was partially supported by the NIH under grants NS051622-05, NS052344-05, and EB000473-10.
References
- 1.Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95(4):1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO(2) during rat forepaw stimulation. Magn Reson Med. 1999;42(5):944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A. 1999;96(16):9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kastrup A, Kruger G, Glover GH, Moseley ME. Assessment of cerebral oxidative metabolism with breath holding and fMRI. Magn Reson Med. 1999;42(3):608–611. doi: 10.1002/(sici)1522-2594(199909)42:3<608::aid-mrm26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Kastrup A, Kruger G, Neumann-Haefelin T, Glover GH, Moseley ME. Changes of cerebral blood flow, oxygenation, and oxidative metabolism during graded motor activation. Neuroimage. 2002;15(1):74–82. doi: 10.1006/nimg.2001.0916. [DOI] [PubMed] [Google Scholar]
- 6.Kim SG, Ugurbil K. Comparison of blood oxygenation and cerebral blood flow effects in fMRI: estimation of relative oxygen consumption change. Magn Reson Med. 1997;38(1):59–65. doi: 10.1002/mrm.1910380110. [DOI] [PubMed] [Google Scholar]
- 7.Kim SG, Tsekos NV, Ashe J. Multi-slice perfusion-based functional MRI using the FAIR technique: comparison of CBF and BOLD effects. NMR Biomed. 1997;10(4–5):191–196. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<191::aid-nbm460>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Grubb RL, Jr, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke; a journal of cerebral circulation. 1974;5(5):630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- 9.Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14(7–8):413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Takahashi K, Hatazawa J, Kim SG, Kanno I. Changes in human regional cerebral blood flow and cerebral blood volume during visual stimulation measured by positron emission tomography. J Cereb Blood Flow Metab. 2001;21(5):608–612. doi: 10.1097/00004647-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Jones M, Berwick J, Mayhew J. Changes in blood flow, oxygenation, and volume following extended stimulation of rodent barrel cortex. Neuroimage. 2002;15(3):474–487. doi: 10.1006/nimg.2001.1000. [DOI] [PubMed] [Google Scholar]
- 12.Wu G, Luo F, Li Z, Zhao X, Li SJ. Transient relationships among BOLD, CBV, and CBF changes in rat brain as detected by functional MRI. Magn Reson Med. 2002;48(6):987–993. doi: 10.1002/mrm.10317. [DOI] [PubMed] [Google Scholar]
- 13.Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage. 2001;13(6 Pt 1):1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- 14.Lee SP, Duong TQ, Yang G, Iadecola C, Kim SG. Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: implications for BOLD fMRI. Magn Reson Med. 2001;45(5):791–800. doi: 10.1002/mrm.1107. [DOI] [PubMed] [Google Scholar]
- 15.Kida I, Rothman DL, Hyder F. Dynamics of changes in blood flow, volume, and oxygenation: implications for dynamic functional magnetic resonance imaging calibration. J Cereb Blood Flow Metab. 2007;27(4):690–696. doi: 10.1038/sj.jcbfm.9600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin T, Kim SG. Cortical layer-dependent dynamic blood oxygenation, cerebral blood flow and cerebral blood volume responses during visual stimulation. Neuroimage. 2008;43(1):1–9. doi: 10.1016/j.neuroimage.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito H, Kanno I, Ibaraki M, Hatazawa J, Miura S. Changes in human cerebral blood flow and cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab. 2003;23(6):665–670. doi: 10.1097/01.WCB.0000067721.64998.F5. [DOI] [PubMed] [Google Scholar]
- 18.Rostrup E, Knudsen GM, Law I, Holm S, Larsson HB, Paulson OB. The relationship between cerebral blood flow and volume in humans. Neuroimage. 2005;24(1):1–11. doi: 10.1016/j.neuroimage.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 19.Glielmi CB, Schuchard RA, Hu XP. Estimating cerebral blood volume with expanded vascular space occupancy slice coverage. Magn Reson Med. 2009;61(5):1193–1200. doi: 10.1002/mrm.21979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffeth VE, Buxton RB. A theoretical framework for estimating cerebral oxygen metabolism changes using the calibrated-BOLD method: modeling the effects of blood volume distribution, hematocrit, oxygen extraction fraction, and tissue signal properties on the BOLD signal. Neuroimage. 2011;58(1):198–212. doi: 10.1016/j.neuroimage.2011.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, Bacskai BJ, Dale AM, Boas DA. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007;35(1):89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim T, Hendrich KS, Masamoto K, Kim SG. Arterial versus total blood volume changes during neural activity-induced cerebral blood flow change: implication for BOLD fMRI. J Cereb Blood Flow Metab. 2007;27(6):1235–1247. doi: 10.1038/sj.jcbfm.9600429. [DOI] [PubMed] [Google Scholar]
- 23.Chen JJ, Pike GB. BOLD-specific cerebral blood volume and blood flow changes during neuronal activation in humans. NMR Biomed. 2009;22(10):1054–1062. doi: 10.1002/nbm.1411. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez AL, Fukuda M, Tasker ML, Masamoto K, Kim SG. Changes in cerebral arterial, tissue and venous oxygenation with evoked neural stimulation: implications for hemoglobin-based functional neuroimaging. J Cereb Blood Flow Metab. 2010;30(2):428–439. doi: 10.1038/jcbfm.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefanovic B, Hutchinson E, Yakovleva V, Schram V, Russell JT, Belluscio L, Koretsky AP, Silva AC. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab. 2008;28(5):961–972. doi: 10.1038/sj.jcbfm.9600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian P, Teng IC, May LD, Kurz R, Lu K, Scadeng M, Hillman EM, De Crespigny AJ, D'Arceuil HE, Mandeville JB, Marota JJ, Rosen BR, Liu TT, Boas DA, Buxton RB, Dale AM, Devor A. Cortical depth-specific microvascular dilation underlies laminar differences in. Proc Natl Acad Sci U S A. 2010;107(34):15246–15251. doi: 10.1073/pnas.1006735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieger SN, Streicher MN, Trampel R, Turner R. Cerebral blood volume changes during brain activation. J Cereb Blood Flow Metab. 2012;32(8):1618–1631. doi: 10.1038/jcbfm.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JJ, Pike GB. MRI measurement of the BOLD-specific flow-volume relationship during hypercapnia and hypocapnia in humans. Neuroimage. 2010;53(2):383–391. doi: 10.1016/j.neuroimage.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Daniel DG, Mathew RJ, Wilson WH. Sex roles and regional cerebral blood flow. Psychiatry Res. 1989;27(1):55–64. doi: 10.1016/0165-1781(89)90009-7. [DOI] [PubMed] [Google Scholar]
- 30.Esposito G, Van Horn JD, Weinberger DR, Berman KF. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nucl Med. 1996;37(4):559–564. [PubMed] [Google Scholar]
- 31.Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, Naumann E. Resting cerebral blood flow, attention, and aging. Brain Res. 2009;1267:77–88. doi: 10.1016/j.brainres.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 32.Kastrup A, Li TQ, Glover GH, Kruger G, Moseley ME. Gender differences in cerebral blood flow and oxygenation response during focal physiologic neural activity. J Cereb Blood Flow Metab. 1999;19(10):1066–1071. doi: 10.1097/00004647-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Zhu X, Feinberg D, Guenther M, Gregori J, Weiner MW, Schuff N. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med. 2012;68(3):912–922. doi: 10.1002/mrm.23286. [DOI] [PubMed] [Google Scholar]
- 34.Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med. 2004;51(4):736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- 35.Devous MD, Sr, Stokely EM, Chehabi HH, Bonte FJ. Normal distribution of regional cerebral blood flow measured by dynamic single-photon emission tomography. J Cereb Blood Flow Metab. 1986;6(1):95–104. doi: 10.1038/jcbfm.1986.12. [DOI] [PubMed] [Google Scholar]
- 36.Podreka I, Baumgartner C, Suess E, Muller C, Brucke T, Lang W, Holzner F, Steiner M, Deecke L. Quantification of regional cerebral blood flow with IMP-SPECT. Reproducibility and clinical relevance of flow values. Stroke. 1989;20(2):183–191. doi: 10.1161/01.str.20.2.183. [DOI] [PubMed] [Google Scholar]
- 37.Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113(Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 38.Buijs PC, Krabbe-Hartkamp MJ, Bakker CJ, de Lange EE, Ramos LM, Breteler MM, Mali WP. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology. 1998;209(3):667–674. doi: 10.1148/radiology.209.3.9844657. [DOI] [PubMed] [Google Scholar]
- 39.Ciris PA, Qiu M, Constable RT. Non-invasive quantification of absolute cerebral blood volume during functional activation applicable to the whole human brain. [2013 Mar 8];Magn Reson Med. 2013 doi: 10.1002/mrm.24694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donahue MJ, Lu H, Jones CK, Edden RA, Pekar JJ, van Zijl PC. Theoretical and experimental investigation of the VASO contrast mechanism. Magn Reson Med. 2006;56(6):1261–1273. doi: 10.1002/mrm.21072. [DOI] [PubMed] [Google Scholar]
- 41.Scouten A, Constable RT. Applications and limitations of whole-brain MAGIC VASO functional imaging. Magn Reson Med. 2007;58(2):306–315. doi: 10.1002/mrm.21273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herscovitch P, Raichle ME. What is the correct value for the brain--blood partition coefficient for water? J Cereb Blood Flow Metab. 1985;5(1):65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- 43.Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med. 1994;32(6):749–763. doi: 10.1002/mrm.1910320610. [DOI] [PubMed] [Google Scholar]
- 44.Yablonskiy DA. Quantitation of intrinsic magnetic susceptibility-related effects in a tissue. Magn Reson Med. 1998;39(3):417–428. doi: 10.1002/mrm.1910390312. [DOI] [PubMed] [Google Scholar]
- 45.He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen. Magn Reson Med. 2007;57(1):115–126. doi: 10.1002/mrm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52(3):679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- 47.Silvennoinen MJ, Clingman CS, Golay X, Kauppinen RA, van Zijl PC. Comparison of the dependence of blood R2 and R2* on oxygen saturation at 1.5 and 4.7 Tesla. Magn Reson Med. 2003;49(1):47–60. doi: 10.1002/mrm.10355. [DOI] [PubMed] [Google Scholar]
- 48.Donahue MJ, Hua J, Pekar JJ, van Zijl PC. Effect of inflow of fresh blood on vascular-space-occupancy (VASO) contrast. Magn Reson Med. 2009;61(2):473–480. doi: 10.1002/mrm.21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao JM, Clingman CS, Narvainen MJ, Kauppinen RA, van Zijl PC. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn Reson Med. 2007;58(3):592–597. doi: 10.1002/mrm.21342. [DOI] [PubMed] [Google Scholar]
- 50.Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJ. Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility. Magn Reson Med. 2001;45(4):533–542. doi: 10.1002/mrm.1072. [DOI] [PubMed] [Google Scholar]
- 51.Gu H, Lu H, Ye FQ, Stein EA, Yang Y. Noninvasive quantification of cerebral blood volume in humans during functional activation. Neuroimage. 2006;30(2):377–387. doi: 10.1016/j.neuroimage.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 52.Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41(6):1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 53.Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10(4–5):237–249. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 54.Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage. 2002;15(3):488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003;49(5):796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- 56.Gahan PB. In: Life: the science of biology. 7th edn. Purves WK, Sadava D, Orians GH, Heller HC, editors. Vol. 1121. W. H. Freeman & Co; 2004. ISBN 0-7167-9856-5. [Google Scholar]
- 57.Sharan M, Jones MD, Jr, Koehler RC, Traystman RJ, Popel AS. A compartmental model for oxygen transport in brain microcirculation. Ann Biomed Eng. 1989;17(1):13–38. doi: 10.1007/BF02364271. [DOI] [PubMed] [Google Scholar]
- 58.Lu H, Nagae-Poetscher LM, Golay X, Lin D, Pomper M, van Zijl PC. Routine clinical brain MRI sequences for use at 3.0 Tesla. J Magn Reson Imaging. 2005;22(1):13–22. doi: 10.1002/jmri.20356. [DOI] [PubMed] [Google Scholar]
- 59.Clare S, Jezzard P. Rapid T(1) mapping using multislice echo planar imaging. Magn Reson Med. 2001;45(4):630–634. doi: 10.1002/mrm.1085. [DOI] [PubMed] [Google Scholar]
- 60.Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;9(4):531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 61.van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, Kauppinen RA. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med. 1998;4(2):159–167. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]
- 62.Lu H, Golay X, van Zijl PC. Intervoxel heterogeneity of event-related functional magnetic resonance imaging responses as a function of T(1) weighting. Neuroimage. 2002;17(2):943–955. [PubMed] [Google Scholar]
- 63.Oja JM, Gillen JS, Kauppinen RA, Kraut M, van Zijl PC. Determination of oxygen extraction ratios by magnetic resonance imaging. J Cereb Blood Flow Metab. 1999;19(12):1289–1295. doi: 10.1097/00004647-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Lacadie C, Fulbright R, Arora J, Constable R, Papademetris X. Brodmann Areas defined in MNI space using a new Tracing Tool in BioImage Suite. 2008 [Google Scholar]
- 65.Donahue MJ, Sideso E, MacIntosh BJ, Kennedy J, Handa A, Jezzard P. Absolute arterial cerebral blood volume quantification using inflow vascular-space-occupancy with dynamic subtraction magnetic resonance imaging. J Cereb Blood Flow Metab. 2010;30(7):1329–1342. doi: 10.1038/jcbfm.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hua J, Qin Q, Pekar JJ, van Zijl PC. Measurement of absolute arterial cerebral blood volume in human brain without using a contrast agent. NMR Biomed. 2011;24(10):1313–1325. doi: 10.1002/nbm.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grandin CB, Bol A, Smith AM, Michel C, Cosnard G. Absolute CBF and CBV measurements by MRI bolus tracking before and after acetazolamide challenge: repeatabilily and comparison with PET in humans. NeuroImage. 2005;26(2):525–535. doi: 10.1016/j.neuroimage.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 68.Scouten A. Optimization and Application of Whole-Brain Cerebral Blood Volume Functional MRI. New Haven, CT, USA: Yale University; 2007. [Google Scholar]
- 69.Giovacchini G, Chang MC, Channing MA, Toczek M, Mason A, Bokde AL, Connolly C, Vuong BK, Ma Y, Der MG, Doudet DJ, Herscovitch P, Eckelman WC, Rapoport SI, Carson RE. Brain incorporation of [11C]arachidonic acid in young healthy humans measured with positron emission tomography. J Cereb Blood Flow Metab. 2002;22(12):1453–1462. doi: 10.1097/01.WCB.0000033209.60867.7A. [DOI] [PubMed] [Google Scholar]
- 70.Lu H, Law M, Johnson G, Ge Y, van Zijl PC, Helpern JA. Novel approach to the measurement of absolute cerebral blood volume using vascular-space-occupancy magnetic resonance imaging. Magn Reson Med. 2005;54(6):1403–1411. doi: 10.1002/mrm.20705. [DOI] [PubMed] [Google Scholar]
- 71.Hua J, Qin Q, Donahue MJ, Zhou J, Pekar JJ, van Zijl PC. Inflow-based vascular-space-occupancy (iVASO) MRI. Magn Reson Med. 2011;66(1):40–56. doi: 10.1002/mrm.22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li TQ, Haefelin TN, Chan B, Kastrup A, Jonsson T, Glover GH, Moseley ME. Assessment of hemodynamic response during focal neural activity in human using bolus tracking, arterial spin labeling and BOLD techniques. Neuroimage. 2000;12(4):442–451. doi: 10.1006/nimg.2000.0634. [DOI] [PubMed] [Google Scholar]
- 73.Francis ST, Pears JA, Butterworth S, Bowtell RW, Gowland PA. Measuring the change in CBV upon cortical activation with high temporal resolution using look-locker EPI and Gd-DTPA. Magn Reson Med. 2003;50(3):483–492. doi: 10.1002/mrm.10547. [DOI] [PubMed] [Google Scholar]
- 74.Christen T, Ni W, Qiu D, Schmiedeskamp H, Bammer R, Moseley M, Zaharchuk G. High-resolution cerebral blood volume imaging in humans using the blood pool contrast agent ferumoxytol. [2012 Sep 21];Magn Reson Med. 2012 doi: 10.1002/mrm.24500. [DOI] [PubMed] [Google Scholar]
- 75.Uh J, Lin AL, Lee K, Liu P, Fox P, Lu H. Validation of VASO cerebral blood volume measurement with positron emission tomography. Magn Reson Med. 2011;65(3):744–749. doi: 10.1002/mrm.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu H, van Zijl PC. Experimental measurement of extravascular parenchymal BOLD effects and tissue oxygen extraction fractions using multi-echo VASO fMRI at 1.5 and 3.0 T. Magn Reson Med. 2005;53(4):808–816. doi: 10.1002/mrm.20379. [DOI] [PubMed] [Google Scholar]
- 77.Asllani I, Habeck C, Borogovac A, Brown TR, Brickman AM, Stern Y. Separating function from structure in perfusion imaging of the aging brain. Hum Brain Mapp. 2009;30(9):2927–2935. doi: 10.1002/hbm.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feng CM, Narayana S, Lancaster JL, Jerabek PA, Arnow TL, Zhu F, Tan LH, Fox PT, Gao JH. Neuroimage. Volume 22. United States: 2004. CBF changes during brain activation: fMRI vs. PET; pp. 443–446. [DOI] [PubMed] [Google Scholar]
- 79.Mark CI, Pike GB. J Cereb Blood Flow Metab. Volume 32. United States: 2012. Indication of BOLD-specific venous flow-volume changes from precisely controlled hyperoxic vs. hypercapnic calibration; pp. 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Francis ST, Bowtell R, Gowland PA. Modeling and optimization of Look-Locker spin labeling for measuring perfusion and transit time changes in activation studies taking into account arterial blood volume. Magn Reson Med. 2008;59(2):316–325. doi: 10.1002/mrm.21442. [DOI] [PubMed] [Google Scholar]
- 81.Rapoport SI, Ohno K, Pettigrew KD. Brain Res. Volume 172. Netherlands: 1979. Drug entry into the brain; pp. 354–359. [DOI] [PubMed] [Google Scholar]
- 82.MacIntosh BJ, Filippini N, Chappell MA, Woolrich MW, Mackay CE, Jezzard P. Assessment of arterial arrival times derived from multiple inversion time pulsed arterial spin labeling MRI. Magn Reson Med. 2010;63(3):641–647. doi: 10.1002/mrm.22256. [DOI] [PubMed] [Google Scholar]
- 83.Piechnik SK, Chiarelli PA, Jezzard P. Neuroimage. Volume 39. United States: 2008. Modelling vascular reactivity to investigate the basis of the relationship between cerebral blood volume and flow under CO2 manipulation; pp. 107–118. [DOI] [PubMed] [Google Scholar]
- 84.Ito H, Ibaraki M, Kanno I, Fukuda H, Miura S. Changes in cerebral blood flow and cerebral oxygen metabolism during neural activation measured by positron emission tomography: comparison with blood oxygenation level-dependent contrast measured by functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2005;25(3):371–377. doi: 10.1038/sj.jcbfm.9600030. [DOI] [PubMed] [Google Scholar]
- 85.Shen Q, Ren H, Duong TQ. CBF, BOLD, CBV, and CMRO(2) fMRI signal temporal dynamics at 500-msec resolution. J Magn Reson Imaging. 2008;27(3):599–606. doi: 10.1002/jmri.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zong X, Kim T, Kim SG. Contributions of dynamic venous blood volume versus oxygenation level changes to BOLD fMRI. Neuroimage. 2012;60(4):2238–2246. doi: 10.1016/j.neuroimage.2012.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim T, Kim SG. Cortical layer-dependent arterial blood volume changes: improved spatial specificity relative to BOLD fMRI. Neuroimage. 2010;49(2):1340–1349. doi: 10.1016/j.neuroimage.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim T, Kim SG. Temporal dynamics and spatial specificity of arterial and venous blood volume changes during visual stimulation: implication for BOLD quantification. J Cereb Blood Flow Metab. 2011;31(5):1211–1222. doi: 10.1038/jcbfm.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schutz SL. Oxygen saturation monitoring by pulse oximetry. In: Lynn-McHale DJ, Carlson KK, editors. AACN procedure manual for critical care. 4 ed. St. Louis, Mo: Elsevier Saunders; 2001. [Google Scholar]